Abstract
Exposure to environmental toxicants and stressors, radiation, pharmaceutical drugs, inflammation, cellular respiration, and routine DNA metabolism all lead to the production of cytotoxic DNA strand breaks. Akin to splintered wood, DNA breaks are not “clean”. Rather, DNA breaks typically lack DNA 5'-phosphate and 3'-hydroxyl moieties required for DNA synthesis and DNA ligation. Failure to resolve damage at DNA ends can lead to abnormal DNA replication and repair, and is associated with genomic instability, mutagenesis, neurological disease, ageing and carcinogenesis. An array of chemically heterogeneous DNA termini arises from spontaneously generated DNA single-strand and double-strand breaks (SSBs and DSBs), and also from normal and/or inappropriate DNA metabolism by DNA polymerases, DNA ligases and topoisomerases. As a front line of defense to these genotoxic insults, eukaryotic cells have accrued an arsenal of enzymatic first responders that bind and protect damaged DNA termini, and enzymatically tailor DNA ends for DNA repair synthesis and ligation. These nucleic acid transactions employ direct damage reversal enzymes including Aprataxin (APTX), Polynucleotide kinase phosphatase (PNK), the tyrosyl DNA phosphodiesterases (TDP1 and TDP2), the Ku70/80 complex and DNA polymerase β (POLβ). Nucleolytic processing enzymes such as the MRE11/RAD50/NBS1/CtIP complex, Flap endonuclease (FEN1) and the apurinic endonucleases (APE1 and APE2) also act in the chemical "cleansing" of DNA breaks to prevent genomic instability and disease, and promote progression of DNA- and RNA-DNA damage response (DDR and RDDR) pathways. Here, we provide an overview of cellular first responders dedicated to the detection and repair of abnormal DNA termini.
INTRODUCTION
Spontaneous chemical processes generating DNA strand breaks create damaged DNA termini. Modified non-canonical DNA termini are also generated during base excision repair reactions, from aberrant DNA ligation [Tumbale et al. 2014], and from failed topoisomerase–DNA transactions [Deweese and Osheroff 2009; Nitiss 2009; Pommier 2013]. Emerging evidence also indicates that the contamination of RNA in DNA created by polymerase misincorporation of ribonucleotides (rNMPs) into genomic DNA is also an important, poorly understood source of non-canonical DNA termini, whose efficient repair is required to prevent replication stress and genomic instability [Nick McElhinny et al. 2010; Reijns et al. 2012; Williams et al. 2013; Tumbale et al. 2014]. To exacerbate these primary challenges to genomic integrity, the enzymatic conversion of existing damage by DNA metabolic processes can generate additional forms of compound DNA-end damage. Furthermore, collisions of damaged DNA strand breaks with the DNA and RNA metabolic enzymes can collapse DNA replication forks, impact the cellular transcription apparatus, and create recombinogenic and carcinogenic DNA double strand breaks (DSBs).
Eukarya are equipped with an equally diverse set of enzymatic processing activities for detecting and processing damaged DNA termini. These enzymes include direct chemical reversal mechanisms yielding “canonical” 5′–phosphate (5′–P) and 3′–hydroxyl (3′–OH) termini, as well as multi-purpose genome maintenance nucleolytic enzymes. These enzymatic activities are integral to major DNA strand break repair pathways including DNA base excision repair (BER), single strand break repair (SSBR) [Caldecott 2007; Caldecott 2008] and DNA double strand break repair (DSBR) [Williams et al. 2007; Ciccia and Elledge 2010]. We divide this overview broadly into two parts: first we summarize sources and the chemical nature of DNA termini at strand breaks (see Figures 1 and 2) and second, we survey molecular mechanisms for recognition and enzymatic “healing” of damage at DNA ends, with a focus on factors for which molecular structure information is available.
Figure 1. Chemically heterogeneous, “dirty”, DNA end structures.
Repair of strand breaks by DNA ligases requires 5′–phosphate and 3′–hydroxyl groups. Dirty ends have chemical modifications (red) at either the 5′– or 3′–ends that will interfere with ligation. Sources of specific types of damage and enzymes that can repair them are indicated.
Figure 2.
Map of DNA end cleansing pathways. Networks of end-cleansing proteins repair specific types of end damage before DNA ligase can complete the final break–sealing reaction. Unrepaired DNA damage can interfere with the function of enzymes such as DNA topoisomerases or DNA ligase to create compound metabolized DNA damage (see blue brackets and arrows).
SOURCES OF MODIFIED DNA TERMINI
IR and ROS induced DNA end damage
DNA damage is unavoidable. Radiation from various natural and unnatural sources along with the generation of reactive oxygen species (ROS) during normal cellular metabolism or during inflammation mount a constant assault on our genetic material, and can produce DNA strand breaks. ROS modification of DNA is estimated to generate DNA base damage and strand break lesions on the order of 10,000–100,000 events per mammalian cell per day [Lindahl and Nyberg 1972; Drinkwater et al. 1980; Nakamura and Swenberg 1999; Roberts et al. 2006]. Ionizing radiation (IR) triggers production of SSBs and DSBs bearing "dirty" DNA ends by inducing free radical attack on the deoxyribose sugar backbone [Dedon 2008; Weinfeld et al. 2011; Povirk 2012]. The stable end products on 3′ termini of IR-induced breaks and those created by endogenous ROS or oxidants such as hydrogen peroxide (H2O2) include 3′-phosphate (3′-P) and 3′-phosphosphoglycolate (3′-PG) moieties (Figure 1). In oxic conditions, IR generated strand breaks harbor 3′-PG and 3′-P in an approximate 1.6:1 3′-PG:3′-P ratio [Buchko and Weinfeld 1993]. At the 5′ terminus of breaks, abundant stable products of IR insult and oxidation are 5′ phosphate (5′-P) groups, however 5′ -aldehyde and 5′ hydroxyl (5′-OH) moieties also constitute significant measurable fractions of damaged [Dedon 2008; Weinfeld et al. 2011; Povirk 2012].
DNA glycosylase sources of modified termini
DNA base excision repair (BER) acts in the removal of damaged DNA base lesions in mammalian cells [Friedberg et al. 2006; Liu and Wilson 2012; Sykora et al. 2013]. The DNA processing reactions carried out by BER monofunctional and bifunctional DNA glycosylases create variable modified DNA ends. In removing damaged bases via hydrolysis of the N-glycosidic bond, monofunctional DNA glycosylases generate abasic or apurinic (AP) sites. Spontaneous depurination of DNA also creates AP sites in large numbers in cells. Following glycosylase action, endonucleolytic incision at the 5′ side of the AP site by AP endonuclease 1 (APE1) during BER yields DNA strand breaks bearing 5′-deoxyribose-phosphate (5′-dRP) groups and 3′-OH ends [Wilson and Barsky 2001; Demple and Sung 2005]. By comparison, following damaged base removal, β,δ-elimination or β-elimination reactions of the bifunctional DNA glycosylase-lyases yield DNA breaks bearing 3′-phosphate or 3′-phospho-α, β-unsaturated aldehyde groups [Wallace 2013].
