Abstract
Pituitary adenomas, monoclonal in origin, are the most common intracranial neoplasms. Altered gene expression as well as somatic mutations is detected frequently in pituitary adenomas. The purpose of this study was to detect differentially expressed genes (DEGs) and biological processes during tumor formation of pituitary adenomas. We performed an integrated analysis of publicly available GEO datasets of pituitary adenomas to identify DEGs between pituitary adenomas and normal control (NC) tissues. Gene function analysis including Gene Ontology (GO), Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis, and protein-protein interaction (PPI) networks analysis was conducted to interpret the biological role of those DEGs. In this study we detected 3994 DEGs (2043 upregulated and 1951 downregulated) in pituitary adenoma through an integrated analysis of 5 different microarray datasets. Gene function analysis revealed that the functions of those DEGs were highly correlated with the development of pituitary adenoma. This integrated analysis of microarray data identified some genes and pathways associated with pituitary adenoma, which may help to understand the pathology underlying pituitary adenoma and contribute to the successful identification of therapeutic targets for pituitary adenoma.
1. Introduction
Pituitary adenomas account for 10–15% of all intracranial neoplasms. Most pituitary adenomas are benign, although they may cause significant morbidity through mass effects and/or the improper secretion of pituitary hormones, indicating that the development of pituitary adenomas is a complex multistep process. Pituitary adenomas usually occur sporadically and are grouped into functioning and nonfunctioning adenomas (NFAs) according to hormonal status and further subdivided into microadenomas (<1 cm) and macroadenomas (≥1 cm) based on tumor size [1].
Despite massive research, the pathogenesis of pituitary adenomas still remains unclear. However, advances in molecular biology such as microarray technique enable the identification of new genes associated with pituitary tumor genesis. The microarray technique, which allows the simultaneous analysis of thousands of genes at the transcript expression level in a single experiment [2], has greatly facilitated the investigation of gene expression differences between normal pituitary and pituitary adenomas. Recently, researchers have used this powerful technique to compare gene expression between normal pituitary tissues and pituitary adenomas of different origins and have also identified many genes associated with certain tumor types [3–6]. However, there are inconsistencies among these studies due to limitations of different sample sources, microarray platforms, and analysis techniques [7]. Towards this end, we performed a systematic integration of gene expression data from multiple sources, to increase statistical power for detecting differentially expressed genes (DEGs) [8, 9]. Now in this study we use this method to identify DEGs and biological processes associated with pituitary adenomas to provide some insights into molecular mechanisms underlying the pathogenesis of pituitary adenomas and many guided further therapies for this disease.
2. Material and Methods
2.1. Identification of Eligible Gene Expression Datasets
Expression profiling studies of pituitary adenomas were identified by searching the Gene Expression Omnibus database (GEO, http://www.ncbi.nlm.nih.gov/geo/) [12]. We only collected original experimental articles that analyzed gene expression profiling between pituitary adenoma and normal control (NC) tissues. Nonhuman studies, review articles, and integrated analysis of expression profiles were excluded.
2.2. Data Preprocessing
Normalization is very important to compare multiple microarray datasets accurately. The heterogeneity of multiple datasets resulted from different platforms, and clinical samples may make it difficult to compare the results directly. Consequently a global normalization approach should be included to minimize the heterogeneity. For this purpose, we first preprocessed the raw microarray data of each study by log2 transformation, then the Z-score transformation was applied for calculation of expression intensities of each probe, and Z-scores were calculated following the formula
| (1) |
where x i indicates raw intensity data for each gene; indicates the average intensity of the gene in a single experiment, and δ indicates standard deviation (SD) of all the measured intensities.
2.3. Statistical Analysis
The significance analysis of microarray (SAM) software was used to determine the DEGs between pituitary adenoma and NC tissues. Gene specific t-tests were carried out, outputting a “relative difference” score or d value which was defined as the average expression change of each gene from different expression levels to the SD of measurements. The genes with at least 1.5-fold change and a false discovery rate (FDR) less than 0.05 were selected as DEGs [13].
