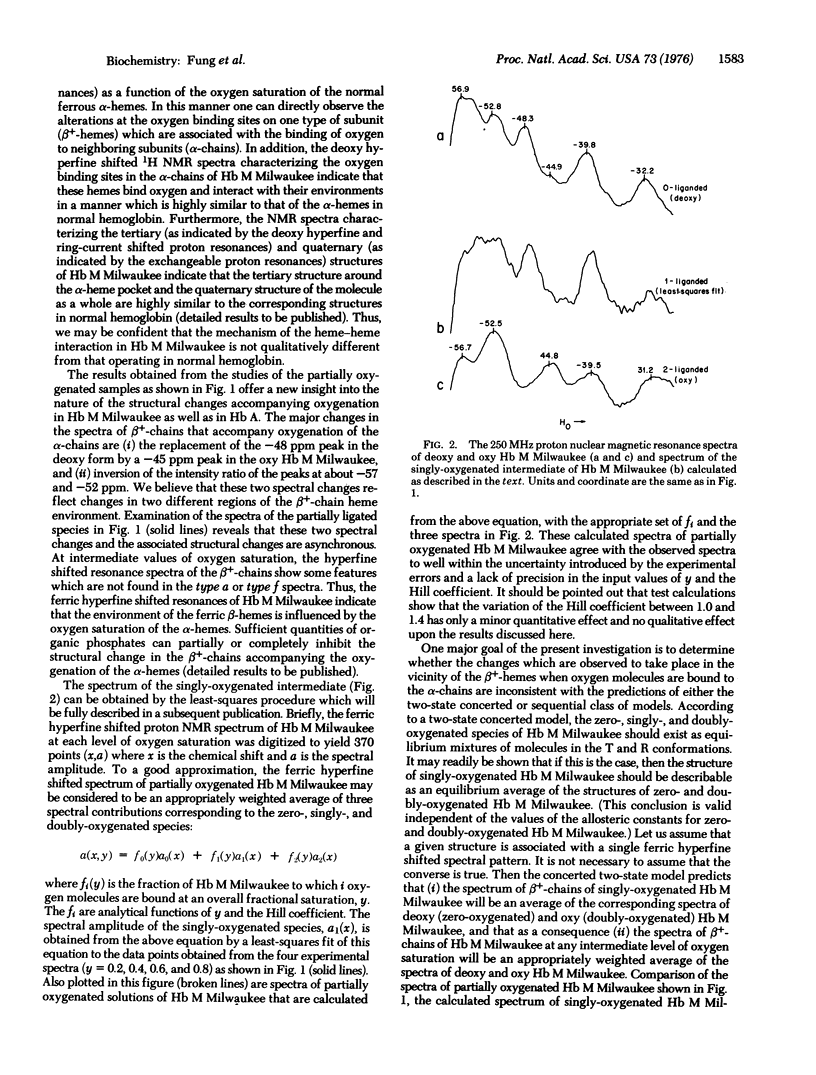
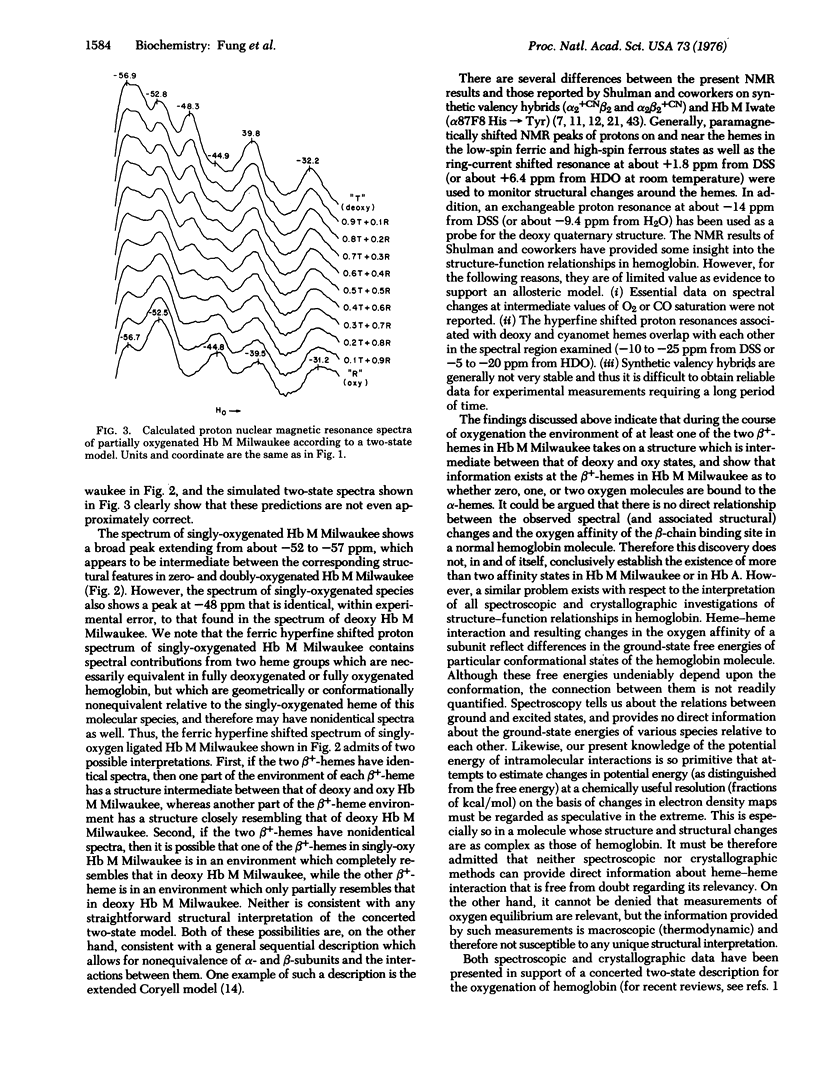
Abstract
Hemoglobin M Milwaukee (beta 67E11 val leads to Glu) is a naturally occurring valency hybrid containing two permanently oxidized hemes in the beta-chains. In this mutant, the two abnormal beta-chains cannot combine with oxygen, whereas the two alpha-chains are normal and can combine with oxygen cooperatively with a Hill coefficient of approximately 1.3. High-resolution proton nuclear magnetic resonance spectroscopy at 250 MHz has been used to investigate the hyperfine shifted resonances of the abnormal ferric beta-chains of Hb M Milwaukee over the spectral region from -30 to -60 parts per million from water at pD 7 and 30 degrees.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin J. M. Structure and function of haemoglobin. Prog Biophys Mol Biol. 1975;29(3):225–320. doi: 10.1016/0079-6107(76)90024-9. [DOI] [PubMed] [Google Scholar]
- Banerjee R., Cassoly R. Oxygen equilibria of human hemoglobin valency hybrids. Discussion on the intrinsic properties of alpha and beta chains in the native protein. J Mol Biol. 1969 Jun 14;42(2):351–361. doi: 10.1016/0022-2836(69)90048-5. [DOI] [PubMed] [Google Scholar]
- Berman M., Benesch R., Benesch R. E. The removal of organic phosphates from hemoglobin. Arch Biochem Biophys. 1971 Jul;145(1):236–239. doi: 10.1016/0003-9861(71)90031-2. [DOI] [PubMed] [Google Scholar]
- Brunori M., Amiconi G., Antonini E., Wyman J. Artificial intermediates in the reaction of haemoglobin. Functional and conformational properties of the cyanmet intermediates. J Mol Biol. 1970 Apr 28;49(2):461–471. doi: 10.1016/0022-2836(70)90257-3. [DOI] [PubMed] [Google Scholar]
- Byckova V., Wajcman H., Labie D., Travers F. Hemoglobin M Saskatoon: further data on biophysics and oxygen equilibrium. Biochim Biophys Acta. 1971 Jul 25;243(1):117–125. doi: 10.1016/0005-2795(71)90045-6. [DOI] [PubMed] [Google Scholar]
- Cassoly R., Gibson Q. H., Ogawa S., Shulman R. G. Effects of phosphate upon CO binding kinetics and NMR spectra of hemoglobin valency hybrids. Biochem Biophys Res Commun. 1971 Sep;44(5):1015–1021. doi: 10.1016/s0006-291x(71)80187-0. [DOI] [PubMed] [Google Scholar]
