Abstract
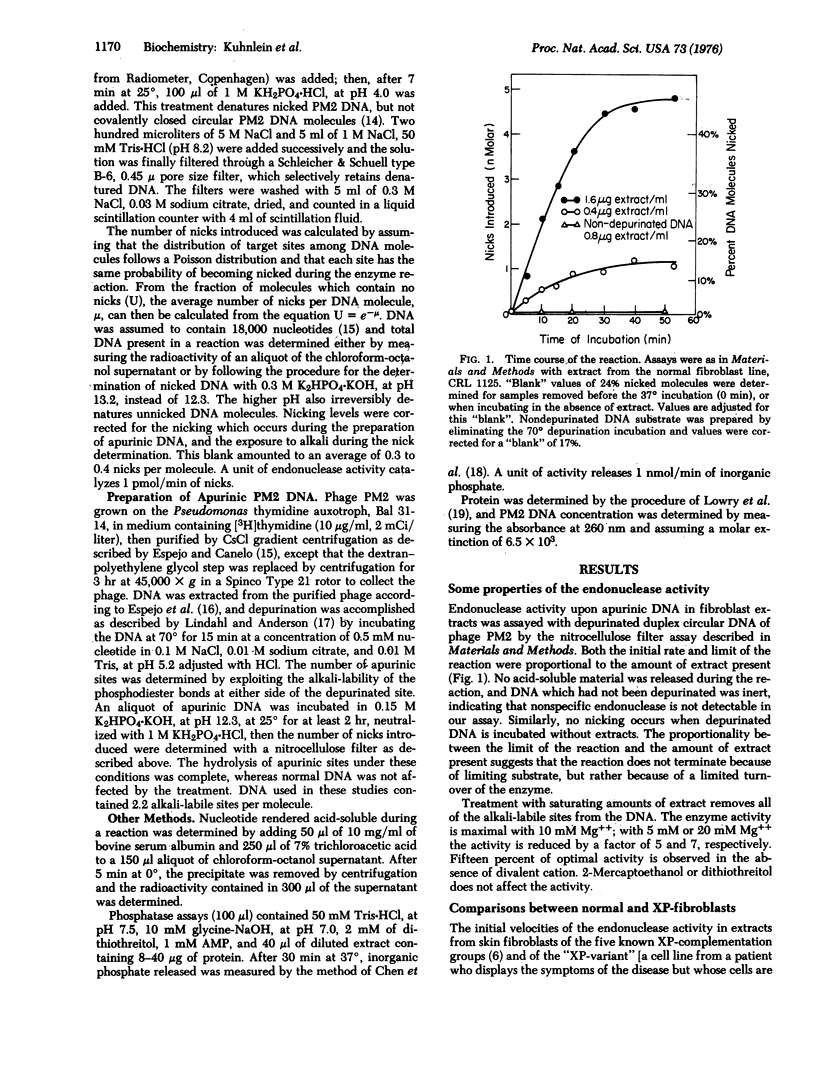
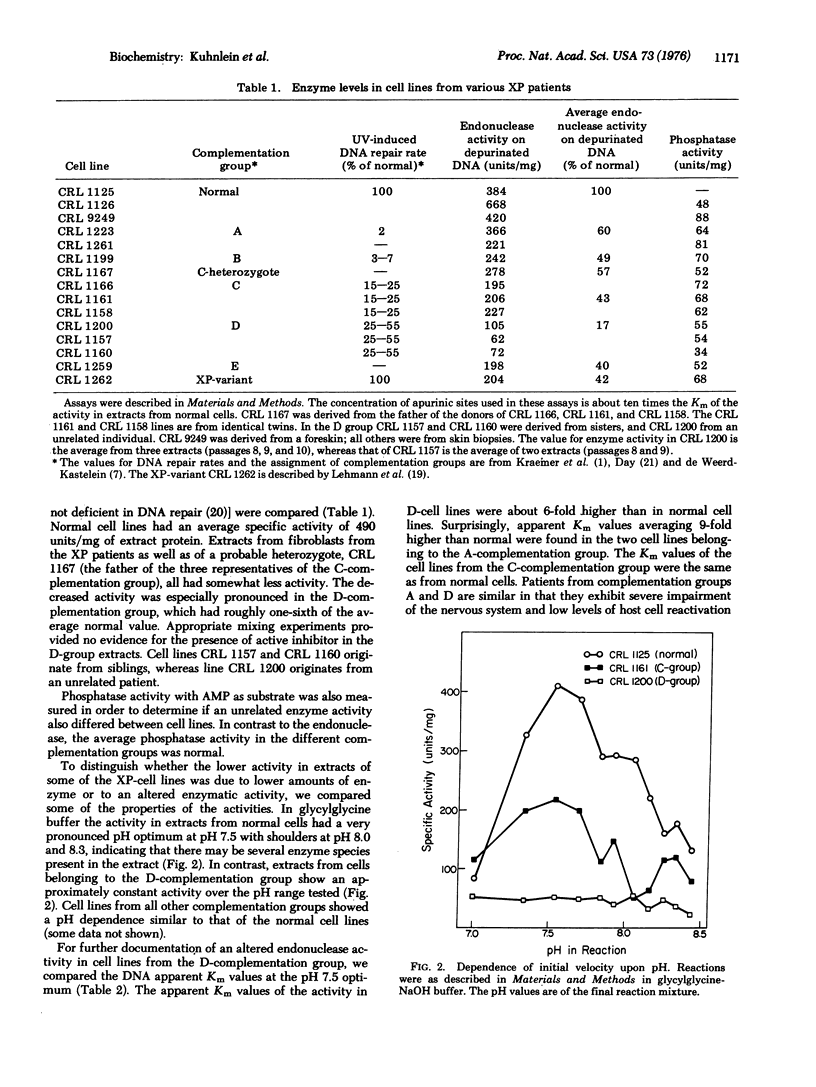
Endonuclease activity upon depurinated DNA was measured in extracts of cultured fibroblasts from xeroderma pigmentosum patients. Cell lines from complementation groups A, B, C, E, and the XP-variant had slightly reduced levels of activity, but cell lines from complementation group D had one-sixth of the normal activity. An altered pH dependence and a higher apparent Km for substrate in D-cell lines indicate that the remaining activity is also qualitatively different from the activity found in normal cells. A higher Km was also found in cell lines from the A-complementation group but not in cell lines from the C-complementation group. These defects in apurinic DNase might account for the neurological disorders in patients from the D- and the A-complementation groups.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacchetti S., van der Plas A., Veldhuisen G. A UV-specific endonucleolytic activity present in human cell extracts. Biochem Biophys Res Commun. 1972 Aug 7;48(3):662–669. doi: 10.1016/0006-291x(72)90399-3. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968 May 18;218(5142):652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- Cleaver J. E., Trosko J. E. Absence of excision of ultraviolet-induced cyclobutane dimers in xeroderma pigmentosum. Photochem Photobiol. 1970 Jun;11(6):547–550. doi: 10.1111/j.1751-1097.1970.tb06025.x. [DOI] [PubMed] [Google Scholar]
- Day R. S., 3rd Studies on repair of adenovirus 2 by human fibroblasts using normal, xeroderma pigmentosum, and xeroderma pigmentosum heterozygous strains. Cancer Res. 1974 Aug;34(8):1965–1970. [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties of bacteriophage PM2: a lipid-containing bacterial virus. Virology. 1968 Apr;34(4):738–747. doi: 10.1016/0042-6822(68)90094-9. [DOI] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S., Sinsheimer R. L. DNA of bacteriophage PM2: a closed circular double-stranded molecule. