Summary
Whether MCP-1 contributes to chronic musculoskeletal pain is unknown. We provide evidence that MCP-1 induces acute muscle hyperalgesia and a state of chronic nociceptive sensitization.
While raised levels of monocyte chemoattractant protein 1 (MCP-1) have been observed in patients with chronic muscle pain, direct evidence for its role as an algogen in skeletal muscle is still lacking. In the rat, MCP-1 induces a dose-dependent mechanical hyperalgesia lasting for up to 6 weeks. Following recovery, rats exhibited a markedly prolonged hyperalgesia to an intramuscular injection of prostaglandin E2, hyperalgesic priming. Intrathecal pre-treatment with isolectin B4 (IB4)-saporin, which selectively destroys IB4-positive (IB4+) nociceptors, markedly decreased MCP-1 induced hyperalgesia and prevented the subsequent development of priming. To evaluate the involvement of MCP-1 in stress-induced chronic pain we administered, intrathecally (i.t.), antisense (AS) or mismatch (MM) oligodeoxynucleotides directed against CCR2 (the canonical receptor for MCP-1) mRNA, during the exposure to water avoidance stress, a model of stress-induced persistent muscle pain. The AS treatment attenuated this hyperalgesia, whereas IB4-saporin abolished water avoidance stress-induced muscle hyperalgesia and prevented stress-induced hyperalgesic priming. These results indicate that MCP-1 induces persistent muscle hyperalgesia and a state of latent chronic sensitization to other algogens, by action on its cognate receptor on IB4+ nociceptors. Since MCP-1 also contributes to stress-induced widespread chronic muscle pain, it should be considered as a player in chronic musculoskeletal pain syndromes.
Keywords: Stress, myalgia, nociceptor, fibromyalgia, inflammation
1. Introduction
Chronic muscle pain syndromes are very common and disabling, producing a large burden of economic losses related to health care expenses, decreased work productivity and disability compensation [24]. It is often observed in the absence of a previous injury, and may exhibit features of a widespread pain syndrome. Remarkably, stress is well-established to trigger and aggravate chronic muscle pain [24]. In spite of these distinctive clinical features, the mechanisms underlying chronic muscle pain still remain obscure, precluding the development of rational analgesic strategies.
The search for potential biomarkers and proalgesic agents of chronic muscle pain has identified a correlation between reported pain levels and raised plasma/local levels of some pro-inflammatory mediators. For example, a small (13 kDa) chemokine, the monocyte chemoattractant protein 1 (MCP-1, also known as chemokine (C-C motif) ligand 2 or CCL2), has been identified as a candidate algogen involved in fibromyalgia [7; 19; 67] and polymyalgia rheumatica [41], which are characterized by intense widespread chronic muscle pain. In addition, some viral diseases, such as Chikungunya fever, Dengue fever, Influenza A and vesicular stomatitis, exhibit a widespread muscle pain concomitant to a marked rise in circulating levels of MCP-1 [10; 21; 44; 55]. Since MCP-1 plays an important role as a chemotactic agent for monocyte/macrophages at the site of injury acting on its cognate receptor, CCR2 [63], an indirect contribution of MCP-1 to pain by means of inflammatory cells is plausible. More recently, however, a direct proalgesic role for MCP-1 has been identified: indeed, intradermal injection of MCP-1 produces primary mechanical hyperalgesia with a rapid-onset [16], whereas the application of MCP-1 increases the excitability of C-fibers [54] and cultured dorsal root ganglion (DRG) neurons [38]. Indeed, while both nociceptors and dorsal horn neurons express MCP-1 only nociceptors express CCR2 [66], further supporting the view that MCP-1 is a proalgesic mediator that acts directly on nociceptors.
Interestingly, plasma levels of MCP-1 are increased in women exposed to prolonged psychosocial stress [9]. This is also observed in rodents after repeated exposure to different stressors [27; 31; 47]. This phenomenon appears to be dependent on the release of catecholamines and beta-adrenoceptor activation [31], a hallmark in models of stress-induced widespread muscle pain [5; 40]. Furthermore, paclitaxel chemotherapy, which produces robust muscle pain in humans [28; 33; 43] and rodents [3; 22], is associated with a marked increase in serum levels of MCP-1 in humans [52] and enhanced expression of MCP-1 in nociceptors and dorsal horn neurons in rodents [66]. While these observations strongly suggest a role for MCP-1 as a pro-nociceptive mediator in skeletal muscle, evidence supporting this view is still lacking. Here we provide direct evidence for a role of MCP-1 in the induction of acute and chronic muscle pain.
2. Methods
2.1 Animals
Adult male Sprague Dawley rats (240–250 g at arrival; Charles River, Hollister, CA, USA) were used in these experiments. They were housed in the Animal Care Facility at the University of California San Francisco, under environmentally controlled conditions (lights on 07:00–19:00 h; room temperature 21–23°C) with food and water available ad libitum. Upon completion of experiments, rats were euthanized by inhalation of CO2 followed by bilateral thoracotomy. Animal care and use conformed to NIH guidelines (NIH Guide for the Care and Use of Laboratory Animals) and to the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain. The University of California San Francisco Committee on Animal Research approved all experimental protocols. Concerted effort was made to minimize number and suffering of experimental animals.
2.2 Chemicals
Unless otherwise stated, all chemicals used in these experiments were obtained from Sigma-Aldrich (St. Louis, MO, USA). The stock solution of rat recombinant MCP-1 (rrMCP-1, Fitzgerald Industries International, Concord, MA, USA) was made by dissolving it in 0.9% NaCl containing 0.5% BSA (1 μg/μl) and stored at −20°C. The stock solution of prostaglandin E2 (PGE2) was made by dissolving it in 100% ethanol (1 μg/μl), and stored at −80°C. The stock solutions were diluted in 0.9% NaCl immediately before injection.
2.3 Local injections
We performed intramuscular (i.m.) injections of rrMCP-1 to explore the direct proalgesic effect of this mediator, whereas PGE2 was administered to assess the presence of hyperalgesic priming, a latent state where hyperalgesia induced by inflammatory cytokines is prolonged, indicative of the induction of chronic neuroplastic adaptation [53].
Rats were briefly anesthetized with 2.5% isoflurane to facilitate the injection (20 μl) of either rrMCP-1 or PGE2 into the belly of the gastrocnemius muscle. The skin overlying the injection site was previously shaved and scrubbed with alcohol. Immediately after i.m. injections the site at which the skin was punctured was marked with a fine-tip indelible ink pen, so that the mechanical nociceptive threshold at the underlying i.m. injection site in the muscle could be repeatedly measured.
