Abstract
Central nervous system (CNS) complications resulting from HIV infection remain a major public health problem as individuals live longer due to the success of combined antiretroviral therapy (cART). As many as 70% of HIV infected people have HIV associated neurocognitive disorders (HAND). Many HIV infected individuals abuse drugs, such as cocaine, heroin or methamphetamine, that may be important cofactors in the development of HIV CNS disease. Despite different mechanisms of action, all drugs of abuse increase extracellular dopamine in the CNS. The effects of dopamine on HIV neuropathogenesis are not well understood, and drug induced increases in CNS dopamine may be a common mechanism by which different types of drugs of abuse impact the development of HAND. Monocytes and macrophages are central to HIV infection of the CNS and to HAND. While T cells have not been shown to be a major factor in HIV-associated neuropathogenesis, studies indicate that T cells may play a larger role in the development of HAND in HIV infected drug abusers. Drug induced increases in CNS dopamine may dysregulate functions of, or increase HIV infection in, monocytes, macrophages and T cells in the brain. Thus, characterizing the effects of dopamine on these cells is important for understanding the mechanisms that mediate the development of HAND in drug abusers.
Keywords: Macrophage, monocyte, T cell, HIV-associated neurological disorders, dopamine, drug abuse, neuroinflammation
Overview
HIV-associated neurocognitive disorders, termed HAND (Antinori et al. 2007), are present in a large percentage of the HIV infected population, despite successful antiretroviral therapy. Many HIV infected individuals abuse drugs that may have a significant impact on the neuropathogenesis of HIV infection. These drugs increase extracellular dopamine in the CNS, and several studies have examined correlations between changes in the CNS dopaminergic system and HAND (Nath et al. 2000; Purohit et al. 2011; Koutsilieri et al. 2002a; Kieburtz et al. 1991). Much of this research evaluated neuropsychological performance or global alterations in brain function (Gelman et al. 2012; Chang et al. 2008; Koutsilieri et al. 2002b; Wang et al. 2004; Kumar et al. 2011; Berger and Arendt 2000; Ferris et al. 2008). This review has a different focus, specifically on the effects of dopamine on monocytes and macrophages, the predominant cell types involved in the pathogenesis of HAND, as well as on T cells, in the context of the HIV infected CNS. We will first review briefly the pathology of HAND, the effects of drug abuse on the development of HAND, and relevant dopamine biology. We will then detail what is known about the effects of dopamine on monocytes, macrophages and T cells, and discuss how these effects may alter the development of neuroinflammation and HAND.
Background
Neuroinflammation, HAND and Drug Abuse
HIV enters the CNS within two weeks of peripheral infection (Davis et al. 1992). Entry is mediated by transmigration of infected monocytes across the blood brain barrier (BBB) in response to chemokines (Peluso et al. 1985; Epstein and Gendelman 1993; Buckner et al. 2006; Williams et al. 2012). Infected monocytes and macrophages within the CNS produce virus that infects and activates additional macrophages, as well as other CNS cells, including microglia and astrocytes (Cosenza et al. 2002; Eugenin and Berman 2007; Herbein and Varin 2010; Koenig et al. 1986; Wiley et al. 1986). Infected cells release viral proteins that can cause neuronal damage and apoptosis and exacerbate neuroinflammation (Mocchetti et al. 2012; Eugenin et al. 2007; Guha et al. 2012; Rappaport et al. 1999; Kanmogne et al. 2007). They also produce cytokines that activate resident CNS cells, as well as recruit additional monocytes and perhaps T cells into the CNS through increased chemokine expression (Herbein and Varin 2010; Xing et al. 2009; McManus et al. 2000; Roberts et al. 2004; Gaskill et al. 2012). These cytokines include IL-6, IL-10, IL-1β, TNF-α and the chemokines CCL2 and CXCL12. The effects of these and other cytokines on HIV neuropathogenesis are discussed in detail in other publications (Brabers and Nottet 2006; Tyor et al. 1992; Merrill et al. 1989; Fischer-Smith and Rappaport 2005; Kraft-Terry et al. 2009; Dhillon et al. 2008; Persidsky et al. 2000). CNS monocytes, macrophages, and other cells, both infected and uninfected, exposed to these cytokines and/or viral proteins are activated and produce additional mediators (Kraft-Terry et al. 2009; Williams and Hickey 2002; Fischer-Smith and Rappaport 2005; Nuovo and Alfieri 1996). Even in individuals on cART, low levels of immune activation, cytokine production, chronic inflammation, and HIV infection persist within the CNS (Valcour et al. 2011; Anthony et al. 2005; Kamat et al. 2012).
Chronic neuroinflammation coupled with persistent low level CNS infection create a neurotoxic environment, often causing neuronal damage and death (Wiley et al. 1986) that can lead to HAND. HAND is a spectrum of cognitive impairments and functional abnormalities that occur in approximately 40% to 70% of HIV infected individuals (Cysique et al. 2004; Heaton et al. 2010; Tozzi et al. 2005; Simioni et al. 2010; Robertson et al. 2007). Although the advent of cART has dramatically reduced systemic viral replication, improved cognitive function, and greatly increased the lifespan of HIV infected individuals, it has not eradicated HAND (Sacktor 2002; Cysique et al. 2009; Everall et al. 2005; Robertson et al. 2007; Heaton et al. 2010). Prior to cART, more individuals developed HIV-associated dementia, the most severe form of HAND. However, advances in antiretroviral therapy have shifted the presentation of HAND so that currently milder forms represent the majority of neurocognitive and motor disorders in HIV infected individuals on cART (Heaton et al. 2011; Valcour et al. 2011). As these individuals live longer, the prevalence of CNS damage and HAND is increasing (Sacktor 2002; Ances and Ellis 2007; Bhavan et al. 2008). Higher concentrations of CNS dopamine due to drug abuse may alter the neuropathogenesis of HAND.
A significant number of HIV infected individuals use illicit drugs (Sohler et al. 2007; Shurtleff and Lawrence 2012; Turner et al. 2001; Mathers et al. 2008; Beyrer et al. 2010). Several studies suggested that, prior to the advent of cART, HIV infected drug abusers developed accelerated neurocognitive dysfunction and/or neuropathologic changes as compared to the non-drug abusing HIV infected population (Langford et al. 2003; Kousik et al. 2012; Rippeth et al. 2004; Starace et al. 1998; Nath et al. 2002), or to non-HIV infected drug abusers (Gray et al. 1992; Makrigeorgi-Butera et al. 1996). These drug related changes were often characterized by increased infiltration of lymphocytes and/or CD8+ T cells into the CNS in addition to monocytes (Tomlinson et al. 1999; Bell et al. 1993; Anthony et al. 2003). This T cell influx was not observed in the absence of drug abuse, suggesting that the contribution of T cells to HIV neuropathogenesis in drug abusers is distinct. One study of drug abusers on antiretroviral therapy demonstrated that the amount of T cell infiltration was lower than that seen in individuals not on cART, but that T cells were still present and were distributed in different brain regions (Anthony et al. 2005).
Studies examining the effects of drug abuse on HAND in the era of cART described contradictory findings, which were further complicated by the possibility that drug abusers may not have adhered rigorously to their cART regimens (Mellins et al. 2009; Binford et al. 2012; Nahvi et al. 2012; Wood et al. 2003). Some studies reported that drug abuse increased neurocognitive impairment with HIV infection (Meade et al. 2011b; Meade et al. 2011a), while others indicated that it had no effect on HAND (Byrd et al. 2011; Basso and Bornstein 2003).
All classes of illegal drugs increase extracellular dopamine in the CNS (Kimmel et al. 2005; Desai et al. 2010; Di Chiara and Imperato 1988; Koob 1992a; Pierce and Kumaresan 2006; Koob and Bloom 1988; Carboni et al. 1989). This review will examine the effects of dopamine, as a model of drug abuse, on monocytes, macrophages and T cells within the HIV infected CNS. Before addressing this, we will briefly review some aspects of dopamine biology pertinent to the studies described later in this review.
Dopamine
Dopamine is a catecholamine neurotransmitter that regulates a number of physiological functions including locomotion, cognition and reward (Missale et al. 1998; Di Chiara and Bassareo 2007; Iversen and Iversen 2007). Dopamine also affects immune cells including monocytes, macrophages and T cells (Levite 2008; Gaskill et al. 2009; Gaskill et al. 2012; Besser et al. 2005). Dopamine signals primarily through dopamine receptors (DR), members of the G protein coupled receptor (GPCR) superfamily of membrane proteins. Although dopamine may also signal through other receptors (Lin et al. 2008; Hasko et al. 2002), this review focuses on dopamine signaling mediated by DR. Dopamine receptors are divided into two subclasses, the D1-like DR, D1R and D5R, and the D2-like DR, D2R, D3R and D4R. Although D1R and D2R are the most common, all DR are present in the CNS and each DR exhibits distinct expression patterns (Missale et al. 1998; Beaulieu and Gainetdinov 2011).
Dopamine receptor signaling is most often mediated by G proteins. The D1-like DR couple to Gαs or Gαolf and stimulate cAMP through activation of PKA and DARPP-32, while D2-like DR couple to Gαi or Gαq and inhibit cAMP production by blocking PKA activation. Both D1-like and D2-like DR also signal through G protein independent pathways by activation of factors such as ERK kinases, phospholipase C, or β-arrestins. Many pharmacologic agonists and antagonists have been developed to study different DR subtypes. The dissociation constants (Ki) of the ligands used by studies referenced in this review are listed in Table 1. It is important to note that this table details the Ki values only for dopamine receptors and does not list those for other receptors to which these ligands may bind. Dopamine receptors also heterodimerize, either with other DR or other GPCR, and activate additional signaling pathways (Missale et al. 1998; Girault and Greengard 2004; Beaulieu and Gainetdinov 2011; Hasbi et al. 2011; Rashid et al. 2007; Guo et al. 2008; Javitch 2004). It is unclear which or how many of these pathways mediate the effects of dopamine on monocytes, macrophages and T cells. This is an area for further investigation.
Table 1.
| Ligand | D1R | D5R | D2R | D3R | D4R | Reference |
|---|---|---|---|---|---|---|
| DR Agonists | ||||||
| Dopamine | 2313.6 | 114.45 | 1017 | 30.8 | 27.1 | 2, 3, 6, 7, 13 |
| SKF38393 | 200.4 | ~ 34 | 4927.5 | ~ 7500 | ~ 900 | 3, 6, 15 |
| SKF82958 | 11.25 | N.A. | 136 | 68 | N.A. | 3, 15 |
| Quinpirole | ~ 7300 | > 10000 | 952.4 | 21 | 619.8 | 2, 3, 6, 13, 14 |
| Bromocriptine | 968.3 | 480.3 | 4.1 | 9.7 | 251.6 | 3, 6, 13, 14 |
| Pergolide | 567.5 | 475.6 | ~ 26 | ~ 3 | 58.4 | 3, 6 |
| 7-OH-DPAT | ~ 5000 | N.A. | 255.3 | 7.2 | 694.8 | 3, 6, 12, 13 |
| U99194 | N.A. | N.A. | 2281 | 223 | > 10000 | 12 |
| PD168077 | N.A. | N.A. | 2810 | 3740 | 12.5 | 6, 11 |
| ABT 724 | N.A. | N.A. | N.A. | N.A. | 45.1 | 7–9 |
| DR Antagonists | ||||||
| SCH23390 | 10.6 | 11.9 | 913 | ~ 3425 | ~ 3720 | 2, 3, 6 |
| Sulpiride | > 10000 | > 10000 | ~ 141 | ~ 91 | ~ 709 | 2, 3, 6 |
| Chlorpromazine | ~ 112 | ~ 132 | 5 | 3.4 | 15.7 | 3, 6 |
| Spiperone | ~ 220 | ~ 4000 | 0.22 | 0.38 | 2 | 3, 6 |
| Metaclopramide | N.A | N.A | 27 | 51 | 90 | 5 |
| Domperidone | N.A. | N.A. | 1.1 | 5.2 | N.A | 2–4 |
| Eticlopride | < 10000 | < 10000 | 0.1 | 0.1 | 27 | 3 |
| L750667 | N.A | N.A | > 1700 | > 4500 | 0.51 | 1, 2 |
All Ki values are in nM and were determined by averaging the Ki values from multiple studies performed in human cells with cloned DR. All of the studies used to determine Ki values used competition binding assays with radiolabeled antagonists. The affinity states of the dopamine receptors are undefined in many of these studies and therefore values may have been determined from both low and high affinity states of each DR.
Many of the Ki determinations were generously provided by the National Institute of Mental Health’s Psychoactive Drug Screening Program, Contract # HHSN-271-2008-00025-C (NIMH PDSP). The NIMH PDSP is Directed by Bryan L. Roth MD, PhD at the University of North Carolina at Chapel Hill and Project Officer Jamie Driscol at NIMH, Bethesda MD, USA.
The ligands listed in this table may also interact with receptors other than DR.
Bibliography
Kulagowski JJ, Broughton HB, Curtis NR, Mawer IM, Ridgill MP, Baker R, Emms F, Freedman SB, Marwood R, Patel S, Patel S, Ragan CI, Leeson PD: 3-((4-(4-Chlorophenyl)piperazin-1-yl)-methyl)-1H-pyrrolo-2,3-b-pyridine: an antagonist with high affinity and selectivity for the human dopamine D4 receptor, Journal of medicinal chemistry 1996, 39:1941–1942
Patel S, Patel S, Marwood R, Emms F, Marston D, Leeson PD, Curtis NR, Kulagowski JJ, Freedman SB: Identification and pharmacological characterization of [125I]L-750,667, a novel radioligand for the dopamine D4 receptor, Molecular pharmacology 1996, 50:1658–1664
Hohman M, Gregory K, Chibale K, Smith PJ, Ekins S, Bunin B: Novel web-based tools combining chemistry informatics, biology and social networks for drug discovery, Drug discovery today 2009, 14:261–270
Seeman P, Tallerico T, Ko F: Dopamine displaces [3H]domperidone from high-affinity sites of the dopamine D2 receptor, but not [3H]raclopride or [3H]spiperone in isotonic medium: Implications for human positron emission tomography, Synapse 2003, 49:209–215
Sokoloff P, Andrieux M, Besancon R, Pilon C, Martres MP, Giros B, Schwartz JC: Pharmacology of human dopamine D3 receptor expressed in a mammalian cell line: comparison with D2 receptor, European journal of pharmacology 1992, 225:331–337
Seeman P, Van Tol HH: Dopamine receptor pharmacology, Trends in pharmacological sciences 1994, 15:264–270
Brioni JD, Moreland RB, Cowart M, Hsieh GC, Stewart AO, Hedlund P, Donnelly-Roberts DL, Nakane M, Lynch JJ, 3rd, Kolasa T, Polakowski JS, Osinski MA, Marsh K, Andersson KE, Sullivan JP: Activation of dopamine D4 receptors by ABT-724 induces penile erection in rats, Proceedings of the National Academy of Sciences of the United States of America 2004, 101:6758–6763
Cowart M, Latshaw SP, Bhatia P, Daanen JF, Rohde J, Nelson SL, Patel M, Kolasa T, Nakane M, Uchic ME, Miller LN, Terranova MA, Chang R, Donnelly-Roberts DL, Namovic MT, Hollingsworth PR, Martino BR, Lynch JJ, 3rd, Sullivan JP, Hsieh GC, Moreland RB, Brioni JD, Stewart AO: Discovery of 2-(4-pyridin-2-ylpiperazin-1-ylmethyl)-1H-benzimidazole (ABT-724), a dopaminergic agent with a novel mode of action for the potential treatment of erectile dysfunction, Journal of medicinal chemistry 2004, 47:3853–3864
Stewart AO, Cowart MD, Moreland RB, Latshaw SP, Matulenko MA, Bhatia PA, Wang X, Daanen JF, Nelson SL, Terranova MA, Namovic MT, Donnelly-Roberts DL, Miller LN, Nakane M, Sullivan JP, Brioni JD: Dopamine D4 ligands and models of receptor activation: 2-(4-pyridin-2-ylpiperazin-1-ylmethyl)-1H-benzimidazole and related heteroarylmethylarylpiperazines exhibit a substituent effect responsible for additional efficacy tuning, Journal of medicinal chemistry 2004, 47:2348–2355
Glase SA, Akunne HC, Georgic LM, Heffner TG, MacKenzie RG, Manley PJ, Pugsley TA, Wise LD: Substituted [(4-phenylpiperazinyl)-methyl]benzamides: selective dopamine D4 agonists, Journal of medicinal chemistry 1997, 40:1771–1772
Matulenko MA, Hakeem AA, Kolasa T, Nakane M, Terranova MA, Uchic ME, Miller LN, Chang R, Donnelly-Roberts DL, Namovic MT, Moreland RB, Brioni JD, Stewart AO: Synthesis and functional activity of (2-aryl-1-piperazinyl)-N-(3-methylphenyl)acetamides: selective dopamine D4 receptor agonists, Bioorganic & medicinal chemistry 2004, 12:3471–3483
Audinot V, Newman-Tancredi A, Gobert A, Rivet JM, Brocco M, Lejeune F, Gluck L, Desposte I, Bervoets K, Dekeyne A, Millan MJ: A comparative in vitro and in vivo pharmacological characterization of the novel dopamine D3 receptor antagonists (+)-S 14297, nafadotride, GR 103,691 and U 99194, The Journal of pharmacology and experimental therapeutics 1998, 287:187–197
Sautel F, Griffon N, Levesque D, Pilon C, Schwartz JC, Sokoloff P: A functional test identifies dopamine agonists selective for D3 versus D2 receptors, Neuroreport 1995, 6:329–332
Mierau J, Schneider FJ, Ensinger HA, Chio CL, Lajiness ME, Huff RM: Pramipexole binding and activation of cloned and expressed dopamine D2, D3 and D4 receptors, European journal of pharmacology 1995, 290:29–36
Qandil AM, Lewis MM, Jassen A, Leonard SK, Mailman RB, Nichols DE: Synthesis and pharmacological evaluation of substituted naphth[1,2,3-de]isoquinolines (dinapsoline analogues) as D1 and D2 dopamine receptor ligands, Bioorganic & medicinal chemistry 2003, 11:1451–1464
Many other proteins also regulate the effects of dopamine. These include the dopamine transporter (DAT), vesicular monoamine transporter 2 (VMAT2) and the enzymes tyrosine hydroxylase (TH), aromatic-amino acid decarboxylase (AADC), monoamine oxidase (MAO) and catechol-O-methyl-transferase (COMT). Dopamine transporter and VMAT2 recycle dopamine from the extracellular space (Horn 1990; Weihe and Eiden 2000). Monoamine oxidase and COMT catabolize dopamine in the cytoplasm after reuptake by transporters (Kopin 1994; Napolitano et al. 1995). Tyrosine hydroxylase and AADC are necessary for catecholamine biosynthesis (Daubner et al. 2011; Molinoff and Axelrod 1971). These proteins, in addition to dopamine receptors, regulate dopamine production, uptake and signaling within the CNS (Gonon and Buda 1985; Rouge-Pont et al. 2002; Missale et al. 1998; Beaulieu and Gainetdinov 2011).
