Abstract
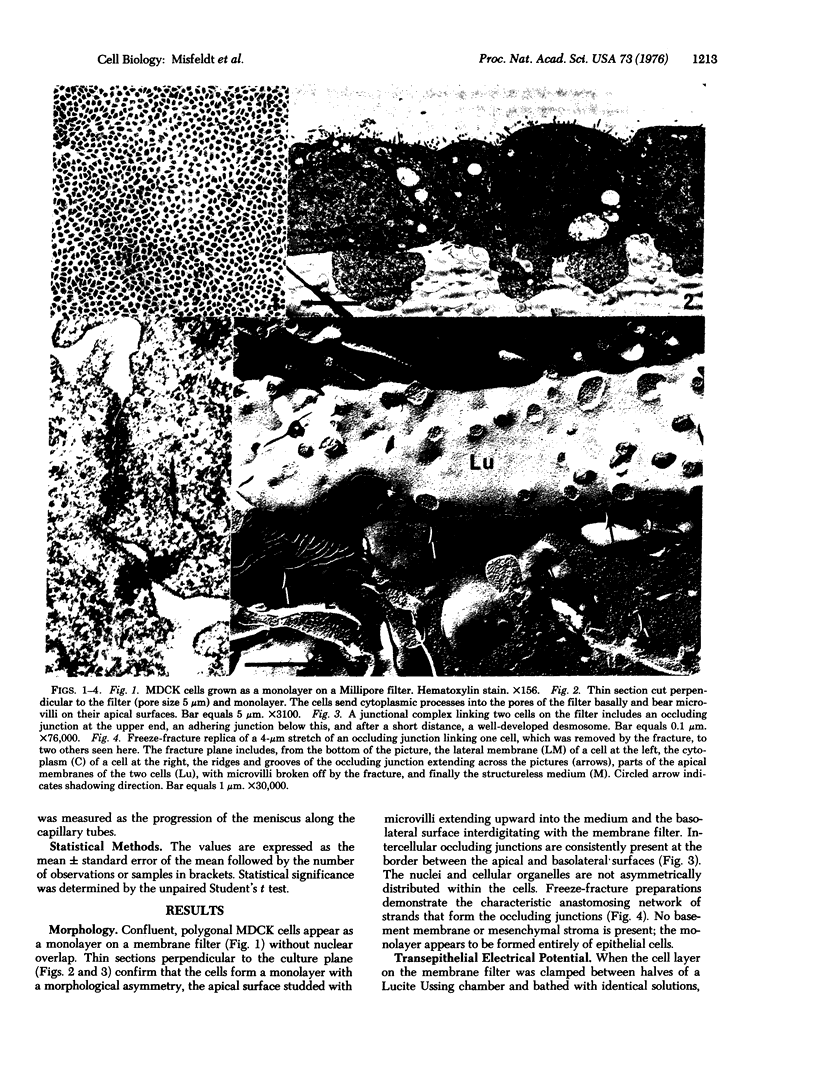
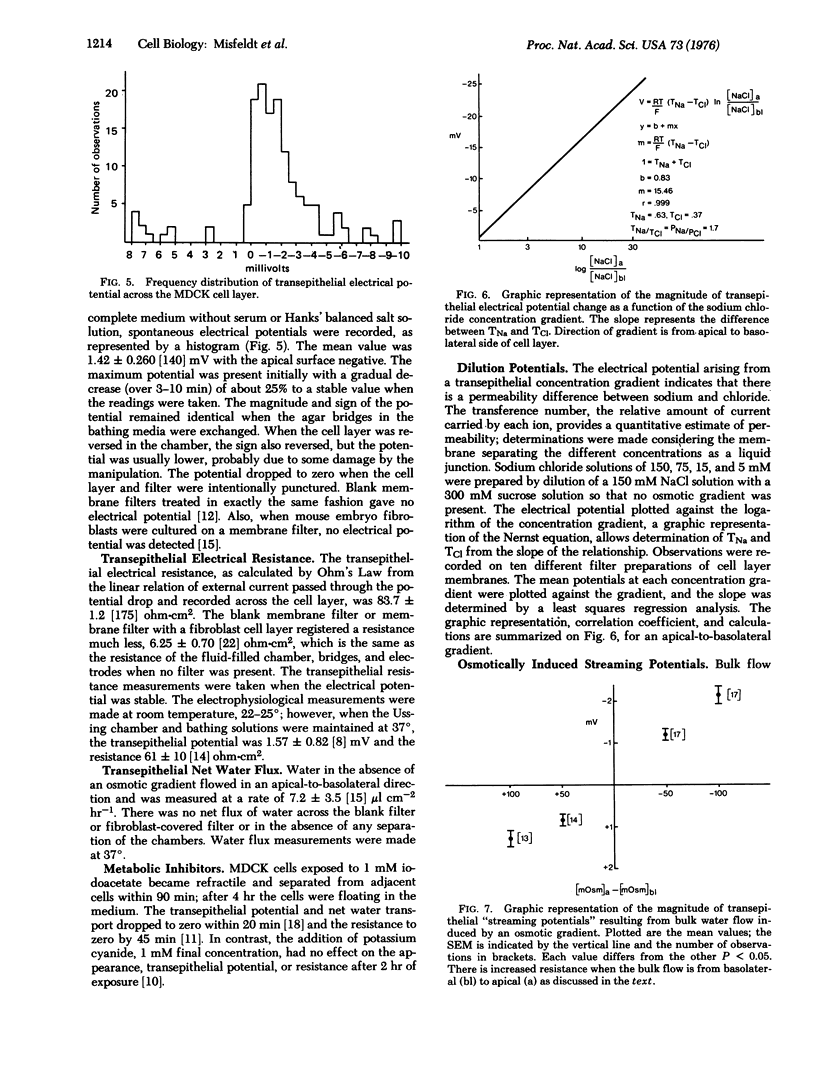
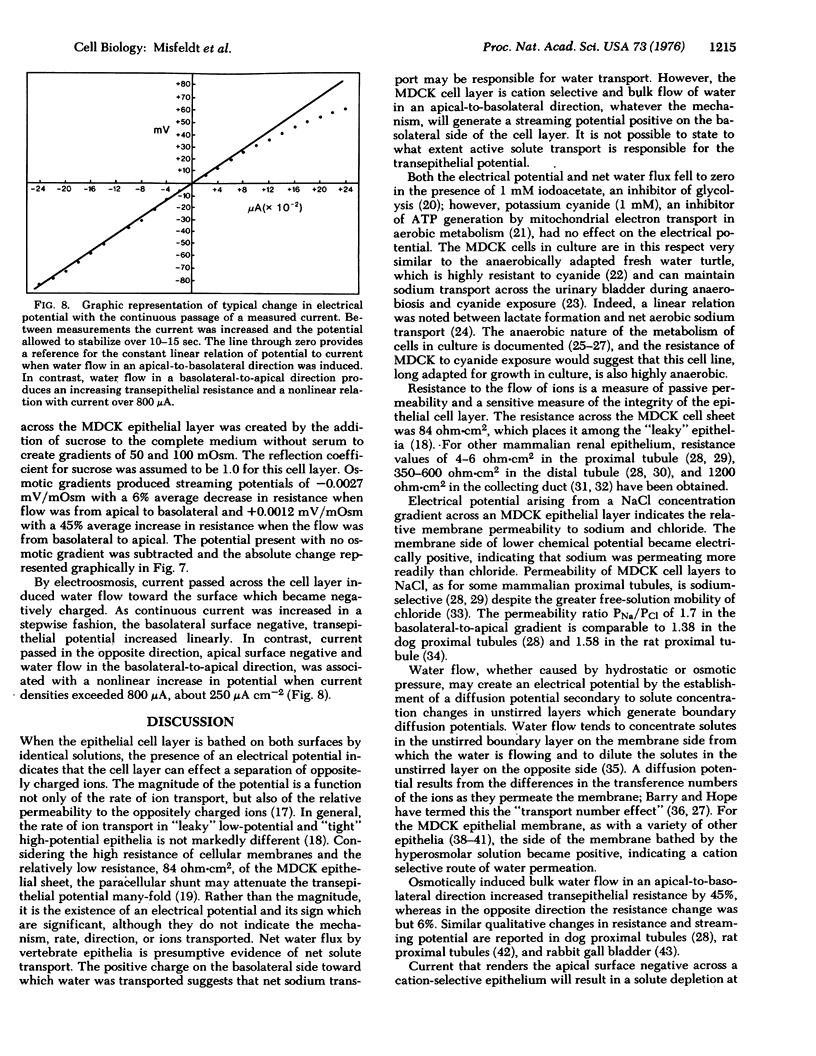
In cell culture a kidney epithelial cell line MDCK, forms a continuous sheet of identically oriented asymmetrical cells joined by circumferential occluding junctions. The reconstructed epithelial membrane has transport and permeability qualities of in vivo transporting epithelia. The cell layer can be readily manipulated when cultured on a freely permeable membrane filter and, when placed in an Ussing chamber, electrophysiological measurements can be taken. In the absence of a chemical gradient, the cell layer generates an electrical potential of 1.42 mV, the apical surface negative. It is an effective permeability barrier and lacks significant shunting at the clamped edge, as indicated by a resistance of 84 ohms-cm2, which increased when bulk flow from basolateral to apical was induced by an osmotic gradient or electroosmosis. The MDCK cell layer is cation selective with a relative permeability ratio, PNa/PCl, of 1.7. Net water flux, apical to basolateral, was 7.3 mul cm-2 hr-1 in the absence of a chemical gradient. The morphological and functional qualities of a transporting epithelium are stable in cell culture, and the potential use of a homogeneous cell population in cell culture would enhance studies of epithelial transport at the cellular and subcellular levels.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auersperg N. Histogenetic behavior of tumors. I. Morphologic variation in vitro and in vivo of two related human carcinoma cell lines. J Natl Cancer Inst. 1969 Jul;43(1):151–173. [PubMed] [Google Scholar]