2.4. Functional Annotation of DEGs
To interpret the biological functions of the DEGs, we performed Gene Ontology (GO) enrichment analysis to explore functional distribution of DEGs in pituitary adenoma. GO provides a common descriptive framework and functional annotation of the gene sets data. Furthermore we also performed Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis for DEGs to find important pathways involved in pituitary adenoma. KEGG pathway database is a recognized and comprehensive database including all kinds of biochemistry pathways [14]. The online based software GENECODIS was utilized in this analysis [15].
2.5. PPI Network Construction
The protein-protein interactions (PPIs) analysis was conducted to investigate the functions of proteins at the molecular level [16]. The identification of protein interactions in a genome-wide scale is important to uncover the cellular regulation mechanisms [17]. Biological General Repository for Interaction Datasets (BioGRID) (http://thebiogrid.org/) was used to construct the PPI network, and then Cytoscape software was used to visualize the distribution characteristics of the top 10 up- and downregulated DEGs in the PPI network [18].
3. Results
3.1. Short Overview of the Studies Included
In this study, we obtained a total of 5 expression profiles of pituitary adenoma in GEO database; it contained 44 samples of pituitary adenoma and 12 samples of controls. The individual studies for analyzing are displayed in Table 1. Several types of pituitary adenomas were included in our study such as NFPA, growth hormone pituitary adenomas, and prolactin adenomas.
Table 1.
Characteristics of the individual studies.
| GEO ID | Author | Platform | Samples (N : P) | Year |
|---|---|---|---|---|
| GSE51618 | Feng J | GPL6480 Agilent-014850 4x44K G4112F | 3 : 7 | 2013 |
| GSE46311 | Lekva T | GPL6244 Affymetrix Human Gene 1.0 ST Array | 0 : 16 | 2013 |
| GSE36314 | Oyesiku [10] | GPL8300 Affymetrix Human Genome U95 Version 2 Array | 3 : 4 | 2012 |
| GSE22812 | Wierinckx et al. [11] | GPL2895 GE Healthcare/Amersham Biosciences CodeLink Bioarray [11] | 0 : 13 | 2011 |
| GSE4237 | Hussaini IM | GPL570 Affymetrix Human Genome U133 Plus 2.0 Array | 6 : 4 | 2006 |
3.2. Detecting Genes Associated with Pituitary Adenoma
After global normalization, we adopted SAM software to identify DEGs between pituitary adenomas and control samples. With FDR ≤0.05 and a minimal fold change of 1.5, a total of 3994 genes were found to show aberrant expression in samples of pituitary adenoma compared with NC tissues, among which 2043 DEGs were upregulated and 1951 were downregulated. A list of the top 10 most significantly up- or downregulated genes was presented in Table 2.
Table 2.
The top 10 most significantly up- or down-regulated DEGs.