Dopamine concentrations in the human brain and the precise effects of drugs of abuse on CNS dopamine are not well defined. In animal models, studies using both pulse and cyclic voltammetry found basal dopamine levels to be in the low nanomolar range (Parsons and Justice 1992; Venton et al. 2003; Gonon and Buda 1985). In rodents and monkeys, extracellular dopamine was significantly elevated by drugs of abuse, increasing concentrations to the low micromolar range (Zachek et al. 2010; Schiffer et al. 2003; Kimmel et al. 2005; Carboni et al. 1989; Di Chiara and Imperato 1988). Intraperitoneal injection of methamphetamine into rats increased striatal dopamine by as much as six thousand percent above basal levels (Xi et al. 2009). Drug induced increases in extracellular dopamine resulted in its efflux from the synapse into the surrounding tissue (Cragg and Rice 2004; Venton et al. 2003; Garris et al. 1994), exposing monocytes, macrophages, and T cells in the CNS to increased dopamine. The effects of dopamine on immune cells are not well characterized. How these effects might impact the development of HAND is the focus of this review.
Dopamine and HIV in Monocytes and Macrophages
Monocytes and Dopamine in HIV Neuropathogenesis
Monocytes play a major role in the development of HAND and are the primary cell type responsible for bringing HIV into the CNS (Peluso et al. 1985; Epstein and Gendelman 1993; Buckner et al. 2006). Few studies examined DR expression in monocytes and the effects of dopamine on these cells. One described low expression of D2R, D3R, D4R and D5R on the cell surface of human monocytes by flow cytometry (McKenna et al. 2002), while another showed that human monocytes expressed D4R mRNA (Watanabe et al. 2006). One report showed that dopamine treatment of human cells of the monocyte lineage that were isolated by adherence decreased proliferation and significantly suppressed LPS induced binding of NF-κB to the TNF-α promoter (Bergquist et al. 2000). Studies in rats showed that dopamine decreased infiltration of monocytes into grafted kidneys (Hoeger et al. 2008).
Two broadly defined human monocyte subpopulations have been described based on their expression of CD14, the LPS receptor, and CD16, the FcγIII receptor. In healthy individuals, 90 – 95% of circulating monocytes are only CD14+, while 5–10% are CD14+ and CD16+ (Ziegler-Heitbrock et al. 1993), which are considered to be more mature. In HIV infected individuals (Nockher et al. 1994; Thieblemont et al. 1995) and SIV infected monkeys (Kim et al. 2010), CD14+CD16+ monocytes were increased in the circulation. Studies showed that these cells were preferentially infected with HIV or SIV when compared to CD14+CD16− cells (Ancuta et al. 2006; Ellery et al. 2007; Williams et al. 2005; Pulliam et al. 1997). Even in individuals on cART, HIV proviral DNA was detected in CD14+CD16+ monocytes (Crowe et al. 2003; Delobel et al. 2005; Lopez et al. 2010). There was a correlation between the amount of HIV proviral DNA in these cells and the severity of neurocognitive impairment in individuals on cART (Shiramizu et al. 2005; Valcour et al. 2010). In other studies, the expansion of the CD14+CD16+ population in the periphery also correlated with CNS disease pathogenesis in both HIV and SIV infection (Pulliam et al. 1997; Fischer-Smith et al. 2001; Burdo et al. 2010), and increased numbers of CD14+CD16+ monocytes in the brain tissues of HIV infected humans (Fischer-Smith et al. 2001) and SIV infected monkeys (Clay et al. 2007) correlated with the presence of CNS virus. These data indicate that CD14+CD16+ monocytes transport HIV across the BBB into the CNS in vivo, and that these cells are central to the pathogenesis of HAND. Our laboratory demonstrated that HIV infected CD14+CD16+ cells preferentially transmigrate across a tissue culture model of the human BBB in response to the chemokine CCL2 (Buckner et al. 2011; Williams et al. 2012).
The studies described at the beginning of this section were performed on the total peripheral blood monocyte population and did not distinguish between monocyte subsets. It is not known how dopamine mediates the functions of CD14+CD16+ monocytes. Our laboratory is studying the effects of dopamine on this population. These studies will address which DR are expressed on CD14+CD16+ cells, how dopamine mediates their functions and contributes to their transmigration across a model of the BBB. Characterizing the effects of dopamine on this mature monocyte population is important to understanding the impact of drug abuse on HIV infection of the CNS and the development of HAND.
Macrophages in HIV Neuropathogenesis
When mature CD14+CD16+ monocytes transmigrate across the BBB into the CNS parenchyma, they differentiate into macrophages. These cells are central to the development of HAND. They are the primary target for HIV infection in the CNS and have a major regulatory role in neuroinflammation (Williams and Hickey 2002; Williams et al. 2001; Glass et al. 1995; Koenig et al. 1986). Infected macrophages within the CNS elaborate viral proteins that are neurotoxic (Eugenin et al. 2007; King et al. 2010; Guha et al. 2012) and produce virus that infects additional macrophages as well as microglia and, to a lesser extent, astrocytes (Eugenin et al. 2011; Eugenin and Berman 2007; Cosenza et al. 2002). Infected macrophages have altered cytokine production, as do uninfected cells, in response to mediators produced by infected or activated cells. This promotes neuroinflammation and the development of HAND (Yadav and Collman 2009; Williams and Hickey 2002; D’Aversa et al. 2008; Zheng et al. 2001; Witwer et al. 2009). In the brains of drug abusers, these processes occur in the presence of elevated dopamine. However, the effects of dopamine on macrophages in the HIV infected CNS have not been examined. We will first describe the proteins expressed by macrophages that enable them to respond to dopamine. Second, we will detail the effects of dopamine on HIV infection of macrophages and on macrophage functions, including cytokine production, phagocytosis, and production of nitric oxide (NO) and hydrogen peroxide (H2O2), and how these effects may contribute to the development of HAND.
Dopaminergic Protein Expression in Macrophages
Our laboratory showed that human monocyte-derived macrophages express mRNA for all DR subtypes and protein for D1R, D2R, D3R and D4R in the plasma membrane, and for DAT, VMAT2, TH and AADC (Gaskill et al. 2009; Gaskill et al. 2012). Another study also found D1R in human macrophages (Liang et al. 2008). One report in human promyelocytic U937 cells showed enzymatically active AADC (Kokkinou et al. 2009), while another found TH and VMAT2 in LPS stimulated U937 cells but not in LPS stimulated THP-1 cells (Capellino et al. 2010). The second study also showed TH and VMAT2 in CD163+ macrophages from arthritic but not normal tissues. In contrast to these findings are data that showed VMAT2 to be absent from all human macrophages except Langerhans cells (Anlauf et al. 2006). Studies in rodent microglia demonstrated mRNA for D1R, D2R, D4R and D5R in rat cells and mRNA for D1R and D5R in mouse cells (Farber et al. 2005). Tyrosine hydroxylase was detected in mouse macrophage cell lines, and TH mRNA was increased in LPS treated RAW 264.7 cells (Brown et al. 2003; Flierl et al. 2007). Both MAO and COMT were found in murine alveolar macrophages, and expression of these enzymes was altered by LPS (Flierl et al. 2007). One study in RAW 264.7 macrophages found no DAT expression (Rudd et al. 2005). Differences in the expression of these proteins among experiments may be due to the assays used, the cell type, species, variations inherent in primary cells, and/or to differential regulation of expression by inflammation or other environmental stimuli. Despite differences, these findings demonstrate that macrophages express the proteins necessary for dopaminergic signaling.
Dopamine Mediated Changes in HIV Infection of Macrophages
Our data showed that treatment with dopamine significantly increased HIV replication in primary human macrophages. Treatment of macrophages with the D2-like DR agonist quinpirole but not the D1-like DR agonist SKF82958 also increased viral replication (Gaskill et al. 2009), suggesting that the effect was mediated specifically through a D2-like DR. However, additional data from our laboratory indicated that treatment of macrophages with the D1-like DR agonist SKF38393 also significantly increased HIV replication (unpublished data). We are performing further analyses of macrophage DR to define more completely the receptors that mediate changes in HIV replication. Dopamine also increased the number of macrophages infected with HIV, which is one mechanism by which dopamine increases HIV replication in these cells (Gaskill et al. 2009).
In SIV infected rhesus macaques, treatment with L-DOPA, the biosynthetic precursor of dopamine, or selegiline, which inhibits dopamine catabolism by blocking MAO activity, increased brain viral load (Czub et al. 2001; Czub et al. 2004). The increased viral load suggests an increase in the number of SIV infected CNS macrophages in these animals. Selegiline also significantly increased the number of SIV mRNA expressing cells in the frontal cortex, hippocampus and basal ganglia (Czub et al. 2001). Together, these findings indicate that elevated dopamine in the CNS may increase HIV replication and that this may be due, at least in part, to an increase in the number of infected macrophages within the brain.
Effects of Dopamine on Macrophage Functions
Cytokines and Chemokines
There are few studies that examine the effects of dopamine on human macrophages. Our data demonstrated that dopamine increased secretion of IL-6 and CCL2 by human macrophages and increased IL-6, CCL2, IL-10 and CXCL8 in these cells when treated with LPS (Gaskill et al. 2012). Elevated IL-10 was shown to increase HIV infection of macrophages by upregulating surface expression of CD4 and CCR5 (Sozzani et al. 1998; Wang et al. 2002; Tuttle et al. 1998). IL-6, CCL2 and CXCL8 may promote neuroinflammation by activating resident CNS cells, as well as by damaging the BBB and increasing the transmigration of monocytes into the CNS (Eugenin et al. 2006; Gouwy et al. 2008; McManus et al. 2000; Kossmann et al. 1997; Gruol and Nelson 1997; Roberts et al. 2012). Conversely, increases in IL-10 and CCL2 could be neuroprotective and inhibit the development of inflammation (Eugenin et al. 2003; Knoblach and Faden 1998). We showed previously that CCL2, when expressed early during a neurotoxic process, protects neurons from HIV tat induced apoptosis (Eugenin et al. 2003) and therefore can protect against cell death as well as be inflammatory.
Dopamine significantly decreased production of TNF-α in LPS treated human macrophages (Gaskill et al. 2012). Another group showed a similar finding in U937 cells treated with reserpine, which inhibits vesicular uptake of dopamine by blocking VMAT. In that study, a reserpine-mediated increase in cytoplasmic dopamine in LPS- or PMA- treated U937 cells also significantly decreased TNF-α (Capellino et al. 2010). These findings indicate that dopamine may decrease TNF-α production in macrophages activated in response to inflammatory stimuli. However, the previously discussed study in selegiline treated monkeys showed an increase in TNF-α mRNA in microglia in brains with increased dopamine (Czub et al. 2004). In contrast to the other studies, this finding suggests that dopamine may increase macrophage TNF-α secretion. Based on these data, it is difficult to determine how increased dopamine might alter macrophage TNF-α production, and how this would affect the development of HAND. A decrease in this cytokine could reduce neuronal death (McCoy et al. 2006) but also decrease the anti-viral activity of TNF-α (Lane et al. 1999; Bailer et al. 2000), while an increase could promote more inflammation and neurotoxicity (Belarbi et al. 2012; Buscemi et al. 2007).
Other studies examining the effects of dopamine on cytokines used murine macrophages and rodent models, often with LPS or other stimuli. In mice with staphylococcal enterotoxin B induced sepsis, treatment with the pan DR antagonist chlorpromazine induced IL-10, an effect that was negated using the D1-like DR agonist SKF38393 but not the D2-like DR agonist quinpirole. This effect was seen in athymic and SCID mice but not phagocyte-depleted mice, indicating that chlorpromazine was acting specifically on macrophages (Tarazona et al. 1995). Experiments in LPS treated mouse peritoneal macrophages demonstrated that dopamine significantly decreased IL-12 and significantly increased IL-10. These effects were not blocked by either D1-like or D2-like DR antagonists, but were partially inhibited by the β-adrenergic receptor antagonist propanolol (Hasko et al. 2002), suggesting the effects may occur, at least in part, through non-DR mediated signaling. Another study in LPS treated primary rodent microglia showed that dopamine did not significantly alter production of either IL-6 or TNF-α (Farber et al. 2005).
These studies demonstrate that dopamine mediated regulation of macrophage cytokine production is complex and may involve signaling pathways mediated by receptors other than DR. The effects of dopamine may also vary with the experimental system and types of stimuli used. The differences in the results among these studies underscore the need to be cautious in the interpretation of data obtained with the use of pharmacological agonists and antagonists. The functions of many of these ligands were defined over short time periods in transfected cell lines that overexpress DR. Therefore, the use of these agents for longer time periods in experimental systems using primary cells or animal models may produce off-target effects or pharmacological activities that had not been previously attributed to these molecules.
Despite these caveats, the data indicate that dopamine mediates cytokine and chemokine production in macrophages. The specific effects of dopamine would depend on its concentration, the kinetics of its release and location in which it is encountered. The effects of the cytokines produced will similarly depend on their localization and timing of secretion. Overall, dopamine mediated changes in macrophage cytokine production may be significant in development of HAND in drug abusers.
Phagocytosis
There are no studies directly examining the effects of dopamine on the phagocytic activity of human macrophages. In vitro studies using murine and chicken macrophages showed that activation of D2-like DR increased IFN-γ induced phagocytosis and phagocytosis of IgG-coated sheep red blood cells (RBC) and E. coli. In murine macrophages, phagocytosis was blocked by the D2-like DR antagonist spiperone, but not by other DR antagonists (Sternberg et al. 1987). In chicken macrophages, phagocytosis was inhibited by the D2-like DR antagonist metaclopromide (Ali et al. 1994). In a study in guinea pigs, treatment with DR agonists showed improved clearance of radiolabeled IgG-coated RBC, whereas DR antagonists decreased clearance. The decrease was greatest when animals were treated with D2-like DR antagonists (Gomez et al. 1999). These studies demonstrate that dopamine alters macrophage phagocytosis, possibly through activation of D2-like DR.