2.4 Intrathecal treatments
2.4.1 IB4-saporin
The destruction of the isolectin B4-positive (IB4+) population of dorsal root ganglion neurons by intrathecal injection of a cytotoxin, saporin, conjugated to IB4 (IB4-saporin) has been established previously [16; 35; 51; 59]. The selective neurotoxic effect of the IB4-saporin conjugate has also been previously shown; in contrast to unconjugated saporin, intrathecal injection of IB4-saporin produces a dramatic decrease in the IB4 labeling in lamina II of the dorsal horn [35]. The results obtained by our group are fully consistent with those observed by Nishiguchi and colleagues [51] in DRGs after intrathecal injection of IB4-saporin. Indeed, intrathecal IB4-saporin abolishes glial cell-derived neurotrophic factor (GDNF), but not nerve growth factor (NGF)-induced hyperalgesia compatible with a selective effect on IB4+ nociceptors [2; 16; 18; 36].
IB4-saporin (Advanced Targeting Systems, San Diego, CA, USA) was diluted with saline and a dose of 3.2 μg in 20 μl administered intrathecally 10 days prior to other experimental interventions [2; 4; 35; 36]. Control treatment consisted of intrathecal injection of saline (20 μl).
2.4.2 Antisense oligodeoxynucleotides
To assess the role of MCP-1 in muscle pain observed in a preclinical model of stress-induced hyperalgesia, the expression of its cognate receptor (CCR2) was disrupted by intrathecal injection of antisense oligodeoxynucleotides (AS ODN) to CCR2 mRNA. This procedure not only modulates behavioral nociceptive responses, but also produces reversible inhibition of the expression of different proteins in DRG neurons (for a review see [56]). The AS ODN sequence, 5′-ACTCGGTCTGCTGTCTCCCTA-3′, was directed against a unique sequence in rat CCR2 mRNA (GenBank number NM021866). This sequence has been shown to produce a knock-down of CCR2 protein in DRG neurons [66]. The mismatch (MM) ODN sequence, 5′-ACACGCTGTCCTGTCAGCCTA-3′, corresponds to the CCR2 AS sequence with 7 mismatched bases (denoted by bold letters). A search of the NCBI database to Rattus norvegicus identified no other homologous sequences. Rats were briefly anesthetized with 2.5% isoflurane in 97.5% O2 and received daily intrathecal injections (40 μg/20 μl), with ODN either AS or MM to CCR2 mRNA, for 4 consecutive days. The AS and MM ODN primers were synthetized by Invitrogen (San Francisco, CA, USA).
To perform intrathecal injections rats were briefly anaesthetized with 2.5% isoflurane (Phoenix Pharmaceuticals, St. Joseph, MO, USA) in 97.5% O2. Then, a 29-gauge hypodermic needle was inserted into the subarachnoid space on the midline, between the L4 and L5 vertebrae. Proper intrathecal injection was systematically confirmed by observation of a tail-flick [49].
2.5 Measurement of mechanical hyperalgesia
Mechanical nociceptive threshold in the gastrocnemius muscle was quantified using a digital force transducer (Chatillon DFI2; Amtek Inc., Largo, FL, USA) with a custom-made 7 mm-diameter probe [2-6]. Rats were lightly restrained in a cylindrical acrylic holder with lateral slats that allow for easy access to the hind limb and application of the force transducer probe to the site of injection in the belly of the gastrocnemius muscle. The nociceptive threshold was defined as the force, in mN, required to produce a flexion reflex in the hind leg. Baseline withdrawal threshold was defined as the mean of 3 readings taken at 5 min intervals and magnitude of hyperalgesia calculated as percentage decrease from the baseline withdrawal threshold.
2.6 Water avoidance stress
Water-avoidance induced stress, which produces mechanical hyperalgesia, is considered a model of widespread muscle pain [29] is associated with increased firing in nociceptors innervating the gastrocnemius muscle [23]. To induce water avoidance stress, rats were placed on a 10 cm high acrylic platform (8 × 8 cm) in the center of a clear plastic tank (45 cm length × 25 cm width × 25 cm height) filled with room temperature tap water to a depth of 9 cm, for 1 h/day, for 10 consecutive days. This protocol produces psychological stress as indicated by a large increase in ACTH and glucocorticoids within 30 min of the start of stress exposure [20]. Experiments on water avoidance stress were performed 1 day after the last stress exposure.
2.7 Statistical analysis
Group data are expressed as mean ± SEM of n independent observations. Statistical comparisons were made using GraphPad Prism 5.0 statistical software (GraphPad Software, Inc., La Jolla, CA, USA). Comparisons were made by means of one- or two-way repeated measures analysis of variance (ANOVA) followed by Bonferroni's multiple comparisons test. P < 0.05 was considered statistically significant.
3. Results
3.1 MCP-1 induces mechanical hyperalgesia and hyperalgesic priming
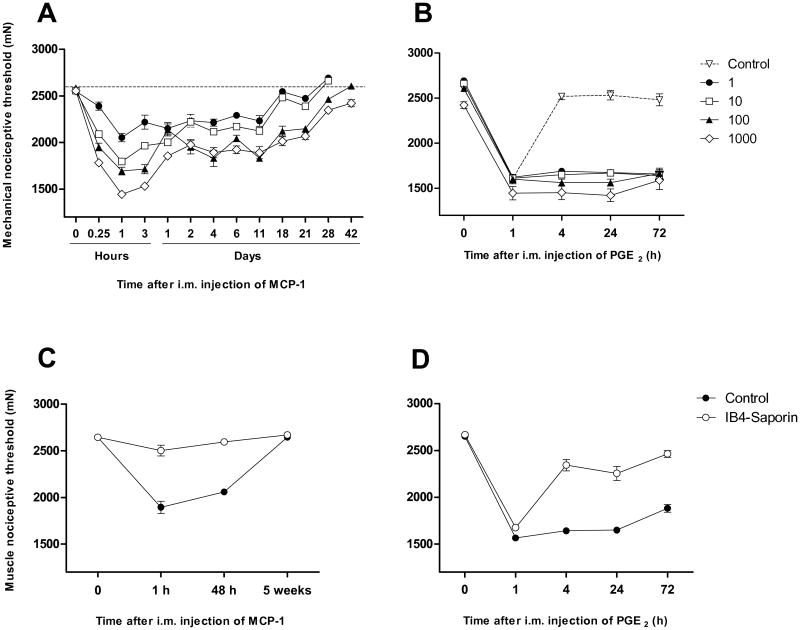
The injection of MCP-1 into the gastrocnemius muscle produced a dose- (1-1000 ng/20 μl) and time-dependent decrease in mechanical nociceptive threshold. Fifteen min after its injection MCP-1 produced a significant decrease in mechanical nociceptive threshold, reaching a peak at 1 h for all doses studied (Fig. 1A). One day after injection MCP-1 continued to produce a persistent mechanical hyperalgesia, albeit at lower magnitude, that remained unattenuated for 10 to 20 days depending on dose (Fig. 1A). The mechanical threshold then progressively increased to reach baseline values, with the highest dose assayed producing a mechanical hyperalgesia lasting up to 6 weeks (Fig. 1A).