- Enoki Y., Tomita S. Oxygen equilibria of half cyanomet hybrids of human and canine haemoglobins. J Mol Biol. 1968 Feb 28;32(1):121–134. doi: 10.1016/0022-2836(68)90150-2. [DOI] [PubMed] [Google Scholar]
- Fermi G. Three-dimensional fourier synthesis of human deoxyhaemoglobin at 2-5 A resolution: refinement of the atomic model. J Mol Biol. 1975 Sep 15;97(2):237–256. doi: 10.1016/s0022-2836(75)80037-4. [DOI] [PubMed] [Google Scholar]
- GERALD P. S., EFRON M. L. Chemical studies of several varieties of Hb M. Proc Natl Acad Sci U S A. 1961 Nov 15;47:1758–1767. doi: 10.1073/pnas.47.11.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson Q. H., Heller P., Yakulis V. The rate of reaction of carbon monoxide with hemoglobins M. J Biol Chem. 1966 Apr 10;241(7):1650–1651. [PubMed] [Google Scholar]
- Haber J. E., Koshland D. E., Jr The effect of 2,3-diphosphoglyceric acid on the changes in - interactions in hemoglobin during oxygenation. J Biol Chem. 1971 Dec 25;246(24):7790–7793. [PubMed] [Google Scholar]
- Hayashi A., Suzuki T., Shimizu A., Imai K., Morimoto H. Some observations on the physicochemical properties of hemoglobin M-Hyde Park. Arch Biochem Biophys. 1968 Jun;125(3):895–901. doi: 10.1016/0003-9861(68)90528-6. [DOI] [PubMed] [Google Scholar]
- Hayashi A., Suzuki T., Shimizu A., Yamamura Y. Properties of hemoglobin M. Unequivalent nature of the alpha and beta subunits in the hemoglobin molecule. Biochim Biophys Acta. 1968 Oct 21;168(2):262–273. doi: 10.1016/0005-2795(68)90149-9. [DOI] [PubMed] [Google Scholar]
- Ho C., Lindstrom T. R., Baldassare J. J., Breen J. J. Magnetic resonance studies of human hemoglobins and their implications to the structure-function relationships in human normal and abnormal hemoglobins. Ann N Y Acad Sci. 1973 Dec 31;222:21–39. doi: 10.1111/j.1749-6632.1973.tb15250.x. [DOI] [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- Kurland R. J., Davis D. G., Ho C. Paramagnetic proton nuclear magnetic resonance shifts of metmyoglobin, methemoglobin, and hemin derivatives. J Am Chem Soc. 1968 May 8;90(10):2700–2701. doi: 10.1021/ja01012a048. [DOI] [PubMed] [Google Scholar]