Activation of D2-like DR inhibits cAMP (Beaulieu and Gainetdinov 2011; Missale et al. 1998), suggesting that inhibition of cAMP increases phagocytosis. However, wall lizard macrophages treated with the phosphodiesterase inhibitor IBMX, which blocks cAMP production, showed decreased phagocytosis (Roy and Rai 2004). In rat peritoneal macrophages, treatment with the D2-like DR antagonist domperidone increased phagocytosis of S. aureus (Carvalho-Freitas et al. 2011). This suggests that the D2-like DR mediated effects may occur through more than one signaling pathway and use a mechanism that does not involve cAMP, such as activation of β-arrestin 2. The wall lizard study also found that dopamine both increased and decreased macrophage phagocytic activity, depending on the concentration used (Roy and Rai 2004). In the chicken macrophage study, a longer exposure to dopamine reversed the effect and reduced phagocytosis of sheep RBC (Ali et al. 1994). These results demonstrate the complexity of dopaminergic regulation of phagocytosis, suggesting that dopamine may both stimulate and inhibit this process depending on its concentration and the kinetics of exposure.
An additional mechanism by which dopamine may mediate phagocytosis is by altering FC receptor expression (Aderem and Underhill 1999). Both the chicken and guinea pig studies demonstrated by flow cytometry that dopamine increased macrophage surface expression of different Fcγ receptors (Ali et al. 1994; Gomez et al. 1999). In the guinea pig, the increase in Fc receptors was primarily mediated through D2-like DR (Gomez et al. 1999). In the context of HAND, dopaminergic alterations in macrophage phagocytosis could disrupt scavenging of apoptotic cells and cell debris, as well as inhibit the macrophage response to HIV associated opportunistic pathogens. A dopamine mediated increase in phagocytosis could also interfere with regulated tissue repair resulting in damage to healthy cells and tissue (Laskin et al. 2011).
Nitric Oxide and Hydrogen Peroxide
Reactive nitrogen and oxygen species, including NO and H2O2, are crucial to macrophage function, as they enable cytotoxic and antimicrobial activity (MacMicking et al. 1997; Pacelli et al. 1995; Nathan 1983). A study using N9 murine microglial cells showed that dopamine significantly decreased LPS induced NO as measured using Greiss reagent. In this study, dopamine also minimally reduced the production of iNOS (Chang and Liu 2000). In a separate study in murine microglia using similar techniques, dopamine, D1-like, and D2-like DR agonists also decreased NO release. This study used patch-clamping to show that dopamine, D1-like and D2-like agonists reduced the inward rectifying potassium conductance Kir and sometimes induced an outward current (Farber et al. 2005). This suggests that DR may mediate some of their effects through changes in potassium conductance. Other studies using rat peritoneal macrophages examined changes in oxidative bursts mediated by the D2-like DR antagonist domperidone. Using flow cytometric analysis of 2′,7′-dichlorofluorescein diacetate and fluorescent S. aureus to measure production of H2O2 and phagocytosis respectively, these studies found that domperidone increased spontaneous oxidative bursts, while decreasing those induced by phagocytosis. It is unclear why domperidone affected the two types of oxidative bursts differently, although the authors suggest its effects may be related to changes in in vivo prolactin levels (Carvalho-Freitas et al. 2008; Carvalho-Freitas et al. 2011). Dopaminergic changes in production of NO and H2O2 in the CNS of HIV infected individuals may interfere with macrophage antimicrobial activity, enabling neuroinvasion by opportunistic pathogens (Tan et al. 2012).
Dopamine and HIV in T Cells
T Cells in HIV Neuropathogenesis
While monocytes and macrophages are central to the neuropathogenesis of HIV, the contributions of T cells to HIV-induced CNS disease are less clear and few studies have reported T cells within the infected CNS. In one study examining hippocampal brain sections from individuals with AIDS, increased numbers of CD4+ and CD8+ T cells were detected in sections from infected individuals with HIV encephalitis (HIVE) as compared to those without HIVE, regardless of whether they received cART (Petito et al. 2003). Another study reported that CD8+ T cells were detected in prefrontal cortex and frontal white matter brain sections from HIV infected individuals on cART (Nguyen et al. 2010). T cell infiltration is also important in another HIV related pathology, immune reconstitution inflammatory syndrome (IRIS). In a small number of HIV infected individuals, partial to full restoration of the immune system by cART initiates an inflammatory response in the CNS that is characterized as a CD8+ T cell encephalitis, termed CNS-IRIS (Johnson and Nath 2011).
The importance of CD8+ T cells in the immune response to viral infection was demonstrated by increased peripheral viral load and accelerated disease progression, including SIV encephilitis, in some SIV-infected monkeys depleted of these cells (Schmitz et al. 1999; Madden et al. 2004; Marcondes et al. 2008). In macaques with normal T cell profiles, SIV-specific CD8+ cytotoxic T cells (CTL) were detected in the CNS soon after infection (von Herrath et al. 1995) and in another study using this model, T cell influx correlated with impairment of CNS function (Marcondes et al. 2001). These data suggest that an immune response in the early stages of SIV infection of the CNS, characterized by an influx of activated CD8+ CTL, is generated to eliminate virus but that this response also contributes to CNS damage and neurocognitive impairments. However, anti-viral immune functions of T cells are also beneficial in minimizing viral load and HIV pathogenesis in both the periphery and the CNS (Schmitz et al. 1999).
In cART-naïve HIV infected individuals, drug abuse was postulated to induce significant T cell infiltration of the CNS. Postmortem studies on the brains of HIV infected drug abusers in the pre-cART era reported more pathologic changes when compared to HIV infected individuals with no history of drug abuse. HIV neuropathogenesis in drug abusers was characterized by a significant level of CD8+ T cell infiltration into the CNS (Anthony et al. 2003; Tomlinson et al. 1999; Bell et al. 1993). The role of T cells in HIV infected drug abusers in the era of cART is less clear. In an autopsy study, CD8+ T cell infiltration of the basal ganglia and hippocampus was quantified in drug abusers pre-cART and in HIV infected individuals on cART, the majority of which were drug abusers. The number of CD8+ T cells in these regions was significantly decreased with cART; however, in some areas of the white matter, lymphocytic infiltration was similar to that seen in pre-cART drug abusers (Anthony et al. 2005). These data indicate that T cells are present in the CNS of HIV infected drug abusers on cART, but that the magnitude and areas of T cell involvement are different when compared to the neuropathology in pre-cART studies. It has been suggested that T cell influx may be a component of the immune response generated to control HIV within the CNS during the early stages of infection, similar to SIV infection of the CNS. However, a dysregulated T cell immune response in HIV-infected drug abusers may result in bystander CNS damage and neuroinflammation, contributing to an increase in cognitive deficits.
Elevated dopamine may mediate T cell influx into the brain of HIV infected drug abusers. This is supported by data obtained from SIV infected monkeys given selegiline or L-DOPA to increase CNS dopamine. In these animals, lymphocytic encephalitic lesions and perivascular accumulation of lymphocytes were increased in the CNS during the asymptomatic phase of SIV neuropathogenesis (Czub et al. 2004; Czub et al. 2001). Thus, increased extracellular dopamine in the CNS during the early stages of infection may contribute to increased transmigration of lymphocytes across the BBB.
To delineate the effects of dopamine on T cells, we will first describe dopaminergic protein expression in these cells. We will then discuss how activation of DR alters the functions of specific T cell subsets, followed by a description of CNS-IRIS, and a possible role for dopamine in the pathogenesis of this T cell encephilitis in HIV infected drug abusers. Dopamine mediated changes in HIV infection of T cells will also be addressed.
Dopaminergic Protein Expression in T Cells
Many studies showed T cells expressed D2R, D3R, D4R and D5R (Santambrogio et al. 1993; McKenna et al. 2002; Ghosh et al. 2003; Besser et al. 2005; Sarkar et al. 2006; Watanabe et al. 2006). The expression of D1R is less clear, as conflicting studies reported its presence or absence in these cells (McKenna et al. 2002; Boneberg et al. 2006; Watanabe et al. 2006; Cosentino et al. 2007; Kipnis et al. 2004; Nakano et al. 2008; Basu et al. 2010). Other dopaminergic proteins, including TH, VMAT2 and COMT, were also expressed by T cells (Tsao et al. 1998; Cosentino et al. 2007; Bidart et al. 1983). While MAOs have not been demonstrated specifically in T cells, these cells do contain catecholamine metabolites, suggesting that active MAOs are present (Cosentino et al. 2000). All of these proteins are differentially expressed by specific T cell subsets, and expression is dependent on the activation state and/or differentiation of these cells.
T cells are divided into two main groups characterized by their expression of CD4 or CD8. CD4+ T cells can differentiate into multiple subsets including Tregs or T helper 1 (Th1), Th2 or Th17 effector T cells (Teffs) (Zhu and Paul 2008; Mosmann et al. 1986). Tregs are also CD25+ FoxP3+, expand in the presence of TGF-β and secrete several cytokines, including IL-10 and TGF-β. The main role of Tregs is to suppress a variety of immune functions effected by mature/differentiated CD4+ and CD8+ T cells, including suppression of activation-induced proliferation of Teffs (Vignali et al. 2008; Peterson 2012). Th1 cells are characterized by the secretion of IFN-γ and are involved in cellular immunity elicited to control intracellular pathogens and tumor cells. Th2 cells are characterized by the secretion of IL-4 and are involved in the immune response to extracellular pathogens. Th17 cells are characterized by the secretion of IL-17, participate in the immune response to extracellular pathogens and have been implicated in autoimmune diseases (Cua et al. 2003; Chen et al. 2006; Komiyama et al. 2006; Langrish et al. 2005; Peterson 2012; Bettelli et al. 2008). CD8+ T cells can differentiate into several subsets including CTL effector cells (Williams and Bevan 2007).
Human resting CD4+ T cells expressed low levels of D2R and D3R mRNA. Upon activation with Con A, D2R message increased while D3R message was totally downregulated. In human resting CD8+ T cells, D3R message and protein were highly expressed, while D4R mRNA was at low levels. Upon activation with PHA, protein and mRNA for both D3R and D4R were completely downregulated (Watanabe et al. 2006). A study using murine cells showed that Tregs expressed significantly more D1R and D5R mRNA than mature CD4+ Teffs (Kipnis et al. 2004). Another study in human cells showed that Tregs expressed more D2R, D3R, D4R and D5R mRNA than Teffs. The same study demonstrated that Tregs and CD4+ Teffs both expressed VMAT2 mRNA, and Tregs expressed mRNA for TH, which was minimally present in CD4+ Teffs (Cosentino et al. 2007).
T cells were shown to produce dopamine (Bergquist et al. 1994; Cosentino et al. 2000), which mediated autocrine stimulation of dopamine receptors (Bergquist et al. 1994; Cosentino et al. 2007). While both T effs and Tregs stored dopamine in intracellular compartments, Tregs stored significantly more (Cosentino et al. 2007). This indicates that these cells are more likely to secrete dopamine to impact their own functions and those of nearby cells that express DR.
In general, T cell exposure to dopamine was immunostimulatory or immunosuppressive depending on the activation state and/or differentiation of different T cell subsets and on the activation of specific DR expressed by these different subsets. Both Tregs and Teffs, may accumulate at sites of inflammation, and autocrine or paracrine exposure to dopamine may directly affect these cells by interacting with specific DR, altering immune responses elicited at inflammatory sites within the CNS. In the following sections, we will focus on the immunostimulatory and immunosuppressive effects of dopamine on specific resting and activated T cell subsets, the DR implicated in these effects, and how dopamine-mediated regulation of T cell function may contribute to the pathogenesis of HAND in HIV infected drug abusers.
Immunostimulatory Effects of Dopamine on T Cells
Cytokines
The effects of dopamine on cytokine secretion in vitro are dependent on the T cell subset, the method of cellular activation, and the specific DR activated. In resting T cells, dopamine stimulation of D3R significantly increased TNF-α secretion (Besser et al. 2005). CD8+ T cells expressed significantly more D3R mRNA and protein as compared to naïve CD4+ T cells (Watanabe et al. 2006), suggesting that TNF-α production may be mediated by D3R on resting CD8+ T cells.
Dopamine mediated changes in cytokine secretion were also studied in activated T cells. T cell activation/differentiation is induced in vitro by a variety of methods including T cell receptor activation with antigenic peptides or anti-CD3/CD28 antibodies, or treatment with PHA or Con A. Cytokines can also regulate T cell activation/differentiation. In human PBMC activated by anti-CD3/CD28 antibodies, dopamine increased the number of CD4+ and CD8+ T cells producing IFN-γ, TNF-α and IL-6 (Torres et al. 2005). In another study, mRNA for D3R, IL-4 and IL-10 increased in human T lymphoblasts generated by incubation of resting cells with PHA and IL-2, while IFN-γ mRNA remained undetectable. In these cells, the D2-like DR agonist quinpirole reduced IL-4 and IL-10 and increased IFN-γ, although it had no effect on cytokine mRNA expression in resting T cells (Ilani et al. 2004). Since D3R is upregulated in T lymphoblasts, the effects of quinpirole were attributed to activation of D3R. This study also performed experiments on activated, purified CD4+ and CD8+ T cells. Treatment of activated CD4+ T lymphoblasts with quinpirole reduced IL-4 and IL-10 but increased IFN-γ mRNA. In activated CD8+ T lymphoblasts, quinpirole treatment only increased IFN-γ mRNA (Ilani et al. 2004). These results suggest that in the presence of dopamine, activated CD4+ T cells undergo a Th2 to Th1 shift and this effect may be mediated by D3R. Activation of D3R may also induce IFN-γ in activated CD8+ T cells. In another study, different results were seen in CD4+ T cells activated with anti-CD3/CD28. In these cells, dopamine increased a Th2 shift, as measured by increased IL-4 production (Nakano et al. 2009).
Thus, dopamine mediated changes in the production of specific cytokines by resting and activated T cell subsets may impact CNS inflammatory responses that occur with HIV infection and drug abuse. Alterations in cytokine expression may result in neuronal damage, contributing to HAND.
Chemotaxis
In the CNS, there is low level, chemokine mediated constitutive transmigration of activated T cells across the BBB. Resting T cells also enter the CNS but to a lesser extent (Hickey et al. 1991). In vitro, dopamine selectively induced low level chemotaxis of naïve human and murine CD8+ T cells, but did not affect the migration of naïve human and murine CD4+ T cells, or activated CD4+ and CD8+ T cells (Watanabe et al. 2006). In this study, suboptimal concentrations of the chemokines CCL19 (MIP-3β), CCL21 (6Ckine) or CXCL12 (SDF-1) alone did not induce significant migration of naïve human CD4+ or CD8+ T cells. The addition of dopamine with these chemokines increased the migration of CD8+, but not CD4+, naïve T cells when compared to dopamine alone, and this was inhibited by the D3R antagonist U99194 (Watanabe et al. 2006). In another study, dopamine also increased the migration of human resting CD8+ T cells, but not T cells activated with anti-CD3/CD28 (Strell et al. 2009). These data indicate that dopamine may increase the ability of resting CD8+ T cells to transmigrate into the CNS in response to specific chemokines. Resting CD8+ T cells express significantly more D3R than resting CD4+ T cells (Watanabe et al. 2006), indicating that dopamine activation of D3R may increase the transmigration of this T cell subset across the BBB.
Activation of integrins on the T cell surface mediates T cell transmigration across the vasculature in response to chemokines (Springer 1994), and has been implicated in the chemotaxis of T cells in response to dopamine. Dopamine or the D3R agonist, 7-OH-DPAT, induced adhesion of naïve CD8+ T cells to fibronectin through activation of the integrins VLA-4 and VLA-5 and to ICAM-1 through LFA-1 (Watanabe et al. 2006). Another study reported that dopamine, 7-OH-DPAT, and the D2-like DR agonists bromocriptine and pergolide increased the binding of resting human T cells to fibronectin, which was mediated by activation of VLA-4 and VLA-5 (Levite et al. 2001). In a flow-through adhesion assay with human microvascular EC, resting CD8+ T cells adhered at very low rates to EC and had a high rolling activity, while anti-CD3/CD28 activated CD8+ T cells exhibited higher adhesion and lower rolling. Dopamine increased the adhesion of resting CD8+ T cells to EC while it had no effect on the rolling of these cells. The adhesion and rolling of activated CD8+ T cells was not affected by dopamine (Strell et al. 2009). Increased adhesion facilitates diapedesis (Muller 2011), indicating that dopamine mediated activation of integrins on the surface of resting CD8+ T cells may increase their transmigration across the BBB in response to a chemokine gradient. This may contribute to increased accumulation of this T cell subset in inflammatory sites within the CNS of HIV infected drug abusers.
