Abstract
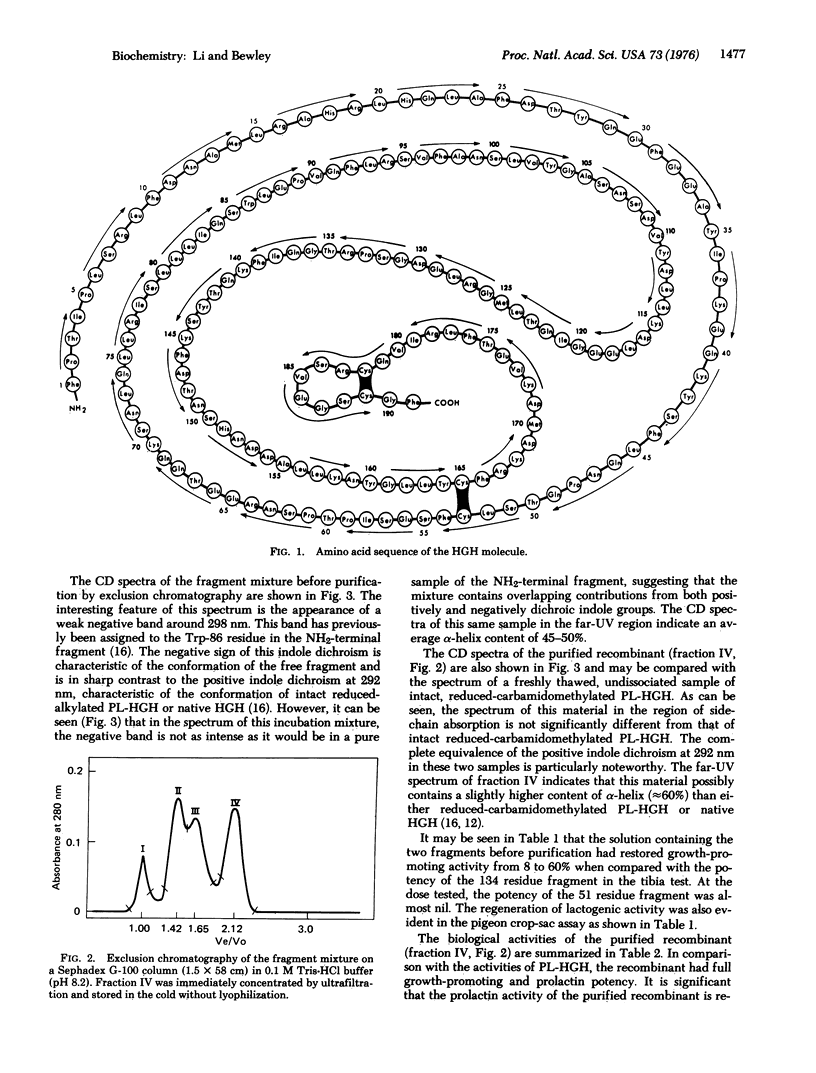
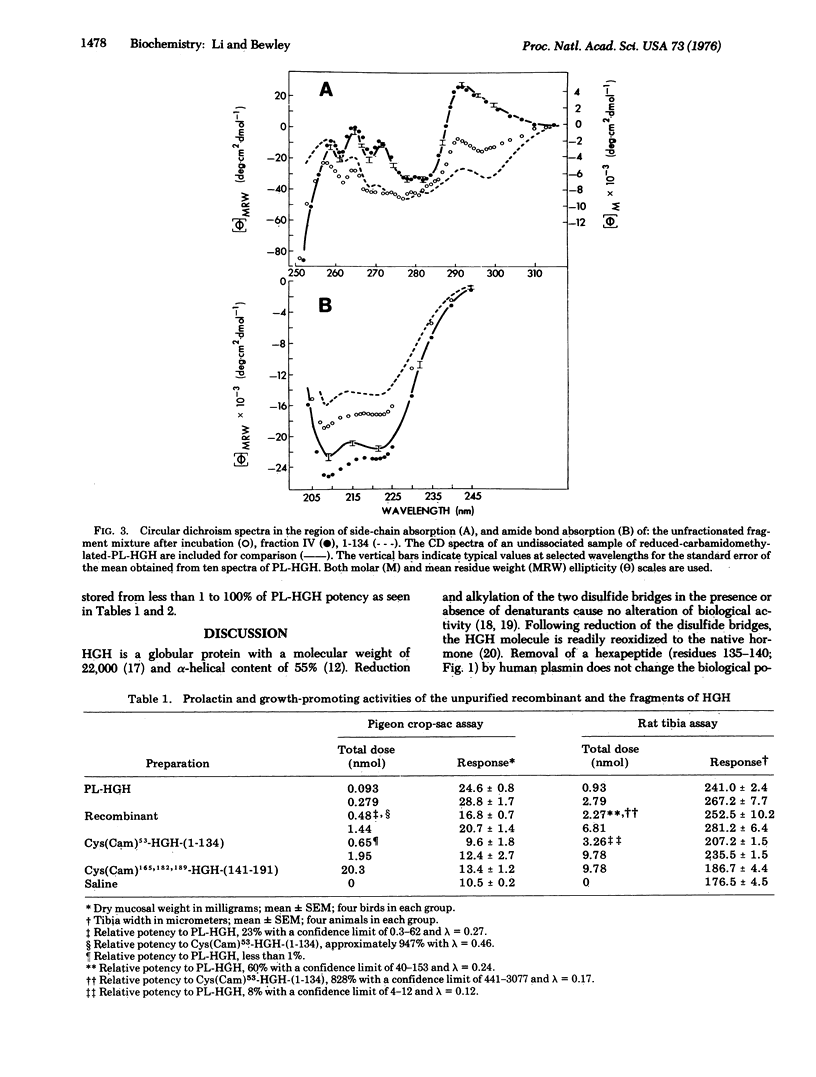
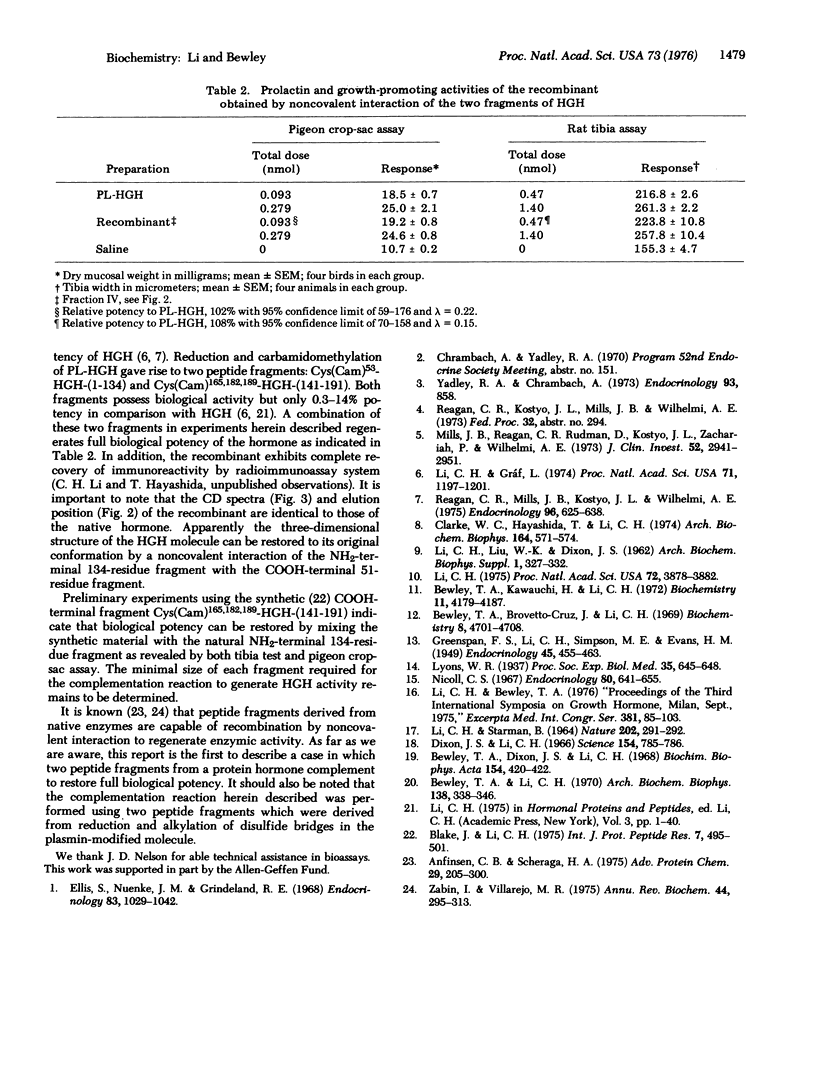
The NH2-terminal 134 amino-acid fragment of the reduced-carbamidomethylated human somatropin molecule is found to react noncovalently with the COOH-terminal 51 amino-acid fragment in solutions of pH 8.4 at 2 degrees to restore full biological activity as evidenced by the rat tibia and pigeon crop-sac assays. In addition, circular dichroism spectra of the recombinant show the conformation to be completely repaired in comparison with that of the native hormone.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anfinsen C. B., Scheraga H. A. Experimental and theoretical aspects of protein folding. Adv Protein Chem. 1975;29:205–300. doi: 10.1016/s0065-3233(08)60413-1. [DOI] [PubMed] [Google Scholar]
- Bewley T. A., Brovetto-Cruz J., Li C. H. Human pituitary growth hormone. Physicochemical investigations of the native and reduced-alkylated protein. Biochemistry. 1969 Dec;8(12):4701–4708. doi: 10.1021/bi00840a007. [DOI] [PubMed] [Google Scholar]
- Bewley T. A., Dixon J. S., Li C. H. Human pituitary growth hormone. XVI. Reduction with dithiothreitol in the absence of urea. Biochim Biophys Acta. 1968 Feb 19;154(2):420–422. doi: 10.1016/0005-2795(68)90116-5. [DOI] [PubMed] [Google Scholar]
- Bewley T. A., Kawauchi H., Li C. H. Comparative studies of the single tryptophan residue in human chorionic somatomammotropin and human pituitary growth hormone. Biochemistry. 1972 Oct 24;11(22):4179–4187. doi: 10.1021/bi00772a023. [DOI] [PubMed] [Google Scholar]
- Bewley T. A., Li C. H. Human pituitary growth hormone. XXII. The reduction and reoxidation of the hormone. Arch Biochem Biophys. 1970 May;138(1):338–346. doi: 10.1016/0003-9861(70)90315-2. [DOI] [PubMed] [Google Scholar]
