Abstract
TLR-mediated activation of dendritic cells (DCs) is associated with a metabolic transition in which mitochondrial oxidative phosphorylation is inhibited by endogenously synthesized NO and the cells become committed to glucose and aerobic glycolysis for survival. We show that inhibition of mechanistic target of rapamycin (mTOR) extends the lifespan of TLR-activated DCs by inhibiting the induction of NO production, thereby allowing the cells to continue to use their mitochondria to generate ATP, and allowing them the flexibility to use fatty acids or glucose as nutrients to fuel core metabolism. These data provide novel mechanistic insights into how mTOR modulates DC metabolism and cellular longevity following TLR activation and provide an explanation for previous findings that mTOR inhibition enhances the efficacy of DCs in autologous vaccination.
Dendritic cells (DCs) are professional APCs that respond to infection or immunization and initiate adaptive immune responses (1, 2). TLRs are a class of receptors expressed by DCs that allow them to recognize pathogen-associated molecular patterns during either natural infection or following immunization with vaccines that contain pathogen-associated molecular pattern as adjuvants (3, 4). In response to TLR agonists, DCs become activated, a process characterized by marked changes in expression of hundreds of genes encoding a broad array of proteins such as cytokines, chemokines, and costimulatory molecules (5) that affect the way that DCs interact with other cells, most notably T cells.
TLR agonists stimulate bone marrow–derived DCs grown in GMCSF (GM-DCs) to undergo a striking metabolic transition that results in the cells becoming reliant on aerobic glycolysis to meet their bioenergetics needs (6, 7). We recently showed that this metabolic shift is a response to the inhibition of oxidative phosphorylation (OXPHOS) by NO, a toxic gas made by GM-DCs following activation (6). Once GM-DCs express inducible NO synthase (iNOS; NOS type 2 [NOS2]) and begin producing NO, mitochondrial respiration declines and they become reliant on glycolysis for ATP.
Consistent with this, glucose restriction severely inhibits the activation and lifespan of GM-DCs following TLR stimulation (6, 7).
The metabolic switch to glycolysis induced by TLR agonists is prevented by the inhibition of mechanistic target of rapamycin (mTOR) (7, 8), which regulates a central nutrient-sensing pathway in cells, controlling a diverse array of cellular responses, including cell activation, metabolism, and survival (9–12). We previously reported that inhibition of mTOR expression or function prolongs the postactivation lifespan of GM-DCs while simultaneously enhancing their activation and T cell stimulatory capacity (8). These effects were consistent with the improved ability of rapamycin (RAP)-treated GM-DCs to induce immunity in a mouse therapeutic anticancer autologous DC vaccine (8).
The underlying mechanism responsible for the beneficial effects of mTOR inhibition on the lifespan and function of TLR-activated GM-DCs has remained unclear. In this study, we demonstrate that mTOR inhibition results in the retention of mitochondrial functions in GM-DCs activated by TLR agonists. Because mTOR-inhibited DCs can still use OXPHOS, they have a reduced requirement for glucose and exhibit the metabolic flexibility to use other nutrients to generate ATP. We show that the attenuation of iNOS expression and reduced NO production are the central mechanisms for this observation in mTOR-inhibited cells. These data provide a mechanistic explanation for how mTOR inhibition modulates GMDC metabolism and cellular longevity following TLR activation. Additionally, these studies provide a more detailed understanding of how mTOR inhibition in DCs may augment DC immune stimulatory potential for cellular vaccination approaches.
Materials and Methods
Mice and reagents
C57BL/6 mice were from The Jackson Laboratory and were maintained under specific pathogen-free conditions under protocols approved by Institutional Animal Care and Use Committees. Femurs from Atgflox/flox and Atgflox × CD11c-cre mice were a gift from David Lieb (Geisel School of Medicine, Dartmouth College, Hanover, NH). LPS (Escherichia coli sero-type 0111:B4) was from Sigma-Aldrich and used at 100 ng/ml. Etomoxir and 6-diazo-5-oxo-l-norleucine were purchased from Sigma-Aldrich. NOS inhibitor S-ethyl-isothiourea (SEITU, 500 μM) was purchased from Cayman Chemical. RAP (100 nM) was purchased from InvivoGen. KU 0063794 (KU, 100 nM) was purchased from Tocris Bioscience. 7-Aminoactinomycin D (7-AAD) and all Abs for FACS analysis were from BD Biosciences except for anti-CD40, which was purchased from eBioscience.
Mouse DC culture and activation
Bone marrow–derived DCs were generated as described (13). Briefly, bone marrow cells were differentiated in the presence of GM-CSF (20 ng/ml) in complete DC media (RPMI 1640 containing 10% FCS, 100 U/ml penicillin/ streptomycin, and 2 mM l-glutamine) for 6 d. On day 6 of culture, DCs were washed in complete DC media and pulsed as indicated with media alone, mTOR inhibitor, LPS, or mTOR inhibitior plus LPS. Where indicated, after DC differentiation, cells were switched to glucose-free media or glucose-free media supplemented with galactose.
Real-Time PCR of NOS2 expression
RNA isolations were done using the RNeasy kit (Qiagen, Valencia, CA) and single-strand cDNA was synthesized using a high-capacity cDNA reverse transcription kit (Applied Biosystems, Carlsbad, CA). Primers were purchased from Applied Biosystems and real-time PCR was performed by the TaqMan method using an Applied Biosystems 7500 sequence detection system. The expression levels of mRNA were normalized to the expression of a housekeeping gene (β-actin).
In vitro T cell responses
DCs were treated as indicated for 24 h, after which media were changed to complete medium without agonists or inhibitors and cultured with daily medium changes for 3 d, after which they were cocultured, at a 1:5 ratio, with CFSE-labeled MACS-selected CD8+ OT-I cells for 5 d. T cell proliferation was measured as CFSE dilution by flow cytometry.
In vivo T cell responses
DCs pulsed with either LPS plus OVA or RAP plus LPS plus OVA for 6 h were washed and transferred into the rear footpads of C57BL/6 mice (5 × 105/footpad). Seven days later, peripheral blood was harvested and analyzed by Kb-Ova tetramer staining and flow cytometry for endogenous OVA-specific CD8+ T cells.