Topoisomerase-DNA crosslinks
Key cellular processes including transcription, DNA replication, and cell division are impossible without a mechanism to relieve DNA topological strain and DNA entanglement generated by nuclear DNA compaction and translocation of DNA and RNA polymerases. To achieve this, DNA topoisomerases generate transient and reversible SSBs (type I topoisomerases) or DSBs (type II topoisomerases), [Nitiss 2009a; Nitiss 2009b; Deweese and Osheroff 2009; Pommier 2013]. During these transactions, the formation of enzyme-phosphotyrosyl-DNA linkages with DNA 5′ and 3′ termini by topoisomerases can lead to protein-DNA adducts in cases where the topoisomerase reactions are perturbed (Figures 1 and 2).
Type I topoisomerases (eg. TOP1) relieve topological strain by a mechanism that uses an active-site tyrosine residue to introduce a single-strand break, thereby linking the enzyme covalently to the 3′–phosphate side of the nick (TOP1–cleavage complex) [Pommier 2013]. This bond ensures that the enzyme remains bound to the nick while the DNA is allowed to rotate and relieve topological strain before the nick is re-sealed. However, this reaction is sensitive to a variety of compounds and pre-existing DNA damage that can “poison” the topoisomerase by preventing re-ligation and release of TOP1 from DNA. Camptothecin and its derivatives are TOP1 poisons and are potent chemotherapeutics [Pommier 2009]. TOP1 incision adjacent to a DNA mismatch, AP site, or a nick also leads to irreversible TOP1-DNA cleavage complex formation [Pourquier et al. 1997; Lebedeva et al. 2006; Pourquier et al. 1997]. The presence of oxidative damage (e.g. 8-oxoG) near the TOP1–cleavage site can also impair re-ligation, and extend the lifetime of the TOP1-cleavage complex [Pourquier et al. 1997; Pourquier et al. 1999]. TOP1 and TOP2 adducts lead to detrimental effects such as blocking transcription and creating replication fork collapse [Nitiss 2009a; Nitiss 2009b].
TOP2 alters supercoiling of DNA and relaxes tangles or knots by creating paired DNA strand cleavages spaced 4 base–pairs apart [Liu et al. 1983], gating passage of duplex DNA through the break, then resealing the break to restore genome integrity [Roca and Wang 1994]. This reaction is driven by a trans-esterification of a DNA backbone phosphate to an active–site tyrosine in TOP2, creating a covalently linked TOP2–DNA cleavage complex [Rowe et al. 1984]. TOP2-DNA intermediates pose unique challenges to genomic integrity. TOP2 reactions are sensitive to both genetic and environmental perturbations that can accelerate TOP2 DNA cleavage or stall topoisomerase re-ligation. As is the case for TOP1, these changes poison the reaction cycle by altering the cleavage/ligation equilibrium towards production of excessive DSBs in the form of TOP2 cleavage complexes and protein linked strand breaks. Anticancer chemotherapeutic TOP2 poisons such as etoposide target this vulnerability by creating genomic instability leading to cell death [Hande 1998; Baldwin and Osheroff 2005; Pommier 2013]. Additional TOP2 poisons include dietary bioflavonoids like genistein [Okura et al. 1988], which is prominent in soy, or EGCG [Bandele and Osheroff 2008] from green tea, as well as metabolic products of the environmental toxicants poly-chloro biphenyls (PCBs) [Bender et al. 2006], benzene (1,4 benzoquinone) [Bender et al. 2007], and drug metabolites of acetaminophen (NAPQI) [Bender et al. 2004]. Significantly, DNA base damage repair intermediates including AP-sites and DNA SSBs can be converted to DSBs when TOP2 engages these structures and processes them to protein-linked strand breaks [Wilstermann and Osheroff 2001].
Damage generated by DNA ligases
The final critical step of DNA replication, recombination and repair is DNA ligation, during which the chemical joining of broken DNA strands occurs. The eukaryotic DNA ligases (DNA Ligases I, III and IV) catalyze ligation in a 3-step, ATP-dependent DNA nick sealing reaction. In the first step, an active-site ligase lysine is adenylated. In step two, the AMP moiety is transferred to the 5′ phosphate of the DNA substrate to facilitate step 3, phosphodiester bond formation and sealing of the DNA backbone by nucleophilic attack of the 3'-OH on the 5'-adenylate [Pascal et al. 2004; Tomkinson et al. 2006]. Like many key biological processes, ligation can fail. Environmental and metabolic sources of DNA damage trigger this failure causing DNA ligase-dependent production of 5′-adenylated DNA adducts (5'-AMP) (Figures 1 and 2).
Structural studies of ligase–DNA complexes revealed how the nick sealing reactions are orchestrated by the complete encirclement of the DNA nick by the conserved 3-domain architecture in DNA Ligase I and Ligase III [Cotner-Gohara et al. 2010; Pascal et al. 2004]. Like topoisomerase reactions, the essential genome maintenance functions of DNA ligases are also prone to DNA damage-induced side reactions. During a process termed “abortive ligation”, 5′-AMP strand breaks can arise if DNA ligases engage damaged DNA structures including direct oxidative single-strand breaks [Ahel et al. 2006], DNA nicks bearing 3′ AP sites, oxidative DNA base damage (e.g 8-oxo-dG) [Harris et al. 2009], 5′-dRP groups generated in BER [Rass et al. 2007], or RNA-DNA junctions arising during ribonucleotide excision repair (RER) [Tumbale et al. 2014] (see below).
Premature detachment of DNA ligases from the 5′-AMP reaction intermediate is problematic because under physiological ATP concentrations eukaryotic DNA ligases are predicted to exist predominantly in the enzyme-AMP state [Tomkinson et al. 2006]. Ligase-AMP cannot perform the nick sealing reactions, or deadenylate 5′-termini. Thus, while typically viewed as the ultimate step in DNA repair pathways, DNA ligases paradoxically can also generate unwanted toxic intermediates in the form of adenylated DNA. While the molecular mechanisms underlying abortive ligation are currently ill defined, presumably substrate-induced distortions of damaged substrates in the ligase active site permit 5′ adenylation, but reduce the ligase nick-sealing reaction efficiency such that completion of this step is not ensured before ligase dissociates from its substrate.