| Gene ID | Gene symbol | Official full name | P value | Fold change |
|---|---|---|---|---|
| Up-regulated genes | ||||
| 219557 | C7orf62 | Chromosome 7 open reading frame 62 | 3.33E − 16 | 2.1819 |
| 57519 | STARD9 | StAR-related lipid transfer (START) domain containing 9 | 1.28E − 14 | 1.8589 |
| 89792 | GAL3ST3 | Galactose-3-O-sulfotransferase 3 | 1.29E − 13 | 1.9935 |
| 57650 | KIAA1524 | KIAA1524 | 1.09E − 12 | 1.351 |
| 114757 | CYGB | Cytoglobin | 4.85E − 12 | 1.8788 |
| 157983 | C9orf66 | Chromosome 9 open reading frame 66 | 1.73E − 11 | 1.4951 |
| 341880 | SLC35F4 | Solute carrier family 35, member F4 | 1.91E − 11 | 2.2741 |
| 57121 | LPAR5 | Lysophosphatidic acid receptor 5 | 3.26E − 11 | 1.3792 |
| 257044 | C1orf101 | Chromosome 1 open reading frame 101 | 3.63E − 11 | 1.2287 |
| 145581 | LRFN5 | Leucine rich repeat and fibronectin type III domain containing 5 | 3.80E − 11 | 1.9332 |
|
| ||||
| Down-regulated genes | ||||
| 157506 | RDH10 | Retinol dehydrogenase 10 (all-trans) | 0 | −2.3698 |
| 8324 | FZD7 | Frizzled class receptor 7 | 0 | −2.6494 |
| 140576 | S100A16 | S100 calcium binding protein A16 | 3.00E − 15 | −1.8248 |
| 84952 | CGNL1 | Cingulin-like 1 | 2.40E − 14 | −2.1091 |
| 85375 | KIAA1661 | KIAA1661 protein | 4.95E − 14 | −1.896 |
| 55276 | PGM2 | Phosphoglucomutase 2 | 7.29E − 14 | −1.5698 |
| 55300 | PI4K2B | Phosphatidylinositol 4-kinase type 2 beta | 9.64E − 14 | −1.4987 |
| 84899 | TMTC4 | Transmembrane and tetratricopeptide repeat containing 4 | 1.20E − 13 | −1.4509 |
| 345557 | PLCXD3 | Phosphatidylinositol-specific phospholipase C, X domain containing 3 | 3.01E − 13 | −3.3246 |
| 5570 | PKIB | Protein kinase (cAMP-dependent, catalytic) inhibitor beta | 1.00E − 12 | −2.3052 |
The upregulated gene with the lowest P value was C7orf62, whose function has been unclear. The downregulated gene with the lowest P value was RDH10, which is essential for synthesis of embryonic retinoic acid and limb, craniofacial, and organ development. The full list of these genes was provided as Supplementary Table 1 in Supplementary Material available online at http://dx.doi.org/10.1155/2015/164087.
3.3. Functional Annotation
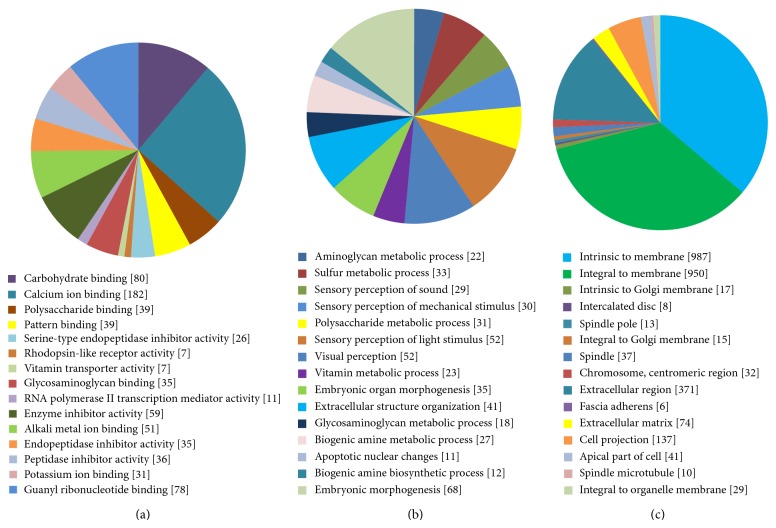
To understand the biological roles of the DEGs from pituitary adenomas, we conducted GO categories and KEGG pathway enrichment analysis. GO categories are separated into three groups: biological process, cellular component, and molecular function. We examined GO categories separately. The significantly enriched GO terms for molecular functions were carbohydrate binding (GO: 0030246, P = 1.93E − 03) and calcium ion binding (GO: 0005509, P = 3.10E − 03) for molecular functions, while for biological processes they were aminoglycan metabolic process (GO: 0006022, P = 9.76E − 04) and sulfur metabolic process (GO: 0006790, P = 1.07E − 03), and for cellular component they were intrinsic to membrane (GO: 0031224, P = 2.76E − 04) and integral to membrane (GO: 0016021, P = 7.43E − 04) (Figure 1).
