Abstract
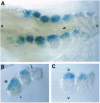
myf-5 is the only member of the MyoD family of myogenic regulatory genes to be expressed in the mouse embryo prior to muscle cell differentiation. We have used the developing limb as a model in which to follow the formation of peripheral muscle, to address the question of whether myogenic precursor cells are already present in the limb bud before expression of myf-5. The lacZ reporter gene has been introduced into the myf-5 gene by homologous recombination so that its expression is under the control of the endogenous myf-5 locus. beta-Galactosidase (beta-gal) coloration provides a sensitive assay for myf-5+ cells. Embryos were generated from embryonic stem cells carrying this mutation, and the appearance of beta-gal+ (myf-5+) cells was followed during limb development in vivo. Limb buds, at a stage when they are beta-gal-, were cultured in vitro. After several days, beta-gal+ cells accumulated in the premuscle mass. We conclude that determined muscle precursor cells in the limb bud do not initially express any member of the MyoD family. Furthermore, myogenic precursor cells in the somite, which, according to the avian model, migrate from the ventral/lateral edge of the dermomyotome to form peripheral muscle masses, are also negative for these factors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beddington R. S., Martin P. An in situ transgenic enzyme marker to monitor migration of cells in the mid-gestation mouse embryo. Somite contribution to the early forelimb bud. Mol Biol Med. 1989 Aug;6(4):263–274. [PubMed] [Google Scholar]
- Braun T., Rudnicki M. A., Arnold H. H., Jaenisch R. Targeted inactivation of the muscle regulatory gene Myf-5 results in abnormal rib development and perinatal death. Cell. 1992 Oct 30;71(3):369–382. doi: 10.1016/0092-8674(92)90507-9. [DOI] [PubMed] [Google Scholar]
- Buckingham M. Making muscle in mammals. Trends Genet. 1992 Apr;8(4):144–148. doi: 10.1016/0168-9525(92)90373-C. [DOI] [PubMed] [Google Scholar]
- Buonanno A., Apone L., Morasso M. I., Beers R., Brenner H. R., Eftimie R. The MyoD family of myogenic factors is regulated by electrical activity: isolation and characterization of a mouse Myf-5 cDNA. Nucleic Acids Res. 1992 Feb 11;20(3):539–544. doi: 10.1093/nar/20.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T. C., Hanley T. A., Mudd J., Merlie J. P., Olson E. N. Mapping of myogenin transcription during embryogenesis using transgenes linked to the myogenin control region. J Cell Biol. 1992 Dec;119(6):1649–1656. doi: 10.1083/jcb.119.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier A., Kieny M., Mauger A. Limb-somite relationship: origin of the limb musculature. J Embryol Exp Morphol. 1977 Oct;41:245–258. [PubMed] [Google Scholar]
- Christ B., Jacob H. J., Jacob M. Experimental analysis of the origin of the wing musculature in avian embryos. Anat Embryol (Berl) 1977 Mar 30;150(2):171–186. doi: 10.1007/BF00316649. [DOI] [PubMed] [Google Scholar]
- Cossu G., Ranaldi G., Senni M. I., Molinaro M., Vivarelli E. 'Early' mammalian myoblasts are resistant to phorbol ester-induced block of differentiation. Development. 1988 Jan;102(1):65–69. doi: 10.1242/dev.102.1.65. [DOI] [PubMed] [Google Scholar]
- Dienstman S. R., Biehl J., Holtzer S., Holtzer H. Myogenic and chondrogenic lineages in developing limb buds grown in vitro. Dev Biol. 1974 Jul;39(1):83–95. doi: 10.1016/s0012-1606(74)80010-2. [DOI] [PubMed] [Google Scholar]
- Doetschman T. C., Eistetter H., Katz M., Schmidt W., Kemler R. The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol. 1985 Jun;87:27–45. [PubMed] [Google Scholar]
- Hannon K., Smith C. K., 2nd, Bales K. R., Santerre R. F. Temporal and quantitative analysis of myogenic regulatory and growth factor gene expression in the developing mouse embryo. Dev Biol. 1992 May;151(1):137–144. doi: 10.1016/0012-1606(92)90221-2. [DOI] [PubMed] [Google Scholar]