Genomic RNA-derived intermediates
During DNA replication in eukaryotic cells, replicative DNA polymerases Pol ɛ and Pol δ incorporate ribonucleotides (ribonucleotide monophosphates, rNMPs) into genomic DNA. It is estimated that greater than 10,000 rNMPs are incorporated by replicative DNA polymerases each cell cycle in budding yeast [Nick McElhinny et al. 2010, Williams et al. 2013; Nick McElhinny et al. 2010], and greater than ~1,000,000 rNMPs are incorporated each cell division in murine cells [Reijns et al. 2012; Hiller et al. 2012]. These numbers overshadow classically viewed common forms of DNA damage such as AP sites, which are produced ~10,000 times per mammalian cell per day [Lindahl, 2000]. Overall, the transient contamination of nuclear genomic DNA by RNA is estimated to outnumber all other known types of DNA damage combined [Caldecott 2014]. Further, Pol α Primase synthesizes ~ 5% of the nascent lagging strand using rNTPs. Ribonucleotides are also incorporated into mitochondrial DNA [Kasiviswanathan and Copeland 2011; Yang et al. 2002], and rNTPs are utilized by DNA repair Polymerases µ and λ [Nick McElhinny and Ramsden 2003]. In non-cycling cells where dNTP concentrations are decreased relative to rNTPs [Chabes et al. 2003; Ferraro et al. 2010], elevated rNTP:dNTP ratios [Ferraro et al. 2010] may favor increased ribonucleotide incorporation by DNA repair polymerases [Nick McElhinny and Ramsden 2003]. The presence of ribonucleotides in genomic DNA poses unique challenges to genomic stability. Ribonucleotides, unlike deoxyribonucleotides, bear a reactive 2′-OH on the ribose sugar that renders the sugar-phosphate backbone prone to hydrolysis in physiological conditions, creating strand breaks with 2′-3′-cyclic phosphate and 5′-OH groups (Figure 1). A major enzymatic removal pathway for ribonucleotides is RER [Sparks et al. 2012]. RER is initiated when RNase H2, a heterotrimeric enzyme, cleaves on the 5′ side of a DNA embedded ribonucleotide. These RER strand break intermediates bear a 5′-P ribonucleotide and 3′-OH end.
The fate of ribonucleotides, and their impact on genomic integrity is highly relevant to the current topic of damaged DNA end repair, and is the least explored in the field. Significantly, ribonucleotides introduce local geometric distortions into DNA [Egli et al. 1993; Ban et al. 1994; DeRose et al. 2012] that can influence enzymatic transactions of DNA metabolic enzymes engaging ribonucleotide containing RNA-DNA [Kim et al. 2011] or nicked RER intermediates generated by RNase H2 [Tumbale et al. 2014]. In the case of ribonucleotides embedded in DNA, TOP1 incision on the 3′ side of a ribonucleotide, like hydrolysis, can generate the end-blocking 2′-3′-cyclic phosphate group when the ribonucleotide 2′-OH attacks the RNA 3′-phosphotyrosyl-linked TOP1-cleavage complex [Sekiguchi and Shuman 1997; Shuman 1998; Kim et al. 2011]. These events are also associated with mutagenic TOP1 processing of rNMPs in genomic DNA, and the generation of 2–5 bp deletions in repetitive sequences [Kim et al. 2011; Cho et al. 2013; Williams et al. 2013]. In a second example of metabolism of rNMPs leading to non-canonical DNA termini, it has been demonstrated recently that the incised RNA-DNA junctions from RER are potent triggers for abortive ligation by DNA ligases. In this context, the misincorporation of a ribonucleotide by a DNA polymerase, followed by abortive DNA ligase processing of the RER intermediate generates adenylated RNA-DNA, or so called “RNA-DNA damage” [Tumbale et al. 2014].
DNA END PROCESSING AND REPAIR MACHINERIES
Just as there are multiple forms of damaged DNA termini, a number of direct reversal DNA end processing enzymes exist to convert damaged DNA ends to ligatable substrates. These proteins factors are described below.
Reversal of 5′-adenylation by Aprataxin (APTX)
Analogous to the correction of nucleotide misincorporation errors made by DNA polymerases, DNA ligation errors in the form of adenylation DNA damage are “proofread” [Ahel et al. 2006]. Eukaryotic Aprataxin (APTX) is the only known enzyme to catalyze direct reversal of DNA or RNA-DNA 5′-adenylation to generate a 5′-phosphorylated terminus, [Ahel et al. 2006; Reynolds et al. 2009; Harris et al. 2009; Tumbale et al. 2014]. DNA damage repair roles for APTX in BER, SSBR, and DSBR are underlined by the facts that APTX deficient AOA1 cells show elevated levels of oxidative DNA damage [Harris et al. 2009; Hirano et al. 2007] and have increased DNA damage following treatment with the anticancer topoisomerase inhibitor camptothecin [Mosesso et al. 2005]. Although direct quantitative measures of genomic DNA adenylation have not been reported, genetic studies link the budding yeast APTX homolog Hnt3p to RNA-triggered abortive ligation during RER [Tumbale et al. 2014], as well as pathways for repair of alkylation and oxidative DNA damage [Daley et al. 2010]. Identification and characterization of a mitochondrial isoform of mammalian APTX suggests these activities are also critical for maintenance of mitochondrial DNA [Sykora et al. 2011].
X-ray structures of Schizosaccharomyces pombe and human APTX deadenylases bound to DNA and RNA-DNA targets revealed a conserved two-domain architecture that constructs APTX deadenylases [Tumbale et al. 2011; Tumbale et al. 2013] (Figure 3). APTX belongs to the functionally diverse histidine triad (HIT) family of nucleotide hydrolases and transferases. APTX is unlike other HIT family members, such as the fragile histidine triad (FHIT) protein [Lima et al. 1997a; Lima et al. 1997b], which self-associate via an extended HIT domain β-sheet interaction, and function as dimeric molecules. Rather, APTX is monomeric and the HIT domains mixed α-β fold catalytic core is fused to an APTX-specific C2H2 (C2HE in S. pombe Aptx) zinc-finger (ZnF) domain that confers DNA damage recognition and substrate specificity for adenylated polynucleotide substrates [Rass et al. 2008; Tumbale et al. 2011; Tumbale et al. 2013]. Vertebrate APTX orthologs also contain a forkhead associated domain (FHA) that mediates APTX interactions with multi-protein DNA repair complexes via phosphorylation regulated protein-protein interactions with XRCC1 [Clements et al. 2004; Luo et al. 2004], XRCC4 [Clements et al. 2004] and MDC1 [Becherel et al. 2010]. Additional APTX interactions with PCNA suggest roles for APTX in DNA replication and/or long patch BER [Hirano et al. 2007].
Figure 3. Structural basis for repair of 5′-DNA ends by end–cleansing enzymes.
