Abstract
Background
Treatment of allergic patients with omalizumab results in a paradoxical increase in their basophil histamine release response, ex vivo, to crosslinking anti-IgE antibody. It is not known whether this change in response is associated with an increase in intrinsic cellular sensitivity, which would be a paradoxical response.
Objective
To determine if the increase in response to anti-IgE Ab is a reflection of an increased cellular sensitivity, expressed as molecules of antigen-specific IgE per basophil required to produce a 50% of maximal response.
Methods
Patients were treated with omalizumab or placebo agent for 12 weeks (NCT01003301 at ClinicalTrials.gov) and the metric of basophil sensitivity was assessed at 4 time points, baseline, 6–8 weeks, 12 weeks (after which treatment stopped) and 24 weeks (12 weeks after the end of treatment).
Results
As observed previously, treatment with omalizumab resulted in a marked increase in the maximal histamine release induced by crosslinking anti-IgE Ab. This change was accompanied by a marked shift in intrinsic basophil sensitivity, ranging from 2.5 to 125 fold, with an average of 6 fold at the midpoint of the treatment to 12 fold after 12 weeks. The magnitude of the increase in cellular sensitivity was inversely related to the starting sensitivity or the starting maximum histamine release. The increased cellular sensitivity also occurred when using LTC4 secretion as a metric of the basophil response. 12 weeks after the end of treatment, cellular sensitivity was found to shift towards the baseline level although the return to baseline was not yet complete at this time point.
Conclusions
Treatment with omalizumab results in a markedly increased sensitivity of basophils to IgE-mediated stimulation, in terms of the number of IgE molecules required to produce a given response. These results provide a better quantitative sense of the phenotypic change that occurs in basophils during omalizumab treatment which has implications both mechanistic and clinical.
Keywords: Human, Basophil, Allergy, Fc Receptors
Introduction
Omalizumab is a recent addition to the therapies being used to treat asthma. It works by reducing free IgE levels sufficiently low to reduce the presence of IgE on the cell surface of mast cells, basophils and any other cell expressing IgE receptors. It has been established for many years that one consequence of the reduction in free IgE is a parallel reduction in FceRI on cells that express this receptor1–4. Reduction in FcεRI occurs because bound IgE stabilizes FcεRI on the cell surface; unbound receptor is internalized and degraded5, 6. The combined reduction in free IgE and FcεRI is necessary to blunt IgE-mediated secretion from mast cells and basophils because these cells are exquisitely sensitive to stimulation. For example, fewer than 2000 antigen-specific IgE molecules are needed for a half-maximal response to antigen but even 100–500 molecules are sufficient to initiate some secretion7. With starting densities of 150,000 IgE/basophil, considerable reduction in free IgE and FceRI is required to achieve <500 molecules of IgE per cell. A parameter that is highly relevant to achieving suppression of cell surface antigen-specific IgE is the ratio of specific/total IgE; low ratios allow for more rapid suppression of cell surface densities8, 9.
However, recent studies have identified an unexpected effect occurring during treatment with omalizumab. Two clinical studies have now shown that peripheral blood basophils respond better to stimulation with a pan-specific cross-linking anti-IgE antibody during omalizumab treatment10, 11. One reason for not observing a decreased response to a cross-linking anti-IgE antibody is that the dosing table for omalizumab isn’t designed to strongly reduce free IgE and this condition produces a basophil with densities of total cell surface IgE sufficient for a maximal response. But this only explains the absence of reduction in response to anti-IgE antibody, not an increase. An increased response suggests that other changes are occurring. Previous studies have established a modest linkage between the maximal amount of histamine release that can be induced by an optimal stimulus and the sensitivity of the cell to stimulation7. Cellular sensitivity is defined as the number of antigen-specific IgE molecules per basophil required to induce 50% of the maximal response (when stimulated with an optimal concentration of antigen). Although there is some linkage between these two parameters of IgE-mediated secretion in basophils, the linkage is weak and can be broken by exposing the cells to certain conditions. For example, maximum release can be increased when basophils are exposed to cytokines12–14 and based on the signaling involved, increased without significant increases in cellular sensitivity15–19, so that it is possible that the weak linkage that is seen in the general population7 doesn’t apply when are subjects are being treated with biological or pharmacological agents. Furthermore, a determination of the magnitude of the shift in sensitivity would also have additional implications regarding the underlying mechanisms of the change. On a more practical level, the metric of cellular sensitivity is the more interesting number for the purposes of developing a quantitative sense of what is happening to basophils (or mast cells) during omalizumab treatment and for predicting how the competing effects of cell surface IgE reduction and changes in cellular sensitivity will effect the cell’s response11. Therefore, it was of interest to determine whether treatment with omalizumab resulted in an increase in cellular sensitivity (fewer molecules needed for release) as a first step in a mechanistic study of the underlying basis for the change in maximal response.
Material and Methods
Reagents & Buffers
Common materials are purchased as described in the online repository. PIPES-albumin-glucose (PAG) buffer consisted of 25 mM PIPES, 110 mM NaCl, 5 mM KCl, 0.1% glucose, and 0.003% HSA. PAGCM was PAG supplemented with 1 mM CaCl2 and 1 mM MgCl2. Labeling with antibodies for flow cytometry was conducted in PAG with 1 mM EDTA containing 0.25% BSA in place of 0.003% HSA. Countercurrent elutriation was conducted in PAG containing 0.25% BSA in place of 0.003% HSA. Lactic acid buffer for removing endogenous cell bound IgE; 0.01 M lactic acid, 0.14 M NaCl, 0.005 M KCl, pH 3.97, 20.