- Blake J., Li C. H. The synthesis and biological activity of (165, 182, 189-S-carbamidomethylcysteine)-human growth hormone-(140-191). Int J Pept Protein Res. 1975;7(6):495–501. doi: 10.1111/j.1399-3011.1975.tb02471.x. [DOI] [PubMed] [Google Scholar]
- Clarke W. C., Hayashida T., Li C. H. Human pituitary growth hormone: immunochemical investigations of biologically active fragments. Arch Biochem Biophys. 1974 Oct;164(2):571–574. doi: 10.1016/0003-9861(74)90068-x. [DOI] [PubMed] [Google Scholar]
- Dixon J. S., Li C. H. Retention of the biological potency of human pituitary growth hormone after reduction and carbamidomethylation. Science. 1966 Nov 11;154(3750):785–786. doi: 10.1126/science.154.3750.785. [DOI] [PubMed] [Google Scholar]
- Ellis S., Nuenke J. M., Grindeland R. E. Identity between the growth hormone degrading activity of the pituitary gland and plasmin. Endocrinology. 1968 Nov;83(5):1029–1042. doi: 10.1210/endo-83-5-1029. [DOI] [PubMed] [Google Scholar]
- GREENSPAN F. S., LI C. H. Bioassay of hypophyseal growth hormone; the tibia test. Endocrinology. 1949 Nov;45(5):455-63, illust. doi: 10.1210/endo-45-5-455. [DOI] [PubMed] [Google Scholar]
- LI C. H., LIU W. K., DIXON J. S. Human pituitary growth hormone. VI. Modified procedure of isolation and NH2-terminal amino acid sequence. Arch Biochem Biophys. 1962 Sep;Suppl 1:327–332. [PubMed] [Google Scholar]
- LI C. H., STARMAN B. MOLECULAR WEIGHT OF SHEEP PITUITARY INTERSTITIAL CELL-STIMULATING HORMONE. Nature. 1964 Apr 18;202:291–292. doi: 10.1038/202291b0. [DOI] [PubMed] [Google Scholar]