- Kaehn K., Jacob H. J., Christ B., Hinrichsen K., Poelmann R. E. The onset of myotome formation in the chick. Anat Embryol (Berl) 1988;177(3):191–201. doi: 10.1007/BF00321131. [DOI] [PubMed] [Google Scholar]
- Kato K., Gurdon J. B. Single-cell transplantation determines the time when Xenopus muscle precursor cells acquire a capacity for autonomous differentiation. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1310–1314. doi: 10.1073/pnas.90.4.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieny M., Pautou M. P., Chevallier A. On the stability of the myogenic cell line in avian limb bud development. Arch Anat Microsc Morphol Exp. 1981;70(2):81–90. [PubMed] [Google Scholar]
- Lallemand Y., Brûlet P. An in situ assessment of the routes and extents of colonisation of the mouse embryo by embryonic stem cells and their descendants. Development. 1990 Dec;110(4):1241–1248. doi: 10.1242/dev.110.4.1241. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Milaire J. Contribution cellulaire des somites a la genèse des bourgeons de membres postérieurs chez la souris. Arch Biol (Liege) 1976;87(3):315–343. [PubMed] [Google Scholar]
- Montarras D., Chelly J., Bober E., Arnold H., Ott M. O., Gros F., Pinset C. Developmental patterns in the expression of Myf5, MyoD, myogenin, and MRF4 during myogenesis. New Biol. 1991 Jun;3(6):592–600. [PubMed] [Google Scholar]
- Ontell M., Ontell M. P., Sopper M. M., Mallonga R., Lyons G., Buckingham M. Contractile protein gene expression in primary myotubes of embryonic mouse hindlimb muscles. Development. 1993 Apr;117(4):1435–1444. doi: 10.1242/dev.117.4.1435. [DOI] [PubMed] [Google Scholar]
- Ordahl C. P., Le Douarin N. M. Two myogenic lineages within the developing somite. Development. 1992 Feb;114(2):339–353. doi: 10.1242/dev.114.2.339. [DOI] [PubMed] [Google Scholar]
- Ott M. O., Bober E., Lyons G., Arnold H., Buckingham M. Early expression of the myogenic regulatory gene, myf-5, in precursor cells of skeletal muscle in the mouse embryo. Development. 1991 Apr;111(4):1097–1107. doi: 10.1242/dev.111.4.1097. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Walldorf U., Eldridge J., Dübendorfer A., Frasch M., Gehring W. J. The Drosophila homologue of vertebrate myogenic-determination genes encodes a transiently expressed nuclear protein marking primary myogenic cells. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3782–3786. doi: 10.1073/pnas.88.9.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rong P. M., Teillet M. A., Ziller C., Le Douarin N. M. The neural tube/notochord complex is necessary for vertebral but not limb and body wall striated muscle differentiation. Development. 1992 Jul;115(3):657–672. doi: 10.1242/dev.115.3.657. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoon D., Lyons G., Wright W. E., Lin V., Lassar A., Weintraub H., Buckingham M. Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature. 1989 Sep 28;341(6240):303–307. doi: 10.1038/341303a0. [DOI] [PubMed] [Google Scholar]
- Selleck M. A., Stern C. D. Fate mapping and cell lineage analysis of Hensen's node in the chick embryo. Development. 1991 Jun;112(2):615–626. doi: 10.1242/dev.112.2.615. [DOI] [PubMed] [Google Scholar]
- Stockdale F. E. Myogenic cell lineages. Dev Biol. 1992 Dec;154(2):284–298. doi: 10.1016/0012-1606(92)90068-r. [DOI] [PubMed] [Google Scholar]
- Vivarelli E., Cossu G. Neural control of early myogenic differentiation in cultures of mouse somites. Dev Biol. 1986 Sep;117(1):319–325. doi: 10.1016/0012-1606(86)90374-x. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Davis R., Tapscott S., Thayer M., Krause M., Benezra R., Blackwell T. K., Turner D., Rupp R., Hollenberg S. The myoD gene family: nodal point during specification of the muscle cell lineage. Science. 1991 Feb 15;251(4995):761–766. doi: 10.1126/science.1846704. [DOI] [PubMed] [Google Scholar]
- Wright W. E. Muscle basic helix-loop-helix proteins and the regulation of myogenesis. Curr Opin Genet Dev. 1992 Apr;2(2):243–248. doi: 10.1016/s0959-437x(05)80280-1. [DOI] [PubMed] [Google Scholar]