Figure 1.
MCP-1 induces muscle hyperalgesia and hyperalgesic priming dependent on IB4+ nociceptors. (A) Effect of a single injection of rrMCP-1 (1-1000 ng/20 μl, n=6/group) into the gastrocnemius muscle. The dotted line indicates the average mechanical nociceptive threshold observed in naïve rats. (B) Effect of PGE2 (1 μg/20 μl) injected into the gastrocnemius muscle of rats previously with rrMCP-1 (ng/20 μl, n=6/group) or saline (n=6). (C) Effect of treatment with intrathecal IB4-saporin (n=6) on baseline nociceptive threshold compared to control treatment (i.t. saline, n=6), and muscle hyperalgesia induced by i.m. rrMCP-1 (100 ng/20 μl). (D) Effect of treatment with intrathecal IB4-saporin and control treatment on PGE2 (1 μg/20 μl) induced hyperalgesia in rats previously injected with rrMCP-1.
Once rats fully recovered (i.e., reaching baseline mechanical threshold) the presence of hyperalgesic priming was assessed by injecting PGE2 (1 μg/20 μl) at the site previously injected with MCP-1. As shown in figure 1B, regardless of the dose previously injected, all the animals exposed to MCP-1 exhibited prolonged PGE2-induced mechanical hyperalgesia (i.e. significantly longer than 4 h), when compared to naïve control rats.
3.2 IB4-saporin attenuates MCP-1 hyperalgesia
Since our previous studies have pointed to an involvement of IB4+ nociceptors in MCP-1 induced hyperalgesia [16], we assessed their role in MCP-1 hyperalgesia by i.t. injection of the neurotoxin saporin conjugated to isolectin B4, which selectively destroys IB4+ nociceptors [59], or its vehicle (saline), to naïve rats. One hour after an i.m. injection of MCP-1 (100 ng/20 μl), rats pre-treated with IB4-saporin (i.t.) exhibited a small, albeit statistically significant, decrease in mechanical nociceptive threshold (P < 0.05, n=6, Fig. 1C). Compared to control-treated (i.t. saline) rats, the change in mechanical nociceptive threshold in IB4-saporin treated rats was markedly attenuated (-28.3 ± 2.4% versus -5.4 ± 1.8%, respectively, n=6/group, P < 0.01, Fig. 1C). Hyperalgesia was fully recovered by 48 h after i.m. injection of MCP-1 in IB4-saporin treated, but not in control rats, the differences between these two treatments being statistically significant (-1.9 ± 1.0% versus -22.1 ± 1.0%, n=6/group, respectively, P < 0.001, Fig. 1C). Five weeks later, mechanical threshold reached baseline levels in both groups, at which time rats were injected with PGE2 (1 μg/20 μl) at the site previously injected with MCP-1. One hour after i.m. injection of PGE2 rats from both groups exhibited a comparable mechanical hyperalgesia. At later time points, control but not IB4-saporin treated rats displayed a persistent hyperalgesia lasting for at least 72 h (Fig. 1D).
3.3 IB4-saporin attenuates water avoidance stress-induced muscle hyperalgesia
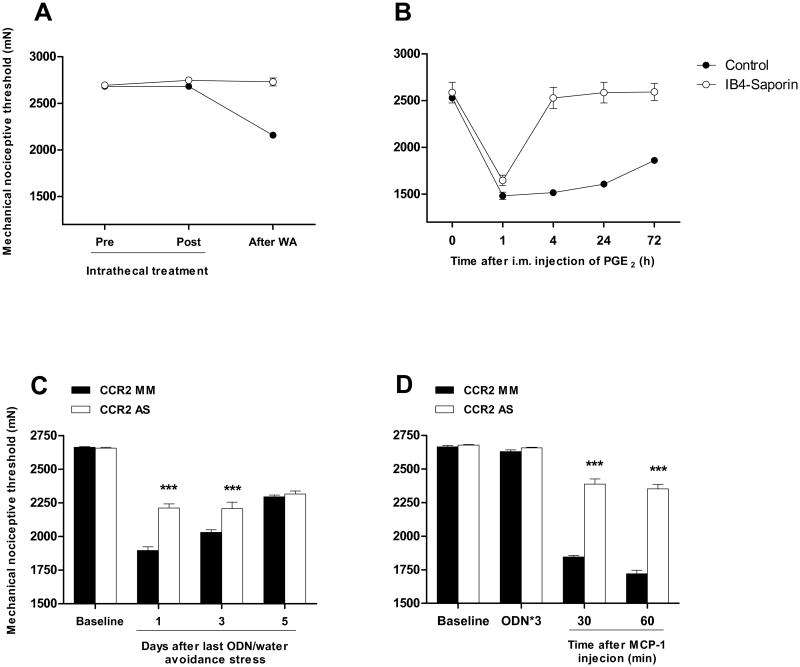
Given that sustained exposure to stressful stimuli can produce hyperalgesic priming in skeletal muscle [29; 30; 40] and that IB4-saporin treatment attenuated MCP-1 induced hyperalgesic priming, we assessed whether this stress-induced hyperalgesic priming depends on IB4+ nociceptors. We explored this in rats previously injected i.t. with IB4-saporin (10 days before) in the water avoidance stress paradigm, which is known to produce hyperalgesic priming [29] and raised plasma levels of MCP-1 [27]. Ten days after i.t. treatment, no differences in nociceptive mechanical threshold were observed between control rats and rats treated with IB4-saporin (P > 0.05, n=6/group, Fig. 2A). However, consistent with our previous observations [29], 10 days of exposure to water avoidance stress produced a significant decrease in mechanical nociceptive threshold in control rats (∼24% compared to baseline; P < 0.001, n=6, Fig. 2A). In contrast, IB4-saporin treated rats did not exhibit changes in mechanical nociceptive threshold after water avoidance stress (P > 0.05, n=6, Fig. 2A). Thirteen days after the last exposure to water stress, PGE2 (1 μg/20 μl) was injected into the gastrocnemius muscle (Fig. 2B). While control and IB4-saporin treated rats exhibited similar acute hyperalgesia (i.e., measured 1 h after injections of PGE2), a later phase of mechanical hyperalgesia (i.e., 4 to 72 h) was only observed in control rats (Fig. 2B).