Figure 1.
The top 15 enriched GO terms of DEGs. (a) Molecular functions for DEGs (P value ≤ 4.61E − 03); (b) biological process for DEGs (P value ≤ 6.54E − 03); (c) cellular component for DEGs (P value ≤ 1.95E − 03).
Hypergeometric test with P value <0.05 was used as the criteria for pathway detection (Table 3). The most significant pathway in our analysis was neuroactive ligand-receptor interaction (P = 3.97E − 03). Furthermore, tryptophan metabolism (P = 2.42E − 02) and cardiac muscle contraction (P = 5.38E − 02) are also highly enriched.
Table 3.
The enriched KEGG pathway of DEGs.
| KEGG pathway | Number of genes |
P value |
|---|---|---|
| Neuroactive ligand-receptor interaction | 54 | 3.97E − 03 |
| Tryptophan metabolism | 12 | 2.42E − 02 |
| Cardiac muscle contraction | 18 | 5.38E − 02 |
| O-Glycan biosynthesis | 9 | 6.01E − 02 |
| Taste transduction | 13 | 6.67E − 02 |
| TGF-beta signaling pathway | 19 | 7.43E − 02 |
| Arrhythmogenic right ventricular cardiomyopathy (ARVC) | 17 | 7.83E − 02 |
| Basal cell carcinoma | 13 | 9.46E − 02 |
| Colorectal cancer | 18 | 9.56E − 02 |
| Amino sugar and nucleotide sugar metabolism | 11 | 9.71E − 02 |
3.4. Protein-Protein Interaction (PPI) Network Construction
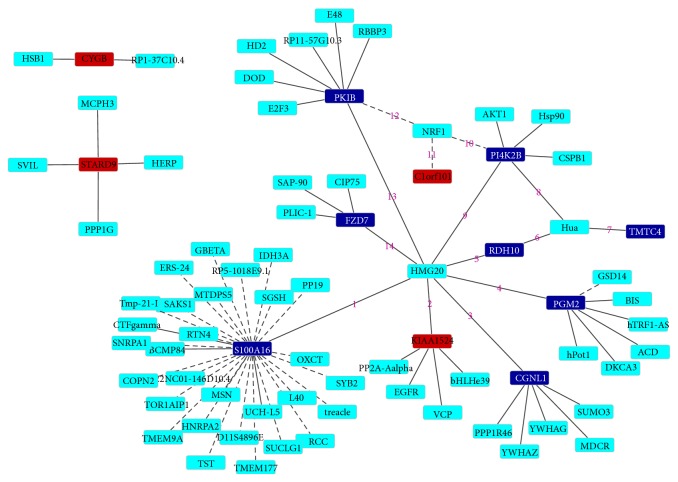
The PPI networks of the top 10 upregulated and downregulated DEGs were established by Cytoscape software including 77 nodes, 76 edges. In the PPI network the nodes with high degree are defined as hub protein, and degrees are defined to measure how many neighbors a node directly connects to. The significant hub proteins in our PPI networks contained S100A16 (Degree = 27), PKIB (Degree = 8), and PGM2 (Degree = 7) (Figure 2).
Figure 2.
The constructed PPI network for the top 10 up- and downregulated DEGs. Nodes represent proteins; edges represent interactions between two proteins. Red- and blue-color nodes represent products of up- and downregulated DEGs, respectively. Green nodes denote products of genes predicted to interact with the DEGs.