Dendritic Cell Activation of T Cells
Presentation of antigen by dendritic cells results in the differentiation of naïve T cells into specialized effector cells (Steinman 2007). In basal conditions the CNS is believed to lack detectable dendritic cells. However, recent studies suggested that there are dendritic cells in the CNS, specifically during neuroinflammation, injury, and infection with protozoans, bacteria or viruses (D’Agostino et al. 2012; Colton 2012). The presence of dendritic cells in the CNS may contribute to T cell mediated neuroinflammation, as occurs during multiple sclerosis. In experimental autoimmune encephalomyelitis (EAE), a murine model of multiple sclerosis, studies reported that dendritic cells in the CNS contribute to the development of EAE by regulating the differentiation and activation of Th17 cells (Bailey-Bucktrout et al. 2008; Bailey et al. 2007). Th17 cells were shown to alter tight junction expression and disrupt the BBB, resulting in their transmigration into the CNS. Th17 cell localization within the CNS resulted in damage to neurons and increased neuroinflammation through CD4+ T cell recruitment (Kebir et al. 2007; Huppert et al. 2010).
Dendritic cells stored dopamine in secretory vesicles and secreted it during antigen-specific adhesion and activation of T cells (Nakano et al. 2009). Treatment of human dendritic cells with L750667, a D2-like DR antagonist, increased dendritic cell-mediated Th17 differentiation of CD4+ T cells while the D1-like DR antagonist SCH23390 inhibited Th17 differentiation (Nakano et al. 2008). In another experiment from this study, SCH23390 completely blocked the development of EAE clinical symptoms in vivo, while sulpiride, a D2-like DR antagonist, induced an accelerated, hyperacute form of EAE (Nakano et al. 2008). Antagonizing D1-like DR on dendritic cells inhibited Th17 differentiation and blocked the development of EAE, an inflammatory disease of the CNS. These data suggest that autocrine and/or paracrine stimulation of D1-like DR on dendritic cells increases the ability of these cells to induce CD4+ T cell differentiation into Th17 cells, increasing the severity neuroinflammation. Thus, activation of DR on dendritic cells may have effects on CNS inflammatory responses mediated by altered Th17 differentiation. The role of Th17 cells in HIV neuropathogenesis in drug abusers has not been characterized and is an important area for future study.
Treg Function
Tregs suppress the immune activity of Teffs. These immunosuppressive effects are necessary to inhibit inflammatory responses and autoimmunity (Vignali et al. 2008). Treg suppression of CD4+ Teff proliferation induced by PHA or anti-CD3/CD28 in both human and murine T cells was inhibited by dopamine and the D1-like DR agonist, SKF38393, while the dopamine-mediated inhibition was blocked by SCH23390, a D1-like DR antagonist. Dopamine also inhibited IL-10 and TGF-β production, which are the main immunosuppressive cytokines produced by Tregs, and SCH23390 also blocked this effect (Kipnis et al. 2004; Cosentino et al. 2007). The expression of D1R/D5R in Tregs was significantly higher as compared to Teffs (Kipnis et al. 2004), suggesting that D1-like DR activation by dopamine is more likely to occur in Tregs as opposed to Teffs. These data suggest that D1R/D5R activation in Tregs reduced IL-10 and TGF-β production by this T cell subset, and blocked the ability of these cells to inhibit activation induced Teff proliferation. As a result of dopamine mediated inhibition of Treg function, an enhanced and/or prolonged Teff immune response may occur.
Dopamine may also inhibit the migration of Tregs to inflammatory sites. As compared with Teffs, Tregs preferentially expressed the chemokine receptors CCR4 and CCR8 (Sebastiani et al. 2001). Dopamine inhibited the migration of murine Tregs to the CCR4 ligand, CCL22 (MDC) (Kipnis et al. 2004). Dopamine can not only inhibit Treg function, but may also inhibit CCL22-mediated migration of Tregs to inflammatory sites, which may contribute further to an immunostimulatory effect of dopamine in the CNS. In a murine model of HIV encephalitis, T regs exhibited anti-inflammatory and neuroprotective functions (Liu et al. 2009; Gong et al. 2011). Dopamine mediated inhibition of Treg function in the CNS may reduce this protective role, contributing to an increase in neuroinflammation and neurotoxicity in HIV infected drug abusers.
As previously discussed, CD8+ CTL entered the CNS of SIV infected monkeys during the early phase of infection, which correlated with impairment of CNS function (von Herrath et al. 1995; Marcondes et al. 2001). However, accelerated disease progression and increased peripheral viral loads occurred in SIV infected monkeys lacking CD8+ T cells (Schmitz et al. 1999; Madden et al. 2004). These data suggest that effector functions of CD8+ T cells in the CNS are necessary to inhibit viral pathogenesis, but that neurological damage results from CD8+ CTL-mediated immune responses. Tregs may function as suppressors of HIV specific CD8+ T cell effector functions in the CNS, decreasing viral loads while limiting immune mediated damage. Thus, dopamine mediated suppression of T reg function in HIV infected drug abusers may contribute to increased CNS damage.
Immunosuppressive Effects of Dopamine in T Cells
The inhibition of activation induced T cell proliferation and effector function by dopamine may contribute to the suppression of a T cell mediated immune response, which may increase viral load in the CNS of HIV infected drug abusers. Dopamine inhibited the proliferation of purified human CD4+ and CD8+ T cells activated with anti-CD3 and IL-2. Additionally, dopamine inhibited CD8+ CTL killing of target K562 cells, and these suppressive effects on T cell function were blocked by the D1-like DR antagonist SCH23390 (Saha et al. 2001).
In human CD8+ T cells activated with anti-CD3/CD28, dopamine decreased the number of activated cells and also decreased IL-2 production in these cells (Strell et al. 2009). Another study showed activation of resting human T cells with anti-CD3/CD28 and the D4R agonists PD168077 and ABT 724 inhibited T cell proliferation, suggesting that D4R mediated this effect. Production of IL-2 was also inhibited by PD168077 (Sarkar et al. 2006). In these experiments, quiescence in T cells was regulated by the transcription factor, Krüppel-like Factor-2 (KLF-2), and expression of KLF-2 in resting T cells was reduced significantly upon activation with anti-CD3/CD28. When activation was performed in the presence of the D4R agonists, KLF-2 did not change, suggesting that D4R stimulation induced T cell quiescence by inhibiting activation induced suppression of KLF-2 (Sarkar et al. 2006).
Tregs produce IL-10, a major immunosuppressive cytokine of Teff function (Vignali et al. 2008; Peterson 2012). Dopamine, the D1-like DR agonist SKF38393 and the D2-like DR agonist quinpirole, but not the D3R agonist 7-OH-DPAT or the D4R agonist PD168077, significantly increased IL-10 secretion by resting human T cells (Besser et al. 2005). In another study, resting CD4+ T cells expressed D2R mRNA, which was absent in CD8+ T cells, suggesting that activation of D2-like DR in CD4+ T cells contributed to the dopamine-induced increase in IL-10 (Watanabe et al. 2006). In human PBMC activated by anti-CD3/CD28 in the presence of dopamine, there was an increase in CD4+ cells producing IL-10. However, dopamine also increased the number of CD4+ and CD8+ T cells producing IFN-γ, TNF-α and IL-6 in PBMC activated by anti-CD3/CD28 (Torres et al. 2005), indicating that dopamine can have both immunostimulatory and immunosuppressive effects.
The method of T cell activation contributes to the effects of dopamine on T cell proliferation and cytokine secretion. In contrast to anti-CD3/CD28 activation, dopamine inhibited mitogen-induced proliferation and differentiation of T cells in PBMC cultures. Dopamine inhibited proliferation and the expression of IL-2, IFN-γ and IL-4 in T cells treated with anti-CD3, a method of Treg activation in vitro. Dopamine also suppressed Lck and Fyn, components of the T cell receptor complex. The effects of dopamine on cytokine production were mediated by activation of D2R or D3R, as they were negated by the D2R and D3R antagonists eticlopride and U99194, but not by antagonists for D1-like DR or D4R (Ghosh et al. 2003). These results are in contrast to anti-CD3/CD28 activation of PBMC, in which dopamine increased IFN-γ and IL-4 (Torres et al. 2005). Dopamine did not alter human Treg suppression of TNF-α and IFN-γ production by activated Teffs (Cosentino et al. 2007). However, dopamine did inhibit the suppressive effect of Tregs on PHA or anti-CD3/CD28 stimulated Teff proliferation through activation of D1-like DR (Kipnis et al. 2004; Cosentino et al. 2007). All of these data underscore the immunostimulatory and immunosuppressive effects of dopamine on T cell function.
Immune Reconstitution Inflammatory Syndrome
Ten to thirty percent of HIV infected individuals beginning cART develop IRIS (Shelburne et al. 2005; Puthanakit et al. 2006; Meintjes and Boulle 2012; Haddow et al. 2012). One study of a Canadian cohort found an estimated 1% developed CNS-related IRIS (McCombe et al. 2009), while in a South African cohort the rate of CNS involvement was 28% (Asselman et al. 2010). CNS-IRIS is a T cell-mediated encephalitis that occurs after partial to full restoration of the immune system in HIV infected individuals beginning cART. This hyperacute immune response can be initiated by opportunistic infections of the CNS (Woods et al. 1998; Jenny-Avital and Abadi 2002; Tan et al. 2009; Nelson and Zunt 2011; Tsambiras et al. 2001; Martin-Blondel et al. 2011; Post et al. 2012a, b). These infections may have been present and treated in HIV infected individuals before their initiation of cART, resulting in an exacerbated immune response to residual infection upon reconstitution of the immune system. In other individuals, an undetected opportunistic infection is unmasked after cART, but in many cases of CNS-IRIS there is no identifiable infectious pathogen. These cases may be due to an enhanced immune response directed against the HIV reservoir within the CNS, an autoimmune response directed against CNS proteins, or a nonspecific inflammatory response generated within the CNS (Post et al. 2012b).
CNS-IRIS is characterized by acute infiltration of the CNS by activated CD8+ T cells, although a more chronic type of T cell encephalitis is also believed to occur in the absence of opportunistic infections. Chronic CNS-IRIS in HIV infected individuals may contribute to the development of HAND due to CNS damage caused by prolonged immune activation (reviewed in (Johnson and Nath 2011)). HIV infected individuals who do not mount an effective Treg response after cART initiation may be more prone to develop IRIS due to ineffectual suppression of hyperimmune responses after reconstitution of the immune system (Shankar et al. 2008; Martin-Blondel et al. 2012). During the chronic form of CNS-IRIS, elevated extracellular dopamine as a result of drug abuse may inhibit Treg function within the CNS, exacerbating this chronic immune activation and the development of HAND. The effect of dopamine on the development of CNS-IRIS has not been characterized. Based on data from previous studies on the effects of dopamine on CD8+ T cells and CD4+ Tregs, we suggest that dopamine may contribute to CNS-IRIS in HIV infected drug abusers. This is an area for further study.
Dopamine Mediated Changes in HIV Infection of T Cells
Both CD8+ T cells and CD4+ Tregs may play a role in the neuropathogenesis of HIV. CD4+ Tregs are able not only to alter immune functions in CD8+ T cells, but may also transport HIV into the CNS later in the course of HIV/AIDS. Previous studies showed that dopamine increased HIV replication in Jurkat T cells and primary human T cells (Rohr et al. 1999a). Transcriptional activity of NF-κB and Sp1 binding sites in the HIV LTR was increased by dopamine and involved the transcription factors CREB and Coup-TF (Rohr et al. 1999b; Rohr et al. 1999a). Dopamine mediated increases in transcription and replication were potentiated by TNF-α, which was increased in resting T cells treated with dopamine (Besser et al. 2005). This indicates that dopamine may act on several pathways to increase HIV replication in T cells.
Dopamine also activated HIV replication in chronically infected ACH-2 T lymphoblasts. These effects were inhibited by glutathione and N-acetyl cysteine, suggesting they were due to oxidative stress induced by dopamine oxidation products (Scheller et al. 2000). These studies indicate that infected CD4+ T cells, once within the CNS of drug abusers, may have increased viral replication in response to elevated dopamine. Additionally, if the CD4+ T cells are latently infected, interaction with elevated dopamine or dopamine oxidation products in the CNS of drug abusers may reactivate viral replication. Uninfected and/or infected CD4+ T cells have not been shown to play a major role in the neuropathogenesis of HIV but transmigration of peripheral CD4+ Tregs into the CNS in a murine model of HIV encephalitis has been reported (Liu et al. 2009). Once in the CNS, HIV replication in infected Tregs may also be increased by dopamine.
Conclusion
Drug abuse is a common cofactor in HIV infection (Sohler et al. 2007; Shurtleff and Lawrence 2012; Turner et al. 2001; Mathers et al. 2008). Despite different mechanisms of action, all types of illicit drugs increase CNS dopamine (Koob 1992b; Pierce and Kumaresan 2006; Di Chiara and Imperato 1988; Carboni et al. 1989; Kimmel et al. 2005; Desai et al. 2010; Koob and Bloom 1988). This elevated dopamine in HIV infected drug abusers may increase HIV infection and/or dysregulate several functions of monocytes, macrophages, and T cells, impacting CNS damage associated with HIV infection. These changes are depicted in Figures 1 and 2. Figure 1 illustrates the mechanisms by which monocytes, macrophages and T cells contribute to neuroinflammation and HAND. Figure 2 postulates how these mechanisms are altered by higher concentrations of dopamine in the CNS of drug abusers, increasing neuroinflammation and thereby exacerbating HAND. Understanding the effects of dopamine on monocytes, macrophages and T cells in the context of HIV infection of the CNS is important for characterizing the mechanisms that mediate the development of HAND in HIV infected drug abusers. This may indicate new targets for therapeutic intervention.
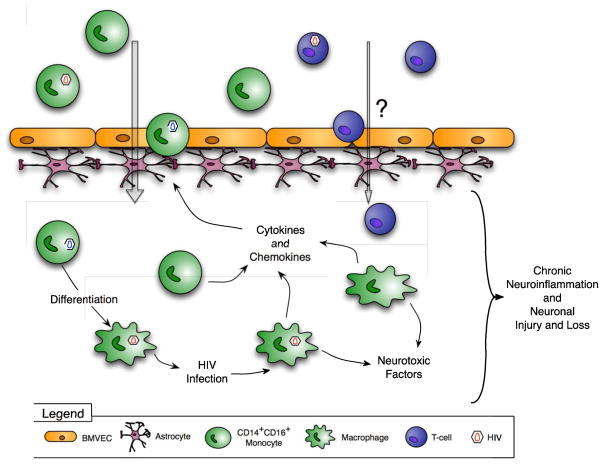
Figure 1. Mechanisms of HIV CNS inflammation and the development of HAND.
HIV enters the CNS within infected CD14+CD16+ monocytes that transmigrate across the BBB in response to chemokines. These cells produce virus and inflammatory mediators and differentiate into infected macrophages. HIV infected cells release neurotoxic viral proteins, and both infected and uninfected cells elaborate cytokines and chemokines in response to CNS infection. These factors activate CNS cells, recruit additional monocytes into the CNS, mediate ongoing neuroinflammation and promote the development of HAND.
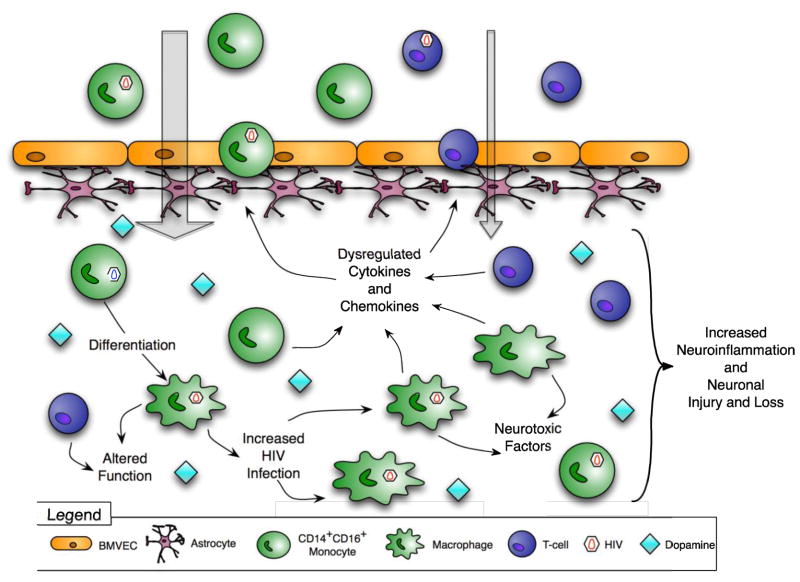
Figure 2. Postulated mechanisms by which dopamine increases CNS inflammation and contributes to HAND.