Metabolic analysis
Nitrate levels in cell culture media were determined using a Griess reagent kit for nitrite determination (Invitrogen) according the manufacturer's instructions. Real-time changes in extracellular acidification rates (ECARs, as a measure of lactate production) and oxygen consumption rates (OCRs, as a measure of OXPHOS) were analyzed using extracellular flux analysis (Seahorse Bioscience, North Billerica, MA). In brief, DCs were plated in XF-24 cell culture plates (2 × 105 cells/well in 200 μl) and either left unstimulated or stimulated with indicated conditions. At indicated time points, DCs were washed and analyzed in XF running buffer (unbuffered RPMI 1640, 10 mM glucose, 10% FCS, 100 U/ml penicillin/streptomycin, 2 mM l-glutamine, and 20 ng/ml GMCSF) per the manufacturer's instructions to obtain real-time measurements of OCRs and ECARs. Where indicated, ECARs and/or OCRs were analyzed in response to 1 μM oligomycin, 1.5 μM fluoro-carbonyl cyanide phenylhydrazone, and 100 nM rotenone plus 1 μM antimycin A (all from Sigma-Aldrich), or 500 μM SEITU as indicated. For ATP analysis, DCs were treated as indicated, after which cells were washed and resuspended in 100 μl PBS. Samples were boiled for 5 min and relative ATP levels were determined using an ATP determination kit (Invitrogen, Grand Island, NY) per the manufacturer's instructions. For Biolog assays, cells were treated as indicated for 24 h and then plated overnight at 5 × 104 cells per well onto PMM Tox-1 plates (Biolog) in MC-0 media (Biolog IF-M1 media [glucose free], 5% FCS, 0.3 mM l-gluta-mine). Biolog MA redox dye was added the next morning and dye reduction was measured at 540 nm absorbance at the indicated times. Data were normalized to absorbance readings at time 0.
Statistical analysis
For statistical analysis, a two-way ANOVA with a Tukey posttest was performed on data where appropriate to determine data significance (p < 0.05).
Results
mTOR inhibition attenuates LPS-mediated GM-DC death and augments GM-DC activation in vitro and in vivo
We have previously reported that mTOR inhibition in GM-DCs prolongs cell survival and improves DC vaccine therapy in the B16 mouse melanoma model (8). Consistent with these findings, inhibiting mTOR with either the canonical mTOR inhibitor RAP or the ATP-competitive inhibitor KU during TLR stimulation diminished activation-associated DC death (Fig. 1A) (8). Concomitantly, GM-DCs activated in the presence of mTOR inhibitors were substantially more capable of activating OT-I T cells in vitro (Fig. 1B). Furthermore, autologous transfer of GM-DCs pulsed with Ag plus LPS in the presence of RAP resulted in the development of enhanced Ova-specific CD8+ T cell responses in vivo (Fig. 1C). This effect was apparent in peripheral blood (Fig. 1C), and similar trends were observed for draining popliteal lymph nodes and spleen (data not shown).
FIGURE 1.
mTOR inhibition of GM-DCs enhances cell survival and augments their ability to activate CD8+ T cells in vitro and in vivo. (A) GM-DCs were either left unstimulated or activated with LPS in the presence or absence of the mTOR inhibitors RAP or KU. Cell viability of GM-DCs at 4 d after DC activation by LPS was measured by FACS analysis of 7-AAD+ cells. Graph represents mean values ± SD of at least three independent experiments. (B) DCs were treated as indicated for 24 h, after which cells were washed and replaced with normal media. Three days after activation, DCs were cocultured at a 1:5 ratio with CFSE-labeled OTI CD8+ T cells for 4 d. T cell proliferation was determined by CFSE dilution within the CD8+ cell population. Graph represents mean values ± SD of three independent experiments. (C) Mice per group were immunized s.c. with DCs stimulated in vitro for 6 h with LPS only, LPS plus OVA, or RAP plus LPS plus OVA. Seven days later, peripheral blood was harvested and frequencies of Kb-OVA tetramer+CD8+ cells were determined. Each datum point represents tetramer frequencies in an individual mouse. Graph represents pooled results from two independent experiments. *p < 0.05.
LPS-mediated GM-DC death depends on NOS2 expression and function
We have previously shown that mTOR inhibition during TLR stimulation is associated with a key metabolic change, namely decreased induction of glycolytic metabolism, in mTOR-inhibited GM-DCs compared with cells activated with TLR agonists alone (6–8). Because mTOR has been identified as a regulator of iNOS expression in other cell types (14–16), we hypothesized that mTOR inhibition may exert its beneficial effects on postactivation GM-DC lifespan through its regulation of NO production. Consistent with our previous work (8), we found that LPS-activated GM-DCs died within 4 d of activation whereas unstimulated DCs survived well during this time period (Fig. 2A). An inhibitor of iNOS, SEITU, reversed the phenotype of activation-associated GM-DC death without impairing GM-DC activation (Fig. 2A and data not shown). Additionally, iNOS-deficient GM-DCs did not exhibit the postactivation cell death observed in wild-type (WT) GM-DCs stimulated with LPS (Fig. 2B). NO was not produced by unstimulated DCs, but it was made in increasing amounts during the first 48 h following activation (Fig. 2C). Blocking iNOS function at time 0 and at 24 h after LPS stimulation prevented activation-associated GM-DC death, whereas inhibiting iNOS at 48 or 72 h after LPS stimulation had little effect on activation-associated DC death (Fig. 2D). These data demonstrate that iNOS expression and function are central regulators of GM-DC survival following TLR activation.
FIGURE 2.
LPS-mediated death in GM-DC cultures is dependent on NOS2 expression and NO production. (A) WT GM-DCs were either left unstimulated or activated with LPS in the presence or absence of the iNOS inhibitor SEITU and monitored daily for viability by FACS analysis of 7-AAD+ cells. (B) NOS2−/− GM-DCs were either left unstimulated or activated with LPS and monitored daily for viability by FACS analysis of 7-AAD+ cells. (C) Nitrite levels were measured by Griess reaction during 72 h in unstimulated or LPS-activated GM-DCs. (D) GM-DCs were either left unstimulated or activated with LPS, and the iNOS inhibitor SEITU was added to cultures at the time of activation or 1, 2, or 3 d after activation. Cell viability of GM-DCs at 4 d after GM-DC activation by LPS was measured by FACS analysis of 7-AAD+ cells. All graphs in this figure represent mean values ± SD of at least three independent experiments. *p < 0.05.
mTOR function regulates LPS-mediated NOS2 expression and NO production in GM-DCs
The rates of nitrite production and maximum nitrite accumulation in culture media were significantly reduced in GM-DCs activated with LPS in the presence of mTOR inhibitors than in cells activated with LPS alone (Fig. 3A). To determine whether mTOR signaling is involved in regulating iNOS expression in GM-DCs, we analyzed NOS2 mRNA levels in DCs treated with LPS in the presence or absence of RAP. GM-DCs stimulated with LPS in the presence of RAP exhibited a 50% reduction in NOS2 mRNA levels compared with GM-DCs stimulated with LPS alone (Fig. 3B) and a concomitant reduction in iNOS protein expression 24 h following activation (Fig. 3C), apparent as both a reduced frequency of iNOS+ GM-DCs following activation (Fig. 3D, left) and reduced iNOS protein levels in iNOS+ GM-DCs (Fig. 3D, right) compared with LPS-stimulated controls.