Basophil Histamine Release
Venous blood was drawn into syringes containing PBS-EDTA and basophils were isolated using a two-step Percoll-based density-gradient. A portion of the enriched basophils were treated with lactic acid (see above) for 10 seconds to remove some of the endogenously bound IgE, thus exposing unoccupied receptors for greater sensitization20. Both lactic acid-treated cells and non-treated cells were divided into aliquots for serial concentrations of sensitizing IgE antibody. The concentrations of sensitizing antibody were chosen to provide a large range of IgE densities as well as provide some overlap in the IgE densities found on cells treated with lactic acid vs. those that were not treated with lactic acid. This was to ensure that the treatment with lactic acid did not alter the sensitivity/function of the treated basophils, as noted previously7. In general, overlap was accomplished with the chosen sensitization concentrations and also demonstrated that the lactic acid treatment did not significantly alter the sensitivity of the cells. The cells were sensitized with dilutions of an antibody specific for a peptide of HIV protein gp120 in a buffer containing 10 µg/ml heparin (which aids sensitization) and 1 mM EDTA at 4°C for 1 hour. For cells not treated with lactic acid, the concentrations of gp120-specific IgE were 5 µg/ml, 2.5 µg/ml, 1.25 µg/ml and 0.625 µg/ml and for the lactic acid-treated cells, 0.625 µg/ml, 0.31 µg/ml and 0.16 µg/ml. One aliquot of cells was not sensitized; these cells provided background binding for the flow cytometric measurements of bound gp120-specific IgE and were also used for testing two concentrations of anti-IgE Ab (6061). The gp120-specific antibody was biotinylated. The biotinylation provided a way to both assess the gp120-specific IgE levels of the cells after sensitization by flow cytometry and the same strepavidin-alexa647 used for flow cytometry (see online repository for additional details of flow cytometry) was also used to stimulate the basophils because streptavidin is naturally tetrameric. After washing the sensitized cells, a portion were fixed for subsequent flow cytometry. The remaining cells from each sensitization condition were stimulated in PAGCM buffer with either an optimal concentration of streptavidin-alexa647 (1/500,000 dilution of stock reagent, as determined in pilot studies) or anti-IgE (HP6061, Hybridoma Reagent Laboratory, 0.5 µg/mL) for 45 minutes at 37°C. Automated fluorometry was used to measure histamine in cell free supernatants21. Results for each stimulus are reported as a percentage of the total histamine content found in an aliquot of perchloric acid-lysed leukocytes after subtraction of spontaneous BHR from cells in buffer alone [(stimulated HR – spontaneous release)/(lysed HR - spontaneous HR)].
Omalizumab study
The details of this study are very similar to a previously published study9. The current study is registered at ClinicalTrials.gov under the identifier, NCT1003301. Briefly, adults, 18–50 years old, were chosen for their sensitivity to cat allergen. Informed consent was obtained via a protocol approved by the Johns Hopkins Hospital Institutional Review Board and the National Institute of Allergy and Infectious Diseases’ Data Safety Monitoring Boards. After testing for inclusion criteria, adults were entered into the study after a 2 week interval and randomized 1:1 omalizumab to placebo treatment. Omalizumab was administered according to the manufacturer’s dosing table. The basophil sensitivity assay was performed prior to drug administration, repeated at 6–8 weeks, after 12 weeks and in some individuals, 3 months after the cessation of treatment with omalizumab.. After the study was initiated, it was decided that basophils would also be assessed for both FcεRI and syk expression by flow cytometry and LTC4 release was measured in supernatants used for histamine analysis. These later subjects also returned for an assessment 3 months after the cessation of treatment.
Results
Since its initial development22, the method for measuring intrinsic basophil sensitivity has been improving as a metric of the basophil IgE-mediated response23. Notably, the method has become based on flow cytometric measurements of the antigen-specific IgE densities (rather than elution and measurement in RAST assays). For this study, additional refinements were added in order to reduce the requirement for larger amounts of blood and to potentially allow the method to be used with more generally available reagents. The basis of the method is to sensitize basophils with a specific IgE at different densities, and measure both the amount of IgE actually bound to FcεRI as well as the basophil’s response to antigenic stimulation following sensitization. Sensitization with the same IgE throughout a study eliminates concerns that the natural IgE might change its characteristics (e.g., affinity and epitope-profile) during treatment. The online repository describes the changes incorporated into the current method.