Drug abuse increases extracellular dopamine in the CNS. Dopamine, alone or with chemokines, increases the transmigration of CD14+CD16+ monocytes across the BBB and may cause T cell influx into the CNS. Elevated dopamine increases HIV replication in macrophages and dysregulates production of cytokines and chemokines. Macrophage and T cell functions are also altered. All of these effects increase neuroinflammation and contribute to HAND.
The effects of dopamine on neuroinflammation are highly relevant in the era of cART. HIV enters the CNS soon after peripheral infection, before most individuals know that they are infected and begin therapy. Prior to initiation of antiretroviral therapy, elevated CNS dopamine due to drug abuse may accelerate brain infection, resulting in increased brain viral load. In addition, dopamine is likely to continue to affect immune cells due to repeated drug use by HIV infected individuals. Even if the alterations resulting from each exposure to increased CNS dopamine are small, the cumulative effects of these changes on the infection and/or functions of monocytes, macrophages, and T cells within the CNS of HIV infected individuals may increase neuroinflammation, interfere with effective host defense, and accelerate disease progression beyond that which occurs in the CNS not periodically exposed to increased dopamine. Dopamine may also contribute to CNS-IRIS through interference with Treg function. Thus, we propose that the effects of dopamine on monocytes, macrophages, and T cells will contribute to the development of HIV-associated CNS disease in drug abusers. A more complete understanding of these effects is essential to treating HAND in this population.
Acknowledgments
We thank all of the members of Dr. Joan W. Berman’s laboratory at Einstein, especially Lillie Lopez and Dionna Williams, for important discussions, and Dr. Jonathan A. Javitch, at Columbia University Medical Center, for assistance with Table 1. We also thank Dr. Brad Poulos and the Fetal Tissue Repository, the Einstein Flow Cytometry Facility and the Einstein Center For AIDS Research (AI-051519) for their assistance. These studies were funded by grants from the National Institutes of Drug Abuse, DA029476 (PJG) and DA025567 (JSC, TMC and JWB), and the National Institutes of Mental Health, MH090958 (TMC and JWB) and MH075679 (TMC and JWB).
Footnotes
Competing Interests
The authors declare that they have no conflict of interest.
Bibliography
- Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annual review of immunology. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- Ali RA, Qureshi MA, McCorkle FM. Profile of chicken macrophage functions after exposure to catecholamines in vitro. Immunopharmacology and immunotoxicology. 1994;16(4):611–625. doi: 10.3109/08923979409019742. [DOI] [PubMed] [Google Scholar]
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Seminars in neurology. 2007;27(1):86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Ancuta P, Kunstman KJ, Autissier P, Zaman T, Stone D, Wolinsky SM, Gabuzda D. CD16+ monocytes exposed to HIV promote highly efficient viral replication upon differentiation into macrophages and interaction with T cells. Virology. 2006;344(2):267–276. doi: 10.1016/j.virol.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Anlauf M, Schafer MK, Schwark T, von Wurmb-Schwark N, Brand V, Sipos B, Horny HP, Parwaresch R, Hartschuh W, Eiden LE, Kloppel G, Weihe E. Vesicular monoamine transporter 2 (VMAT2) expression in hematopoietic cells and in patients with systemic mastocytosis. The journal of histochemistry and cytochemistry: official journal of the Histochemistry Society. 2006;54(2):201–213. doi: 10.1369/jhc.5A6739.2005. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Crawford DH, Bell JE. B lymphocytes in the normal brain: contrasts with HIV-associated lymphoid infiltrates and lymphomas. Brain: a journal of neurology. 2003;126(Pt 5):1058–1067. doi: 10.1093/brain/awg118. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-related CNS disease and neuroinflammation. Journal of neuropathology and experimental neurology. 2005;64(6):529–536. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Clifford DB, Cinque P, Epstein LG, Goodkin K, Gisslen M, Grant I, Heaton RK, Joseph J, Marder K, Marra CM, McArthur JC, Nunn M, Price RW, Pulliam L, Robertson KR, Sacktor N, Valcour V, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asselman V, Thienemann F, Pepper DJ, Boulle A, Wilkinson RJ, Meintjes G, Marais S. Central nervous system disorders after starting antiretroviral therapy in South Africa. AIDS. 2010;24(18):2871–2876. doi: 10.1097/QAD.0b013e328340fe76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailer RT, Lee B, Montaner LJ. IL-13 and TNF-alpha inhibit dual-tropic HIV-1 in primary macrophages by reduction of surface expression of CD4, chemokine receptors CCR5, CXCR4 and post-entry viral gene expression. European journal of immunology. 2000;30(5):1340–1349. doi: 10.1002/(SICI)1521-4141(200005)30:5<1340::AID-IMMU1340>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nature immunology. 2007;8(2):172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- Bailey-Bucktrout SL, Caulkins SC, Goings G, Fischer JA, Dzionek A, Miller SD. Cutting edge: central nervous system plasmacytoid dendritic cells regulate the severity of relapsing experimental autoimmune encephalomyelitis. J Immunol. 2008;180(10):6457–6461. doi: 10.4049/jimmunol.180.10.6457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso MR, Bornstein RA. Effects of past noninjection drug abuse upon executive function and working memory in HIV infection. Journal of clinical and experimental neuropsychology. 2003;25(7):893–903. doi: 10.1076/jcen.25.7.893.16489. [DOI] [PubMed] [Google Scholar]
- Basu B, Sarkar C, Chakroborty D, Ganguly S, Shome S, Dasgupta PS, Basu S. D1 and D2 dopamine receptor-mediated inhibition of activated normal T cell proliferation is lost in jurkat T leukemic cells. The Journal of biological chemistry. 2010;285(35):27026–27032. doi: 10.1074/jbc.M110.144022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacological reviews. 2011;63(1):182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Belarbi K, Jopson T, Tweedie D, Arellano C, Luo W, Greig NH, Rosi S. TNF-alpha protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. Journal of neuroinflammation. 2012;9:23. doi: 10.1186/1742-2094-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JE, Busuttil A, Ironside JW, Rebus S, Donaldson YK, Simmonds P, Peutherer JF. Human immunodeficiency virus and the brain: investigation of virus load and neuropathologic changes in pre-AIDS subjects. The Journal of infectious diseases. 1993;168(4):818–824. doi: 10.1093/infdis/168.4.818. [DOI] [PubMed] [Google Scholar]
- Berger JR, Arendt G. HIV dementia: the role of the basal ganglia and dopaminergic systems. J Psychopharmacol. 2000;14(3):214–221. doi: 10.1177/026988110001400304. [DOI] [PubMed] [Google Scholar]
- Bergquist J, Ohlsson B, Tarkowski A. Nuclear factor-kappa B is involved in the catecholaminergic suppression of immunocompetent cells. Annals of the New York Academy of Sciences. 2000;917:281–289. doi: 10.1111/j.1749-6632.2000.tb05394.x. [DOI] [PubMed] [Google Scholar]
- Bergquist J, Tarkowski A, Ekman R, Ewing A. Discovery of endogenous catecholamines in lymphocytes and evidence for catecholamine regulation of lymphocyte function via an autocrine loop. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(26):12912–12916. doi: 10.1073/pnas.91.26.12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser MJ, Ganor Y, Levite M. Dopamine by itself activates either D2, D3 or D1/D5 dopaminergic receptors in normal human T-cells and triggers the selective secretion of either IL-10, TNFalpha or both. Journal of neuroimmunology. 2005;169(1–2):161–171. doi: 10.1016/j.jneuroim.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453(7198):1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyrer C, Wirtz AL, Baral S, Peryskina A, Sifakis F. Epidemiologic links between drug use and HIV epidemics: an international perspective. J Acquir Immune Defic Syndr. 2010;55(Suppl 1):S10–16. doi: 10.1097/QAI.0b013e3181f9c0c9. [DOI] [PubMed] [Google Scholar]
- Bhavan KP, Kampalath VN, Overton ET. The aging of the HIV epidemic. Current HIV/AIDS reports. 2008;5(3):150–158. doi: 10.1007/s11904-008-0023-3. [DOI] [PubMed] [Google Scholar]
- Bidart JM, Motte P, Assicot M, Bohuon C, Bellet D. Catechol-O-methyltransferase activity and aminergic binding sites distribution in human peripheral blood lymphocyte subpopulations. Clinical immunology and immunopathology. 1983;26(1):1–9. doi: 10.1016/0090-1229(83)90167-8. [DOI] [PubMed] [Google Scholar]
- Binford MC, Kahana SY, Altice FL. A Systematic Review of Antiretroviral Adherence Interventions for HIV-Infected People Who Use Drugs. Current HIV/AIDS reports. 2012;9(4):287–312. doi: 10.1007/s11904-012-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boneberg EM, von Seydlitz E, Propster K, Watzl H, Rockstroh B, Illges H. D3 dopamine receptor mRNA is elevated in T cells of schizophrenic patients whereas D4 dopamine receptor mRNA is reduced in CD4+-T cells. Journal of neuroimmunology. 2006;173(1–2):180–187. doi: 10.1016/j.jneuroim.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Brabers NA, Nottet HS. Role of the pro-inflammatory cytokines TNF-alpha and IL-1beta in HIV-associated dementia. European journal of clinical investigation. 2006;36(7):447–458. doi: 10.1111/j.1365-2362.2006.01657.x. [DOI] [PubMed] [Google Scholar]
- Brown SW, Meyers RT, Brennan KM, Rumble JM, Narasimhachari N, Perozzi EF, Ryan JJ, Stewart JK, Fischer-Stenger K. Catecholamines in a macrophage cell line. Journal of neuroimmunology. 2003;135(1–2):47–55. doi: 10.1016/s0165-5728(02)00435-6. [DOI] [PubMed] [Google Scholar]
- Buckner CM, Calderon TM, Willams DW, Belbin TJ, Berman JW. Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood-brain barrier and infection by HIV: implications for NeuroAIDS. Cellular immunology. 2011;267(2):109–123. doi: 10.1016/j.cellimm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner CM, Luers AJ, Calderon TM, Eugenin EA, Berman JW. Neuroimmunity and the blood-brain barrier: molecular regulation of leukocyte transmigration and viral entry into the nervous system with a focus on neuroAIDS. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2006;1(2):160–181. doi: 10.1007/s11481-006-9017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdo TH, Soulas C, Orzechowski K, Button J, Krishnan A, Sugimoto C, Alvarez X, Kuroda MJ, Williams KC. Increased monocyte turnover from bone marrow correlates with severity of SIV encephalitis and CD163 levels in plasma. PLoS pathogens. 2010;6(4):e1000842. doi: 10.1371/journal.ppat.1000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscemi L, Ramonet D, Geiger JD. Human immunodeficiency virus type-1 protein Tat induces tumor necrosis factor-alpha-mediated neurotoxicity. Neurobiology of disease. 2007;26(3):661–670. doi: 10.1016/j.nbd.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd DA, Fellows RP, Morgello S, Franklin D, Heaton RK, Deutsch R, Atkinson JH, Clifford DB, Collier AC, Marra CM, Gelman B, McCutchan JA, Duarte NA, Simpson DM, McArthur J, Grant I. Neurocognitive impact of substance use in HIV infection. J Acquir Immune Defic Syndr. 2011;58(2):154–162. doi: 10.1097/QAI.0b013e318229ba41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capellino S, Cosentino M, Wolff C, Schmidt M, Grifka J, Straub RH. Catecholamine-producing cells in the synovial tissue during arthritis: modulation of sympathetic neurotransmitters as new therapeutic target. Annals of the rheumatic diseases. 2010;69(10):1853–1860. doi: 10.1136/ard.2009.119701. [DOI] [PubMed] [Google Scholar]
- Carboni E, Imperato A, Perezzani L, Di Chiara G. Amphetamine, cocaine, phencyclidine and nomifensine increase extracellular dopamine concentrations preferentially in the nucleus accumbens of freely moving rats. Neuroscience. 1989;28(3):653–661. doi: 10.1016/0306-4522(89)90012-2. [DOI] [PubMed] [Google Scholar]
- Carvalho-Freitas MI, Anselmo-Franci JA, Maiorka PC, Palermo-Neto J, Felicio LF. Prolactin differentially modulates the macrophage activity of lactating rats: possible role of reproductive experience. Journal of reproductive immunology. 2011;89(1):38–45. doi: 10.1016/j.jri.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Carvalho-Freitas MI, Rodrigues-Costa EC, Nasello AG, Palermo-Neto J, Felicio LF. In vitro macrophage activity: biphasic effect of prolactin and indirect evidence of dopaminergic modulation. Neuroimmunomodulation. 2008;15(2):131–139. doi: 10.1159/000148196. [DOI] [PubMed] [Google Scholar]
- Chang JY, Liu LZ. Catecholamines inhibit microglial nitric oxide production. Brain research bulletin. 2000;52(6):525–530. doi: 10.1016/s0361-9230(00)00291-4. [DOI] [PubMed] [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. NeuroImage. 2008;42(2):869–878. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Langrish CL, McKenzie B, Joyce-Shaikh B, Stumhofer JS, McClanahan T, Blumenschein W, Churakovsa T, Low J, Presta L, Hunter CA, Kastelein RA, Cua DJ. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. The Journal of clinical investigation. 2006;116(5):1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay CC, Rodrigues DS, Ho YS, Fallert BA, Janatpour K, Reinhart TA, Esser U. Neuroinvasion of fluorescein-positive monocytes in acute simian immunodeficiency virus infection. Journal of virology. 2007;81(21):12040–12048. doi: 10.1128/JVI.00133-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA. Immune Heterogeneity in Neuroinflammation: Dendritic Cells in the Brain. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2012 doi: 10.1007/s11481-012-9414-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino M, Bombelli R, Ferrari M, Marino F, Rasini E, Maestroni GJ, Conti A, Boveri M, Lecchini S, Frigo G. HPLC-ED measurement of endogenous catecholamines in human immune cells and hematopoietic cell lines. Life sciences. 2000;68(3):283–295. doi: 10.1016/s0024-3205(00)00937-1. [DOI] [PubMed] [Google Scholar]
- Cosentino M, Fietta AM, Ferrari M, Rasini E, Bombelli R, Carcano E, Saporiti F, Meloni F, Marino F, Lecchini S. Human CD4+CD25+ regulatory T cells selectively express tyrosine hydroxylase and contain endogenous catecholamines subserving an autocrine/paracrine inhibitory functional loop. Blood. 2007;109(2):632–642. doi: 10.1182/blood-2006-01-028423. [DOI] [PubMed] [Google Scholar]
- Cosenza MA, Zhao ML, Si Q, Lee SC. Human brain parenchymal microglia express CD14 and CD45 and are productively infected by HIV-1 in HIV-1 encephalitis. Brain Pathol. 2002;12(4):442–455. doi: 10.1111/j.1750-3639.2002.tb00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ, Rice ME. DAncing past the DAT at a DA synapse. Trends in neurosciences. 2004;27(5):270–277. doi: 10.1016/j.tins.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Crowe S, Zhu T, Muller WA. The contribution of monocyte infection and trafficking to viral persistence, and maintenance of the viral reservoir in HIV infection. Journal of leukocyte biology. 2003;74(5):635–641. doi: 10.1189/jlb.0503204. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, Zurawski S, Wiekowski M, Lira SA, Gorman D, Kastelein RA, Sedgwick JD. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421(6924):744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Maruff P, Brew BJ. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. Journal of neurovirology. 2004;10(6):350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Vaida F, Letendre S, Gibson S, Cherner M, Woods SP, McCutchan JA, Heaton RK, Ellis RJ. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology. 2009;73(5):342–348. doi: 10.1212/WNL.0b013e3181ab2b3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czub S, Czub M, Koutsilieri E, Sopper S, Villinger F, Muller JG, Stahl-Hennig C, Riederer P, Ter Meulen V, Gosztonyi G. Modulation of simian immunodeficiency virus neuropathology by dopaminergic drugs. Acta neuropathologica. 2004;107(3):216–226. doi: 10.1007/s00401-003-0801-3. [DOI] [PubMed] [Google Scholar]
- Czub S, Koutsilieri E, Sopper S, Czub M, Stahl-Hennig C, Muller JG, Pedersen V, Gsell W, Heeney JL, Gerlach M, Gosztonyi G, Riederer P, ter Meulen V. Enhancement of central nervous system pathology in early simian immunodeficiency virus infection by dopaminergic drugs. Acta neuropathologica. 2001;101(2):85–91. doi: 10.1007/s004010000313. [DOI] [PubMed] [Google Scholar]
