Abstract
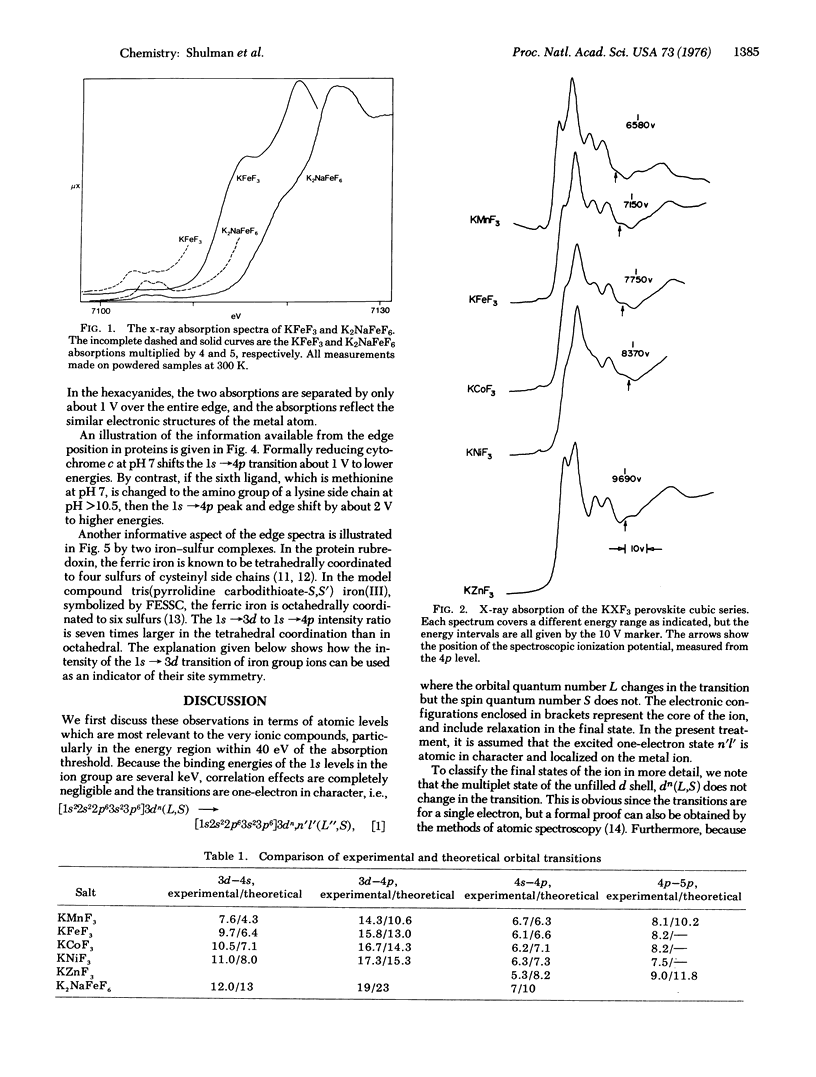
X-ray absorption spectra near the Kalpha edge have been measured in various iron group compounds using the intense synchrotron radiation at the Stanford Synchrotron Research Project. In the cubic compounds KMF3 where M = Mn+2, Fe+2, Co+2, Ni+2, and Zn+2, well resolved lines were observed and assigned to the 1s leads to 3d, 1s leads to 4s, and 1s leads to 4p transitions. The observed energies agreed rather well with the spectroscopic energy levels of the Z + 1 ion and the intensities are shown to agree with those expected on the basis of one electron transitions of the form Z 1s2dn(L,S) leads to (Z + 1)1s2dnn'l'(L",S). The energies of the intense 1s leads to 4p transition increase by about 5 V going from KFeF3 to K2NaFeF6, but only by about 1 V from K4Fe(CN)6 to K3Fe(CN)6. The transitions confirm that upon oxidation of the hexacyanides the iron electronic structure barely changes. In the iron sulfur protein rubredoxin, where the iron is bound to a tetrahedron of sulfurs, the 1s leads to 3d transition was about seven times more intense than the same transition in an octahedrally coordinated compound. These intensities parallel those observed in the d-d transitions of optical spectra, because in both types of spectra the intensities depend upon 4p admixture. In the heme protein cytochrome c, upon oxidation the 1s leads to 4p transition shifts only about 1 V to higher energies similar to the iron hexacyanides. These results are discussed in terms of covalent bonding.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmayer H., Piette L. H., Yasunobu K. T., Whiteley H. R. The binding sites of iron in rubredoxin from Micrococcus aerogenes. Proc Natl Acad Sci U S A. 1967 Jan;57(1):122–127. doi: 10.1073/pnas.57.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman R. G., Eisenberger P., Blumberg W. E., Stombaugh N. A. Determination of the iron-sulfur distances in rubredoxin by x-ray absorption spectroscopy. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4003–4007. doi: 10.1073/pnas.72.10.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]