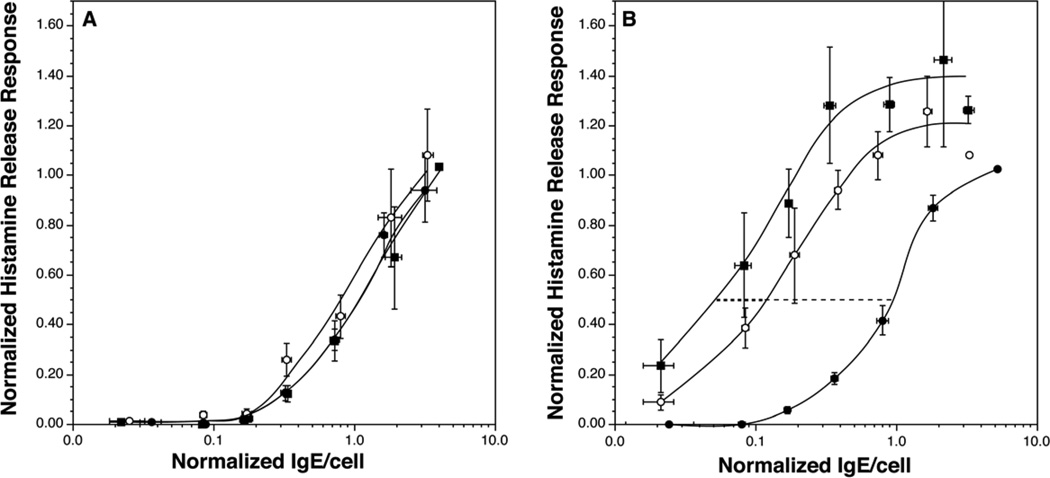
Since individuals’ basophils display different cellular sensitivity values22, 23 and we were interested in detecting the average shift in sensitivity in a population of individuals, an analytical approach was developed that optimally detected the shift and allowed averaging of disparate starting values of sensitivity. The online repository describes the two analytical methods used to evaluate the results. Figure 1 shows the results for sensitivity measurements made during the omalizumab study. For the 9 subjects that completed the study on placebo, there is no statistical difference in the sensitivity curves when comparing the starting curve to the curves generated at the midpoint of the study or its final visit during treatment. In contrast, there were marked shifts in sensitivity in the basophils of subjects treated with omalizumab. The sensitivity shifted 6 fold at the mid-study assessment and 12 fold at the final on-treatment assessment. Sigmoid fit values of the ED50 of 1.03±0.11, 0.18±0.06, and 0.08±0.03 at baseline, midpoint and final, respectively. Figure E2, in the online repository shows individual data for the treated group and the curve fit (+fit envelopes) for both treated and placebo groups. Likewise, the maximum histamine release response increased from 1.0 at baseline to 1.55 ± 0.21 and 1.55±0.14 at midpoint and final measurements, respectively (this corresponded to 58%, 89% and 89% histamine release for a maximum in absolute terms).
Figure 1.
Sensitivity curves for subjects treated with omalizumab or placebo. Panel A; placebo treated subjects (n=9), ( ) average baseline sensitivity curve, (
) average baseline sensitivity curve, ( ) average sensitivity curve at the midpoint of the study (approximately 6–8 weeks) and (
) average sensitivity curve at the midpoint of the study (approximately 6–8 weeks) and ( ) average sensitivity curve at approximately 12 weeks, just prior to cessation of drug dosing. Panel B; omalizumab treated subjects (n=10), (
) average sensitivity curve at approximately 12 weeks, just prior to cessation of drug dosing. Panel B; omalizumab treated subjects (n=10), ( ) average baseline sensitivity curve, (
) average baseline sensitivity curve, ( ) average sensitivity curve at the midpoint of the study (approximately 6–8 weeks) and (
) average sensitivity curve at the midpoint of the study (approximately 6–8 weeks) and ( ) average sensitivity curve at approximately 12 weeks. The dotted line provides visual reference for the change in sensitivity.
) average sensitivity curve at approximately 12 weeks. The dotted line provides visual reference for the change in sensitivity.
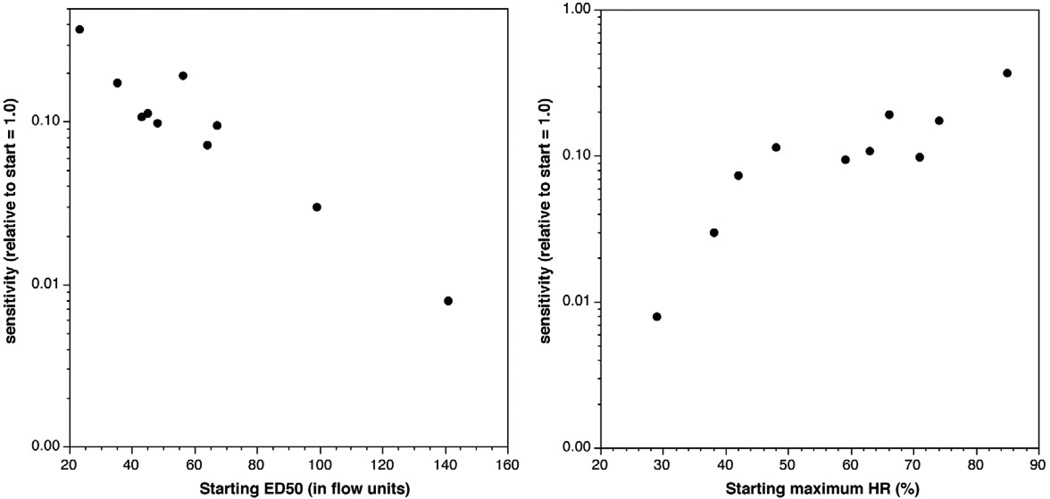
In previous studies10, the fold increase in histamine release to anti-IgE Ab was inversely correlated with the starting response to anti-IgE Ab. Likewise, the shift in sensitivity was inversely related to the starting absolute sensitivity (figure 2A). The shift in sensitivity was also related to the starting maximal response (figure 2B).
Figure 2.
Relationships in change in sensitivity and starting sensitivity or starting maximum histamine release. Panel A; starting sensitivity (ED50) as measured in units of MFI from the flow cytometric measurements. Panel B; relationship between starting maximum histamine release and change in sensitivity.