- D’Agostino PM, Gottfried-Blackmore A, Anandasabapathy N, Bulloch K. Brain dendritic cells: biology and pathology. Acta neuropathologica. 2012 doi: 10.1007/s00401-012-1018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Aversa TG, Eugenin EA, Berman JW. CD40-CD40 ligand interactions in human microglia induce CXCL8 (interleukin-8) secretion by a mechanism dependent on activation of ERK1/2 and nuclear translocation of nuclear factor-kappaB (NFkappaB) and activator protein-1 (AP-1) Journal of neuroscience research. 2008;86(3):630–639. doi: 10.1002/jnr.21525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daubner SC, Le T, Wang S. Tyrosine hydroxylase and regulation of dopamine synthesis. Archives of biochemistry and biophysics. 2011;508(1):1–12. doi: 10.1016/j.abb.2010.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42(9):1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- Delobel P, Sandres-Saune K, Cazabat M, L’Faqihi FE, Aquilina C, Obadia M, Pasquier C, Marchou B, Massip P, Izopet J. Persistence of distinct HIV-1 populations in blood monocytes and naive and memory CD4 T cells during prolonged suppressive HAART. AIDS. 2005;19(16):1739–1750. doi: 10.1097/01.aids.0000183125.93958.26. [DOI] [PubMed] [Google Scholar]
- Desai RI, Paronis CA, Martin J, Desai R, Bergman J. Monoaminergic psychomotor stimulants: discriminative stimulus effects and dopamine efflux. The Journal of pharmacology and experimental therapeutics. 2010;333(3):834–843. doi: 10.1124/jpet.110.165746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhillon NK, Williams R, Callen S, Zien C, Narayan O, Buch S. Roles of MCP-1 in development of HIV-dementia. Frontiers in bioscience: a journal and virtual library. 2008;13:3913–3918. doi: 10.2741/2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn’t do. Current opinion in pharmacology. 2007;7(1):69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(14):5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellery PJ, Tippett E, Chiu YL, Paukovics G, Cameron PU, Solomon A, Lewin SR, Gorry PR, Jaworowski A, Greene WC, Sonza S, Crowe SM. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178(10):6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- Epstein LG, Gendelman HE. Human immunodeficiency virus type 1 infection of the nervous system: pathogenetic mechanisms. Annals of neurology. 1993;33(5):429–436. doi: 10.1002/ana.410330502. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, Berman JW. Gap junctions mediate human immunodeficiency virus-bystander killing in astrocytes. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27(47):12844–12850. doi: 10.1523/JNEUROSCI.4154-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, D’Aversa TG, Lopez L, Calderon TM, Berman JW. MCP-1 (CCL2) protects human neurons and astrocytes from NMDA or HIV-tat-induced apoptosis. Journal of neurochemistry. 2003;85(5):1299–1311. doi: 10.1046/j.1471-4159.2003.01775.x. [DOI] [PubMed] [Google Scholar]
- Eugenin EA, King JE, Hazleton JE, Major EO, Bennett MV, Zukin RS, Berman JW. Differences in NMDA receptor expression during human development determine the response of neurons to HIV-tat-mediated neurotoxicity. Neurotoxicity research. 2011;19(1):138–148. doi: 10.1007/s12640-010-9150-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, King JE, Nath A, Calderon TM, Zukin RS, Bennett MV, Berman JW. HIV-tat induces formation of an LRP-PSD-95-NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(9):3438–3443. doi: 10.1073/pnas.0611699104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26(4):1098–1106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everall IP, Hansen LA, Masliah E. The shifting patterns of HIV encephalitis neuropathology. Neurotoxicity research. 2005;8(1–2):51–61. doi: 10.1007/BF03033819. [DOI] [PubMed] [Google Scholar]
- Farber K, Pannasch U, Kettenmann H. Dopamine and noradrenaline control distinct functions in rodent microglial cells. Molecular and cellular neurosciences. 2005;29(1):128–138. doi: 10.1016/j.mcn.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Mactutus CF, Booze RM. Neurotoxic profiles of HIV, psychostimulant drugs of abuse, and their concerted effect on the brain: current status of dopamine system vulnerability in NeuroAIDS. Neuroscience and biobehavioral reviews. 2008;32(5):883–909. doi: 10.1016/j.neubiorev.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. Journal of neurovirology. 2001;7(6):528–541. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- Fischer-Smith T, Rappaport J. Evolving paradigms in the pathogenesis of HIV-1-associated dementia. Expert reviews in molecular medicine. 2005;7(27):1–26. doi: 10.1017/S1462399405010239. [DOI] [PubMed] [Google Scholar]
- Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, Zetoune FS, McGuire SR, List RP, Day DE, Hoesel LM, Gao H, Van Rooijen N, Huber-Lang MS, Neubig RR, Ward PA. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 2007;449(7163):721–725. doi: 10.1038/nature06185. [DOI] [PubMed] [Google Scholar]
- Garris PA, Ciolkowski EL, Pastore P, Wightman RM. Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1994;14(10):6084–6093. doi: 10.1523/JNEUROSCI.14-10-06084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Calderon TM, Luers AJ, Eugenin EA, Javitch JA, Berman JW. Human immunodeficiency virus (HIV) infection of human macrophages is increased by dopamine: a bridge between HIV-associated neurologic disorders and drug abuse. The American journal of pathology. 2009;175(3):1148–1159. doi: 10.2353/ajpath.2009.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskill PJ, Carvallo L, Eugenin EA, Berman JW. Characterization and function of the human macrophage dopaminergic system: implications for CNS disease and drug abuse. Journal of neuroinflammation. 2012;9(1):203. doi: 10.1186/1742-2094-9-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Lisinicchia JG, Chen T, Johnson KM, Jennings K, Freeman DH, Jr, Soukup VM. Prefrontal dopaminergic and enkephalinergic synaptic accommodation in HIV-associated neurocognitive disorders and encephalitis. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2012;7(3):686–700. doi: 10.1007/s11481-012-9345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh MC, Mondal AC, Basu S, Banerjee S, Majumder J, Bhattacharya D, Dasgupta PS. Dopamine inhibits cytokine release and expression of tyrosine kinases, Lck and Fyn in activated T cells. International immunopharmacology. 2003;3(7):1019–1026. doi: 10.1016/S1567-5769(03)00100-0. [DOI] [PubMed] [Google Scholar]
- Girault JA, Greengard P. The neurobiology of dopamine signaling. Archives of neurology. 2004;61(5):641–644. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Annals of neurology. 1995;38(5):755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Gomez F, Ruiz P, Briceno F, Rivera C, Lopez R. Macrophage Fcgamma receptors expression is altered by treatment with dopaminergic drugs. Clin Immunol. 1999;90(3):375–387. doi: 10.1006/clim.1998.4665. [DOI] [PubMed] [Google Scholar]
- Gong N, Liu J, Reynolds AD, Gorantla S, Mosley RL, Gendelman HE. Brain ingress of regulatory T cells in a murine model of HIV-1 encephalitis. Journal of neuroimmunology. 2011;230(1–2):33–41. doi: 10.1016/j.jneuroim.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonon FG, Buda MJ. Regulation of dopamine release by impulse flow and by autoreceptors as studied by in vivo voltammetry in the rat striatum. Neuroscience. 1985;14(3):765–774. doi: 10.1016/0306-4522(85)90141-1. [DOI] [PubMed] [Google Scholar]
- Gouwy M, Struyf S, Noppen S, Schutyser E, Springael JY, Parmentier M, Proost P, Van Damme J. Synergy between coproduced CC and CXC chemokines in monocyte chemotaxis through receptor-mediated events. Molecular pharmacology. 2008;74(2):485–495. doi: 10.1124/mol.108.045146. [DOI] [PubMed] [Google Scholar]
- Gray F, Lescs MC, Keohane C, Paraire F, Marc B, Durigon M, Gherardi R. Early brain changes in HIV infection: neuropathological study of 11 HIV seropositive, non-AIDS cases. Journal of neuropathology and experimental neurology. 1992;51(2):177–185. doi: 10.1097/00005072-199203000-00007. [DOI] [PubMed] [Google Scholar]
- Gruol DL, Nelson TE. Physiological and pathological roles of interleukin-6 in the central nervous system. Molecular neurobiology. 1997;15(3):307–339. doi: 10.1007/BF02740665. [DOI] [PubMed] [Google Scholar]
- Guha D, Nagilla P, Redinger C, Srinivasan A, Schatten GP, Ayyavoo V. Neuronal apoptosis by HIV-1 Vpr: contribution of proinflammatory molecular networks from infected target cells. Journal of neuroinflammation. 2012;9(1):138. doi: 10.1186/1742-2094-9-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Urizar E, Kralikova M, Mobarec JC, Shi L, Filizola M, Javitch JA. Dopamine D2 receptors form higher order oligomers at physiological expression levels. The EMBO journal. 2008;27(17):2293–2304. doi: 10.1038/emboj.2008.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddow LJ, Moosa MY, Mosam A, Moodley P, Parboosing R, Easterbrook PJ. Incidence, Clinical Spectrum, Risk Factors and Impact of HIV-Associated Immune Reconstitution Inflammatory Syndrome in South Africa. PloS one. 2012;7(11):e40623. doi: 10.1371/journal.pone.0040623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A, O’Dowd BF, George SR. Dopamine D1–D2 receptor heteromer signaling pathway in the brain: emerging physiological relevance. Molecular brain. 2011;4:26. doi: 10.1186/1756-6606-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasko G, Szabo C, Nemeth ZH, Deitch EA. Dopamine suppresses IL-12 p40 production by lipopolysaccharide-stimulated macrophages via a beta-adrenoceptor-mediated mechanism. Journal of neuroimmunology. 2002;122(1–2):34–39. doi: 10.1016/s0165-5728(01)00459-3. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of neurovirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbein G, Varin A. The macrophage in HIV-1 infection: from activation to deactivation? Retrovirology. 2010;7:33. doi: 10.1186/1742-4690-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey WF, Hsu BL, Kimura H. T-lymphocyte entry into the central nervous system. Journal of neuroscience research. 1991;28(2):254–260. doi: 10.1002/jnr.490280213. [DOI] [PubMed] [Google Scholar]
- Hoeger S, Reisenbuechler A, Gottmann U, Doyon F, Braun C, Kaya Z, Seelen MA, van Son WJ, Waldherr R, Schnuelle P, Yard BA. Donor dopamine treatment in brain dead rats is associated with an improvement in renal function early after transplantation and a reduction in renal inflammation. Transplant international: official journal of the European Society for Organ Transplantation. 2008;21(11):1072–1080. doi: 10.1111/j.1432-2277.2008.00725.x. [DOI] [PubMed] [Google Scholar]
- Horn AS. Dopamine uptake: a review of progress in the last decade. Progress in neurobiology. 1990;34(5):387–400. doi: 10.1016/0301-0082(90)90033-d. [DOI] [PubMed] [Google Scholar]
- Huppert J, Closhen D, Croxford A, White R, Kulig P, Pietrowski E, Bechmann I, Becher B, Luhmann HJ, Waisman A, Kuhlmann CR. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010;24(4):1023–1034. doi: 10.1096/fj.09-141978. [DOI] [PubMed] [Google Scholar]
- Ilani T, Strous RD, Fuchs S. Dopaminergic regulation of immune cells via D3 dopamine receptor: a pathway mediated by activated T cells. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2004;18(13):1600–1602. doi: 10.1096/fj.04-1652fje. [DOI] [PubMed] [Google Scholar]
- Iversen SD, Iversen LL. Dopamine: 50 years in perspective. Trends in neurosciences. 2007;30(5):188–193. doi: 10.1016/j.tins.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Javitch JA. The ants go marching two by two: oligomeric structure of G-protein-coupled receptors. Molecular pharmacology. 2004;66(5):1077–1082. doi: 10.1124/mol.104.006320. [DOI] [PubMed] [Google Scholar]
- Jenny-Avital ER, Abadi M. Immune reconstitution cryptococcosis after initiation of successful highly active antiretroviral therapy. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2002;35(12):e128–133. doi: 10.1086/344467. [DOI] [PubMed] [Google Scholar]
- Johnson T, Nath A. Immune reconstitution inflammatory syndrome and the central nervous system. Current opinion in neurology. 2011;24(3):284–290. doi: 10.1097/WCO.0b013e328346be57. [DOI] [PubMed] [Google Scholar]
- Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, Gabuzda D. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. 2012;60(3):234–243. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanmogne GD, Schall K, Leibhart J, Knipe B, Gendelman HE, Persidsky Y. HIV-1 gp120 compromises blood-brain barrier integrity and enhances monocyte migration across blood-brain barrier: implication for viral neuropathogenesis. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27(1):123–134. doi: 10.1038/sj.jcbfm.9600330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nature medicine. 2007;13(10):1173–1175. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieburtz KD, Epstein LG, Gelbard HA, Greenamyre JT. Excitotoxicity and dopaminergic dysfunction in the acquired immunodeficiency syndrome dementia complex. Therapeutic implication. Archives of neurology. 1991;48(12):1281–1284. doi: 10.1001/archneur.1991.00530240087028. [DOI] [PubMed] [Google Scholar]
- Kim WK, Sun Y, Do H, Autissier P, Halpern EF, Piatak M, Jr, Lifson JD, Burdo TH, McGrath MS, Williams K. Monocyte heterogeneity underlying phenotypic changes in monocytes according to SIV disease stage. Journal of leukocyte biology. 2010;87(4):557–567. doi: 10.1189/jlb.0209082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HL, Ginsburg BC, Howell LL. Changes in extracellular dopamine during cocaine self-administration in squirrel monkeys. Synapse. 2005;56(3):129–134. doi: 10.1002/syn.20135. [DOI] [PubMed] [Google Scholar]
- King JE, Eugenin EA, Hazleton JE, Morgello S, Berman JW. Mechanisms of HIV-tat-induced phosphorylation of N-methyl-D-aspartate receptor subunit 2A in human primary neurons: implications for neuroAIDS pathogenesis. The American journal of pathology. 2010;176(6):2819–2830. doi: 10.2353/ajpath.2010.090642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipnis J, Cardon M, Avidan H, Lewitus GM, Mordechay S, Rolls A, Shani Y, Schwartz M. Dopamine, through the extracellular signal-regulated kinase pathway, downregulates CD4+CD25+ regulatory T-cell activity: implications for neurodegeneration. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24(27):6133–6143. doi: 10.1523/JNEUROSCI.0600-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblach SM, Faden AI. Interleukin-10 improves outcome and alters proinflammatory cytokine expression after experimental traumatic brain injury. Experimental neurology. 1998;153(1):143–151. doi: 10.1006/exnr.1998.6877. [DOI] [PubMed] [Google Scholar]
- Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, Janotta F, Aksamit A, Martin MA, Fauci AS. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233(4768):1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Kokkinou I, Fragoulis EG, Vassilacopoulou D. The U937 macrophage cell line expresses enzymatically active L-Dopa decarboxylase. Journal of neuroimmunology. 2009;216(1–2):51–58. doi: 10.1016/j.jneuroim.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177(1):566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends in pharmacological sciences. 1992a;13(5):177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Koob GF. Neural mechanisms of drug reinforcement. Annals of the New York Academy of Sciences. 1992b;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science. 1988;242(4879):715–723. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- Kopin IJ. Monoamine oxidase and catecholamine metabolism. Journal of neural transmission Supplementum. 1994;41:57–67. doi: 10.1007/978-3-7091-9324-2_7. [DOI] [PubMed] [Google Scholar]
- Kossmann T, Stahel PF, Lenzlinger PM, Redl H, Dubs RW, Trentz O, Schlag G, Morganti-Kossmann MC. Interleukin-8 released into the cerebrospinal fluid after brain injury is associated with blood-brain barrier dysfunction and nerve growth factor production. Journal of cerebral blood flow and metabolism: official journal of the International Society of Cerebral Blood Flow and Metabolism. 1997;17(3):280–289. doi: 10.1097/00004647-199703000-00005. [DOI] [PubMed] [Google Scholar]