Figure 2.
Water avoidance stress produces a persistent muscle hyperalgesia dependent on CCR2 expressed by IB4+ nociceptors. (A) Effect of intrathecal pre-treatment with IB4-saporin (n=6) or control treatment (saline 20 μl, n=6) on water avoidance stress-induced muscle hyperalgesia. (B) Effect of intrathecal IB4-saporin (open circle, n=6) or control treatment (i.t. saline 20 μl, n=6, solid circle) on water avoidance stress-induced hyperalgesic priming. PGE2 (1 μg/20 μl) was injected into the gastrocnemius muscle of rats previously exposed to water avoidance stress. (C) Effect of CCR2 AS/MM (n=6/group) in water avoidance stress-induced muscle pain. (D) Effect of CCR2 AS/MM (n=6/group) in rrMCP-1 (100 ng/20 μl) induced muscle hyperalgesia in naïve rats.
3.4 Knock-down of CCR2 attenuates stress and rrMCP-1 induced muscle hyperalgesia
To determine whether MCP-1 is involved in water avoidance stress-induced hyperalgesia, by action at its cognate receptor on muscle nociceptors we explored the effect of i.t. AS ODN treatment against mRNA for CCR2, the cognate MCP-1 receptor, in nociceptors (Zhang et al., 2013). Rats received daily i.t. injections of either AS or MM for three consecutive days, starting on day 8 of the 10 day water avoidance protocol. One day after the last ODN injection, a significant inhibition in water avoidance-induced hyperalgesia was observed in the AS treated rats, compared to MM control (-16.7 ± 1.1% vs -28.8 ± 1%, respectively; P < 0.01, n=6/group, Fig. 2C). Such an inhibition was significant up to day 3 after the last ODN injection (-16.9 ± 1.8% vs -23.8 ± 0.7%, respectively; P < 0.01, n=6/group, Fig. 2C) but absent by day 5 (-12.8 ± 1% vs -13.8 ± 0.6%, respectively; P < 0.01, n=6/group, Fig. 2C).
Using the same AS/MM treatments, we assessed the role of CCR2 expressed by nociceptors on muscle hyperalgesia induced by peripherally administered MCP-1. One day after the last ODN injection, no significant differences in baseline mechanical nociceptive threshold was observed between AS and MM treated rats (-0.8 ± 0.3% vs -1.3 ± 0.2% change compared to pre-treatment baseline, respectively; P < 0.01, n=6/group, Fig. 2D). The mechanical hyperalgesia induced by rrMCP-1 (100 ng/20 μl) was however significantly attenuated by AS treatment measured at 30 min (-10.8 ± 1.5% vs -30.7 ± 0.5%, respectively; P < 0.01, n=6/group, Fig. 2D) and 1 h (-12.2 ± 1.2% vs -35.4 ± 1%, respectively; P < 0.01, n=6/group, Fig. 2D) after i.m. injection of MCP-1.
5. Discussion
Our results indicate that MCP-1 not only produces long-lasting mechanical hyperalgesia but also induces a state of chronic sensitization to other algogens, by acting at its cognate receptor CCR2, located on IB4+ nociceptors. We also observed that MCP-1 is involved in the induction of persistent muscle pain observed after repeated exposure to stressful stimuli.
5.1 MCP-1 acts on nociceptors to produce persistent muscle hyperalgesia
While correlative evidence suggests that MCP-1 is involved in musculoskeletal pain syndromes [7; 19; 41; 67], a direct test of this hypothesis as well as the site of its proalgesic action have been elusive. For instance, MCP-1 acts as a chemotactic agent for monocytes and macrophages that may in turn contribute several inflammatory mediators to the induction of persistent pain [63], and also increases the excitability of nociceptors [13; 39; 66]. On the other hand, intradermal injection of rrMCP-1 produces primary hyperalgesia [16]. In the present experiments we observed a robust fast-onset, and persistent mechanical hyperalgesia. Furthermore, i.t. AS treatment that knocks down CCR2 in DRG neurons [66] markedly attenuated MCP-1 induced hyperalgesia. While there is no consensus regarding the subpopulation of nociceptors expressing CCR2 [13; 66], it is not expressed by dorsal horn neurons [66]. While, some studies have failed to detect the expression of CCR2 [14; 37; 38; 50], others have provided evidence by means of Western blot [61; 65] and immunofluorescence [8; 13; 66; 68] that CCR2 is constitutively expressed in DRG neurons of naïve rats. Furthermore, in vitro electrophysiological studies performed in DRG neurons obtained from control rats have shown that MCP-1, but not vehicle, activates between 6 to 10% of those neurons [57; 62]. And, exposure to MCP-1 induces an increase in intracellular calcium concentration in 5.8 to 7.9% of acutely dissociated DRG neurons from control rats [14]. Finally, recombinant MCP-1 induces a dose-dependent increase in markers of neuronal activation (Fos) in cultured trigeminal ganglion neurons, which is sensitive to a CCL2 neutralizing antibody [65]. These observations support the suggestion that functional CCR2 is constitutively expressed in sensory neurons. And, since MCP-1 was injected into the gastrocnemius muscle, the only cell at the site of its injection that was exposed to the AS is the sensory neuron innervating that muscle. While the contribution of a potential indirect effect of MCP-1 cannot be completely excluded in these results, our observations indicate that the exposure of skeletal muscle nociceptors to MCP-1 is sufficient to produce mechanical hyperalgesia.