4. Discussion
Pituitary adenomas are monoclonal in origin and most of them are benign, slow-growing neoplasms, and so it is acceptable that many common oncogenes or tumor-suppressor genes which have been confirmed to be involved in human cancers have been indiscoverable in pituitary adenomas. However, advances in molecular biology make it possible to fast-track the search for candidate tumourigenic genes of pituitary adenomas. Some researchers have compared gene expression profiling and identified DEGs between normal human pituitary and pituitary adenomas of different cell origins via microarray technique. Previous microarray studies of human pituitary adenomas have detected many novel candidate genes, including PTTG [19], GADD45 [20], MEG3a [21], and BMP-4 [22].
In this paper, we chose an integrated analysis approach by which 5 microarray datasets were combined to highlight genes that were consistently expressed differentially with statistical significance, performed GO and KEGG pathway enrichment analysis for these genes, and finally constructed PPI networks of the top 10 upregulated and downregulated DEGs. In our integrated analysis, a total of 3994 genes were found to show altered expression in samples of pituitary adenoma compared with NC tissues (2043 upregulated and 1951 downregulated genes). The upregulated gene with the lowest P value was C7orf62, whose function has been unclear. The downregulated gene with the lowest P value was RDH10, a member of short chain dehydrogenase-reductase (SDR) family, which is essential for synthesis of embryonic retinoic acid. Additionally RDH10 was reported to be necessary for limb, craniofacial, and organ development by inducing proliferation arrest, differentiation, and apoptosis [23, 24]. Bankovic et al. found that patients of non-small-cell lung cancer with mutated RDH10 had shorter survival than those without mutated RDH10, confirming its importance in tumor progression. Previous study also identified that RDH10 played an important role in tumors with lymph node invasion [25]. The role and association with pituitary adenoma have not yet been reported.
In line with previous findings, some genes identified in our integrated analysis have been closely related to the tumorigenesis of pituitary adenomas, such as GADD45G, GADD45B, MEG3 [26], POU1F1 [27], IGFBP3 [28], and CCNB1 [29]. GADD45B and GADD45G belong to GADD45 gene family, and loss of GADD45 expression has been observed in various human cancers [30]. It has been proved that loss of or significantly reduced GADD45G expression is found in most of the pituitary adenomas due to promoter methylation [31]. GADD45B was displayed to be downregulated in pituitary adenomas (-68-fold) through microarray data, subsequently verified by qPCR and immunoblotting, and in vitro experiments identified its role as a tumor suppressor [32]. Cheunsuchon et al. indicated that MEG3 was specifically lost in NFAs, suggesting that its inactivation might be involved in the development of NFAs [33]. The other genes including POU1F1, IGFBP3, and CCNB1 were also reported to correlate with pituitary adenomas.
In order to reveal the biological roles of the DEGs from pituitary adenoma, we performed functional annotation for these genes. Neuroactive ligand-receptor interaction, tryptophan metabolism, and cardiac muscle contraction are found to be the top 3 significantly enriched pathways. Many signal transduction pathways are involved in the development of pituitary adenoma including TGF-β signal pathway, Wnt signal pathway, and MAPK signal pathway. Interestingly, we noted that the most significant pathway in our analysis was neuroactive ligand-receptor interaction, which is physiologically associated with the neuronal functions. The neuroactive ligand-receptor interaction pathway, which is a collection of neuroactive receptors located on the plasma membranes, is implicated in the stabilization of the neuroendocrine system, indicating that neuroactive ligand-receptor interaction pathway may be involved in the development of pituitary adenoma [34, 35].
Furthermore PPI networks of the top 10 upregulated and downregulated DEGs indicated that the significant hub proteins contained S100A16, PKIB, and PGM2. S100A16, a novel member of S100 protein family which is implicated in cognition in the central nervous system [36], is closely associated with brain pathologies [37]. S100A16 has also been shown to be ubiquitously expressed and upregulated in human tumor [38]. However its function in pituitary adenoma is still unknown for lack of related studies.