- Barry P. H., Hope A. B. Electroosmosis in membranes: effects of unstirred layers and transport numbers. I. Theory. Biophys J. 1969 May;9(5):700–728. doi: 10.1016/S0006-3495(69)86413-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry P. H., Hope A. B. Electroosmosis in membranes: effects of unstirred layers and transport numbers. II. Experimental. Biophys J. 1969 May;9(5):729–757. doi: 10.1016/S0006-3495(69)86414-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell M. J., Hatié C., Rubin H. Patterns of glucose metabolism in normal and virus-transformed chick cells in tissue culture. J Natl Cancer Inst. 1972 Aug;49(2):555–565. [PubMed] [Google Scholar]
- Boulpaep E. L., Seely J. F. Electrophysiology of proximal and distal tubules in the autoperfused dog kidney. Am J Physiol. 1971 Oct;221(4):1084–1096. doi: 10.1152/ajplegacy.1971.221.4.1084. [DOI] [PubMed] [Google Scholar]
- Brown A. C., Vick R. L., Jacobson E. D. Ionic determinants of electrical potential in the in vivo small intestine. Gastroenterology. 1970 Mar;58(3):363–370. [PubMed] [Google Scholar]
- CEREIJIDO M., HERRERA F. C., FLANIGAN W. J., CURRAN P. F. THE INFLUENCE OF NA CONCENTRATION ON NA TRANSPORT ACROSS FROG SKIN. J Gen Physiol. 1964 May;47:879–893. doi: 10.1085/jgp.47.5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dermer G. B., Sherwin R. P. Autoradiographic localization of glycoprotein in human breast cancer cells maintained in organ culture after incubation with (3H)fucose or (3H)glucosamine. Cancer Res. 1975 Jan;35(1):63–67. [PubMed] [Google Scholar]
- Diamond J. M., Harrison S. C. The effect of membrane fixed charges on diffusion potentials and streaming potentials. J Physiol. 1966 Mar;183(1):37–57. doi: 10.1113/jphysiol.1966.sp007850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodge D. W., Rubin H. Glucose utilization, pH reduction and density dependent inhibition in cultures of chick embryo fibroblasts. J Cell Physiol. 1975 Jun;85(3):635–642. doi: 10.1002/jcp.1040850316. [DOI] [PubMed] [Google Scholar]