- Kousik SM, Napier TC, Carvey PM. The Effects of Psychostimulant Drugs on Blood Brain Barrier Function and Neuroinflammation. Frontiers in pharmacology. 2012;3:121. doi: 10.3389/fphar.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsilieri E, Sopper S, Scheller C, ter Meulen V, Riederer P. Involvement of dopamine in the progression of AIDS Dementia Complex. J Neural Transm. 2002a;109(3):399–410. doi: 10.1007/s007020200032. [DOI] [PubMed] [Google Scholar]
- Koutsilieri E, Sopper S, Scheller C, ter Meulen V, Riederer P. Parkinsonism in HIV dementia. J Neural Transm. 2002b;109(5–6):767–775. doi: 10.1007/s007020200063. [DOI] [PubMed] [Google Scholar]
- Kraft-Terry SD, Buch SJ, Fox HS, Gendelman HE. A coat of many colors: neuroimmune crosstalk in human immunodeficiency virus infection. Neuron. 2009;64(1):133–145. doi: 10.1016/j.neuron.2009.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. Journal of neurovirology. 2011;17(1):26–40. doi: 10.1007/s13365-010-0003-4. [DOI] [PubMed] [Google Scholar]
- Lane BR, Markovitz DM, Woodford NL, Rochford R, Strieter RM, Coffey MJ. TNF-alpha inhibits HIV-1 replication in peripheral blood monocytes and alveolar macrophages by inducing the production of RANTES and decreasing C-C chemokine receptor 5 (CCR5) expression. J Immunol. 1999;163(7):3653–3661. [PubMed] [Google Scholar]
- Langford D, Adame A, Grigorian A, Grant I, McCutchan JA, Ellis RJ, Marcotte TD, Masliah E. Patterns of selective neuronal damage in methamphetamine-user AIDS patients. J Acquir Immune Defic Syndr. 2003;34(5):467–474. doi: 10.1097/00126334-200312150-00004. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. The Journal of experimental medicine. 2005;201(2):233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskin DL, Sunil VR, Gardner CR, Laskin JD. Macrophages and tissue injury: agents of defense or destruction? Annual review of pharmacology and toxicology. 2011;51:267–288. doi: 10.1146/annurev.pharmtox.010909.105812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levite M. Neurotransmitters activate T-cells and elicit crucial functions via neurotransmitter receptors. Current opinion in pharmacology. 2008;8(4):460–471. doi: 10.1016/j.coph.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Levite M, Chowers Y, Ganor Y, Besser M, Hershkovits R, Cahalon L. Dopamine interacts directly with its D3 and D2 receptors on normal human T cells, and activates beta1 integrin function. European journal of immunology. 2001;31(12):3504–3512. doi: 10.1002/1521-4141(200112)31:12<3504::AID-IMMU3504>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Liang H, Wang X, Chen H, Song L, Ye L, Wang SH, Wang YJ, Zhou L, Ho WZ. Methamphetamine enhances HIV infection of macrophages. The American journal of pathology. 2008;172(6):1617–1624. doi: 10.2353/ajpath.2008.070971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Quartermain D, Dunn AJ, Weinshenker D, Stone EA. Possible dopaminergic stimulation of locus coeruleus alpha1-adrenoceptors involved in behavioral activation. Synapse. 2008;62(7):516–523. doi: 10.1002/syn.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Gong N, Huang X, Reynolds AD, Mosley RL, Gendelman HE. Neuromodulatory activities of CD4+CD25+ regulatory T cells in a murine model of HIV-1-associated neurodegeneration. J Immunol. 2009;182(6):3855–3865. doi: 10.4049/jimmunol.0803330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez CA, Vazquez M, Hill MD, Colon Mdel C, Porrata-Doria T, Johnston IC, Lorenzo E. Characterization of HIV-1 RNA forms in the plasma of patients undergoing successful HAART. Archives of virology. 2010;155(6):895–903. doi: 10.1007/s00705-010-0659-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMicking J, Xie QW, Nathan C. Nitric oxide and macrophage function. Annual review of immunology. 1997;15:323–350. doi: 10.1146/annurev.immunol.15.1.323. [DOI] [PubMed] [Google Scholar]
- Madden LJ, Zandonatti MA, Flynn CT, Taffe MA, Marcondes MC, Schmitz JE, Reimann KA, Henriksen SJ, Fox HS. CD8+ cell depletion amplifies the acute retroviral syndrome. Journal of neurovirology. 2004;10(Suppl 1):58–66. doi: 10.1080/753312754. [DOI] [PubMed] [Google Scholar]
- Makrigeorgi-Butera M, Hagel C, Laas R, Puschel K, Stavrou D. Comparative brain pathology of HIV-seronegative and HIV-infected drug addicts. Clinical neuropathology. 1996;15(6):324–329. [PubMed] [Google Scholar]
- Marcondes MC, Burudi EM, Huitron-Resendiz S, Sanchez-Alavez M, Watry D, Zandonatti M, Henriksen SJ, Fox HS. Highly activated CD8(+) T cells in the brain correlate with early central nervous system dysfunction in simian immunodeficiency virus infection. J Immunol. 2001;167(9):5429–5438. doi: 10.4049/jimmunol.167.9.5429. [DOI] [PubMed] [Google Scholar]
- Marcondes MC, Sopper S, Sauermann U, Burdo TH, Watry D, Zandonatti M, Fox HS. CD4 deficits and disease course acceleration can be driven by a collapse of the CD8 response in rhesus macaques infected with simian immunodeficiency virus. AIDS. 2008;22(12):1441–1452. doi: 10.1097/QAD.0b013e3283052fb5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Blondel G, Alvarez M, Delobel P, Uro-Coste E, Cuzin L, Cuvinciuc V, Fillaux J, Massip P, Marchou B. Toxoplasmic encephalitis IRIS in HIV-infected patients: a case series and review of the literature. Journal of neurology, neurosurgery, and psychiatry. 2011;82(6):691–693. doi: 10.1136/jnnp.2009.199919. [DOI] [PubMed] [Google Scholar]
- Martin-Blondel G, Mars LT, Liblau RS. Pathogenesis of the immune reconstitution inflammatory syndrome in HIV-infected patients. Current opinion in infectious diseases. 2012;25(3):312–320. doi: 10.1097/QCO.0b013e328352b664. [DOI] [PubMed] [Google Scholar]
- Mathers BM, Degenhardt L, Phillips B, Wiessing L, Hickman M, Strathdee SA, Wodak A, Panda S, Tyndall M, Toufik A, Mattick RP. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372(9651):1733–1745. doi: 10.1016/S0140-6736(08)61311-2. [DOI] [PubMed] [Google Scholar]
- McCombe JA, Auer RN, Maingat FG, Houston S, Gill MJ, Power C. Neurologic immune reconstitution inflammatory syndrome in HIV/AIDS: outcome and epidemiology. Neurology. 2009;72(9):835–841. doi: 10.1212/01.wnl.0000343854.80344.69. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Martinez TN, Ruhn KA, Szymkowski DE, Smith CG, Botterman BR, Tansey KE, Tansey MG. Blocking soluble tumor necrosis factor signaling with dominant-negative tumor necrosis factor inhibitor attenuates loss of dopaminergic neurons in models of Parkinson’s disease. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26(37):9365–9375. doi: 10.1523/JNEUROSCI.1504-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna F, McLaughlin PJ, Lewis BJ, Sibbring GC, Cummerson JA, Bowen-Jones D, Moots RJ. Dopamine receptor expression on human T- and B-lymphocytes, monocytes, neutrophils, eosinophils and NK cells: a flow cytometric study. Journal of neuroimmunology. 2002;132(1–2):34–40. doi: 10.1016/s0165-5728(02)00280-1. [DOI] [PubMed] [Google Scholar]
- McManus CM, Weidenheim K, Woodman SE, Nunez J, Hesselgesser J, Nath A, Berman JW. Chemokine and chemokine-receptor expression in human glial elements: induction by the HIV protein, Tat, and chemokine autoregulation. The American journal of pathology. 2000;156(4):1441–1453. doi: 10.1016/S0002-9440(10)65013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade CS, Conn NA, Skalski LM, Safren SA. Neurocognitive impairment and medication adherence in HIV patients with and without cocaine dependence. Journal of behavioral medicine. 2011a;34(2):128–138. doi: 10.1007/s10865-010-9293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade CS, Lowen SB, MacLean RR, Key MD, Lukas SE. fMRI brain activation during a delay discounting task in HIV-positive adults with and without cocaine dependence. Psychiatry research. 2011b;192(3):167–175. doi: 10.1016/j.pscychresns.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes G, Boulle A. Immune reconstitution inflammatory syndrome in a large multicenter cohort study: case definition and comparability. Expert review of anti-infective therapy. 2012;10(7):737–741. doi: 10.1586/eri.12.62. [DOI] [PubMed] [Google Scholar]
- Mellins CA, Havens JF, McDonnell C, Lichtenstein C, Uldall K, Chesney M, Santamaria EK, Bell J. Adherence to antiretroviral medications and medical care in HIV-infected adults diagnosed with mental and substance abuse disorders. AIDS care. 2009;21(2):168–177. doi: 10.1080/09540120802001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill JE, Koyanagi Y, Chen IS. Interleukin-1 and tumor necrosis factor alpha can be induced from mononuclear phagocytes by human immunodeficiency virus type 1 binding to the CD4 receptor. Journal of virology. 1989;63(10):4404–4408. doi: 10.1128/jvi.63.10.4404-4408.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiological reviews. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Mocchetti I, Bachis A, Avdoshina V. Neurotoxicity of human immunodeficiency virus-1: viral proteins and axonal transport. Neurotoxicity research. 2012;21(1):79–89. doi: 10.1007/s12640-011-9279-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinoff PB, Axelrod J. Biochemistry of catecholamines. Annual review of biochemistry. 1971;40:465–500. doi: 10.1146/annurev.bi.40.070171.002341. [DOI] [PubMed] [Google Scholar]
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- Muller WA. Mechanisms of leukocyte transendothelial migration. Annual review of pathology. 2011;6:323–344. doi: 10.1146/annurev-pathol-011110-130224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahvi S, Litwin AH, Heo M, Berg KM, Li X, Arnsten JH. Directly observed antiretroviral therapy eliminates adverse effects of active drug use on adherence. Drug and alcohol dependence. 2012;120(1–3):174–180. doi: 10.1016/j.drugalcdep.2011.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Higashi T, Hashimoto K, Takagi R, Tanaka Y, Matsushita S. Antagonizing dopamine D1-like receptor inhibits Th17 cell differentiation: preventive and therapeutic effects on experimental autoimmune encephalomyelitis. Biochemical and biophysical research communications. 2008;373(2):286–291. doi: 10.1016/j.bbrc.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Nakano K, Higashi T, Takagi R, Hashimoto K, Tanaka Y, Matsushita S. Dopamine released by dendritic cells polarizes Th2 differentiation. International immunology. 2009;21(6):645–654. doi: 10.1093/intimm/dxp033. [DOI] [PubMed] [Google Scholar]
- Napolitano A, Cesura AM, Da Prada M. The role of monoamine oxidase and catechol O-methyltransferase in dopaminergic neurotransmission. Journal of neural transmission Supplementum. 1995;45:35–45. [PubMed] [Google Scholar]
- Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C, Bell J, Hauser KF, Mattson M. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. J Psychopharmacol. 2000;14(3):222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, Cass W, Turchan JT. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S62–69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Nathan CF. Mechanisms of macrophage antimicrobial activity. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1983;77(5):620–630. doi: 10.1016/0035-9203(83)90190-6. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Zunt JR. Tuberculosis of the central nervous system in immunocompromised patients: HIV infection and solid organ transplant recipients. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2011;53(9):915–926. doi: 10.1093/cid/cir508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TP, Soukup VM, Gelman BB. Persistent hijacking of brain proteasomes in HIV-associated dementia. The American journal of pathology. 2010;176(2):893–902. doi: 10.2353/ajpath.2010.090390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nockher WA, Bergmann L, Scherberich JE. Increased soluble CD14 serum levels and altered CD14 expression of peripheral blood monocytes in HIV-infected patients. Clinical and experimental immunology. 1994;98(3):369–374. doi: 10.1111/j.1365-2249.1994.tb05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuovo GJ, Alfieri ML. AIDS dementia is associated with massive, activated HIV-1 infection and concomitant expression of several cytokines. Mol Med. 1996;2(3):358–366. [PMC free article] [PubMed] [Google Scholar]
- Pacelli R, Wink DA, Cook JA, Krishna MC, DeGraff W, Friedman N, Tsokos M, Samuni A, Mitchell JB. Nitric oxide potentiates hydrogen peroxide-induced killing of Escherichia coli. The Journal of experimental medicine. 1995;182(5):1469–1479. doi: 10.1084/jem.182.5.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Justice JB., Jr Extracellular concentration and in vivo recovery of dopamine in the nucleus accumbens using microdialysis. Journal of neurochemistry. 1992;58(1):212–218. doi: 10.1111/j.1471-4159.1992.tb09298.x. [DOI] [PubMed] [Google Scholar]
- Peluso R, Haase A, Stowring L, Edwards M, Ventura P. A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology. 1985;147(1):231–236. doi: 10.1016/0042-6822(85)90246-6. [DOI] [PubMed] [Google Scholar]
- Persidsky Y, Zheng J, Miller D, Gendelman HE. Mononuclear phagocytes mediate blood-brain barrier compromise and neuronal injury during HIV-1-associated dementia. Journal of leukocyte biology. 2000;68(3):413–422. [PubMed] [Google Scholar]
- Peterson RA. Regulatory T-cells: diverse phenotypes integral to immune homeostasis and suppression. Toxicologic pathology. 2012;40(2):186–204. doi: 10.1177/0192623311430693. [DOI] [PubMed] [Google Scholar]
- Petito CK, Adkins B, McCarthy M, Roberts B, Khamis I. CD4+ and CD8+ cells accumulate in the brains of acquired immunodeficiency syndrome patients with human immunodeficiency virus encephalitis. Journal of neurovirology. 2003;9(1):36–44. doi: 10.1080/13550280390173391. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neuroscience and biobehavioral reviews. 2006;30(2):215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Post MJ, Thurnher MM, Clifford DB, Nath A, Gonzalez RG, Gupta RK, Post KK. CNS-Immune Reconstitution Inflammatory Syndrome in the Setting of HIV Infection, Part 1: Overview and Discussion of Progressive Multifocal Leukoencephalopathy-Immune Reconstitution Inflammatory Syndrome and Cryptococcal-Immune Reconstitution Inflammatory Syndrome. AJNR American journal of neuroradiology. 2012a doi: 10.3174/ajnr.A3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post MJ, Thurnher MM, Clifford DB, Nath A, Gonzalez RG, Gupta RK, Post KK. CNS-Immune Reconstitution Inflammatory Syndrome in the Setting of HIV Infection, Part 2: Discussion of Neuro-Immune Reconstitution Inflammatory Syndrome with and without Other Pathogens. AJNR American journal of neuroradiology. 2012b doi: 10.3174/ajnr.A3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349(9053):692–695. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- Purohit V, Rapaka R, Shurtleff D. Drugs of abuse, dopamine, and HIV-associated neurocognitive disorders/HIV-associated dementia. Molecular neurobiology. 2011;44(1):102–110. doi: 10.1007/s12035-011-8195-z. [DOI] [PubMed] [Google Scholar]
- Puthanakit T, Oberdorfer P, Akarathum N, Wannarit P, Sirisanthana T, Sirisanthana V. Immune reconstitution syndrome after highly active antiretroviral therapy in human immunodeficiency virus-infected thai children. The Pediatric infectious disease journal. 2006;25(1):53–58. doi: 10.1097/01.inf.0000195618.55453.9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport J, Joseph J, Croul S, Alexander G, Del Valle L, Amini S, Khalili K. Molecular pathway involved in HIV-1-induced CNS pathology: role of viral regulatory protein, Tat. Journal of leukocyte biology. 1999;65(4):458–465. doi: 10.1002/jlb.65.4.458. [DOI] [PubMed] [Google Scholar]
- Rashid AJ, So CH, Kong MM, Furtak T, El-Ghundi M, Cheng R, O’Dowd BF, George SR. D1–D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(2):654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, Wolfson T, Grant I. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society: JINS. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Roberts ES, Burudi EM, Flynn C, Madden LJ, Roinick KL, Watry DD, Zandonatti MA, Taffe MA, Fox HS. Acute SIV infection of the brain leads to upregulation of IL6 and interferon-regulated genes: expression patterns throughout disease progression and impact on neuroAIDS. Journal of neuroimmunology. 2004;157(1–2):81–92. doi: 10.1016/j.jneuroim.2004.08.030. [DOI] [PubMed] [Google Scholar]