(Left, gray box) Specific examples of 5′–end damage are removed or corrected to leave ligatable ends (5′-phosphate termini). Right: cartoon representations of repair enzymes with α–helices (blue) and β–strands (green) highlighting their catalytic domain bound to DNA (yellow). Surface representation of repair enzymes showing damaged DNA ends (red) engaged in the enzyme active site. Figures were generated from PDB entry 4NDH for APTX, 3ZVN for PNK kinase domain, 4GZ0 for TDP2, 1JEY for Ku70/80, 2P66 for POLβ,
DNA damage sensing by APTX is achieved through two-point nucleic acid binding by both the HIT and Znf domains. Sensing of DNA ends or nicks is regulated by the HIT domain amino terminal α-helix. This helix forms a wedge that binds and interrogates the exposed damaged DNA base stack of DSB end substrates [Tumbale et al. 2011; Tumbale et al. 2013]. The binding mode of blunt-ended nucleic acid substrates in the APTX active site [Tumbale et al. 2011] suggests the α1 helical wedge would clash with the upstream region of nicked or gapped adenylated substrates, and that APTX must significantly distort nicked or gapped DNA to facilitate catalysis. Intriguingly, the APTX active site histidine triad “HIT” (HϕHϕH, where ϕ is a hydrophobic residue) loop and α-helical wedge undergo conformational changes that may gate assembly of the active site, thereby ensuring non-specific hydrolysis of adenine nucleotides is prevented.
Conserved elements from both the HIT domain and Znf converge on the adenylated DNA 5′ terminus, unwind the terminal base pair, and direct the AMP lesion into a deep binding groove. As APTX engages the DNA 5′-end in an A-form conformation, APTX appears engineered to process adenylated 5′-termini of both DNA and RNA-DNA substrates, consistent with functions of APTX in RER [Tumbale et al. 2014]. Structural and biochemical data support a two-step metal-independent catalytic mechanism for DNA-adenylate reversal by APTX. In the first step, an active site nucleophile, His260 of hAPTX, directly attacks the 5′-adenylate phosphoanhydride resulting in the formation of a transient enzyme-AMP intermediate [Rass et al. 2008] and liberation of the DNA 5′–P product. A structure containing a vanadate transition state mimic of RNA-DNA bound hAPTX shows that the histidine triad HIT substrate binding loop completely encircles the AMP lesion to stabilize the transition state during the first step. The second step involves hydrolysis of the ensuing His260-AMP intermediate, and is likely facilitated by activation of a solvent by a His251-Ser281 catalytic dyad [Tumbale et al. 2014]. Whilst the APTX deadenylation reaction appears metal independent, binding of a Na+ ion suggests roles for solvent in stabilization of the charged 5′-phosphate reaction product.
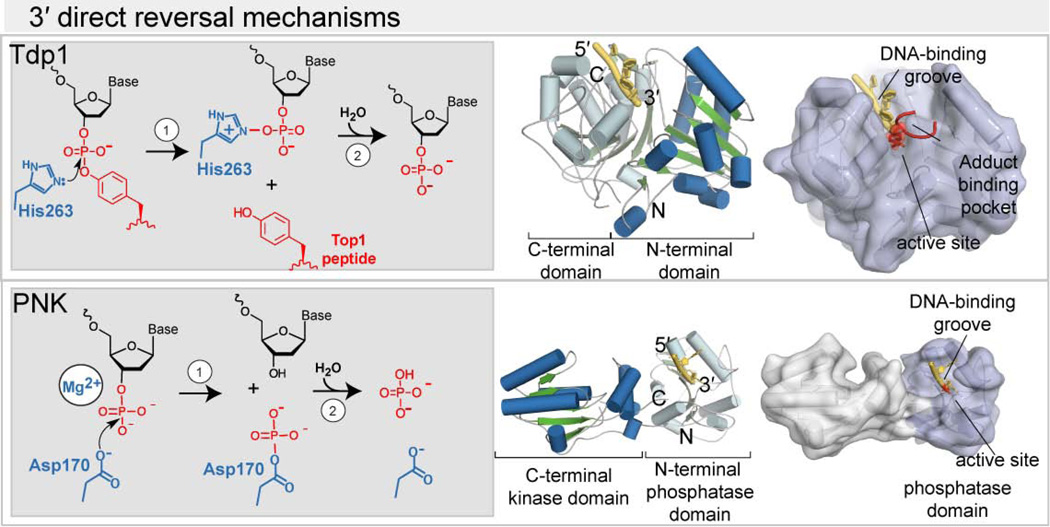
Polynucleotide kinase phosphatase (PNK)
Mammalian Polynucleotide kinase/phosphatase (PNK or PNKP) is a critical DNA end-processing factor participating in BER, SSBR and DSB (Non-homolgous end-joining or NHEJ) repair [Chappell et al. 2002; Wiederhold et al. 2004; Weinfeld et al. 2011]. PNK has two enzymatic activities [Jilani et al. 1999]. It is both a DNA 5′-kinase that phosphorylates 5′-OH termini, and 3′ phosphatase that resolves 3′-phosphorylated termini produced by ROS and DNA glycosylases [Pheiffer et al. 1982]. Clearance of 3′-phosphate moieties generated with TDP1 resolution of TOP1 protein-DNA adducts (see below) is also an important function of PNK [Plo et al. 2003].
X-ray structures of full-length mouse PNK [Bernstein et al. 2005] showed the enzyme adopts a modular architecture characterized by three globular domains; an N-terminal phosphoprotein binding FHA domain, and a C-terminal paired kinase-phosphatase domain. The catalytic domains are fused, and the flexible kinase-phosphatase interface builds a composite DNA interaction interface for substrate binding and catalysis [Garces et al. 2011]. The PNK phosphatase is structurally related to the haloacid dehalogenase (HAD) family of enzymes that includes Phosphoserine phosphatase [Bernstein et al. 2005]. In phosphatase-DNA complex structures [Coquelle 2011; Garces et al. 2011], basic residues lining the phosphatase active site interact with the phosphate backbone. Together with aromatic side chains that stack against the substrate nucleobases, these backbone contacts orient the substrate in a highly extended ssDNA conformation for catalysis. The 3'-P is found in a deep active site pocket evidently well suited for dephosphorylation of 3′-P, but not for accomodating larger modifications such as 3′-phophosphoglycolate [Inamdar et al. 2002]. An active site Asp (Asp170 in mPNK) is proposed to catalyze the Mg2+ assisted nucleophilic attack of the 3′-P to form a phospho-aspartate intermediate that is subsequently hydrolyzed.
A probable mode of substrate binding by the kinase domain has been established from a structure of PNK bound to a self-annealing 5mer ssDNA that assembles into a pseudo-nicked DNA structure occupying the kinase active site. In this work, the 5′ end of the duplex is bound in the kinase active site as a phosphorylated product of the ATP-dependent kinase reaction [Garces et al. 2011]. To recognize the nicked DNA junction, a helical element from the kinase domain wedges into the pseudo-nick duplex, directing the 5′ and 3′ strands to their respective active sites. Such coupling of the phosphatase and kinase substrate interaction sites may provide a mechanism for kinase-phosphatase crosstalk [Garces et al. 2011, Schellenberg and Williams 2011].