The present study has several limitations. First, although global normalization was performed to minimize the heterogeneity of various microarray studies, the heterogeneity cannot be removed completely, which may have distorted the result of analysis. Second, due to limited microarray studies in pituitary adenomas, different subtypes of pituitary adenomas were analyzed, which was not taken into account in our study. Despite these limitations, our findings have important implications for the molecular mechanisms of pituitary adenoma, adding new insights into the future therapy.
In conclusion, by the integrated analysis we have shown the underlying molecular differences between the normal human pituitary and pituitary adenomas of different cell origins and identified DEGs and biological function to contribute to the successful identification of therapeutic targets for pituitary adenoma and the development of effective targeted therapies. Further functional studies may provide additional insights into the role of the differentially regulated genes in the pathophysiology of pituitary adenoma.
Supplementary Material
The gene symbol, P-value, the average expression value in pituitary adenoma and normal control tissues, and fold change of all DEGs were available in the Supplementary Table 1.
Acknowledgments
This study was supported by the National Natural Science Fund of China (No. 30900342), the Research Special Fund for Public Welfare Industry of Health (No. 201402008) and the National High Technology Research and Development Program (“863”Program) of China.
Conflict of Interests
The authors declare that they have no conflict of interests.
References
- 1.Asa S. L., Ezzat S. The cytogenesis and pathogenesis of pituitary adenomas. Endocrine Reviews. 1998;19(6):798–827. doi: 10.1210/edrv.19.6.0350. [DOI] [PubMed] [Google Scholar]
- 2.Lee N. H., Saeed A. I. Protocols for Nucleic Acid Analysis by Nonradioactive Probes. New York, NY, USA: Springer; 2007. Microarrays; pp. 265–300. [Google Scholar]
- 3.Elston M. S., Gill A. J., Conaglen J. V., et al. Wnt pathway inhibitors are strongly down-regulated in pituitary tumors. Endocrinology. 2008;149(3):1235–1242. doi: 10.1210/en.2007-0542. [DOI] [PubMed] [Google Scholar]
- 4.Evans C.-O., Reddy P., Brat D. J., et al. Differential expression of folate receptor in pituitary adenomas. Cancer Research. 2003;63(14):4218–4224. [PubMed] [Google Scholar]
- 5.Morris D. G., Muşat M., Czirják S., et al. Differential gene expression in pituitary adenomas by oligonucleotide array analysis. European Journal of Endocrinology. 2005;153(1):143–151. doi: 10.1530/eje.1.01937. [DOI] [PubMed] [Google Scholar]
- 6.Ruebel K. H., Leontovich A. A., Jin L., et al. Patterns of gene expression in pituitary carcinomas and adenomas analyzed by high-density oligonucleotide arrays, reverse transcriptase-quantitative PCR, and protein expression. Endocrine. 2006;29(3):435–444. doi: 10.1385/ENDO:29:3:435. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqui A. S., Delaney A. D., Schnerch A., Griffith O. L., Jones S. J. M., Marra M. A. Sequence biases in large scale gene expression profiling data. Nucleic Acids Research. 2006;34(12, article e83) doi: 10.1093/nar/gkl404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feichtinger J., Thallinger G. G., McFarlane R. J., Larcombe L. D. Computational Medicine. New York, NY, USA: Springer; 2012. Microarray meta-analysis: from data to expression to biological relationships; pp. 59–77. [Google Scholar]