FIGURE 3.
mTOR inhibitors attenuate LPS-mediated NOS2 expression and NO production in GM-DC cultures. (A) GM-DCs were either left unstimulated or activated with LPS in the presence or absence of the mTOR inhibitors RAP or KU and the kinetics of nitrite accumulation were measured by Griess reaction daily for the first 72 h after GM-DC stimulation. (B) NOS2 mRNA levels from three independent experiments were analyzed by quantitative RT-PCR 4 h after LPS activation in the presence or absence of RAP. Relative gene expression levels are compared with LPS treatment group. (C) GM-DCs were treated as in (A) for 24 h and analyzed for intracellular iNOS protein expression by FACS. FACS plots are gated on CD11c+ cells. (D) GM-DCs were treated as in (A) and the percentages of iNOS+ GM-DCs (left) and the mean fluorescence intensity (MFI) of iNOS+ GM-DCs (right) were quantified by FACS. All graphs in this figure represent mean values ± SD of at least three independent experiments. *p < 0.05.
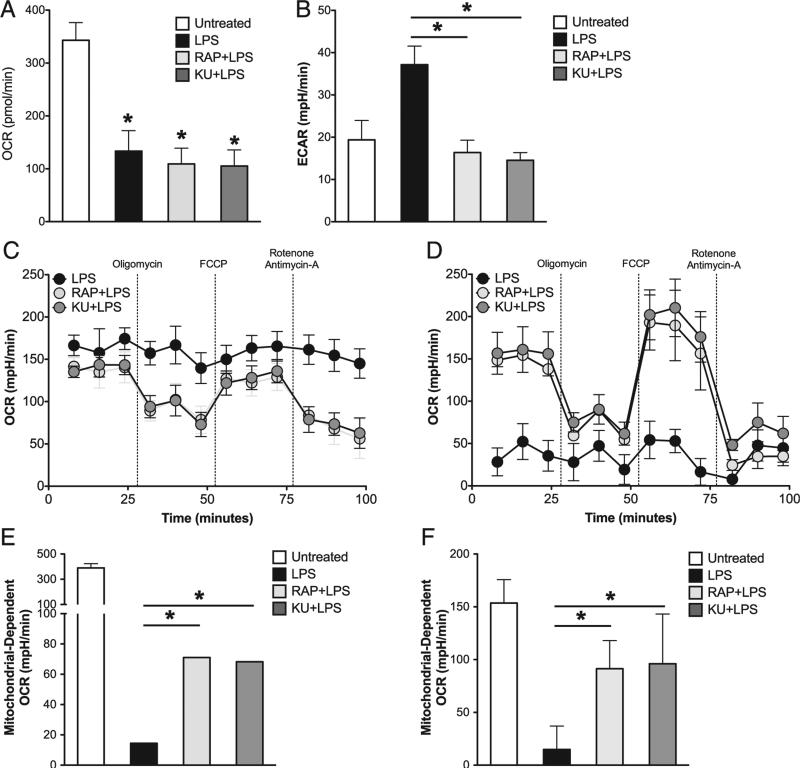
mTOR inhibition preserves mitochondrial OXPHOS in LPS-activated GM-DCs
We have previously reported that upon activation by TLR agonists, GM-DCs commit to aerobic glycolysis as OXPHOS is inhibited by NO (6, 7). Based on the fact that iNOS expression and NO production by LPS-activated GM-DCs are attenuated by mTOR inhibitors (Fig. 3), we were interested in determining whether mTOR inhibition alters the metabolic changes that typically follow TLR activation. Using real-time extracellular flux analysis, we observed that mTOR inhibition did not abrogate the LPS-induced decrease in baseline OXPHOS, measured as OCRs (Fig. 4A), but it did prevent the LPS-induced increase in aerobic glycolysis, measured as ECARs (Fig. 4B). However, despite the fact that baseline OCR was reduced in mTOR-inhibited GM-DCs compared with unactivated cells, these cells retained a measure of mitochondrial fitness, because OCRs declined in response to oligomycin, increased in the additional presence of fluoro-carbonyl cyanide phenylhydrazone, and was inhibited by mitochondrial electron transport chain inhibitors rotenone and antimycin A (Fig. 4C, 4D); this pattern of responsiveness is consistent with active OXPHOS and the production of ATP by this pathway (17). In contrast, these drugs had no measurable effect on OCR in GMDCs activated in the absence of mTOR inhibitors (Fig. 4C, 4D), indicative of the profound inhibition of mitochondrial respiration in these cells. We have previously shown that the oxygen consumption exhibited by GM-DCs stimulated with LPS in the absence of mTOR inhibitors is a reflection of oxygen being consumed for NO production (6). This is consistent with the loss of nonmitochondrial oxygen consumption by 72 h after activation (Fig. 4D), a time point when the cells are no longer making additional measurable NO (Fig. 3A). By subtracting cellular oxygen consumption after treatment with electron transport chain inhibitors from basal oxygen consumption levels, we can determine the cellular oxygen consumption that results specifically from mitochondrial activity. Using this analysis, we found that GM-DCs activated in the presence of mTOR inhibitors exhibited significantly higher levels of mitochondrial respiration compared with those activated with LPS alone at both 24 (Fig. 4E) and 72 h (Fig. 4F) after activation.
FIGURE 4.