Tyrosyl DNA phosphodiesterase 1 (TDP1)
Failure to complete a TOP1 reaction cycle creates a DNA SSB with a 3′covalently linked protein adduct. In the case of stalled TOP1, an initial processing step using the ubiquitin-proteasome system degrades TOP1, yielding a peptide linked to a DNA 3′–P end by a phosphotyrosine linkage [Desai et al. 1997; Lin et al. 2008]. A direct reversal pathway of 3′–TOP1 adducts is catalyzed by TDP1 [Yang et al. 1996].
A series of structures of human TDP1 complexes have been described [Davies et al. 2002; Davies et al. 2003; Davies et al. 2004]. TDP1 is a member of the phospholipase D (PLD) enzyme superfamily. PLD enzymes often function as dimers, but TDP1 contains tandem PLD repeats (Figure 4), with each half of the TDP1 catalytic domain recognizing one side of the substrate phosphodiester. PLD proteins catalyze phosphotransfer reactions with an active site His-Lys-Asp (HKD) motif; in the case of TDP1 the C-terminal aspartate of the motif is not conserved.
Figure 4. Structural basis for repair of 3′-DNA ends by end–cleansing enzymes.
(Left, gray box) Specific examples of 3′–end damage are removed. Right: cartoon representations of repair enzymes with α–helices (blue) and β–strands (green) highlighting their catalytic domain bound to DNA (yellow). (right) Surface representation of repair enzymes showing damaged DNA ends (red) engaged in the enzyme active site. Figures were generated from PDB 1RFF for TDP1, and 3U7F for the PNK phosphatase–DNA complex.
Structures of TDP1 bound to transition-state mimics containing vanadate show that ssDNA binds in an extended conformation in an active site trench, which positions the scissile 3′–P in the active site (Figure 4), where the two conserved HK motifs catalyze a 2–step phosphotyrosine adduct hydrolysis [Davies et al. 2003; Davies et al. 2004; Raymond et al. 2004]. The first step involves metal independent nucleophilic attack by His263 on the scissile phosphate, while His493 protonates the leaving group. Two TDP1 lysine residues provide charge-neutralization of a negatively charged transition state in this reaction. The ensuing DNA phosphohistidyl intermediate is resolved by hydrolysis. The 3′–P product of the TDP1 reaction requires subsequent processing by PNK to yield a 3′-OH end (Figure 1, Figure 3). In a transition–state mimic structure containing a TOP1–derived peptide [Davies et al. 2003], there are few specific interactions from TDP1 with the TOP1 active–site tyrosine. TDP1 also processes other small 3′ adducts such as 3′–phosphoglycolates which although chemically distinct from phosphotyrosine, are also accommodated in the TDP1 active site [Inamdar et al. 2002; Interthal et al. 2005; Akopiants et al. 2013].
A deficiency in TDP1 activity has recently been shown to cause a neurodegenerative disease called spinocerebellar ataxia with axonal neuropathy (SCAN1), caused by mutation of an active–site histidine (H493R) [Interthal et al. 2005]. This mutation has the effect of extending the lifetime of the phosphohistidyl intermediate, thereby linking TDP1 to the 3′–end of DNA in place of the TOP1–derived peptide.
Tyrosyl DNA phosphodiesterase 2, TDP2
Stalled TOP2 cleavage complexes are processed by the proteasome [Alchanati et al. 2009; Ban et al. 2013; Fan et al. 2008], presumably leaving a TOP2 derived peptide linked through a tyrosine to the 5′–end of a DNA break. Although the DSB structure generated in this way has four-nucleotide “sticky” ends, they cannot be directly re-ligated without removal of the 5′–peptide adduct. A vertebrate tyrosyl-DNA phosphodiesterase (TDP2/TTRAP/EapII) was identified, which efficiently processes TOP2-adducts to 5′-phosphorylated termini through a direct reversal mechansism of DSBs [Cortes Ledesma et al. 2009]. TDP2 processing generates a 5′–phosphate, which can be used directly by DNA Ligase IV from NHEJ to seal the break. Consistent with a role in TOP2 processing, targeted RNAi knockdown of TDP2 or knockout sensitizes cells to etoposide, and increases formation of nuclear γH2AX foci, a marker of DSBs [Cortes Ledesma et al. 2009; Gomez-Herreros et al. 2013].
The catalytic domain of TDP2 belongs to the endonuclease/exonuclease/phosphatase (EEP) family of metalloenzymes (Figure 3). High resolution X-ray structures of a series of TDP2-DNA complexes shows TDP2 binds the 5′–end of tyrosylated ssDNA and 5′-overhanging DNA substrates in a narrow trench, similar to TDP1, with direct protein-DNA contacts with the sugar-phosphate backbone and hydrophobic faces of the terminal nucleobases [Schellenberg et al. 2012; Shi et al. 2012] (Figure 3). Although structures of TDP2 containing a TOP2 derived peptide have not yet been reported, a structure containing a non-hydrolysable 5′–6-aminohexanol adduct identified a hydrophobic 5′-adduct binding patch that likely participates in recognition of the peptide half DNA-protein phosphotyrosine substrate [Schellenberg et al. 2012]. The recent discovery that TDP2 can remove the 5′–VPg peptide adduct from picornaviral mRNA [Virgen-Slane et al. 2012], a peptide unrelated to TOP2, suggests that specific TDP2 interactions with the peptide portion of the adduct may be limited to the phosphotyrosine and peptide mainchain.
As with TDP1, the size of the tyrosine-binding pocket is too small to accommodate a deoxyribose-nucleotide, providing a mechanism of excluding DNA from endonucleolytic incision. X-ray structures support a model where TDP2 hydrolyses the 5′–phosphotyrosine bond with a single Mg2+ ion mediated catalytic mechanism, similar to structure-based models for APE1 catalysis [Mol et al. 2000]. In this scheme, a conserved aspartate residue (Asp262, human TDP2 numbering) functions as a catalytic base to activate a water molecule for nucleophilic attack in an SN2 displacement reaction. Bound Mg2+ along with three additional conserved residues (His226, His351 and Ser229) bind the 5′-PO4 and are positioned to interact with the substrate phosphate moiety to stabilize a pentacovalent reaction transition state in this reaction [Schellenberg et al. 2012].