- 9.Ramasamy A., Mondry A., Holmes C. C., Altman D. G. Key issues in conducting a meta-analysis of gene expression microarray datasets. PLoS Medicine. 2008;5(9, article e184) doi: 10.1371/journal.pmed.0050184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tong Y., Zheng Y., Zhou J., Oyesiku N. M., Koeffler H. P., Melmed S. Genomic characterization of human and rat prolactinomas. Endocrinology. 2012;153(8):3679–3691. doi: 10.1210/en.2012-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wierinckx A., Roche M., Raverot G., et al. Integrated genomic profiling identifies loss of chromosome 11p impacting transcriptomic activity in aggressive pituitary PRL tumors. Brain Pathology. 2011;21(5):533–543. doi: 10.1111/j.1750-3639.2011.00476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barrett T., Wilhite S. E., Ledoux P., et al. NCBI GEO: archive for functional genomics data sets—update. Nucleic Acids Research. 2013;41(1):D991–D995. doi: 10.1093/nar/gks1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tusher V. G., Tibshirani R., Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(9):5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Altermann E., Klaenhammer T. R. PathwayVoyager: pathway mapping using the Kyoto Encyclopedia of Genes and Genomes (KEGG) database. BMC Genomics. 2005;6(article 60) doi: 10.1186/1471-2164-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tabas-Madrid D., Nogales-Cadenas R., Pascual-Montano A. GeneCodis3: a non-redundant and modular enrichment analysis tool for functional genomics. Nucleic Acids Research. 2012;40(1):W478–W483. doi: 10.1093/nar/gks402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giot L., Bader J. S., Brouwer C., et al. A protein interaction map of Drosophila melanogaster . Science. 2003;302(5651):1727–1736. doi: 10.1126/science.1090289. [DOI] [PubMed] [Google Scholar]
- 17.Li S., Armstrong C. M., Bertin N., et al. A map of the interactome network of the metazoan C. elegans . Science. 2004;303(5657):540–543. doi: 10.1126/science.1091403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shannon P., Markiel A., Ozier O., et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Research. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pei L., Melmed S. Isolation and characterization of a pituitary tumor-transforming gene (PTTG) Molecular Endocrinology. 1997;11(4):433–441. doi: 10.1210/me.11.4.433. [DOI] [PubMed] [Google Scholar]
- 20.Bahar A., Bicknell J. E., Simpson D. J., Clayton R. N., Farrell W. E. Loss of expression of the growth inhibitory gene GADD45γ, in human pituitary adenomas, is associated with CpG island methylation. Oncogene. 2004;23(4):936–944. doi: 10.1038/sj.onc.1207193. [DOI] [PubMed] [Google Scholar]
- 21.Zhang X., Zhou Y., Mehta K. R., et al. A pituitary-derived MEG3 isoform functions as a growth suppressor in tumor cells. The Journal of Clinical Endocrinology & Metabolism. 2003;88(11):5119–5126. doi: 10.1210/jc.2003-030222. [DOI] [PubMed] [Google Scholar]
- 22.Páez-Pereda M., Giacomini D., Refojo D., et al. Involvement of bone morphogenetic protein 4 (BMP-4) in pituitary prolactinoma pathogenesis through a Smad/estrogen receptor crosstalk. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(3):1034–1039. doi: 10.1073/pnas.0237312100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu B. X., Chen Y., Fan J., Rohrer B., Crouch R. K., Ma J.-X. Cloning and characterization of a novel all-trans retinol short-chain dehydrogenase/reductase from the RPE. Investigative Ophthalmology and Visual Science. 2002;43(11):3365–3372. [PubMed] [Google Scholar]
- 24.Picozzi P., Marozzi A., Fornasari D., et al. Genomic organization and transcription of the human retinol dehydrogenase 10 (RDH10) gene. FEBS Letters. 2003;554(1-2):59–66. doi: 10.1016/s0014-5793(03)01089-5. [DOI] [PubMed] [Google Scholar]