Mitochondrial OXPHOS is preserved in GM-DCs activated by LPS in the presence of mTOR inhibitors. (A and B) GM-DCs were either left unstimulated or activated with LPS for 24 h in the presence or absence of RAP or KU. Basal oxygen OCRs (A) and ECARs (B) for each treatment group were determined by an XF extracellular flux analyzer assay. (C and D) GM-DCs were stimulated with LPS in the presence or absence of RAP or KU for 24 (C) and 72 h (D). Mitochondrial function was assessed by an XF extracellular flux analyzer assay. (E and F) Mitochondrial-dependent OCR was determined for experiments described in (C) and (D) by subtracting residual OCR after antimycin A/rotenone treatment from basal OCR levels. This calculation was performed for GM-DCs after 24 h stimulation (E) and 72 h after stimulation (F). Graphs in this figure represent mean values ± SD of at three independent experiments. *p < 0.05.
mTOR inhibition does not protect GM-DCs from mitochondrial inhibition by paracrine NO production
An important question arising from these studies is whether NO production by activated DCs inhibits mitochondrial respiration only in an autocrine fashion, or also acts on neighboring cells that cannot themselves make NO. To address this question, we compared the mitochondrial respiration and survival of LPS-stimulated WT GM-DCs, NOS2−/− GM-DCs, and a 1:1 mixture of WT and NOS2−/− GM-DCs. LPS-stimulated WT GM-DCs lost mitochondrial activity whereas NOS2−/− DCs did not following 24 h of LPS stimulation (Fig. 5A). However, mitochondrial oxygen consumption following LPS stimulation was completely inhibited in a 1:1 mixture of WT and NOS2−/− GM-DCs, indicating that NO produced by WT is able to inhibit mitochondrial function in NOS2−/− cells (Fig. 5A). Consistent with these results, cell death following LPS stimulation was as high in WT NOS2−/− GM-DC cocultures as in WT GM-DC cultures, whereas NOS2−/− GM-DCs cultured alone survived much longer following LPS stimulation (Fig. 5B). These data indicate that NO made by GM-DCs has paracrine effects on the metabolism and survival of bystander cells. We next tested whether GM-DCs in which mTOR was inhibited were intrinsically protected from the toxic effects of NO, or whether they simply make insufficient amounts of NO to induce death in either themselves or neighboring cells. We cocultured NOS2−/− GM-DCs activated with LPS with WT GM-DCs activated with LPS in the presence or absence of mTOR inhibitors and asked whether the mTOR-inhibited GM-DCs are able to induce NO-dependent cell death in iNOS-deficient cells. We found that LPS-activated WT GM-DCs induced death in iNOS-deficient GM-DCs, but that this effect was inhibited when the WT GM DCs were activated in the presence of mTOR inhibitors (Fig. 5C). These data support the view that mTOR inhibition during GM-DC activation results in sufficiently lowered NO production to prevent paracrine effects on the metabolism of neighboring cells.
FIGURE 5.
The mitochondrial inhibitory effect of LPS-stimulated NO production is a cell-extrinsic effect. (A) WT, NOS2−/−, or a 1:1 mixture of WT/NOS2−/− GM-DCs were stimulated with LPS. After 24 h, mitochondrial function was assessed by an XF extracellular flux analyzer assay. (B) DCs were treated as in (A) and monitored daily for viability by FACS analysis of 7-AAD+ cells. (C) WT GM-DCs were either left unstimulated or activated with LPS for 6 h in the presence or absence of RAP or KU. These cells were then coin-cubated with 6 h LPS-stimulated NOS2−/− DCs at a 1:1 ratio for 4 d. Cell viability was monitored daily by FACS analysis of 7-AAD+ cells. Depicted are the survival kinetics for each coincubation gated on the NOS2−/− GM-DCs in the coculture. (D) CD45.1 and CD45.2 DCs were either left unstimulated or activated with LPS in the presence or absence of KU for 6 h. Indicated combinations of the congenic populations were cocultured at a 1:1 ratio, and cell viability was monitored daily by FACS analysis of 7-AAD+ cells. Depicted are the survival kinetics for the CD45.2+ GMDCs in the coculture. Graphs in this figure represent mean values ± SD of two independent experiments.
An alternative possibility is that mTOR-inhibited cells are protected from damage by a direct mTOR-dependent process unrelated to the attenuation of NO production (18). To explicitly test this, we coincubated GM-DCs activated in the presence of an mTOR inhibitor with congenically marked GM-DCs that were activated with LPS alone. We observed that mTOR-inhibited GMDCs were as susceptible as cells activated without mTOR inhibitors to NO-mediated cell death (Fig. 5D). These data indicate that mTOR inhibition prolongs postactivation DC lifespan primarily by attenuating NO production and not by inducing an intrinsic protective mechanism against oxidative damage.
The protective effects of mTOR inhibition on GM-DCs is independent of macroautophagy
Because mTOR inhibitors are canonical stimulators of macro-autophagy, and autophagic processes have been reported to protect cells from cellular damage and oxidative stress (19), we wanted to assess the role of macroautophagy on the effects of mTOR inhibitors on NO production and cell survival in GM-DCs. Conditional Atg5-deficient GM-DCs were compared with floxed controls and no significant differences were observed between the two genotypes for iNOS protein expression (Fig. 6A), nitrite production (Fig. 6B), and the prolonged survival that is associated with mTOR inhibition (Fig. 6C). These data indicate that the attenuation of iNOS expression and the improved survival of mTOR-inhibited GM-DCs are independent of Atg5-dependent macroautophagy.
FIGURE 6.
The protective effect of mTOR inhibitors on DC survival are independent of macroautophagy. (A) GM-DCs were generated from Atg5flox/flox mice or Atg5flox × CD11c-cre mice and either left unstimulated or activated with LPS for 24 h in the presence or absence of RAP or KU. GMDCs were analyzed for intracellular iNOS protein expression by FACS. FACS plots are gated on CD11c+ cells. (B) GM-DCs were treated as in (A) and total nitrite accumulation was measured by Griess reaction after 48 h of stimulation. (C) GMDCs were treated as in (A) and cell viability of GMDCs at 4 d after DC activation by LPS was measured by FACS analysis of 7-AAD+ cells. Graphs in this figure represent mean values ± SD of two independent experiments. *p < 0.05.