DNA polymerase β AP lyase
The incision of AP sites during base excision repair generates abundant DNA nicks bearing 5′-dRP groups. To resolve these intermediates, mammalian cells employ DNA polymerase β (POLβ) in the single nucleotide BER (SN-BER) as well as long patch BER (LP-BER, also see section below on FEN1) pathways [Liu and Wilson 2012]. The multifunctional 39 kDa POLβ is the smallest of the cellular eukaryotic polymerases, and acts as both a 5′-dRP lyase, and DNA polymerase. In SN-BER, POLβ lyase activity excises the dRP, leaving a gap that is filled by the polymerase domain, resulting in a nick that can be sealed by DNA Ligase III. Polymerase activity resides in the POLβ 31-kDa C-terminal domain, consisting of palm, finger and thumb subdomains, which utilizes a 3-metal ion mechanism to catalyze nucleotidyl transfer [Freudenthal et al. 2013]. The 8-kDa, N-terminal domain harbors the rate-limiting 5′-dRP lyase activity [Matsumoto and Kim 1995; Piersen et al. 1996; Prasad et al. 1998; Bennett et al. 1997] (Figure 3).
POLβ uses a metal-independent 5′-dRP lyase mechanism, involving a lysine mediated nucleophilic attack on C1′ of the sugar to form a Schiff base intermediate, followed by a β-elimination and release of dRP by hydrolysis [Prasad et al. 2005; Matsumoto and Kim 1995; Matsumoto, 1995]. Structures of POLβ bound to nicked and gapped DNA show these interactions bend the DNA 90° across the surface of the protein, positioning the DNA for optimal enzymatic activity [Pelletier et al. 1996; Piersen et al. 1996; Sawaya et al. 1997]. The 5 α-helices of the lyase domain form a lysine rich pocket, that engages the 5′-end, as observed in structures of POLβ bound to both nicked and gapped DNA [Prasad et al. 1994; Pelletier et al. 1994]. Site-directed mutagenesis and mass spectrometry both indicate that Lys72 within the pocket acts as a potential nucleophile in the lyase reaction [Matsumoto et al. 1998]. However, a crystal structure of POLβ bound to nicked DNA bearing the dRP analog tetrahydrofuran, found Lys72 or other potential nucleophiles lie distal from the 5′-dRP. Evidence from the crystal structure suggests that a 120° rotation of dRP about its 3′-phosphate would be required to position the lesion for catalysis.
Ku70/80 AP lyase
While abasic sites generated during BER are processed by POLβ, clustered ROS damage creating DNA strand breaks can also lead to production of AP sites near DSB termini, which are processed by Ku70/80 in NHEJ. Ku70/80 is a heterodimeric, high affinity DSB DNA end binding protein, and is amongst the first proteins to be recruited to DSBs, encircling the DNA ends as a ring structure (Figure 4) [Walker et al. 2001]. The Ku80 C-terminus forms a small helical domain that is required for binding and DNA-dependent activation of the DNA-protein kinase catalytic subunit (DNA-PKcs), a member of the phosphoinositide-3 kinase-related protein kinase family [Hartley et al. 1995; Hammel et al. 2010; Gell and Jackson, 1999; Harris et al., 2004]. In addition to structural DNA end sensing and protein-protein interactions, recent evidence indicates that similar to POLβ, Ku70/80 is a dRP/AP lyase. Ku70/80 removes abasic sites found within 2 nucleotides of a 5′-overhang most efficiently, restricting lyase activity to abasic sites that could potentially directly impact DNA ligation [Roberts et al., 2010; Strande et al. 2012]. Lys31 and Lys160 of the Ku70 monomer are located in the N-terminal α/β domain near the central dimeric pore, and are required for this activity, as K31A/K160A mutants decrease lyase activity 4-fold [Strande et al., 2012].
NUCLEOLYTIC PROCESSING MECHANISMS
Genome maintenance endonucleases and exonucleases are also well suited for clearance of blocked termini, by providing redundant means for DNA end processing of damaged termini. The 5′ nucleases FEN1 and the MRE11/RAD50/NBS1/CtIP complex are the best characterized of these, and have been implicated in alternate pathways for direct reversal of 5′–end blocking lesions. Acting at the 3′–terminus, the AP endonucleases possess activities appropriate for resolving a variety of blocked termini structures. Other DNA damage response nucleases for which we lack structural information include Artemis, Metnase, and Slx1/4 , and have been recently reviewed elsewhere [Povirk 2012].
Flap endonuclease (FEN1)
Varying chemistries on DNA 5′ termini can impair normal processing and clearance of blocked ends by direct reversal processes. For example, during BER if a 5′-dRP group is oxidized [Wong et al. 2010; Jacobs et al. 2011] POLβ lyase activity is impaired. In this case, the polymerase can replace the damaged nucleotide at the 5′ terminus by strand-displacement synthesis (LP-BER). Polymerase displacement then creates a flap structure that is a substrate for FEN1, which recognizes and cleaves the 5′–damaged strand. FEN1 cleavage creates a ligatable nick that is then sealed by ligase. In vitro this general 5′-adduct processing mechanism is also capable of resolving 5′-aldehyde blocked ends, for which there are no known direct reversal pathways [Jung et al. 2011], as well as 5′-ribonucleotides [Sparks et al. 2012] generated by RNaseH2 incision at embedded RNA in DNA during RER. Genetic evidence in budding yeast also implicates the FEN1 homolog Rad27 in resolution of 5′ adenylated termini in vivo, a function that is redundant with Hnt3p, the yeast APTX homolog [Daley et al., 2010]. Thus FEN1, in addition to its critical role in resolving ~50 million flap structures generated during Okazaki fragment maturation [Balakrishnan and Bambara, 2013] is an important damage response enzyme participating in nucleolytic resolution of several 5′ lesions.
FEN1 is the founding member of the FEN family of structure-specific nucleolytic processing enzymes, and is related in structure to EXO1 [Orans et al. 2011], XPG and GEN1 [Williams and Kunkel 2011]. X-ray structures of human FEN1 bound to flap DNA structures revealed that FEN1 overall resembles a left-handed glove, and shows that the enzyme recognizes both the upstream and downstream regions of the flap [Tsutakawa et al. 2011] (Figure 5). At the flap junction, α-helical wedges separate the DNA duplex to segregate 5′ downstream and 3′ upstream DNA. These interactions induce a 100° DNA bend proximal to the cleavage site [Tsutakawa et al. 2011; Chapados et al. 2004], and unpairing of DNA 5' end that provides access to the scissile phosphodiester bond.
Figure 5. Structural basis for repair of DNA ends by nucleolytic processing enzymes.
(Left, gray box) DNA structures (red) recognized and cleaved by nucleolytic enzymes. (Center) Cartoon representations of repair enzymes with α–helices (blue) and β–strands (green) highlighting their catalytic domain bound to DNA (yellow). (Right) Surface representation of repair enzymes showing substrate DNA engaged in the enzyme active site. Figures were generated from PDB entry 3DSD for MRE11, 3Q8L for FEN1, and 1DE9 for APE1.