Several mechanisms can contribute to MCP-1 induced mechanical hyperalgesia; for instance, acting through a pertussis toxin-sensitive mechanism, MCP-1 rapidly increases the density of Nav type 1.8 currents in dissociated DRG neurons, suggesting a role for Gi/o proteins in the signaling from CCR2 to Nav1.8 [13]. In addition, exposure of DRG neurons to MCP-1 produces a delayed increase in levels of mRNA encoding Nav1.8 [39], likely due to MCP-1/CCR2 activation of the PI3K/Akt pathway, which in turn phosphorylates SP1, a transcription factor that binds the proximal promoter region of the Nav1.8 gene [39]. Of note, Nav1.8 plays an essential role in repetitive firing of nociceptors [25] and Nav1.8 null mice exhibit specific deficits in behavioral responses [1] and dorsal horn neuronal firing [48] to encode noxious mechanical, but not thermal, stimuli. Thus, it is tempting to speculate that MCP-1 produces acute and persistent muscle mechanical hyperalgesia by acting on Nav1.8 through CCR2 located on the peripheral terminals of nociceptors.
5.2 MCP-1 mediates stress-induced muscle hyperalgesia
One striking feature of musculoskeletal pain syndromes is that they are typically triggered or aggravated by stress [24]. Since stressful stimuli increase plasma levels of pro-inflammatory cytokines [45], including MCP-1 [19], they have been suggested to contribute to chronic widespread pain syndromes [46; 60]. Furthermore, patients affected by chronic widespread muscle pain also exhibit increased levels of MCP-1 [19; 41; 67]. Here we used a stress protocol that is known to increase plasma levels of pro-inflammatory cytokines, including MCP-1 [27], to assess the role of MCP-1 in the relationship between stress and pain. Coupling this model to an antisense treatment to knock-down CCR2, the high affinity receptor for MCP-1 in nociceptors, we observed a marked, albeit not complete, inhibition of muscle hyperalgesia induced by stress. This suggests that the muscle pain responses to repeated exposure to stressful stimuli depends on increased circulating levels of MCP-1 acting on CCR2 located in muscle nociceptors. The increased circulating levels of MCP-1 have been reported to depend on qualitative and quantitative changes in gut microbiota induced by the exposure to stressful stimuli [12]. Of note, the plasma levels of many other pro-inflammatory cytokines, such as tumor necrosis alpha (TNFα) and interleukin 6 (IL-6), are also enhanced by stress [5; 12], including in the water avoidance stress model [27]. That there is residual hyperalgesia in AS-treated rats suggests a contribution of these other pro-inflammatory cytokines observed in water avoidance stress induced muscle pain.
5.3 IB4+ nociceptors participate in MCP-1 induced muscle hyperalgesia
The IB4+ nociceptors play an important role in persistent mechanical hyperalgesia in skeletal muscle [2; 4] and also in cutaneous hyperalgesia induced by MCP-1 [16]. Furthermore, inflammation induced by Freund's adjuvant dramatically increases the expression of MCP-1 in IB4+ nociceptors [34]. We explored the effect of selective destruction of this nociceptor subpopulation, by means of the neurotoxin saporin conjugated to IB4, on MCP-1 hyperalgesia. Our results are in agreement with these previous reports, which have shown that IB4+ nociceptors are necessary for MCP-1 induced hyperalgesia. The mechanical hyperalgesia induced by water stress was equally inhibited by pre-treatment with IB4-saporin.
Previous studies indicate that IB4+ nociceptor-dependent mechanical hyperalgesia is mediated, to some extent, by PKCε. For example, muscle hyperalgesia induced by ergonomic insults is markedly inhibited by AS treatment against to PKCε mRNA expressed by nociceptors [6; 26]. This seems also to be the case for MCP-1 hyperalgesia, since non-selective inhibitors of PKC block the sensitizing effect of MCP-1 on nociceptors [38]. On the other hand Nav1.8, a well-established downstream target for MCP-1/CCR2 cellular signaling [13; 39], is extensively co-expressed with PKCε in nociceptors [64]. Indeed, it has been suggested that the increase in Nav1.8 current density induced by MCP-1 in small-sized DRG cells is mediated by PKCε [13]. And, proalgesic mediators acting on G-protein coupled receptors, like MCP-1, only induce membrane translocation of PKCε in IB4+ nociceptors [32; 42; 58]. Finally, we have provided evidence that versican, a proteoglycan that confers IB4-binding capacity to nociceptors [17], is critical for MCP-1 induced mechanical hyperalgesia [16].
In addition to acute hyperalgesia, either injection of MCP-1 or exposure to water avoidance stress produced a prolonged hyperalgesic response to PGE2. This hyperalgesic priming response was markedly attenuated by pre-treatment with IB4-saporin.We have previously shown that cutaneous inflammation and diverse muscle insults produce hyperalgesic priming [2; 4; 26; 36]. In this phenomenon, a local injection of PGE2 produces, in addition to short-lasting cAMP/PKA dependent hyperalgesia, a PKCε-dependent abnormally prolonged mechanical hyperalgesia [53]. Hyperalgesic priming is mediated by neuroplastic changes in IB4+ nociceptors, since their destruction abolishes priming [2; 4; 36]. Indeed, IB4+ nociceptors selectively express cytoplasmic polyadenylation element binding protein (CPEB), a mediator that plays a key role in both the induction of hyperalgesic priming [15] and neuroplastic changes involved in long-term memory [11]. And, CPEB is also downstream of PKCε in the signaling cascade for the induction of hyperalgesic priming [15].
In summary, MCP-1 induces long lasting muscle hyperalgesia and a state of latent chronic sensitization to other algogens, hyperalgesic priming, by action on its high affinity receptor, CCR2, located on the peripheral terminals of IB4+ nociceptors. Since MCP-1 was also shown to be involved in stress-induced persistent muscle pain, therapies targeting MCP-1 or CCR2 may be helpful for the treatment of chronic musculoskeletal pain.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (NIH). Authors thank Dr. Oliver Bogen for technical help.