GM-DCs activated in the presence of mTOR inhibitors are more dependent on fatty acid catabolism than glycolysis for cellular ATP
We have previously shown that LPS-activated GM-DCs become highly dependent on glucose and aerobic glycolysis for their postactivation survival and that limiting glucose availability further reduces the lifespan of these cells (6, 7). We hypothesized that because mTOR inhibition preserves mitochondrial function in activated GM-DCs, it should allow cells to use other nutrients (e.g., fatty acids) to fuel the TCA cycle and OXPHOS and thereby diminish reliance on glucose. To test this, we assessed the effect on cellular ATP levels when, at 18 h after activation with LPS with and without mTOR inhibitors, cells were transitioned from glucose-replete media to glucose-free medium or medium containing galactose instead of glucose, which enforces OXPHOS (20–22). We found that cellular ATP levels were reduced by ~70% when LPS-stimulated GM-DCs were cultured for 30 min in galactose-containing media or glucose-free media compared with glucose-replete medium (Fig. 7A). In contrast, ATP levels were unaffected when GM-DCs were activated with LPS in the presence of mTOR inhibitors and cultured in galactose or in glucose-free medium (Fig. 7A). Similarly, whereas transition to galactose-containing media or glucose-free media accelerated the postactivation death of LPS-stimulated GM-DCs (Fig. 7B), these conditions did not promote the death of LPS-activated GM-DCs in which mTOR was inhibited (Fig. 7B). These data show that glucose-derived ATP is not required to meet the energetic demands of activated GM-DCs in which mTOR is inhibited, and they indicate that under these conditions the cells have the flexibility to use other nutrients to support the production of ATP through OXPHOS. To confirm that mTOR-inhibited GM-DCs retain the capacity to generate cellular energy via mitochondrial-dependent processes, we measured the production of the electron transport chain substrate NADH by cells cultured in glucose-free medium supplemented specifically with glucose or with the TCA cycle substrate pyruvate (Fig. 7C, 7D). As expected, we found that DCs could use glucose to generate NADH regardless of whether they were activated with LPS in the presence or absence of mTOR inhibitors (Fig. 7C). This is consistent with the fact that cells are able to generate NADH through glycolysis in a manner that is independent of mitochondrial respiration. However, LPS-activated GM-DCs were unable to use the mitochondrial substrate pyruvic acid to produce NADH unless mTOR was inhibited during activation (Fig. 7D). These data show that mTOR inhibition preserves energy generation by mitochondrial substrates via the TCA cycle, which is in stark contrast to the attenuation of this capability in DCs stimulated with LPS alone. To explore this area further, we focused on fatty acid oxidation and glutaminolysis as two of the major catabolic pathways that, in addition to glycolysis, can fuel the TCA cycle and thereby support OXPHOS (23, 24). To test the importance of these pathways, we activated GM-DCs in the presence or absence of mTOR inhibitors for 24 h and then transferred the cells to glucose-replete media or to glucose-free media containing galactose in the presence or absence of the inhibitor etomoxir that blocks the function of carnitine palmitoyltransferase 1A required for fatty acid import into mitochondria, or the glutaminase inhibitor 6-diazo-5-oxo-l-norleucine (Fig. 7E). In these experiments, inhibition of fatty acid oxidation but not glutamine catabolism significantly impacted ATP levels in mTOR-inhibited GM-DCs. Collectively, these findings show that when mTOR is inhibited, GM-DCs can become activated and they have the flexibility to use glycolysis or fatty acid oxidation to generate ATP to meet their bioenergetic needs (Fig. 7).
FIGURE 7.
GM-DCs activated in the presence of mTOR inhibitors are insensitive to glucose deprivation but are sensitive to disruption of fatty acid catabolism. (A) GM-DCs were either left unstimulated or activated with LPS for 18 h in the presence or absence of RAP or KU. Cultures were washed and then media replaced with complete media, glucose-free media, or glucose-free media supplemented with galactose. The percentage inhibition of ATP levels compared with the complete media group 30 min after media change is depicted. (B) DCs were treated as in (A) and cell viability was monitored 24 h after media change. (C and D) Cells were treated as indicated for 24 h and then plated overnight at 5 × 104 cells per well onto PMM Tox-1 plates containing either glucose (C) or pyruvic acid (D) as the primary carbon substrate in nutrient-restricted media (no glucose, 5% FCS, 0.3 mM l-glutamine). NADH-reactive redox dye was added 18 h later and dye reduction by NADH was measured at 540 nm absorbance at the indicated times. Data are normalized to absorbance readings at time 0. (E) DCs were either untreated or stimulated with LPS in the presence or absence of KU. After 24 h, culture media was changed to glucose-replete media, glucose-free media containing galactose, or glucose-free media containing galactose in the presence of a fatty acid transporter inhibitor (etomoxir [eto]) or a glutamine antagonist (6-diazo-5-oxo-l-norleucine [DON]). Cellular ATP levels were monitored 4 d after media change. Graphs in this figure represent mean values ± SD of technical replicates that are representative of at least two independent experiments. *p < 0.05.
Discussion
We previously reported that TLR-mediated GM-DC activation is characterized by a persistent metabolic shift whereby OXPHOS is suppressed and aerobic glycolysis is significantly increased (6, 7). We have shown that these changes have important implications for GM-DC activation and survival following TLR activation, and that the long-term metabolic changes in these cells are promoted by iNOS-driven NO production, which impairs mitochondrial respiration, thereby driving these cells to rely on glycolytic metabolism for maintaining intracellular ATP levels (6). TLR-mediated metabolic changes in GM-DCs are regulated by PI3K signaling (7), and we have shown that targeted inhibition of mTOR, a key metabolic regulator and downstream target of PI3K, attenuates TLR-mediated commitment to glycolysis and activation-associated death in these cells (8). In this study, we demonstrate that mTOR inhibition reduces the transcription and translation of iNOS in response to LPS stimulation. Because NO production is significantly reduced, autocrine and paracrine inhibition of OXPHOS by NO does not occur and the cells maintain mitochondrial respiration following activation. We reason that preservation of mitochondrial function and the flexibility to use multiple nutrients to support core metabolism allows DCs (in which mTOR has been inhibited) to survive longer after TLR activation and persist under glucose-restricted conditions.
iNOS expression and NO production is an important part of the early inflammatory response initiated by inflammatory DCs stimulated with LPS (25, 26). Additionally, NO has been reported to affect a wide range of DC-mediated inflammatory functions from cellular activation to T cell stimulation (27–31). We have shown that NO production by inflammatory DCs results in auto-crine disruption of mitochondrial respiration (6), presumably through the inhibition of key iron-containing enzymes of the electron transport chain (32–34). Although mTOR signaling has been linked to NO production in cells of the CNS and macrophages (14–16, 35), to our knowledge, this is the first report demonstrating that mTOR signaling directly controls LPS-mediated iNOS induction in DCs. Although work in other cell types has demonstrated that mTOR can regulate iNOS at both the transcriptional and posttranscriptional levels (14–16), our data suggest that in DCs mTOR significantly affects NO production via regulation of iNOS expression. Strikingly, pharmacological inhibition of mTOR during LPS activation of DCs attenuates iNOS expression and NO production without impairing other important features of DC activation such as inflammatory cytokine secretion, costimulatory molecule upregulation, and the capacity to stimulate T cell responses (8). In this context, mTOR inhibition seems to provide specific dampening of the NO-mediated innate immune function of DCs that, in turn, enhances their capacity to activate the adaptive immune response through prolonged cell survival and Ag presentation kinetics (8).