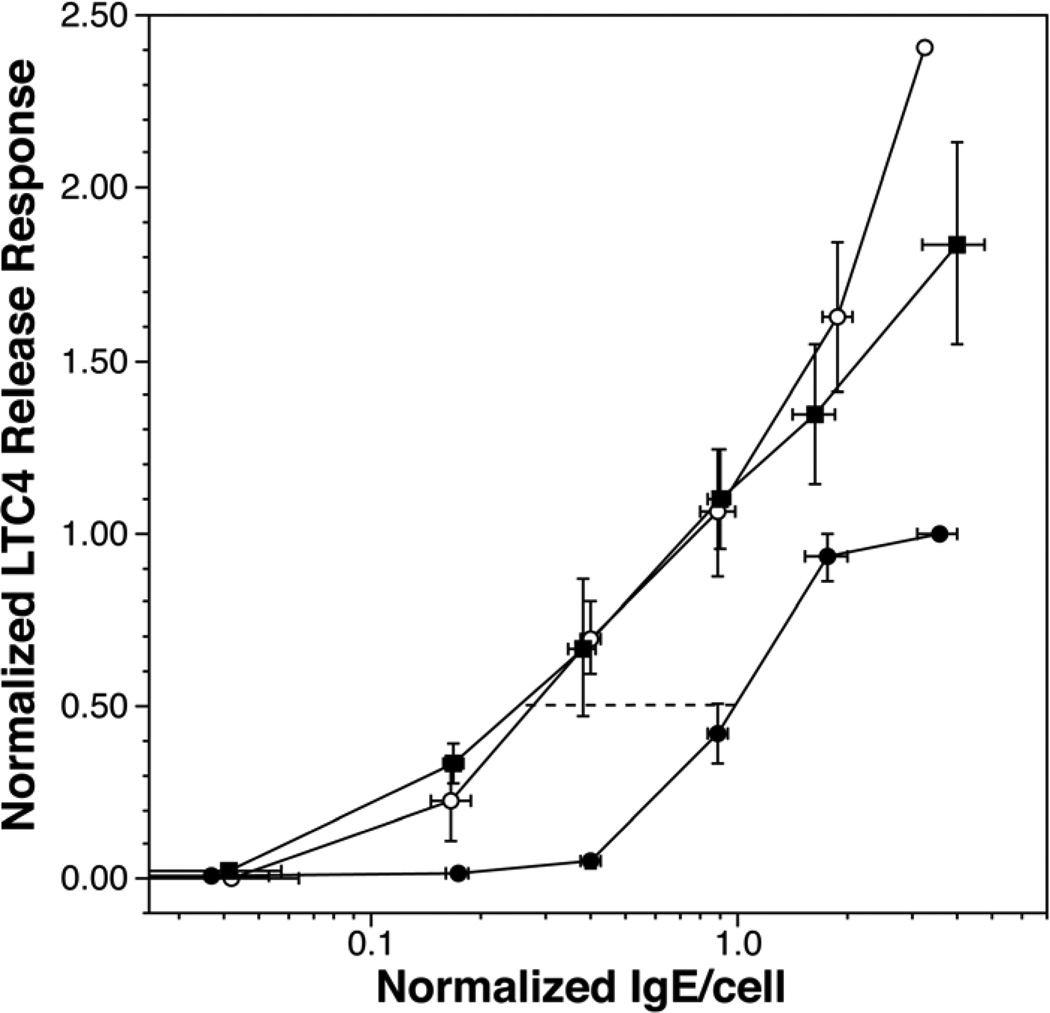
Basophils release several types of mediators but the signal transduction mechanisms differ somewhat in specifics so that correlations in the magnitude of the response for each type of mediator can be somewhat noisy. This is particularly true for the relationship between the release of LTC4 and histamine24. However, as shown in figure 3, the basophil also became more sensitive when LTC4 was the measured outcome. Likewise, the maximum release of LTC4 increased while on treatment. In the same subset of subjects (n=7), FcεRI and syk levels (both determined by flow cytometry) at week 12 were 0.28±0.04, 2.1±0.5 fold of baseline, respectively.
Figure 3.
Sensitivity measurements using LTC4 secretion as the outcome. LTC4 was measured in the same supernatants as used to measure histamine release. The data shows only subjects treated with omalizumab (n=7) and for which data was available at each of the 3 time points (baseline, 6–8 weeks, 12 weeks).
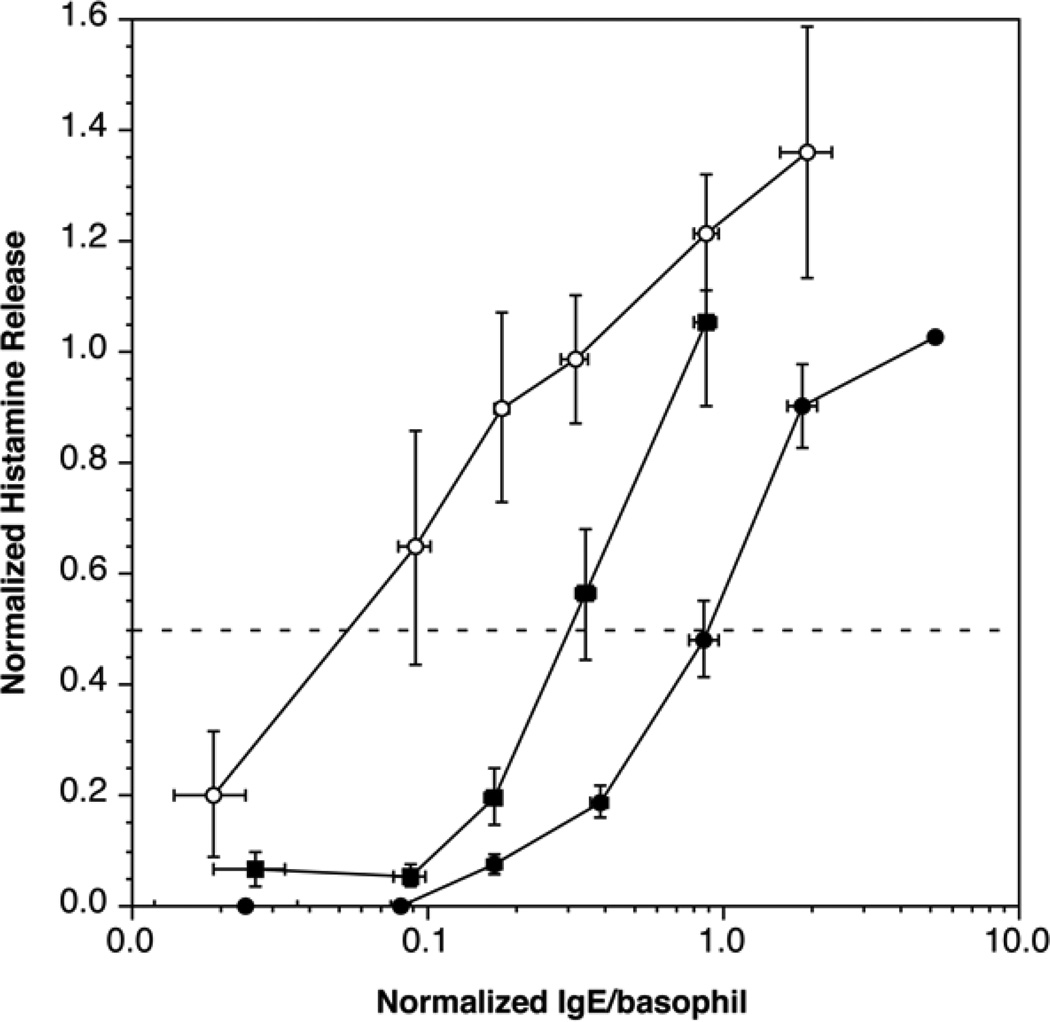
Previous studies have suggested that cessation of omalizumab treatment results in a slow multi-month return of function consistent with the 3–4 week half-life of circulating IgG antibodies2. Figure 4 shows that 3 months after stopping omalizumab, basophil sensitivity has shifted in the direction of the baseline sensitivity although the shift is only about 50% complete.
Figure 4.
Recovery of baseline sensitivity after cessation of omalizumab treatment (n=7); ( ) average baseline sensitivity curve for the 7 subjects that completed the recovery period, (
) average baseline sensitivity curve for the 7 subjects that completed the recovery period, ( ) average sensitivity curve at 12 weeks, (
) average sensitivity curve at 12 weeks, ( ) average sensitivity curve 3 months after cessation of omalizumab.
) average sensitivity curve 3 months after cessation of omalizumab.
Anti-IgE antibody has been used to assess the general basophil IgE-mediated response in previous studies and the concentrations chosen for previous studies were based on pilot work using non-allergic subjects. In those studies, it was surprising to find that both cat and peanut allergic subjects showed a somewhat lower EC50 for the anti-IgE Ab being used and, during treatment, the results from an abbreviated range of concentrations suggested that the point of optimum histamine release shifted. Mechanistically, this was unexpected, so in the current study, in addition to the sensitivity measurements presented above, 3 donors were examined with a much broader dose response curve to anti-IgE Ab. Figure E3 of the online repository shows that response to anti-IgE Ab increased as found previously and there is a shift rightward in the EC50 for the response consistent with shifts found for cat and peanut antigens.
Discussion