References
- 1.Akopian AN, Souslova V, England S, Okuse K, Ogata N, Ure J, Smith A, Kerr BJ, McMahon SB, Boyce S, Hill R, Stanfa LC, Dickenson AH, Wood JN. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nature neuroscience. 1999;2(6):541–548. doi: 10.1038/9195. [DOI] [PubMed] [Google Scholar]
- 2.Alvarez P, Chen X, Bogen O, Green PG, Levine JD. IB4(+) nociceptors mediate persistent muscle pain induced by GDNF. Journal of neurophysiology. 2012;108(9):2545–2553. doi: 10.1152/jn.00576.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alvarez P, Ferrari LF, Levine JD. Muscle pain in models of chemotherapy-induced and alcohol-induced peripheral neuropathy. Annals of neurology. 2011;70(1):101–109. doi: 10.1002/ana.22382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez P, Gear RW, Green PG, Levine JD. IB4-saporin attenuates acute and eliminates chronic muscle pain in the rat. Experimental neurology. 2012;233(2):859–865. doi: 10.1016/j.expneurol.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez P, Green PG, Levine JD. Stress in the adult rat exacerbates muscle pain induced by early-life stress. Biological psychiatry. 2013;74(9):688–695. doi: 10.1016/j.biopsych.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alvarez P, Levine JD, Green PG. Eccentric exercise induces chronic alterations in musculoskeletal nociception in the rat. The European journal of neuroscience. 2010;32(5):819–825. doi: 10.1111/j.1460-9568.2010.07359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ang DC, Moore MN, Hilligoss J, Tabbey R. MCP-1 and IL-8 as pain biomarkers in fibromyalgia: a pilot study. Pain Med. 2011;12(8):1154–1161. doi: 10.1111/j.1526-4637.2011.01179.x. [DOI] [PubMed] [Google Scholar]
- 8.Arms L, Girard BM, Malley SE, Vizzard MA. Expression and function of CCL2/CCR2 in rat micturition reflexes and somatic sensitivity with urinary bladder inflammation. American journal of physiology Renal physiology. 2013;305(1):F111–122. doi: 10.1152/ajprenal.00139.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asberg M, Nygren A, Leopardi R, Rylander G, Peterson U, Wilczek L, Kallmen H, Ekstedt M, Akerstedt T, Lekander M, Ekman R. Novel biochemical markers of psychosocial stress in women. PloS one. 2009;4(1):e3590. doi: 10.1371/journal.pone.0003590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Back AT, Lundkvist A. Dengue viruses - an overview. Infection ecology & epidemiology. 2013;3 doi: 10.3402/iee.v3i0.19839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey CH, Kandel ER, Si K. The persistence of long-term memory: a molecular approach to self-sustaining changes in learning-induced synaptic growth. Neuron. 2004;44(1):49–57. doi: 10.1016/j.neuron.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Bailey MT, Dowd SE, Galley JD, Hufnagle AR, Allen RG, Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: implications for stressor-induced immunomodulation. Brain, behavior, and immunity. 2011;25(3):397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belkouch M, Dansereau MA, Reaux-Le Goazigo A, Van Steenwinckel J, Beaudet N, Chraibi A, Melik-Parsadaniantz S, Sarret P. The chemokine CCL2 increases Nav1.8 sodium channel activity in primary sensory neurons through a Gbetagamma-dependent mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(50):18381–18390. doi: 10.1523/JNEUROSCI.3386-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhangoo S, Ren D, Miller RJ, Henry KJ, Lineswala J, Hamdouchi C, Li B, Monahan PE, Chan DM, Ripsch MS, White FA. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: a mechanism for the development of chronic sensitization of peripheral nociceptors. Molecular pain. 2007;3:38. doi: 10.1186/1744-8069-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bogen O, Alessandri-Haber N, Chu C, Gear RW, Levine JD. Generation of a pain memory in the primary afferent nociceptor triggered by PKCepsilon activation of CPEB. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(6):2018–2026. doi: 10.1523/JNEUROSCI.5138-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bogen O, Dina OA, Gear RW, Levine JD. Dependence of monocyte chemoattractant protein 1 induced hyperalgesia on the isolectin B4-binding protein versican. Neuroscience. 2009;159(2):780–786. doi: 10.1016/j.neuroscience.2008.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bogen O, Dreger M, Gillen C, Schroder W, Hucho F. Identification of versican as an isolectin B4-binding glycoprotein from mammalian spinal cord tissue. The FEBS journal. 2005;272(5):1090–1102. doi: 10.1111/j.1742-4658.2005.04543.x. [DOI] [PubMed] [Google Scholar]
- 18.Bogen O, Joseph EK, Chen X, Levine JD. GDNF hyperalgesia is mediated by PLCgamma, MAPK/ERK, PI3K, CDK5 and Src family kinase signaling and dependent on the IB4-binding protein versican. The European journal of neuroscience. 2008;28(1):12–19. doi: 10.1111/j.1460-9568.2008.06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bote ME, Garcia JJ, Hinchado MD, Ortega E. Inflammatory/stress feedback dysregulation in women with fibromyalgia. Neuroimmunomodulation. 2012;19(6):343–351. doi: 10.1159/000341664. [DOI] [PubMed] [Google Scholar]
- 20.Bradesi S, Schwetz I, Ennes HS, Lamy CM, Ohning G, Fanselow M, Pothoulakis C, McRoberts JA, Mayer EA. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. American journal of physiology Gastrointestinal and liver physiology. 2005;289(1):G42–53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- 21.Bussfeld D, Nain M, Hofmann P, Gemsa D, Sprenger H. Selective induction of the monocyte-attracting chemokines MCP-1 and IP-10 in vesicular stomatitis virus-infected human monocytes. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 2000;20(7):615–621. doi: 10.1089/107999000414781. [DOI] [PubMed] [Google Scholar]