- Frömter E., Diamond J. Route of passive ion permeation in epithelia. Nat New Biol. 1972 Jan 5;235(53):9–13. doi: 10.1038/newbio235009a0. [DOI] [PubMed] [Google Scholar]
- Hegel U., Frömter E., Wick T. Der elektrische Wandwiderstand des proximalen Konvolutes der Rattenniere. Pflugers Arch Gesamte Physiol Menschen Tiere. 1967;294(4):274–290. [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Insulin: interaction with membrane receprots and relationship to cyclic purine nucleotides and cell growth. Fed Proc. 1975 Jun;34(7):1556–1563. [PubMed] [Google Scholar]
- KLAHR S., BRICKER N. S. ENERGETICS OF ANAEROBIC SODIUM TRANSPORT BY THE FRESH WATER TURTLE BLADDER. J Gen Physiol. 1965 Mar;48:571–580. doi: 10.1085/jgp.48.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLAHR S., BRICKER N. S. NA TRANSPORT BY ISOLATED TURTLE BLADDER DURING ANAEROBIOSIS AND EXPOSURE TO KCN. Am J Physiol. 1964 Jun;206:1333–1339. doi: 10.1152/ajplegacy.1964.206.6.1333. [DOI] [PubMed] [Google Scholar]
- Leighton J., Brada Z., Estes L. W., Justh G. Secretory activity and oncogenicity of a cell line (MDCK) derived from canine kidney. Science. 1969 Jan 31;163(3866):472–473. doi: 10.1126/science.163.3866.472. [DOI] [PubMed] [Google Scholar]
- Lewy P. R., Quintanilla A., Levin N. W., Kessler R. H. Renal energy metabolism and sodium reabsorption. Annu Rev Med. 1973;24:365–384. doi: 10.1146/annurev.me.24.020173.002053. [DOI] [PubMed] [Google Scholar]
- McGrath C. M. Replication of mammary tumor virus in tumor cell cultures: dependence on hormone-induced cellular organization. J Natl Cancer Inst. 1971 Aug;47(2):455–467. [PubMed] [Google Scholar]