One of the major unresolved discrepancies in the literature pertaining to mTOR signaling in DCs is the contrasting effects of mTOR inhibition on these cells depending on the source and subset of DCs being examined. A number of reports have demonstrated inhibitory effects of mTOR blockade on differentiation and activation of human monocyte-derived DCs (36–38), which contrasts with some of the literature studying mouse DCs (8, 39–41). Additionally, mTOR inhibitors by themselves have recently been reported to activate key signaling pathways canonically associated with immune activation signals (42). One interesting study demonstrated that mTOR inhibition augmented DC activation in freshly isolated human myeloid DCs but inhibited activation in monocyte-derived DCs differentiated in GM-CSF plus IL-4 (39), which we have also observed (E. Amiel, unpublished observations). We speculate that differential regulation of iNOS and NO production in these different systems may lie at the heart of the discrepancies regarding the effect of mTOR on DCs and think that this is an important area of exploration for future work.
Recent work from our laboratory indicates that long-term commitment to glycolysis in inflammatory DCs, rather than being an intrinsic requirement for their cellular activation, is critical for DC survival by maintaining ATP production in the absence of mitochondrial activity (6). Although glycolytic metabolism provides a short-term metabolic solution for activated, NO-producing DCs, these cells become entirely dependent on glucose availability for their survival, and their rapid postactivation death can only be modestly attenuated by glucose supplementation (7, 8). This is consistent with numerous reports demonstrating that manipulation of glycolytic metabolism can have substantial impact on cellular activation and cell death (43–49). Based on these observations, we would predict that an effective augmentation of the DC response could be achieved by allowing cellular activation while preserving mitochondrial function in these cells. In accordance with this hypothesis, we have recently shown that mTOR-inhibited GM-DCs exhibit significantly improved immunogenic potential in autologous vaccination in a melanoma tumor model (8). In this study, we show that the cellular mechanism for these improved effects is due to attenuation of iNOS expression and the concomitant maintenance of mitochondrial respiration in these cells. Based on the fact that many different immune cell types undergo substantial metabolic transitions as part of their activation and differentiation profiles (6, 50–54), we think that targeted metabolic manipulations in a diverse array of immune cells might provide promising new therapeutic approaches for influencing cellular immune responses.
In attempting to delineate the specific mechanism behind mTOR's influence on the lifespan of GM-DCs, a formal possibility remained that the affect on iNOS expression was merely incidental and not causative in our system. In particular, mTOR inhibitors have been documented to stimulate autophagy, an important cellular survival response to oxidative stress (19) that might allow them to actively protect themselves from NO-mediated mitochondrial inhibition. By demonstrating that LPS-activated GM-DCs are able to accelerate the death of both iNOS-deficient cells and mTOR-inhibited GM-DCs, we have shown that autocrine/paracrine NO toxicity is the prevailing mechanism of postactivation DC death in our system. Furthermore, we show that mTOR-inhibited GM-DCs are not intrinsically protected from NO cytotoxicity as a result of attenuated mTOR signaling and that mTOR-inhibited GM-DCs fail to make enough NO to induce paracrine NO toxicity in iNOS-deficient cells, indicating that attenuation of NO production is the definitive factor responsible for prolonged survival in mTOR-inhibited GM-DCs. The fact that these core phenotypes remain intact in Atg5-deficient DCs suggests that the effects of inhibiting mTOR in this system are independent of macroautophagy and rather depend on the inhibition of iNOS expression. These findings have implications for understanding the activation of T lymphocyte responses by inflammatory DCs, as paracrine NO-mediated effects have been documented to regulate T cell activation and proliferation in certain contexts (28).
There is an increasing appreciation of the important role that cellular metabolism plays in the regulation of immune responses to inflammatory stimuli (55, 56), and there has been a growing effort to use metabolic manipulation to influence the phenotype and function of innate and adaptive cells of the immune system (8, 50, 52, 54). In our laboratory, we have focused on investigating the metabolic requirements for DC activation, focusing specifically on how nutrient usage and energy production shifts between immature and activated DCs, and the functional consequences of these metabolic changes on the immunostimulatory capacity and cellular fates of these cells. Our data indicate that NO production by DCs and other cells in response to inflammatory signals may play a general role in limiting the lifespan of neighboring cells, and in this way be involved in premature aging of cells in inflamed sites (57). Although these questions are fundamentally interesting from a basic cell biology standpoint, understanding the metabolic basis for DC activation also has the potential to permit the development of therapeutic tools for regulating cellular immune responses in clinical settings.
Acknowledgments
We thank Stanley Huang, Marcia Nascimento, and Keke Fairfax for helpful discussions of the data. Additionally, we thank Dr. David Leib (Geisel School of Medicine, Dartmouth College) for donation of femurs from Atg5 conditional KO mice, and Yike Jiang in the Leib Laboratory for technical assistance with using these samples.
This work was supported by National Institutes of Health Grants CA164062, AI053825 (to E.J.P.), CA155823, and AI 091965 (to E.L.P.), as well as by National Institutes of Health Institutional Postdoctoral Training Award AI049823 (to E.A.), University of Vermont start-up funding (to E.A.), and by the Trudeau Institute.