- 22.Chen X, Green PG, Levine JD. Abnormal muscle afferent function in a model of Taxol chemotherapy-induced painful neuropathy. Journal of neurophysiology. 2011;106(1):274–279. doi: 10.1152/jn.00141.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Green PG, Levine JD. Stress enhances muscle nociceptor activity in the rat. Neuroscience. 2011;185:166–173. doi: 10.1016/j.neuroscience.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Diatchenko L, Fillingim RB, Smith SB, Maixner W. The phenotypic and genetic signatures of common musculoskeletal pain conditions. Nature reviews Rheumatology. 2013;9(6):340–350. doi: 10.1038/nrrheum.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dib-Hajj SD, Cummins TR, Black JA, Waxman SG. Sodium channels in normal and pathological pain. Annual review of neuroscience. 2010;33:325–347. doi: 10.1146/annurev-neuro-060909-153234. [DOI] [PubMed] [Google Scholar]
- 26.Dina OA, Joseph EK, Levine JD, Green PG. Mechanisms mediating vibration-induced chronic musculoskeletal pain analyzed in the rat. The journal of pain : official journal of the American Pain Society. 2010;11(4):369–377. doi: 10.1016/j.jpain.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernández-Novoa L, L V, Etcheverría I, Cacabelos R. 11th World Congress of Biological Psychiatry. Kyoto: 2013. The effects of acute and chronic psychological stress on immune function in a rat model. [Google Scholar]
- 28.Garrison JA, McCune JS, Livingston RB, Linden HM, Gralow JR, Ellis GK, West HL. Myalgias and arthralgias associated with paclitaxel. Oncology (Williston Park) 2003;17(2):271–277. discussion 281-272, 286-278. [PubMed] [Google Scholar]
- 29.Green PG, Alvarez P, Gear RW, Mendoza D, Levine JD. Further validation of a model of fibromyalgia syndrome in the rat. The journal of pain : official journal of the American Pain Society. 2011;12(7):811–818. doi: 10.1016/j.jpain.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Green PG, Chen X, Alvarez P, Ferrari LF, Levine JD. Early-life stress produces muscle hyperalgesia and nociceptor sensitization in the adult rat. Pain. 2011;152(11):2549–2556. doi: 10.1016/j.pain.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hanke ML, Powell ND, Stiner LM, Bailey MT, Sheridan JF. Beta adrenergic blockade decreases the immunomodulatory effects of social disruption stress. Brain, behavior, and immunity. 2012;26(7):1150–1159. doi: 10.1016/j.bbi.2012.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hucho TB, Dina OA, Levine JD. Epac mediates a cAMP-to-PKC signaling in inflammatory pain: an isolectin B4(+) neuron-specific mechanism. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(26):6119–6126. doi: 10.1523/JNEUROSCI.0285-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jacobson SD, Loprinzi CL, Sloan JA, Wilke JL, Novotny PJ, Okuno SH, Jatoi A, Moynihan TJ. Glutamine does not prevent paclitaxel-associated myalgias and arthralgias. The journal of supportive oncology. 2003;1(4):274–278. [PubMed] [Google Scholar]
- 34.Jeon SM, Lee KM, Park ES, Jeon YH, Cho HJ. Monocyte chemoattractant protein-1 immunoreactivity in sensory ganglia and hindpaw after adjuvant injection. Neuroreport. 2008;19(2):183–186. doi: 10.1097/WNR.0b013e3282f3c781. [DOI] [PubMed] [Google Scholar]
- 35.Joseph EK, Chen X, Bogen O, Levine JD. Oxaliplatin acts on IB4-positive nociceptors to induce an oxidative stress-dependent acute painful peripheral neuropathy. The journal of pain : official journal of the American Pain Society. 2008;9(5):463–472. doi: 10.1016/j.jpain.2008.01.335. [DOI] [PubMed] [Google Scholar]
- 36.Joseph EK, Levine JD. Hyperalgesic priming is restricted to isolectin B4-positive nociceptors. Neuroscience. 2010;169(1):431–435. doi: 10.1016/j.neuroscience.2010.04.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jung H, Bhangoo S, Banisadr G, Freitag C, Ren D, White FA, Miller RJ. Visualization of chemokine receptor activation in transgenic mice reveals peripheral activation of CCR2 receptors in states of neuropathic pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(25):8051–8062. doi: 10.1523/JNEUROSCI.0485-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. Journal of neurochemistry. 2008;104(1):254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kao DJ, Li AH, Chen JC, Luo RS, Chen YL, Lu JC, Wang HL. CC chemokine ligand 2 upregulates the current density and expression of TRPV1 channels and Nav1.8 sodium channels in dorsal root ganglion neurons. Journal of neuroinflammation. 2012;9:189. doi: 10.1186/1742-2094-9-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(22):5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kreiner F, Langberg H, Galbo H. Increased muscle interstitial levels of inflammatory cytokines in polymyalgia rheumatica. Arthritis and rheumatism. 2010;62(12):3768–3775. doi: 10.1002/art.27728. [DOI] [PubMed] [Google Scholar]
- 42.Kuhn J, Dina OA, Goswami C, Suckow V, Levine JD, Hucho T. GPR30 estrogen receptor agonists induce mechanical hyperalgesia in the rat. The European journal of neuroscience. 2008;27(7):1700–1709. doi: 10.1111/j.1460-9568.2008.06131.x. [DOI] [PubMed] [Google Scholar]
- 43.Kunitoh H, Saijo N, Furuse K, Noda K, Ogawa M. Neuromuscular toxicities of paclitaxel 210 mg m(-2) by 3-hour infusion. British journal of cancer. 1998;77(10):1686–1688. doi: 10.1038/bjc.1998.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lohachanakul J, Phuklia W, Thannagith M, Thonsakulprasert T, Ubol S. High concentrations of circulating interleukin-6 and monocyte chemotactic protein-1 with low concentrations of interleukin-8 were associated with severe chikungunya fever during the 2009-2010 outbreak in Thailand. Microbiology and immunology. 2012;56(2):134–138. doi: 10.1111/j.1348-0421.2011.00417.x. [DOI] [PubMed] [Google Scholar]
- 45.Maes M, Song C, Lin A, De Jongh R, Van Gastel A, Kenis G, Bosmans E, De Meester I, Benoy I, Neels H, Demedts P, Janca A, Scharpe S, Smith RS. The effects of psychological stress on humans: increased production of pro-inflammatory cytokines and a Th1-like response in stress-induced anxiety. Cytokine. 1998;10(4):313–318. doi: 10.1006/cyto.1997.0290. [DOI] [PubMed] [Google Scholar]
- 46.Malhotra D, Saxena AK, Dar SA, Kumar V, Nasare N, Tripathi AK, Banerjee BD. Evaluation of Cytokine Levels in Fibromyalgia Syndrome Patients and its Relationship to the Severity of Chronic Pain. J Musculoskelet Pain. 2012;20(3):164–169. [Google Scholar]