- Kurland R. J., Little R. G., Davis D. G., Ho C. Proton magnetic resonance study of high- and low-spin hemin derivatives. Biochemistry. 1971 Jun 8;10(12):2237–2246. doi: 10.1021/bi00788a009. [DOI] [PubMed] [Google Scholar]
- Lindstrom T. R., Ho C. Functional nonequivalence of and hemes in human adult hemoglobin. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1707–1710. doi: 10.1073/pnas.69.7.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom T. R., Ho C., Pisciotta A. V. Nuclear magnetic resonance studies of haemoglobin M Milwaukee. Nat New Biol. 1972 Jun 28;237(78):263–264. doi: 10.1038/newbio237263a0. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Mayer A., Ogawa S., Schulman R. G., Gersonde K. High-resolution proton nuclear magnetic resonance studies of the quaternary state of hemoglobin M Iwate. J Mol Biol. 1973 Dec 5;81(2):187–197. doi: 10.1016/0022-2836(73)90188-5. [DOI] [PubMed] [Google Scholar]
- McConnell H. M., Ogawa S., Horwitz A. Spin-labelled haemoglobin and the haem-haem interaction. Nature. 1968 Nov 23;220(5169):787–788. doi: 10.1038/220787a0. [DOI] [PubMed] [Google Scholar]
- Minton A. P. Oxygen binding in cyanmet hybrid and normal hemoglobins: applicability of sequential and two-state concerted models. Science. 1974 May 3;184(4136):577–579. doi: 10.1126/science.184.4136.577. [DOI] [PubMed] [Google Scholar]
- Nishikura K., Sugita Y., Nagai M., Yoneyama Y. Ethylisocyanide equilibria of hemoglobins M Iwate, M Boston, M Hyde Park, M Saskatoon, and M Milwaukee-I in half-ferric and fully reduced states. J Biol Chem. 1975 Sep 10;250(17):6679–6685. [PubMed] [Google Scholar]
- Ogata R. T., McConnell H. M. States of hemoglobin in solution. Biochemistry. 1972 Dec 5;11(25):4792–4799. doi: 10.1021/bi00775a024. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Shulman R. G. High resolution nuclear magnetic resonance spectra of hemoglobin. 3. The half-ligated state and allosteric interactions. J Mol Biol. 1972 Sep 28;70(2):315–336. doi: 10.1016/0022-2836(72)90542-6. [DOI] [PubMed] [Google Scholar]