These studies demonstrate that treatment with omalizumab increases intrinsic basophil sensitivity and this occurs for both histamine and LTC4 release. The change in sensitivity was reversible although it appears to require several months since there was only a 50% recovery after 3 months off drug. It should be noted that an increase in sensitivity is generally offset by significant decreases in total and especially allergen-specific IgE antibody on the cell surface so that the final effect on the allergen-specific cell response is often seen as a reduction in the dose response curve for specific allergen9, 25, 26. The change in the allergen full dose response appears to be a good indicator of the efficacy of treatment11, 25, 27–29 because the standard allergen dose response curve captures several changing parameters of the response (reduction in cell surface allergen-specific IgE, any potential changes in IgE affinity, and changes in intrinsic cellular sensitivity). But an allergen dose response curve, in isolation, may mask the potentially important changes to intrinsic cellular function that are also occurring during treatment. A good example of this can be seen in studies of peanut allergy where the concentration of allergen needed to elicit basophil histamine release may shift rightward (higher concentrations, as it does in the current study, data not shown) but the overall dose response curve and its maximum are highly enhanced (also see the anti-IgE dose response curve, figure E3). Recent studies of peanut sensitive subjects demonstrate that the changes in the intrinsic IgE-mediated response (as assessed by increases in the maximum response to anti-IgE Ab) works against the efficacy of omalizumab to modulate the basophil response and may provide a predictor of the clinical response as well11. We have recently determined that there were two factors that contributed to differences in suppression of the basophil response, the ratio of allergen-specific IgE to total IgE and the size of the increase in cellular sensitivity. If the ratio of allergen specific IgE:total IgE is low enough (<4%), even considerable enhancement in cellular sensitivity is not sufficient to prevent suppression when already low densities of allergen-specific IgE are reduced by omalizumab. In addition to these factors, not all rightward shifts in the dose response result from changes in a simple change in IgE density. Changes in the shape of dose response curves could also occur because the ratio of syk and receptor density increases30. Finally, since it is not known how much allergen is found in the environment of basophils or mast cells, it is not yet clear which aspects of the dose response curves are relevant to efficacy. But without knowledge of the change in sensitivity, it is not possible to even guess how the various factors will compete. Therefore, demonstrating that the basophil becomes 3–125 fold more sensitive to antigen-driven secretion provides a framework for future studies of the underlying mechanisms that influence efficacy of this therapeutic approach as well as highlight a potentially important process that regulates natural basophil sensitivity. Studies are underway to determine if the information about the competing effects of sensitivity change and IgE suppression have predictive value for choosing the patients to be treated with omalizumab.