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1164–1168. doi: 10.1073/pnas.63.4.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein G., Scheid M., Hammerling U., Schlesinger D. H., Niall H. D., Boyse E. A. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc Natl Acad Sci U S A. 1975 Jan;72(1):11–15. doi: 10.1073/pnas.72.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadi S. M., Kirtikar D., Goldthwait D. A. Endonuclease II of Escherichia coli. Degradation of double- and single-stranded deoxyribonucleic acid. Biochemistry. 1973 Jul 3;12(14):2747–2754. doi: 10.1021/bi00738a030. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lehmann A. R., Kirk-Bell S., Arlett C. F., Paterson M. C., Lohman P. H., de Weerd-Kastelein E. A., Bootsma D. Xeroderma pigmentosum cells with normal levels of excision repair have a defect in DNA synthesis after UV-irradiation. Proc Natl Acad Sci U S A. 1975 Jan;72(1):219–223. doi: 10.1073/pnas.72.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Andersson A. Rate of chain breakage at apurinic sites in double-stranded deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3618–3623. doi: 10.1021/bi00769a019. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972 Sep 12;11(19):3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Ljungquist S., Lindahl T. A mammalian endonuclease specific for apurinic sites in double-stranded deoxyribonucleic acid. I. Purification and general properties. J Biol Chem. 1974 Mar 10;249(5):1530–1535. [PubMed] [Google Scholar]
- Pedrini A. M., Dalpra L., Ciarrocchi G., Noy G. C., Spadari S., Nuzzo F., Falaschi A. Levels of some enzymes acting on DNA in xeroderma pigmentosum. Nucleic Acids Res. 1974 Feb;1(2):193–202. doi: 10.1093/nar/1.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouwels P. H., Knijnenburg C. M., van Rotterdam J., Cohen J. A. Structure of the replicative form of bacteriphage phi X174. VI. Studies on alkali-denatured double-stranded phi X DNA. J Mol Biol. 1968 Mar 14;32(2):169–182. doi: 10.1016/0022-2836(68)90002-8. [DOI] [PubMed] [Google Scholar]