- Li C. H., Gráf L. Human pituitary growth hormone: isolation and properties of two biologically active fragments from plasmin digests. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1197–1201. doi: 10.1073/pnas.71.4.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. H. Human pituitary growth hormone: a biologically active hendekakaihekaton peptide fragment corresponding to amino-acid residues 15-125 in the hormone molecule. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3878–3882. doi: 10.1073/pnas.72.10.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J. B., Reagan C. R., Rudman D., Kostyo J. L., Zachariah P., Wilhelmi A. E. Metabolic effects of plasmin digests of human growth hormone in the rat and man. J Clin Invest. 1973 Nov;52(11):2941–2951. doi: 10.1172/JCI107491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll C. S. Bio-assay of prolactin. Analysis of the pigeon crop-sac response to local prolactin injection by an objective and quantitative method. Endocrinology. 1967 Apr;80(4):641–655. doi: 10.1210/endo-80-4-641. [DOI] [PubMed] [Google Scholar]
- Reagan C. R., Mills J. B., Kostyo J. L., Wilhelmi A. E. Isolation and biological characterization of fragments of human growth hormone produced by digestion with plasmin. Endocrinology. 1975 Mar;96(3):625–636. doi: 10.1210/endo-96-3-625. [DOI] [PubMed] [Google Scholar]
- Yadley R. A., Chrambach A. Isohormones of human growth hormone. II. Plasmin-catalyzed transformation and increase in prolactin biological activity. Endocrinology. 1973 Oct;93(4):858–865. doi: 10.1210/endo-93-4-858. [DOI] [PubMed] [Google Scholar]
- Zabin I., Villarejo M. R. Protein complementation. Annu Rev Biochem. 1975;44:295–313. doi: 10.1146/annurev.bi.44.070175.001455. [DOI] [PubMed] [Google Scholar]