The FEN1 structure explains efficiency and specificity of the enzyme on double-stranded flap structures. Significantly, extensive protein-nucleic acid contacts are made to the 3′ flap region. These protein-DNA contacts set the endonuclease register for cleavage of the displaced 5′ strand, and ensure generation of a ligatable nick following cleavage. The DNA 5′ strand of the flap is bound using a helix-two turn helix containing (H2TH) potassium-binding fold and two nucleotides are unpaired at the 5′ end, where the flap is “threaded” through a helical arch structure that engulfs the 5′ terminus. Protein disorder-to-order transitions within the helical arch, and a disorder-thread-order mechanism of substrate engagement helps to explain how unique mode of FEN1 substrate binding accommodates diverse 5′ end structures [Patel et al. 2012].
AP Endonucleases
APE1 is a multifunctional protein acting in BER, transcription [Okazaki et al. 1994; Kuninger et al. 2002], and granzyme A-mediated cell death [Fan et al. 2003; Tell et al. 2009]. The catalytic core of APE1 the endonuclease/exonuclease/phosphatase (EEP) fold is related in sequence and structure to vertebrate TDP2, and E. coli Exonuclease III [Mol et al. 2000] (Figure 5). The EEP nucleases adopt a globular α-β fold with a central β-sandwich surrounded by helical elements and surface loops dictating unique substrate interaction specificities [Schellenberg et al. 2012]. In the human APE1 DNA complex structures [Mol et al. 2000] APE1 kinks and completely surrounds the abasic site containing DNA. A substrate recognition arginine interrogates the abasic site for positioning of the scissile phosphate in a metal ion bound active site. In a single metal ion mechanism that is supported by structural studies of human and N. meningitides APE1 [Lu et al. 2012], and similar to a mechanism proposed for TDP2, Asp210 (human APE1 numbering) deprotonates water, which performs nucleophilic attack on scissile phosphate 5′ of the AP site [Mol et al. 2000].
In addition to roles in endonucleolytic processing of abasic sites, APE1 harbors 3′-5′ exonuclease, 3′-phosphodiesterase, 3′-phosphatase, and endoribonuclease activities [Wilson 2003; Kim et al. 2011; Barnes et al. 2009; Barzilay et al. 1995; Chou et al. 2002]. DNA end-processing reactions of APE1 are important during end-healing processes in BER. For example, APE1 phosphodiesterase activity processes 3′-phospho-α,β-unsaturated aldehyde (PUA) generated by β-elimination reactions of glycosylase-lyases, yielding a 3′-OH for repair synthesis and ligation. Presumably APE1 engages damaged 3′-termini in a manner analogous to that observed for the AP-endonuclease reactions (Figure 5), however a detailed structural basis for these transactions is currently undefined.
APE2 (Apn2 in yeast), a related enzyme with sequence homology to APE1, was later identified as an alternate AP endonuclease [Johnson et al. 1998]. However, APE2 exhibits distinct substrate preferences [Hadi et al. 2002; Burkovics et al. 2006; Unk et al. 2000; Unk et al. 2001]. APE2 displays 3′-5′ exonuclease and 3′ phosphodiesterase activity, and weak AP-endonuclease when compared to APE1. Divergence in APE1 and APE2 substrate interaction pocket, DNA binding loops, and active sites suggest substitution of the hydrophobic AP-site interacting groove of APE1 (formed by F266, W280, and L282, human APE1) may underlie differing substrate specificities between the two AP endonucleases [Hadi et al. 2002]. APE2 may play more significant roles in processing of mismatched 3′ nucleotides and damaged 3′ termini. Consistent with roles in clearance of blocked termini, Xenopus APE2 participates in “single strand break end resection” and is required for checkpoint activation in response to oxidative stress in Xenopus extracts [Willis et al. 2013].
MRE11/RAD50/NBS1Xrs2/CtIP Sae2/Ctp1 processing of blocked termini
The MRE11/RAD50/NBS1 (MRN) complex, in conjunction with CtIP Sae2/Ctp1 (CtIP in humans, Ctp1 in S. pombe and Sae2 in S. cerevisiae), generates 3′ ssDNA overhangs required for the initiation of DNA DSBR by homologous recombination (HR) [reviewed in Williams et al. 2007; Williams and Tainer 2005; Mimitou and Symington 2011]. CtIP is also involved in alternative-NHEJ [Bennardo et al., 2008; Zhang et al., 2011; Lee-Theilen et al., 2011]. This multifunctional complex directly tethers DSB DNA termini [Moreno-Herrero et al. 2005; Williams et al. 2008] and initiates 5′-3′ end resection at DNA breaks [Mimitou and Symington 2008; Zhu et al. 2008; Niu et al. 2010]. Resection, as characterized in S. cerevisiae, is coincident at both ends of a DSB [Westmoreland et al. 2013]. MRN also signals DSB damage and regulates cell cycle checkpoints via interactions with ATM [Lee and Paull 2005]. The nucleolytic core of the MRN complex is MRE11. MRE11 directly binds RAD50, NBS1 and DNA. In vitro, MRN possesses 3′-5′ Mn2+-dependent exonuclease activity and ATP-dependent endonuclease activities [Paull and Gellert 1998; Trujillo et al. 1998] that are also modulated by CtIP Sae2/Ctp1 [Nicolette et al. 2010; Sartori et al. 2007]. Evidence in fission yeast [Hartsuiker et al. 2009] and mammalian cells [Quennet et al. 2011] indicates MRN/CtIP is required for nucleolytic processing of etoposide generated TOP2-DNA damage adducts, representing a alternative repair pathway to direct reversal by TDP2. MRN/Ctp1sae2 dependent clearance of protein-blocked 5′-termini is also key during meiosis where the complex functions in removal of Rec12-protein-DNA complex in fission yeast [Rothenberg et al. 2009] or Spo11 in budding yeast [Garcia et al. 2011] to facilitate meiotic strand break processing.
Important insights into MRN/CtIP nucleolytic processing mechanisms have come from X-ray structures of Pyrococcus furiosus (Pf) MRE11-DNA [Williams et al, 2008] and MRE11/RAD50 complexes [Mockel et al. 2012; Williams et al. 2011; Lim et al. 2011] (Figure 5). The PfMRE11 enzyme is a homodimer that associates via a structurally conserved four-helix bundle at the nexus of two N-terminal Calcineurin protein phosphatase related catalytic domains. A mixed α-β fold nuclease “capping domain”, emanates from the nucleolytic cores forming a U-shaped DNA processing cradle, with dsDNA bound in the cleft between the two MRE11 monomers via minor groove sequence-independent interactions in the DNA sugar-phosphate backbone [Williams et al. 2008]. MRE11 splays the duplex apart to access the sugar phosphate backbone for nucleolytic activity. The MRE11 active site consists of five conserved phosphoesterase motifs coordinating two Mn2+ ions [Hopfner et al. 2001; Williams et al. 2008]. Two of these motifs contain histidine residues (His52, His85 of PfMRE11) critical for exo- and endonuclease activity. In the crystal structure bound to duplex DNA, His52 is proposed to participate in positioning the DNA backbone phosphate upstream of the cleavage site, and guides the 3′ terminus into the active site for a 3′-5′ exonucleolytic reaction. His85 likely stabilizes the transition state and Mn2+ acts to align the scissile bond [Hopfner et al. 2001; Williams et al. 2008].