- 47.Maslanik T, Mahaffey L, Tannura K, Beninson L, Greenwood BN, Fleshner M. The inflammasome and danger associated molecular patterns (DAMPs) are implicated in cytokine and chemokine responses following stressor exposure. Brain, behavior, and immunity. 2013;28:54–62. doi: 10.1016/j.bbi.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 48.Matthews EA, Wood JN, Dickenson AH. Na(v) 1.8-null mice show stimulus-dependent deficits in spinal neuronal activity. Molecular pain. 2006;2:5. doi: 10.1186/1744-8069-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mestre C, Pelissier T, Fialip J, Wilcox G, Eschalier A. A method to perform direct transcutaneous intrathecal injection in rats. Journal of pharmacological and toxicological methods. 1994;32(4):197–200. doi: 10.1016/1056-8719(94)90087-6. [DOI] [PubMed] [Google Scholar]
- 50.Miller RE, Tran PB, Das R, Ghoreishi-Haack N, Ren D, Miller RJ, Malfait AM. CCR2 chemokine receptor signaling mediates pain in experimental osteoarthritis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(50):20602–20607. doi: 10.1073/pnas.1209294110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nishiguchi J, Sasaki K, Seki S, Chancellor MB, Erickson KA, de Groat WC, Kumon H, Yoshimura N. Effects of isolectin B4-conjugated saporin, a targeting cytotoxin, on bladder overactivity induced by bladder irritation. The European journal of neuroscience. 2004;20(2):474–482. doi: 10.1111/j.1460-9568.2004.03508.x. [DOI] [PubMed] [Google Scholar]
- 52.Penson RT, Kronish K, Duan Z, Feller AJ, Stark P, Cook SE, Duska LR, Fuller AF, Goodman AK, Nikrui N, MacNeill KM, Matulonis UA, Preffer FI, Seiden MV. Cytokines IL-1beta, IL-2, IL-6, IL-8, MCP-1, GM-CSF and TNFalpha in patients with epithelial ovarian cancer and their relationship to treatment with paclitaxel. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2000;10(1):33–41. doi: 10.1046/j.1525-1438.2000.00003.x. [DOI] [PubMed] [Google Scholar]
- 53.Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends in neurosciences. 2009;32(12):611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richards N, Batty T, Dilley A. CCL2 has similar excitatory effects to TNF-alpha in a subgroup of inflamed C-fiber axons. Journal of neurophysiology. 2011;106(6):2838–2848. doi: 10.1152/jn.00183.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rulli NE, Rolph MS, Srikiatkhachorn A, Anantapreecha S, Guglielmotti A, Mahalingam S. Protection from arthritis and myositis in a mouse model of acute chikungunya virus disease by bindarit, an inhibitor of monocyte chemotactic protein-1 synthesis. The Journal of infectious diseases. 2011;204(7):1026–1030. doi: 10.1093/infdis/jir470. [DOI] [PubMed] [Google Scholar]
- 56.Stone LS, Vulchanova L. The pain of antisense: in vivo application of antisense oligonucleotides for functional genomics in pain and analgesia. Advanced drug delivery reviews. 2003;55(8):1081–1112. doi: 10.1016/s0169-409x(03)00105-4. [DOI] [PubMed] [Google Scholar]
- 57.Sun JH, Yang B, Donnelly DF, Ma C, LaMotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. Journal of neurophysiology. 2006;96(5):2189–2199. doi: 10.1152/jn.00222.2006. [DOI] [PubMed] [Google Scholar]
- 58.Vellani V, Zachrisson O, McNaughton PA. Functional bradykinin B1 receptors are expressed in nociceptive neurones and are upregulated by the neurotrophin GDNF. The Journal of physiology. 2004;560(Pt 2):391–401. doi: 10.1113/jphysiol.2004.067462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vulchanova L, Olson TH, Stone LS, Riedl MS, Elde R, Honda CN. Cytotoxic targeting of isolectin IB4-binding sensory neurons. Neuroscience. 2001;108(1):143–155. doi: 10.1016/s0306-4522(01)00377-3. [DOI] [PubMed] [Google Scholar]
- 60.Wallace DJ, Linker-Israeli M, Hallegua D, Silverman S, Silver D, Weisman MH. Cytokines play an aetiopathogenetic role in fibromyalgia: a hypothesis and pilot study. Rheumatology (Oxford) 2001;40(7):743–749. doi: 10.1093/rheumatology/40.7.743. [DOI] [PubMed] [Google Scholar]
- 61.Wang CH, Zou LJ, Zhang YL, Jiao YF, Sun JH. The excitatory effects of the chemokine CCL2 on DRG somata are greater after an injury of the ganglion than after an injury of the spinal or peripheral nerve. Neuroscience letters. 2010;475(1):48–52. doi: 10.1016/j.neulet.2010.03.044. [DOI] [PubMed] [Google Scholar]
- 62.White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, Lamotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(39):14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White GE, Iqbal AJ, Greaves DR. CC chemokine receptors and chronic inflammation--therapeutic opportunities and pharmacological challenges. Pharmacological reviews. 2013;65(1):47–89. doi: 10.1124/pr.111.005074. [DOI] [PubMed] [Google Scholar]
- 64.Wu DF, Chandra D, McMahon T, Wang D, Dadgar J, Kharazia VN, Liang YJ, Waxman SG, Dib-Hajj SD, Messing RO. PKCepsilon phosphorylation of the sodium channel NaV1.8 increases channel function and produces mechanical hyperalgesia in mice. The Journal of clinical investigation. 2012;122(4):1306–1315. doi: 10.1172/JCI61934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Z, Luo W, Wang J, Tan Y, Fu R, Fang B. Chemokine ligand 2 in the trigeminal ganglion regulates pain induced by experimental tooth movement. The Angle orthodontist. 2014 doi: 10.2319/090213-643.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang H, Boyette-Davis JA, Kosturakis AK, Li Y, Yoon SY, Walters ET, Dougherty PM. Induction of monocyte chemoattractant protein-1 (MCP-1) and its receptor CCR2 in primary sensory neurons contributes to paclitaxel-induced peripheral neuropathy. The journal of pain : official journal of the American Pain Society. 2013;14(10):1031–1044. doi: 10.1016/j.jpain.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Z, Cherryholmes G, Mao A, Marek C, Longmate J, Kalos M, Amand RP, Shively JE. High plasma levels of MCP-1 and eotaxin provide evidence for an immunological basis of fibromyalgia. Exp Biol Med (Maywood) 2008;233(9):1171–1180. doi: 10.3181/0712-RM-328. [DOI] [PubMed] [Google Scholar]
- 68.Zhu X, Cao S, Zhu MD, Liu JQ, Chen JJ, Gao YJ. Contribution of chemokine CCL2/CCR2 signaling in the dorsal root ganglion and spinal cord to the maintenance of neuropathic pain in a rat model of lumbar disc herniation. The journal of pain : official journal of the American Pain Society. 2014 doi: 10.1016/j.jpain.2014.01.492. [DOI] [PubMed] [Google Scholar]