- Robbins J. H., Kraemer K. H., Lutzner M. A., Festoff B. W., Coon H. G. Xeroderma pigmentosum. An inherited diseases with sun sensitivity, multiple cutaneous neoplasms, and abnormal DNA repair. Ann Intern Med. 1974 Feb;80(2):221–248. doi: 10.7326/0003-4819-80-2-221. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Regan J. D., German J., Carrier W. L. Evidence that xeroderma pigmentosum cells do not perform the first step in the repair of ultraviolet damage to their DNA. Proc Natl Acad Sci U S A. 1969 Nov;64(3):1035–1041. doi: 10.1073/pnas.64.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stich H. F., San R. H., Kawazoe Y. Increased sensitivity of xeroderma pigmentosum cells to some chemical carcinogens and mutagens. Mutat Res. 1973 Jan;17(1):127–137. doi: 10.1016/0027-5107(73)90261-3. [DOI] [PubMed] [Google Scholar]
- Sutherland B. M., Rice M., Wagner E. K. Xeroderma pigmentosum cells contain low levels of photoreactivating enzyme. Proc Natl Acad Sci U S A. 1975 Jan;72(1):103–107. doi: 10.1073/pnas.72.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. M., Harnden D. G., Arlett C. F., Harcourt S. A., Lehmann A. R., Stevens S., Bridges B. A. Ataxia telangiectasia: a human mutation with abnormal radiation sensitivity. Nature. 1975 Dec 4;258(5534):427–429. doi: 10.1038/258427a0. [DOI] [PubMed] [Google Scholar]
- Verly W. G., Paquette Y. An endonuclease for depurinated DNA in Escherichia coli B. Can J Biochem. 1972 Feb;50(2):217–224. doi: 10.1139/o72-029. [DOI] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M., Weiss B. Mutations simultaneously affecting endonuclease II and exonuclease III in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Feb;72(2):688–692. doi: 10.1073/pnas.72.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weerd-Kastelein E. A., Keijzer W., Bootsma D. A third complementation group in xeroderma pigmentosum. Mutat Res. 1974 Jan;22(1):87–91. doi: 10.1016/0027-5107(74)90013-x. [DOI] [PubMed] [Google Scholar]