Abbreviations used in this article
- 7-AAD
7-aminoactinomycin D
- DC
dendritic cell
- ECAR
extracellular acidification rate
- GM-DC
bone marrow–derived dendritic cell grown in GM-CSF
- iNOS
inducible NO synthase
- KU
KU 0063794
- mTOR
mechanistic target of rapamycin
- NOS2
NO synthase type 2
- OCR
oxygen consumption rate
- OXPHOS
oxidative phosphorylation
- RAP
rapamycin
- SEITU
S-ethyl-isothiourea
- WT
wild-type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Barton GM, Medzhitov R. Control of adaptive immune responses by Toll-like receptors. Curr. Opin. Immunol. 2002;14:380–383. doi: 10.1016/s0952-7915(02)00343-6. [DOI] [PubMed] [Google Scholar]
- 2.Steinman RM, Hemmi H. Dendritic cells: translating innate to adaptive immunity. Curr. Top. Microbiol. Immunol. 2006;311:17–58. doi: 10.1007/3-540-32636-7_2. [DOI] [PubMed] [Google Scholar]
- 3.Akira S, Takeda K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 4.Takeda K, Akira S. TLR signaling pathways. Semin. Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Amati L, Pepe M, Passeri ME, Mastronardi ML, Jirillo E, Covelli V. Toll-like receptor signaling mechanisms involved in dendritic cell activation: potential therapeutic control of T cell polarization. Curr. Pharm. Des. 2006;12:4247–4254. doi: 10.2174/138161206778743583. [DOI] [PubMed] [Google Scholar]
- 6.Everts B, Amiel E, van der Windt GJ, Freitas TC, Chott R, Yarasheski KE, Pearce EL, Pearce EJ. Commitment to glycolysis sustains survival of NO-producing inflammatory dendritic cells. Blood. 2012;120:1422–1431. doi: 10.1182/blood-2012-03-419747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krawczyk CM, Holowka T, Sun J, Blagih J, Amiel E, DeBerardinis RJ, Cross JR, Jung E, Thompson CB, Jones RG, Pearce EJ. Toll-like receptor-induced changes in glycolytic metabolism regulate dendritic cell activation. Blood. 2010;115:4742–4749. doi: 10.1182/blood-2009-10-249540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amiel E, Everts B, Freitas TC, King IL, Curtis JD, Pearce EL, Pearce EJ. Inhibition of mechanistic target of rapamycin promotes dendritic cell activation and enhances therapeutic autologous vaccination in mice. J. Immunol. 2012;189:2151–2158. doi: 10.4049/jimmunol.1103741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulati P, Thomas G. Nutrient sensing in the mTOR/S6K1 signalling pathway. Biochem. Soc. Trans. 2007;35:236–238. doi: 10.1042/BST0350236. [DOI] [PubMed] [Google Scholar]
- 10.Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meijer AJ, Codogno P. Nutrient sensing: TOR's Ragtime. Nat. Cell Biol. 2008;10:881–883. doi: 10.1038/ncb0808-881. [DOI] [PubMed] [Google Scholar]
- 12.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol. Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inaba K, Inaba M, Deguchi M, Hagi K, Yasumizu R, Ikehara S, Muramatsu S, Steinman RM. Granulocytes, macrophages, and dendritic cells arise from a common major histocompatibility complex class II-negative progenitor in mouse bone marrow. Proc. Natl. Acad. Sci. USA. 1993;90:3038–3042. doi: 10.1073/pnas.90.7.3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin HK, Ahn SH, Yoon JW, Park JW, Lee EK, Yoo JS, Lee JC, Choi WS, Han JW. Rapamycin down-regulates inducible nitric oxide synthase by inducing proteasomal degradation. Biol. Pharm. Bull. 2009;32:988–992. doi: 10.1248/bpb.32.988. [DOI] [PubMed] [Google Scholar]
- 15.Lisi L, Navarra P, Feinstein DL, Dello Russo C. The mTOR kinase inhibitor rapamycin decreases iNOS mRNA stability in astrocytes. J. Neuroinflammation. 2011;8:1–11. doi: 10.1186/1742-2094-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu DY, Liou HC, Tang CH, Fu WM. Hypoxia-induced iNOS expression in microglia is regulated by the PI3-kinase/Akt/mTOR signaling pathway and activation of hypoxia inducible factor-1a. Biochem. Pharmacol. 2006;72:992–1000. doi: 10.1016/j.bcp.2006.06.038. [DOI] [PubMed] [Google Scholar]
- 17.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bian S, Sun X, Bai A, Zhang C, Li L, Enjyoji K, Junger WG, Robson SC, Wu Y. P2X7 integrates PI3K/AKT and AMPK-PRAS40-mTOR signaling pathways to mediate tumor cell death. PLoS ONE. 2013;8:e60184. doi: 10.1371/journal.pone.0060184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee J, Giordano S, Zhang J. Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem. J. 2012;441:523–540. doi: 10.1042/BJ20111451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bustamente E, Morris HP, Pedersen PL. Hexokinase: the direct link between mitochondrial and glycolytic reactions in rapidly growing cancer cells. Adv. Exp. Med. Biol. 1977;92:363–380. doi: 10.1007/978-1-4615-8852-8_15. [DOI] [PubMed] [Google Scholar]
- 21.Chang CH, Curtis JD, Maggi LB, Jr., Faubert B, Villarino AV, O'Sullivan D, Huang SC, van der Windt GJ, Blagih J, Qiu J, et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell. 2013;153:1239–1251. doi: 10.1016/j.cell.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Goffe C, Vallette G, Jarry A, Bou-Hanna C, Laboisse CL. The in vitro manipulation of carbohydrate metabolism: a new strategy for deciphering the cellular defence mechanisms against nitric oxide attack. Biochem. J. 1999;344:643–648. doi: 10.1042/0264-6021:3440643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matés JM, Segura JA, Campos-Sandoval JA, Lobo C, Alonso L, Alonso FJ, Márquez J. Glutamine homeostasis and mitochondrial dynamics. Int. J. Biochem. Cell Biol. 2009;41:2051–2061. doi: 10.1016/j.biocel.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Vats D, Mukundan L, Odegaard JI, Zhang L, Smith KL, Morel CR, Wagner RA, Greaves DR, Murray PJ, Chawla A. Oxidative metabolism and PGC-1b attenuate macrophage-mediated inflammation. Cell Metab. 2006;4:13–24. doi: 10.1016/j.cmet.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao Y. The multiple actions of NO. Pflugers Arch. 2010;459:829–839. doi: 10.1007/s00424-009-0773-9. [DOI] [PubMed] [Google Scholar]
- 26.Wink DA, Hines HB, Cheng RY, Switzer CH, Flores-Santana W, Vitek MP, Ridnour LA, Colton CA. Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 2011;89:873–891. doi: 10.1189/jlb.1010550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adler HS, Simon A, Graulich E, Habermeier A, Bacher N, Friebe A, Closs EI, Steinbrink K. Neuronal nitric oxide synthase modulates maturation of human dendritic cells. J. Immunol. 2010;184:6025–6034. doi: 10.4049/jimmunol.0901327. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman RA, Mahidhara RS, Wolf-Johnston AS, Lu L, Thomson AW, Simmons RL. Differential modulation of CD4 and CD8 T-cell proliferation by induction of nitric oxide synthesis in antigen presenting cells. Transplantation. 2002;74:836–845. doi: 10.1097/00007890-200209270-00018. [DOI] [PubMed] [Google Scholar]