- Ogawa S., Shulman R. G. Observation of allosteric transition in hemoglobin. Biochem Biophys Res Commun. 1971 Jan 8;42(1):9–15. doi: 10.1016/0006-291x(71)90354-8. [DOI] [PubMed] [Google Scholar]
- PISCIOTTA A. V., EBBE S. N., HINZ J. E. Clinical and laboratory features of two variants of methemoglobin M disease. J Lab Clin Med. 1959 Jul;54(1):73–87. [PubMed] [Google Scholar]
- Perutz M. F., Pulsinelli P. D., Ranney H. M. Structure and subunit interaction of haemoglobin M Milwaukee. Nat New Biol. 1972 Jun 28;237(78):259–263. doi: 10.1038/newbio237259a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., TenEyck L. F. Stereochemistry of cooperative effects in hemoglobin. Cold Spring Harb Symp Quant Biol. 1972;36:295–310. doi: 10.1101/sqb.1972.036.01.040. [DOI] [PubMed] [Google Scholar]
- Ranney H. M., Nagel R. L., Heller P., Udem L. Oxygen equilibrium of hemoglobin M-Hyde Park. Biochim Biophys Acta. 1968 May 6;160(1):112–115. doi: 10.1016/0005-2795(68)90070-6. [DOI] [PubMed] [Google Scholar]
- Shulman R. G., Hopfield J. J., Ogawa S. Allosteric interpretation of haemoglobin properties. Q Rev Biophys. 1975 Jul;8(3):325–420. doi: 10.1017/s0033583500001840. [DOI] [PubMed] [Google Scholar]
- Shulman R. G., Ogawa S., Mayer A., Castillo C. L. High-resolution proton NMR studies of low affinity hemoglobins. Ann N Y Acad Sci. 1973 Dec 31;222:9–20. doi: 10.1111/j.1749-6632.1973.tb15249.x. [DOI] [PubMed] [Google Scholar]
- Shulman R. G., Ogawa S., Wüthrich K., Yamane T., Peisach J., Blumberg W. E. The absence of "heme-heme" interactions in hemoglobin. Science. 1969 Jul 18;165(3890):251–257. doi: 10.1126/science.165.3890.251. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Hayashi A., Yamamura Y., Enoki Y., Tyuma I. Functional abnormality of hemoglobin M-Osaka. Biochem Biophys Res Commun. 1965 Jun 9;19(6):691–695. doi: 10.1016/0006-291x(65)90312-8. [DOI] [PubMed] [Google Scholar]
- Udem L., Ranney H. M., Bunn H. F., Pisciotta A. Some observations on the properties of hemoglobin M Milwaukee-1. J Mol Biol. 1970 Mar;48(3):489–498. doi: 10.1016/0022-2836(70)90060-4. [DOI] [PubMed] [Google Scholar]