The basis for an increase in the basophil response during treatment with omalizumab is currently unknown although previous studies of both cat and peanut allergic patients noted an increase in the expression of syk that was correlated with the maximum response to anti-IgE Ab10, 11. A similar change was also noted in the current study. Our previous study (and all subsequent studies including this one) also demonstrated that the response to a non-IgE-dependent stimulus, FMLP, was not changed10, suggesting that the effects of omalizumab are restricted to IgE-mediated signaling steps. It has also been suggested that studies of sustained aggregation during the maturation of basophils from CD34 progenitors might be applicable to this situation31. In previous studies, sustained aggregation during maturation resulted in “basophils” that expressed normal levels of histamine, granules and FcεRI but showed reduced syk expression. It has been speculated that treatment with omalizumab might relieve a state of chronic aggregation in allergic patients, although the nature of chronic aggregation has yet to be identified. The full extent of the changes occurring in basophils both in the CD34 culture studies or in patients treated with omalizumab has not been explored but the changes in syk are consistent with an increase in cellular responsiveness. The current studies now put a number on the change in cellular responsiveness, a more useful number in the context of understanding the magnitude of the change in the cell’s IgE-mediated response. After approximately 12 weeks on omalizumab, basophils have become 12 fold more sensitive but the most extreme change was nearly 125 fold (see figure 2A). Based on previous calibrations of the IgE antibody used for sensitization and an average ED50 of 62 flow units in this study, this value corresponds to approximately 1100 molecules of IgE per cell. This appears somewhat more sensitive than previous baseline estimates of the basophil’s average sensitivity but the antigen in this case, streptavidin, was a somewhat better stimulus than the antigen used in previous studies (see online repository). A 12 fold change in sensitivity implies a cell that is capable of 50% of its maximal response (which has also increased) with only 100 antigen-specific IgE molecules per cell. Our previous modeling of IgE-mediated signaling in basophils32 suggest that a 2 fold change in syk alone (the amount observed in the previous cat and peanut studies as well as this study) cannot readily account for a 12 fold change in sensitivity. The unexpected size of the increase in sensitivity suggests that future studies should examine the basophil for other changes. For example, models of IgE-mediated secretion31 suggest that a change in both syk and a src-family kinase like lyn might be sufficient together to cause this kind of enhancement. There is some support for this possibility because of the 20 different signaling species that have been examined for down-regulation by IgE-dependent aggregation7, only syk, lyn and FceRI expression were altered. The loss of syk is considerably greater than lyn but it is possible that during maturation of basophils (see the discussion above), modulation of lyn might also occur during any sustained aggregation that might be relieved by treatment with omalizumab.
A feature of the changing basophil response that has been consistent in all of our studies is the inverse relationship between the response before treatment and its increase during treatment10, 11. This now applies to the sensitivity metric. Indeed, the correlation between sensitivity before treatment and the fold change in sensitivity was remarkably high. This property of the change supports the concept that the natural (un-modulated) basophil response is some fixed value but that there is heterogeneity of response in the population due to some process that is sensitive to omalizumab. If this process is related to chronic or sustained aggregation (during maturation?), then these results suggest that the un-modulated state of basophils would include an IgE-mediated response that leads to nearly 100% histamine release and a sensitivity on the order 100 molecules/cell of antigen-specific IgE. In our recent study of peanut sensitive patients, there was an indication that this increasing sensitivity mitigated against the efficacy of the drug11. This would stand to reason if the basophil plays a role in expression of food allergies. But these observations suggest that the linkage between basophil function and disease response to omalizumab is possibly different from the linkage between the basophil response and disease severity. The linkage between basophil response and disease severity is not strong33–39. For treatment to reveal that the change in basophil response has an influence on the efficacy of treatment suggests there are other factors involved. One speculation is that the end organ responses in each individual slowly adapt to the basophil’s behavior so that the target organs adapt over time to the individual’s natural basophil responsiveness. This might wash out a relationship between basophil responsiveness and disease expression in a cross-sectional study but make it possible to find the relationship revealed when the basophils’ response is rapidly changed in a longitudinal study.
In summary, this third study of basophil responsiveness during treatment with omalizumab shows that not only is the change in maximum release a repeatable feature of basophil responsiveness but a marked change in the intrinsic sensitivity of the basophil occurs. One interpretation of the various results is that omalizumab alters a process that serves to variably suppress the natural sensitivity and responsiveness of basophils. The magnitude of the increase is greater than current models of IgE-mediated secretion would predict for an increase in syk expression alone.
Key Messages.
Treatment of subjects induces a large increase in the intrinsic sensitivity of basophils to IgE-mediated stimulation, an increase that translates to basophils requiring thousands of allergen-specific IgE per cell to values in the low hundreds to obtain equivalent mediator release.
Based on current understanding about IgE-mediated secretion, the magnitude of the increase in sensitivity is much greater than can be accounted for by the concurrently measured increase in expression of syk, suggesting that there are additional changes in the cell that account for the increase.