- 25.Bankovic J., Stojsic J., Jovanovic D., et al. Identification of genes associated with non-small-cell lung cancer promotion and progression. Lung Cancer. 2010;67(2):151–159. doi: 10.1016/j.lungcan.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Y., Zhang X., Klibanski A. Genetic and epigenetic mutations of tumor suppressive genes in sporadic pituitary adenoma. Molecular and Cellular Endocrinology. 2014;386(1-2):16–33. doi: 10.1016/j.mce.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Araujo R. V., Chang C. V., Cescato V. A. S., et al. PROP1 overexpression in corticotrophinomas: evidence for the role of PROP1 in the maintenance of cells committed to corticotrophic differentiation. Clinics. 2013;68(6):887–891. doi: 10.6061/clinics/2013(06)26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang Z.-Q., Gui S.-B., Zhang Y.-Z. Differential gene expression by fiber-optic beadarray and pathway in adrenocorticotrophin-secreting pituitary adenomas. Chinese Medical Journal. 2010;123(23):3455–3461. doi: 10.3760/cma.j.issn.0366-6999.2010.23.015. [DOI] [PubMed] [Google Scholar]
- 29.Tani Y., Inoshita N., Sugiyama T., et al. Upregulation of CDKN2A and suppression of cyclin D1 gene expressions in ACTH-secreting pituitary adenomas. European Journal of Endocrinology. 2010;163(4):523–529. doi: 10.1530/eje-10-0245. [DOI] [PubMed] [Google Scholar]
- 30.Tamura R. E., de Vasconcellos J. F., Sarkar D., Libermann T. A., Fisher P. B., Zerbini L. F. GADD45 proteins: central players in tumorigenesis. Current Molecular Medicine. 2012;12(5):634–651. doi: 10.2174/156652412800619978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bahar A., Bicknell J. E., Simpson D. J., Clayton R. N., Farrell W. E. Loss of expression of the growth inhibitory gene GADD45γ, in human pituitary adenomas, is associated with CpG island methylation. Oncogene. 2004;23(4):936–944. doi: 10.1038/sj.onc.1207193. [DOI] [PubMed] [Google Scholar]
- 32.Michaelis K. A., Knox A. J., Xu M., et al. Identification of growth arrest and DNA-damage-inducible gene beta (GADD45beta) as a novel tumor suppressor in pituitary gonadotrope tumors. Endocrinology. 2011;152(10):3603–3613. doi: 10.1210/en.2011-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheunsuchon P., Zhou Y., Zhang X., et al. Silencing of the imprinted DLK1-MEG3 locus in human clinically nonfunctioning pituitary adenomas. The American Journal of Pathology. 2011;179(4):2120–2130. doi: 10.1016/j.ajpath.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauss M., Kriegner A., Vierlinger K., Neohammer C. Characterization of the drugged human genome. Pharmacogenomics. 2007;8(8):1063–1073. doi: 10.2217/14622416.8.8.1063. [DOI] [PubMed] [Google Scholar]
- 35.Wang F., Liu W., Jin Y., et al. Transcriptional effects of prenatal and neonatal exposure to PFOS in developing rat brain. Environmental Science and Technology. 2010;44(5):1847–1853. doi: 10.1021/es902799f. [DOI] [PubMed] [Google Scholar]
- 36.Van Eldik L. J., Wainwright M. S. The Janus face of glial-derived S100B: beneficial and detrimental functions in the brain. Restorative Neurology and Neuroscience. 2003;21(3-4):97–108. [PubMed] [Google Scholar]
- 37.Ziegler D. R., Innocente C. E., Leal R. B., Rodnight R., Gonçalves C. A. The S100B protein inhibits phosphorylation of GFAP and vimentin in a cytoskeletal fraction from immature rat hippocampus. Neurochemical Research. 1998;23(10):1259–1263. doi: 10.1023/a:1020740115790. [DOI] [PubMed] [Google Scholar]
- 38.Marenholz I., Heizmann C. W. S100A16, a ubiquitously expressed EF-hand protein which is up-regulated in tumors. Biochemical and Biophysical Research Communications. 2004;313(2):237–244. doi: 10.1016/j.bbrc.2003.11.115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The gene symbol, P-value, the average expression value in pituitary adenoma and normal control tissues, and fold change of all DEGs were available in the Supplementary Table 1.