- 29.Serbina NV, Kuziel W, Flavell R, Akira S, Rollins B, Pamer EG. Sequential MyD88-independent and -dependent activation of innate immune responses to intracellular bacterial infection. Immunity. 2003;19:891–901. doi: 10.1016/s1074-7613(03)00330-3. [DOI] [PubMed] [Google Scholar]
- 30.Serbina NV, Salazar-Mather TP, Biron CA, Kuziel WA, Pamer EG. TNF/iNOS-producing dendritic cells mediate innate immune defense against bacterial infection. Immunity. 2003;19:59–70. doi: 10.1016/s1074-7613(03)00171-7. [DOI] [PubMed] [Google Scholar]
- 31.Sugita K, Kabashima K, Yoshiki R, Ikenouchi-Sugita A, Tsutsui M, Nakamura J, Yanagihara N, Tokura Y. Inducible nitric oxide synthase downmodulates contact hypersensitivity by suppressing dendritic cell migration and survival. J. Invest. Dermatol. 2010;130:464–471. doi: 10.1038/jid.2009.288. [DOI] [PubMed] [Google Scholar]
- 32.Beltrán B, Mathur A, Duchen MR, Erusalimsky JD, Moncada S. The effect of nitric oxide on cell respiration: A key to understanding its role in cell survival or death. Proc. Natl. Acad. Sci. USA. 2000;97:14602–14607. doi: 10.1073/pnas.97.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cleeter MW, Cooper JM, Darley-Usmar VM, Moncada S, Schapira AH. Reversible inhibition of cytochrome c oxidase, the ter minal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 34.Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc. Natl. Acad. Sci. USA. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chou YY, Gao JI, Chang SF, Chang PY, Lu SC. Rapamycin inhibits lipopolysaccharide induction of granulocyte-colony stimulating factor and inducible nitric oxide synthase expression in macrophages by reducing the levels of octamer-binding factor-2. FEBS J. 2011;278:85–96. doi: 10.1111/j.1742-4658.2010.07929.x. [DOI] [PubMed] [Google Scholar]
- 36.Hackstein H, Taner T, Zahorchak AF, Morelli AE, Logar AJ, Gessner A, Thomson AW. Rapamycin inhibits IL-4–induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–4463. doi: 10.1182/blood-2002-11-3370. [DOI] [PubMed] [Google Scholar]
- 37.Sathaliyawala T, O'Gorman WE, Greter M, Bogunovic M, Konjufca V, Hou ZE, Nolan GP, Miller MJ, Merad M, Reizis B. Mammalian target of rapamycin controls dendritic cell development downstream of Flt3 ligand signaling. Immunity. 2010;33:597–606. doi: 10.1016/j.immuni.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Woltman AM, van der Kooij SW, Coffer PJ, Offringa R, Daha MR, van Kooten C. Rapamycin specifically interferes with GM-CSF signaling in human dendritic cells, leading to apoptosis via increased p27KIP1 expression. Blood. 2003;101:1439–1445. doi: 10.1182/blood-2002-06-1688. [DOI] [PubMed] [Google Scholar]
- 39.Haidinger M, Poglitsch M, Geyeregger R, Kasturi S, Zeyda M, Zlabinger GJ, Pulendran B, Hörl WH, Säemann MD, Weichhart T. A versatile role of mammalian target of rapamycin in human dendritic cell function and differentiation. J. Immunol. 2010;185:3919–3931. doi: 10.4049/jimmunol.1000296. [DOI] [PubMed] [Google Scholar]
- 40.Weichhart T, Costantino G, Poglitsch M, Rosner M, Zeyda M, Stuhlmeier KM, Kolbe T, Stulnig TM, Hörl WH, Hengstschläger M, et al. The TSC-mTOR signaling pathway regulates the innate inflammatory response. Immunity. 2008;29:565–577. doi: 10.1016/j.immuni.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 41.Weichhart T, Säemann MD. The multiple facets of mTOR in immunity. Trends Immunol. 2009;30:218–226. doi: 10.1016/j.it.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Rastogi R, Jiang Z, Ahmad N, Rosati R, Liu Y, Beuret L, Monks R, Charron J, Birnbaum MJ, Samavati L. Rapamycin induces mitogen-activated protein (MAP) kinase phosphatase-1 (MKP-1) expression through activation of protein kinase B and mitogen-activated protein kinase kinase pathways. J. Biol. Chem. 2013;288:33966–33977. doi: 10.1074/jbc.M113.492702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leunda-Casi A, Genicot G, Donnay I, Pampfer S, De Hertogh R. Increased cell death in mouse blastocysts exposed to high D-glucose in vitro: implications of an oxidative stress and alterations in glucose metabolism. Diabetologia. 2002;45:571–579. doi: 10.1007/s00125-001-0752-y. [DOI] [PubMed] [Google Scholar]
- 44.MacFarlane M, Robinson GL, Cain K. Glucose—a sweet way to die: metabolic switching modulates tumor cell death. Cell Cycle. 2012;11:3919–3925. doi: 10.4161/cc.21804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madan E, Gogna R, Bhatt M, Pati U, Kuppusamy P, Mahdi AA. Regulation of glucose metabolism by p53: emerging new roles for the tumor suppressor. Oncotarget. 2011;2:948–957. doi: 10.18632/oncotarget.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Maddocks OD, Vousden KH. Metabolic regulation by p53. J. Mol. Med. 2011;89:237–245. doi: 10.1007/s00109-011-0735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson GL, Dinsdale D, Macfarlane M, Cain K. Switching from aerobic glycolysis to oxidative phosphorylation modulates the sensitivity of mantle cell lymphoma cells to TRAIL. Oncogene. 2012;31:4996–5006. doi: 10.1038/onc.2012.13. [DOI] [PubMed] [Google Scholar]
- 48.Shim H, Chun YS, Lewis BC, Dang CV. A unique glucose-dependent apoptotic pathway induced by c-Myc. Proc. Natl. Acad. Sci. USA. 1998;95:1511–1516. doi: 10.1073/pnas.95.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol. Cell. Biol. 2001;21:5899–5912. doi: 10.1128/MCB.21.17.5899-5912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drake CG. Update on prostate cancer vaccines. Cancer J. 2011;17:294–299. doi: 10.1097/PPO.0b013e3182325e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodríguez-Prados JC, Través PG, Cuenca J, Rico D, Aragonés J, Martín-Sanz P, Cascante M, Boscá L. Substrate fate in activated macrophages: a comparison between innate, classic, and alternative activation. J. Immunol. 2010;185:605–614. doi: 10.4049/jimmunol.0901698. [DOI] [PubMed] [Google Scholar]
- 54.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Odegaard JI, Chawla A. The immune system as a sensor of the metabolic state. Immunity. 2013;38:644–654. doi: 10.1016/j.immuni.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jenny NS. Inflammation in aging: cause, effect, or both? Discov. Med. 2012;13:451–460. [PubMed] [Google Scholar]