Acknowledgements
We would like to thank Valerie Alexander for her technical assistance in this study. This study was supported by NIH AI070345.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.MacGlashan JDW, Bochner BS, Adelman DC, Jardieu PM, Togias A, Mckenzie-White J, et al. Down-regulation of FceRI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J. Immunol. 1997;158:1438–1445. [PubMed] [Google Scholar]
- 2.Saini SS, MacGlashan DW, Jr, Sterbinsky SA, Togias A, Adelman DC, Lichtenstein LM, et al. Down-regulation of human basophil IgE and FceRIa surface densities and mediator release by anti-IgE infusions is reversible in vitro and in vivo. J. Immunol. 1999;162:5623–5630. [PubMed] [Google Scholar]
- 3.Prussin C, Griffith DT, Boesel KM, Lin H, Foster B, Casale TB. Omalizumab treatment downregulates dendritic cell FcepsilonRI expression. J Allergy Clin Immunol. 2003;112:1147–1154. doi: 10.1016/j.jaci.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 4.Gomez G, Jogie-Brahim S, Shima M, Schwartz LB. Omalizumab reverses the phenotypic and functional effects of IgE-enhanced Fc epsilonRI on human skin mast cells. J Immunol. 2007;179:1353–1361. doi: 10.4049/jimmunol.179.2.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.MacGlashan DW, Jr, Xia HZ, Schwartz LB, Gong JP. IgE-regulated expression of FceRI in human basophils: Control by regulated loss rather than regulated synthesis. J. Leuk. Biol. 2001;70:207–218. [PubMed] [Google Scholar]
- 6.Borkowski TA, Jouvin MH, Lin SY, Kinet JP. Minimal requirements for IgE-mediated regulation of surface Fc epsilon RI. J Immunol. 2001;167:1290–1296. doi: 10.4049/jimmunol.167.3.1290. [DOI] [PubMed] [Google Scholar]
- 7.MacGlashan DW., Jr Relationship Between Syk and SHIP Expression and Secretion from Human Basophils in the General Population. J. Allergy Clin. Immunol. 2007;119:626–633. doi: 10.1016/j.jaci.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 8.Johansson SG, Nopp A, Oman H, Ankerst J, Cardell LO, Gronneberg R, et al. The size of the disease relevant IgE antibody fraction in relation to 'total-IgE' predicts the efficacy of anti-IgE (Xolair) treatment. Allergy. 2009;64:1472–1477. doi: 10.1111/j.1398-9995.2009.02051.x. [DOI] [PubMed] [Google Scholar]
- 9.Eckman JA, Sterba PM, Kelly D, Alexander V, Liu MC, Bochner BS, et al. Effects of omalizumab on basophil and mast cell responses using an intranasal cat allergen challenge. J Allergy Clin Immunol. 2010;125:889–895. e7. doi: 10.1016/j.jaci.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaidi AK, Saini SS, Macglashan DW., Jr Regulation of Syk kinase and FcRbeta expression in human basophils during treatment with omalizumab. J Allergy Clin Immunol. 2010;125:902–908. e7. doi: 10.1016/j.jaci.2009.12.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.MacGlashan DW, Jr, Savage J, Wood RA, Saini S. Suppression of the Basophil Response to Allergen During Treatment with Omalizumab is Dependent on Two Competing Factors. J Allergy Clin Immunol. 2012;130:1130–1135. doi: 10.1016/j.jaci.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schleimer RP, Derse CP, Friedman B, Gillis S, Plaut M, Lichtenstein LM, et al. Regulation of human basophil mediator release by cytokines. I. Interaction with antiinflammatory steroids. J Immunol. 1989;143:1310–1317. [PubMed] [Google Scholar]
- 13.Kurimoto Y, de Weck AL, Dahinden CA. The effect of interleukin 3 upon IgE-dependent and IgE-independent basophil degranulation and leukotriene generation. Eur J Immunol. 1991;21:361–368. doi: 10.1002/eji.1830210217. [DOI] [PubMed] [Google Scholar]
- 14.MacGlashan D., Jr Subthreshold desensitization of human basophils re-capitulates the loss of Syk and FcepsilonRI expression characterized by other methods of desensitization. Clin Exp Allergy. 2012;42:1060–1070. doi: 10.1111/j.1365-2222.2012.04013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miura K, MacGlashan DW., Jr Dual phase priming by interleukin-3 for leukotriene C4 generation in human basophils. J. Immunol. 2000;164:3026–3034. doi: 10.4049/jimmunol.164.6.3026. [DOI] [PubMed] [Google Scholar]
- 16.Miura K, Lavens-Phillips S, MacGlashan DW., Jr Localizing a control region in the pathway to LTC4 secretion following stimulation of human basophils with anti-IgE antibody. J. Immunol. 2001;167:7027–7037. doi: 10.4049/jimmunol.167.12.7027. [DOI] [PubMed] [Google Scholar]
- 17.Miura K, Saini SS, Gauvreau G, MacGlashan DW., Jr Differences in functional consequences and signal transduction induced by IL-3, IL-5 and NGF in human basophils. J. Immunol. 2001;167:2282–2291. doi: 10.4049/jimmunol.167.4.2282. [DOI] [PubMed] [Google Scholar]
- 18.MacGlashan DW., Jr Two regions of down-regulation in the IgE-mediated signaling pathway in human basophils. J. Immunol. 2003;170:4814–4925. doi: 10.4049/jimmunol.170.10.4914. [DOI] [PubMed] [Google Scholar]
- 19.Vilarino N, Miura K, MacGlashan DW., Jr Acute IL-3 priming up-regulates the stimulus-induced Raf-1-Mek-Erk cascade independently of IL-3-induced activation of Erk. J Immunol. 2005;175:3006–3014. doi: 10.4049/jimmunol.175.5.3006. [DOI] [PubMed] [Google Scholar]