- Nair B. K. Inability of serum to completely reverse density-dependent inhibition of cell growth in culture. J Natl Cancer Inst. 1974 Feb;52(2):513–517. doi: 10.1093/jnci/52.2.513. [DOI] [PubMed] [Google Scholar]
- Owens R. B., Smith H. S., Hackett A. J. Epithelial cell cultures from normal glandular tissue of mice. J Natl Cancer Inst. 1974 Jul;53(1):261–269. doi: 10.1093/jnci/53.1.261. [DOI] [PubMed] [Google Scholar]
- Pickett P. B., Pitelka D. R., Hamamoto S. T., Misfeldt D. S. Occluding junctions and cell behavior in primary cultures of normal and neoplastic mammary gland cells. J Cell Biol. 1975 Aug;66(2):316–332. doi: 10.1083/jcb.66.2.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBIN E. D., VESTER J. W., MURDAUGH H. V., Jr, MILLEN J. E. PROLONGED ANAEROBIOSIS IN A VERTEBRATE: ANAEROBIC METABOLISM IN THE FRESHWATER TURTLE. J Cell Physiol. 1964 Jun;63:287–297. doi: 10.1002/jcp.1030630304. [DOI] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Visser A. S., Prop F. J. "Domes," periodically expanding and collapsing secretory structures in cell cultures of mouse mammary tumors. J Natl Cancer Inst. 1974 Jan;52(1):293–295. doi: 10.1093/jnci/52.1.293. [DOI] [PubMed] [Google Scholar]
- WAYMOUTH C. Rapid proliferation of sublines of NCTC clone 929 (strain L) mouse cells in a simple chemically defined medium (MB 752/1). J Natl Cancer Inst. 1959 May;22(5):1003–1017. doi: 10.1093/jnci/22.5.1003. [DOI] [PubMed] [Google Scholar]
- Watlington C. O. Regulation of sodium transport by alteration of chloride conductance. Biochim Biophys Acta. 1972 Nov 2;288(2):482–485. doi: 10.1016/0005-2736(72)90271-4. [DOI] [PubMed] [Google Scholar]