- Roberts TK, Eugenin EA, Lopez L, Romero IA, Weksler BB, Couraud PO, Berman JW. CCL2 disrupts the adherens junction: implications for neuroinflammation. Laboratory investigation; a journal of technical methods and pathology. 2012;92(8):1213–1233. doi: 10.1038/labinvest.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21(14):1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- Rohr O, Sawaya BE, Lecestre D, Aunis D, Schaeffer E. Dopamine stimulates expression of the human immunodeficiency virus type 1 via NF-kappaB in cells of the immune system. Nucleic acids research. 1999a;27(16):3291–3299. doi: 10.1093/nar/27.16.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr O, Schwartz C, Aunis D, Schaeffer E. CREB and COUP-TF mediate transcriptional activation of the human immunodeficiency virus type 1 genome in Jurkat T cells in response to cyclic AMP and dopamine. Journal of cellular biochemistry. 1999b;75(3):404–413. [PubMed] [Google Scholar]
- Rouge-Pont F, Usiello A, Benoit-Marand M, Gonon F, Piazza PV, Borrelli E. Changes in extracellular dopamine induced by morphine and cocaine: crucial control by D2 receptors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2002;22(8):3293–3301. doi: 10.1523/JNEUROSCI.22-08-03293.2002. 20026322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B, Rai U. Dual mode of catecholamine action on splenic macrophage phagocytosis in wall lizard, Hemidactylus flaviviridis. General and comparative endocrinology. 2004;136(2):180–191. doi: 10.1016/j.ygcen.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Rudd ML, Nicolas AN, Brown BL, Fischer-Stenger K, Stewart JK. Peritoneal macrophages express the serotonin transporter. Journal of neuroimmunology. 2005;159(1–2):113–118. doi: 10.1016/j.jneuroim.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. Journal of neurovirology. 2002;8(Suppl 2):115–121. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- Saha B, Mondal AC, Basu S, Dasgupta PS. Circulating dopamine level, in lung carcinoma patients, inhibits proliferation and cytotoxicity of CD4+ and CD8+ T cells by D1 dopamine receptors: an in vitro analysis. International immunopharmacology. 2001;1(7):1363–1374. doi: 10.1016/s1567-5769(01)00068-6. [DOI] [PubMed] [Google Scholar]
- Santambrogio L, Lipartiti M, Bruni A, Dal Toso R. Dopamine receptors on human T- and B-lymphocytes. Journal of neuroimmunology. 1993;45(1–2):113–119. doi: 10.1016/0165-5728(93)90170-4. [DOI] [PubMed] [Google Scholar]
- Sarkar C, Das S, Chakroborty D, Chowdhury UR, Basu B, Dasgupta PS, Basu S. Cutting Edge: Stimulation of dopamine D4 receptors induce T cell quiescence by up-regulating Kruppel-like factor-2 expression through inhibition of ERK1/ERK2 phosphorylation. J Immunol. 2006;177(11):7525–7529. doi: 10.4049/jimmunol.177.11.7525. [DOI] [PubMed] [Google Scholar]
- Scheller C, Sopper S, Jassoy C, ter Meulen V, Riederer P, Koutsilieri E. Dopamine activates HIV in chronically infected T lymphoblasts. J Neural Transm. 2000;107(12):1483–1489. doi: 10.1007/s007020070012. [DOI] [PubMed] [Google Scholar]
- Schiffer WK, Azmoodeh M, Gerasimov M, Volkow ND, Fowler JS, Dewey SL. Selegiline potentiates cocaine-induced increases in rodent nucleus accumbens dopamine. Synapse. 2003;48(1):35–38. doi: 10.1002/syn.10183. [DOI] [PubMed] [Google Scholar]
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, Ghrayeb J, Forman MA, Montefiori DC, Rieber EP, Letvin NL, Reimann KA. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283(5403):857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- Sebastiani S, Allavena P, Albanesi C, Nasorri F, Bianchi G, Traidl C, Sozzani S, Girolomoni G, Cavani A. Chemokine receptor expression and function in CD4+ T lymphocytes with regulatory activity. J Immunol. 2001;166(2):996–1002. doi: 10.4049/jimmunol.166.2.996. [DOI] [PubMed] [Google Scholar]
- Shankar EM, Vignesh R, Velu V, Murugavel KG, Sekar R, Balakrishnan P, Lloyd CA, Saravanan S, Solomon S, Kumarasamy N. Does CD4+CD25+foxp3+ cell (Treg) and IL-10 profile determine susceptibility to immune reconstitution inflammatory syndrome (IRIS) in HIV disease? J Inflamm (Lond) 2008;5:2. doi: 10.1186/1476-9255-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne SA, Visnegarwala F, Darcourt J, Graviss EA, Giordano TP, White AC, Jr, Hamill RJ. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. AIDS. 2005;19(4):399–406. doi: 10.1097/01.aids.0000161769.06158.8a. [DOI] [PubMed] [Google Scholar]
- Shiramizu B, Gartner S, Williams A, Shikuma C, Ratto-Kim S, Watters M, Aguon J, Valcour V. Circulating proviral HIV DNA and HIV-associated dementia. AIDS. 2005;19(1):45–52. doi: 10.1097/00002030-200501030-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurtleff D, Lawrence D. HIV and Substance Abuse: A Commentary. Current HIV research. 2012 doi: 10.2174/157016212802138760. [DOI] [PubMed] [Google Scholar]
- Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, Calmy A, Chave JP, Giacobini E, Hirschel B, Du Pasquier RA. Cognitive dysfunction in HIV patients despite longstanding suppression of viremia. AIDS. 2010;24(9):1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- Sohler NL, Wong MD, Cunningham WE, Cabral H, Drainoni ML, Cunningham CO. Type and pattern of illicit drug use and access to health care services for HIV-infected people. AIDS patient care and STDs. 2007;21(Suppl 1):S68–76. doi: 10.1089/apc.2007.9985. [DOI] [PubMed] [Google Scholar]
- Sozzani S, Ghezzi S, Iannolo G, Luini W, Borsatti A, Polentarutti N, Sica A, Locati M, Mackay C, Wells TN, Biswas P, Vicenzi E, Poli G, Mantovani A. Interleukin 10 increases CCR5 expression and HIV infection in human monocytes. The Journal of experimental medicine. 1998;187(3):439–444. doi: 10.1084/jem.187.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76(2):301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- Starace F, Baldassarre C, Biancolilli V, Fea M, Serpelloni G, Bartoli L, Maj M. Early neuropsychological impairment in HIV-seropositive intravenous drug users: evidence from the Italian Multicentre Neuropsychological HIV Study. Acta psychiatrica Scandinavica. 1998;97(2):132–138. doi: 10.1111/j.1600-0447.1998.tb09975.x. [DOI] [PubMed] [Google Scholar]
- Steinman RM. Lasker Basic Medical Research Award. Dendritic cells: versatile controllers of the immune system. Nature medicine. 2007;13(10):1155–1159. doi: 10.1038/nm1643. [DOI] [PubMed] [Google Scholar]
- Sternberg EM, Wedner HJ, Leung MK, Parker CW. Effect of serotonin (5-HT) and other monoamines on murine macrophages: modulation of interferon-gamma induced phagocytosis. J Immunol. 1987;138(12):4360–4365. [PubMed] [Google Scholar]
- Strell C, Sievers A, Bastian P, Lang K, Niggemann B, Zanker KS, Entschladen F. Divergent effects of norepinephrine, dopamine and substance P on the activation, differentiation and effector functions of human cytotoxic T lymphocytes. BMC immunology. 2009;10:62. doi: 10.1186/1471-2172-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan IL, Smith BR, von Geldern G, Mateen FJ, McArthur JC. HIV-associated opportunistic infections of the CNS. Lancet neurology. 2012;11(7):605–617. doi: 10.1016/S1474-4422(12)70098-4. [DOI] [PubMed] [Google Scholar]
- Tan K, Roda R, Ostrow L, McArthur J, Nath A. PML-IRIS in patients with HIV infection: clinical manifestations and treatment with steroids. Neurology. 2009;72(17):1458–1464. doi: 10.1212/01.wnl.0000343510.08643.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazona R, Gonzalez-Garcia A, Zamzami N, Marchetti P, Frechin N, Gonzalo JA, Ruiz-Gayo M, van Rooijen N, Martinez C, Kroemer G. Chlorpromazine amplifies macrophage-dependent IL-10 production in vivo. J Immunol. 1995;154(2):861–870. [PubMed] [Google Scholar]
- Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. European journal of immunology. 1995;25(12):3418–3424. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- Tomlinson GS, Simmonds P, Busuttil A, Chiswick A, Bell JE. Upregulation of microglia in drug users with and without pre-symptomatic HIV infection. Neuropathology and applied neurobiology. 1999;25(5):369–379. doi: 10.1046/j.1365-2990.1999.00197.x. [DOI] [PubMed] [Google Scholar]
- Torres KC, Antonelli LR, Souza AL, Teixeira MM, Dutra WO, Gollob KJ. Norepinephrine, dopamine and dexamethasone modulate discrete leukocyte subpopulations and cytokine profiles from human PBMC. Journal of neuroimmunology. 2005;166(1–2):144–157. doi: 10.1016/j.jneuroim.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Serraino D, Bellagamba R, Corpolongo A, Piselli P, Lorenzini P, Visco-Comandini U, Vlassi C, Quartuccio ME, Giulianelli M, Noto P, Galgani S, Ippolito G, Antinori A, Narciso P. Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS research and human retroviruses. 2005;21(8):706–713. doi: 10.1089/aid.2005.21.706. [DOI] [PubMed] [Google Scholar]
- Tsambiras PE, Larkin JA, Houston SH. Case report. Toxoplasma encephalitis after initiation of HAART. The AIDS reader. 2001;11(12):608–610. 615–606. [PubMed] [Google Scholar]
- Tsao CW, Lin YS, Cheng JT. Inhibition of immune cell proliferation with haloperidol and relationship of tyrosine hydroxylase expression to immune cell growth. Life sciences. 1998;62(21):PL 335–344. doi: 10.1016/s0024-3205(98)00170-2. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Fleishman JA, Wenger N, London AS, Burnam MA, Shapiro MF, Bing EG, Stein MD, Longshore D, Bozzette SA. Effects of drug abuse and mental disorders on use and type of antiretroviral therapy in HIV-infected persons. Journal of general internal medicine. 2001;16(9):625–633. doi: 10.1046/j.1525-1497.2001.016009625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuttle DL, Harrison JK, Anders C, Sleasman JW, Goodenow MM. Expression of CCR5 increases during monocyte differentiation and directly mediates macrophage susceptibility to infection by human immunodeficiency virus type 1. Journal of virology. 1998;72(6):4962–4969. doi: 10.1128/jvi.72.6.4962-4969.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyor WR, Glass JD, Griffin JW, Becker PS, McArthur JC, Bezman L, Griffin DE. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Annals of neurology. 1992;31(4):349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- Valcour V, Sithinamsuwan P, Letendre S, Ances B. Pathogenesis of HIV in the central nervous system. Current HIV/AIDS reports. 2011;8(1):54–61. doi: 10.1007/s11904-010-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valcour VG, Shiramizu BT, Shikuma CM. HIV DNA in circulating monocytes as a mechanism to dementia and other HIV complications. Journal of leukocyte biology. 2010;87(4):621–626. doi: 10.1189/jlb.0809571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venton BJ, Zhang H, Garris PA, Phillips PE, Sulzer D, Wightman RM. Real-time decoding of dopamine concentration changes in the caudate-putamen during tonic and phasic firing. Journal of neurochemistry. 2003;87(5):1284–1295. doi: 10.1046/j.1471-4159.2003.02109.x. [DOI] [PubMed] [Google Scholar]
- Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nature reviews Immunology. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Herrath M, Oldstone MB, Fox HS. Simian immunodeficiency virus (SIV)-specific CTL in cerebrospinal fluid and brains of SIV-infected rhesus macaques. J Immunol. 1995;154(10):5582–5589. [PubMed] [Google Scholar]
- Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain: a journal of neurology. 2004;127(Pt 11):2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Wang J, Crawford K, Yuan M, Wang H, Gorry PR, Gabuzda D. Regulation of CC chemokine receptor 5 and CD4 expression and human immunodeficiency virus type 1 replication in human macrophages and microglia by T helper type 2 cytokines. The Journal of infectious diseases. 2002;185(7):885–897. doi: 10.1086/339522. [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Nakayama T, Nagakubo D, Hieshima K, Jin Z, Katou F, Hashimoto K, Yoshie O. Dopamine selectively induces migration and homing of naive CD8+ T cells via dopamine receptor D3. J Immunol. 2006;176(2):848–856. doi: 10.4049/jimmunol.176.2.848. [DOI] [PubMed] [Google Scholar]
- Weihe E, Eiden LE. Chemical neuroanatomy of the vesicular amine transporters. The FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2000;14(15):2435–2449. doi: 10.1096/fj.00-0202rev. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(18):7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DW, Eugenin EA, Calderon TM, Berman JW. Monocyte maturation, HIV susceptibility, and transmigration across the blood brain barrier are critical in HIV neuropathogenesis. Journal of leukocyte biology. 2012;91(3):401–415. doi: 10.1189/jlb.0811394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams K, Westmoreland S, Greco J, Ratai E, Lentz M, Kim WK, Fuller RA, Kim JP, Autissier P, Sehgal PK, Schinazi RF, Bischofberger N, Piatak M, Lifson JD, Masliah E, Gonzalez RG. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. The Journal of clinical investigation. 2005;115(9):2534–2545. doi: 10.1172/JCI22953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Corey S, Westmoreland SV, Pauley D, Knight H, de Bakker C, Alvarez X, Lackner AA. Perivascular macrophages are the primary cell type productively infected by simian immunodeficiency virus in the brains of macaques: implications for the neuropathogenesis of AIDS. The Journal of experimental medicine. 2001;193(8):905–915. doi: 10.1084/jem.193.8.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KC, Hickey WF. Central nervous system damage, monocytes and macrophages, and neurological disorders in AIDS. Annual review of neuroscience. 2002;25:537–562. doi: 10.1146/annurev.neuro.25.112701.142822. [DOI] [PubMed] [Google Scholar]
- Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annual review of immunology. 2007;25:171–192. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- Witwer KW, Gama L, Li M, Bartizal CM, Queen SE, Varrone JJ, Brice AK, Graham DR, Tarwater PM, Mankowski JL, Zink MC, Clements JE. Coordinated regulation of SIV replication and immune responses in the CNS. PloS one. 2009;4(12):e8129. doi: 10.1371/journal.pone.0008129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood E, Montaner JS, Yip B, Tyndall MW, Schechter MT, O’Shaughnessy MV, Hogg RS. Adherence and plasma HIV RNA responses to highly active antiretroviral therapy among HIV-1 infected injection drug users. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 2003;169(7):656–661. [PMC free article] [PubMed] [Google Scholar]
- Woods ML, 2nd, MacGinley R, Eisen DP, Allworth AM. HIV combination therapy: partial immune restitution unmasking latent cryptococcal infection. AIDS. 1998;12(12):1491–1494. doi: 10.1097/00002030-199812000-00011. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Kleitz HK, Deng X, Ladenheim B, Peng XQ, Li X, Gardner EL, Stein EA, Cadet JL. A single high dose of methamphetamine increases cocaine self-administration by depletion of striatal dopamine in rats. Neuroscience. 2009;161(2):392–402. doi: 10.1016/j.neuroscience.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing HQ, Hayakawa H, Izumo K, Kubota R, Gelpi E, Budka H, Izumo S. In vivo expression of proinflammatory cytokines in HIV encephalitis: an analysis of 11 autopsy cases. Neuropathology: official journal of the Japanese Society of Neuropathology. 2009;29(4):433–442. doi: 10.1111/j.1440-1789.2008.00996.x. [DOI] [PubMed] [Google Scholar]
- Yadav A, Collman RG. CNS inflammation and macrophage/microglial biology associated with HIV-1 infection. Journal of neuroimmune pharmacology: the official journal of the Society on NeuroImmune Pharmacology. 2009;4(4):430–447. doi: 10.1007/s11481-009-9174-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachek MK, Takmakov P, Park J, Wightman RM, McCarty GS. Simultaneous monitoring of dopamine concentration at spatially different brain locations in vivo. Biosensors & bioelectronics. 2010;25(5):1179–1185. doi: 10.1016/j.bios.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Thylin MR, Persidsky Y, Williams CE, Cotter RL, Zink W, Ryan L, Ghorpade A, Lewis K, Gendelman HE. HIV-1 infected immune competent mononuclear phagocytes influence the pathways to neuronal demise. Neurotoxicity research. 2001;3(5):461–484. doi: 10.1007/BF03033204. [DOI] [PubMed] [Google Scholar]
- Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112(5):1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock HW, Fingerle G, Strobel M, Schraut W, Stelter F, Schutt C, Passlick B, Pforte A. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. European journal of immunology. 1993;23(9):2053–2058. doi: 10.1002/eji.1830230902. [DOI] [PubMed] [Google Scholar]