- 20.Pruzansky JJ, Grammer LC, Patterson R, Roberts M. Dissociation of IgE from receptors on human basophils. I. Enhanced passive sensitization for histamine release. J. Immunol. 1983;131:1949–1953. [PubMed] [Google Scholar]
- 21.Siraganian RP. An automated continuous-flow system for the extraction and fluorometric analysis of histamine. Anal Biochem. 1974;57:383–394. doi: 10.1016/0003-2697(74)90093-1. [DOI] [PubMed] [Google Scholar]
- 22.MacGlashan DW., Jr Releasability of human basophils: Cellular sensitivity and maximal histamine release are independent variables. J Allergy Clin Immunol. 1993;91:605–615. doi: 10.1016/0091-6749(93)90266-i. [DOI] [PubMed] [Google Scholar]
- 23.MacGlashan DW, Jr, Vilarino N. Nonspecific Desensitization, Functional Memory and the Characteristics of SHIP Phosphorylation Following IgE-Mediated Stimulation of Human Basophils. J. Immunol. 2006;177:1040–1051. doi: 10.4049/jimmunol.177.2.1040. [DOI] [PubMed] [Google Scholar]
- 24.MacGlashan DW, Jr, Peters SP, Warner J, Lichtenstein LM. Characteristics of human basophil sulfidopeptide leukotriene release: releasability defined as the ability of the basophil to respond to dimeric cross-links. J Immunol. 1986;136:2231–2239. [PubMed] [Google Scholar]
- 25.Nopp A, Johansson SG, Ankerst J, Palmqvist M, Oman H. CD-sens and clinical changes during withdrawal of Xolair after 6 years of treatment. Allergy. 2007;62:1175–1181. doi: 10.1111/j.1398-9995.2007.01476.x. [DOI] [PubMed] [Google Scholar]
- 26.Nopp A, Cardell LO, Johansson SG, Oman H. CD-sens: a biological measure of immunological changes stimulated by ASIT. Allergy. 2009;64:811–814. doi: 10.1111/j.1398-9995.2008.01900.x. [DOI] [PubMed] [Google Scholar]
- 27.Dahlen B, Nopp A, Johansson SG, Eduards M, Skedinger M, Adedoyin J. Basophil allergen threshold sensitivity, CD-sens, is a measure of allergen sensitivity in asthma. Clin Exp Allergy. 2011;41:1091–1097. doi: 10.1111/j.1365-2222.2011.03763.x. [DOI] [PubMed] [Google Scholar]
- 28.Glaumann S, Nopp A, Johansson SG, Rudengren M, Borres MP, Nilsson C. Basophil allergen threshold sensitivity, CD-sens, IgE-sensitization and DBPCFC in peanut-sensitized children. Allergy. 2012;67:242–247. doi: 10.1111/j.1398-9995.2011.02754.x. [DOI] [PubMed] [Google Scholar]
- 29.Noga O, Hanf G, Kunkel G, Kleine-Tebbe J. Basophil histamine release decreases during omalizumab therapy in allergic asthmatics. Int Arch Allergy Immunol. 2008;146:66–70. doi: 10.1159/000112504. [DOI] [PubMed] [Google Scholar]
- 30.Nag A, Faeder JR, Goldstein B. Shaping the response: the role of FcepsilonRI and Syk expression levels in mast cell signaling. IET Syst Biol. 2010;4:334–347. doi: 10.1049/iet-syb.2010.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishmael SS, MacGlashan DW., Jr Syk expression in peripheral blood leukocytes, CD34+ progenitors, and CD34-derived basophils. J Leukoc Biol. 2010;87:291–300. doi: 10.1189/jlb.0509336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vilarino N, MacGlashan DW., Jr Actin cytoskeleton-dependent down-regulation of early IgE-mediated signaling in human basophils. J Leukoc Biol. 2004;75:928–937. doi: 10.1189/jlb.0903431. [DOI] [PubMed] [Google Scholar]
- 33.Busse WW, Swenson CA, Sharpe G, Koschat M. Enhanced basophil histamine release to concanavalin A in allergic rhinitis. J Allergy Clin Immunol. 1986;78:90–97. doi: 10.1016/0091-6749(86)90119-3. [DOI] [PubMed] [Google Scholar]
- 34.Gaddy JN, Busse WW. Enhanced IgE-dependent basophil histamine release and airway reactivity in asthma. Am Rev Respir Dis. 1986;134:969–974. doi: 10.1164/arrd.1986.134.5.969. [DOI] [PubMed] [Google Scholar]
- 35.Casolaro V, Spadaro G, Marone G. Human basophil releasability. VI. Changes in basophil releasability in patients with allergic rhinitis or bronchial asthma. Am Rev Respir Dis. 1990;142:1108–1111. doi: 10.1164/ajrccm/142.5.1108. [DOI] [PubMed] [Google Scholar]
- 36.Youssef LA, Schuyler M, Gilmartin L, Pickett G, Bard JD, Tarleton CA, et al. Histamine release from the basophils of control and asthmatic subjects and a comparison of gene expression between "releaser" and "nonreleaser" basophils. J Immunol. 2007;178:4584–4594. doi: 10.4049/jimmunol.178.7.4584. [DOI] [PubMed] [Google Scholar]
- 37.Norman PS, Lichtenstein LM, Ishizaka K. Diagnostic tests in ragweed hay fever. A comparison of direct skin tests, IgE antibody measurements, and basophil histamine release. J Allergy Clin Immunol. 1973;52:210–224. doi: 10.1016/0091-6749(73)90059-6. [DOI] [PubMed] [Google Scholar]
- 38.Bruce CA, Rosenthal RR, Lichtenstein LM, Norman PS. Diagnostic tests in ragweed-allergic asthma. A comparison of direct skin tests, leukocyte histamine release, and quantitative bronchial challenge. J Allergy Clin Immunol. 1974;53:230–239. doi: 10.1016/0091-6749(74)90085-2. [DOI] [PubMed] [Google Scholar]
- 39.Paterniti M, Kelly DC, Eckman JA, Sterba PM, Hamilton RG, Bochner BS, et al. Cat allergen-induced blood basophil reactivity in vitro predicts acute human nasal allergen challenge responses in vivo. Clin Exp Allergy. 2011;41:963–969. doi: 10.1111/j.1365-2222.2011.03719.x. [DOI] [PubMed] [Google Scholar]