Neale and colleagues [Garcia et al. 2011] proposed an attractive bidirectional resection model in which the combined action of S. cerevisiae MRN/Sae2 endonucleolytic nicking creates an entry point for the MRE11 3′-5′ exonuclease activity that tracks towards the 5′ end. This model provides a reasonable explanation for how a 3′-5′ exonuclease paradoxically dictates the resection reaction that is in the opposite polarity (5′-3′) as well as a probable mechanism for MRN dependent clearance of 5′-topoisomerase or Spo11 blocked termini [Garcia et al. 2011]. The nucleolytic transactions in the MRE11 binding groove are also intimately regulated by RAD50 [Deshpande et al. 2014] and NBS1 [Schiller et al., 2012]. NBS1 homologs also bind CtIP/Ctp1 [Sartori et al. 2007; Williams et al. 2009] which influences MRE11 nuclease activity in vitro [Sartori et al. 2007], however, the precise role for these factors in regulating nucleolytic activities awaits definitive co-complex X-ray structure determination of intact MRN/CtIP-DNA complexes.
COORDINATION OF DNA STRAND BREAK PROCESSING REACTIONS
The DNA damage first-responders discussed herein act in coordinated, multistep DNA repair events. The linking together of individual activities via multi-protein interaction hubs or nodes can achieve high specificity, as well as facilitate efficient substrate transfer of the damaged DNA substrates. The employment of hub proteins, generally non-enzymatic interaction polypeptides, enables integrated orchestration of DNA end recognition events with activation of cellular DNA damage signaling, and coordination of multi-step enzymatic processing events to ensure efficient damage recognition, DNA break processing and nick sealing transactions. Hub protein interactions involve: 1) formation of proteinaceous molecular filaments, 2) regulated (eg. phosphorylation-dependent) binding events, 3) multivalent interactions to several proteins and 4) dynamic and flexible scaffold-to-effector linkages. Two examples of scaffolds that link end-processing activities are XRCC1, which acts in SSBR, and XRCC4, which acts in DSB repair (Figures 6, 7).
Figure 6. XRCC1 acts as a strand break repair assembly line.
XRCC1 orchestrates SSBR proteins by serving as an extended protein-protein interaction platform. An example form of complex DNA damage generated by reactive oxygen is displayed (red rectangle). Multistep processing of DNA damage requires. Variable outcomes, including abortive ligation reactions are resolved by FHA domains of DNA end-processing enzymes APTX and PNK (PDB ID 3KT9, 1YJM) associate with phosphorylated XRCC1, localizing the catalytic APTX deadenylase (PDB ID 4NDH) and PNK phosphatase and kinase domains (PDB 1YJ5) to damaged DNA ends. Efficient repair also requires binding of POLβ through the XRCC1 NTD (PDB ID 3K75). XRCC1’s BRCT2 and Ligase III’s BRCT modules (PDB ID 3QVG) link DNA Ligase III’s catalytic domain (PDB ID 3L2P) to the DNA break site for final sealing of the nicked DNA backbone. XRCC1 BRCT1 (PDB ID 2D8M).
Figure 7. XRCC4-XLF coordinates DNA double strand break repair.
A.) XLF occupies binding sites at XRCC4’s NTD (PDB ID 3RWR), forming an elongated, helical filament of alternating XRCC4 and XLF proteins. Both proteins lack enzymatic activity but are proposed to coat DNA strand breaks and align DNA breaks for efficient end processing and ligation. B.) XRCC4-NHEJ repair protein interactions drive DNA end processing and ligation of double-strand breaks. Like XRCC1, XRCC4’s flexible, phosphorylated C-terminal tails mediate binding for both APTX (3KT9) and PNK FHA domains (1YJM). DNA Ligase IV stability is dependent on its association with XRCC4, encasing XRCC4’s helical tails with tandem BRCT domains (PDB ID 3II6) that bind Ligase IV’s catalytic domain (PDB ID 3W5O).
The XRCC1 scaffold in SSBR
SSBR generally requires four coordinated reaction steps to complete the repair: 1) DNA damage detection by XRCC1/Parp1, 2) DNA end processing by PNK, APTX, TDP1 and others, 3) gap filling by POLβ, and 4) final nick sealing by DNA Ligase III. The XRCC1 protein couples these steps by coordinating recruitment and targeting repair factors that catalyze these reactions, thereby accelerating overall rates of repair by acting as a DNA processing assembly line [Whitehouse et al. 2001; Caldecott 2008; Kubota et al. 1996] (Figure 6).
XRCC1 is a multi-domain protein containing three structurally defined regions, the XRCC1 N-terminal domain (NTD) and two BRCA1 Carboxyl Terminal (BRCT) domains. The NTD directly interacts with POLβ near the POLβ thumb domain [Cuneo and London 2010] (Figure 6). Intriguingly, the NTD is capable of adopting dramatically different redox-dependent protein folds that may alter POLβ interactions in response to oxidative stress. X-ray structures show the C-terminal BRCT adopts a canonical BRCT fold characterized by a central 4-stranded β-strand, surrounded by 3 helical elements ( Zhang et al. 1998; Williams et al. 2001; Williams et al. 2004]. The XRCC1 C-terminal BRCT directly interacts with the DNA Ligase III BRCT region [Caldecott et al. 1994] in a heterodimeric BRCT-BRCT interaction [Cuneo et al. 2011] (Figure 6).
Casein kinase 2 (CK2) phosphorylates XRCC1 between the two BRCT domains [Loizou et al. 2004] at multiple sites [Ali et al. 2009], and these modifications promote efficient SSBR [Parsons et al. 2010]. These phosphorylation sites create a docking platform for the FHA domains of APTX and PNK [Clements et al. 2004]. Flexibility is likely a common feature of the FHA-dependent interactions with XRCC1 (and XRCC4, see below). Small angle X-ray scattering (SAXS) [Bernstein et al. 2009] and limited proteolysis experiments [Bernstein et al. 2005] show a flexible region of low structural complexity bridges the PNK FHA domain and phosphatase-kinase C-terminus. Similarly, the linkage between the N-terminal APTX FHA domain, and the catalytic deadenylase HIT-Znf domains is proteolytically labile [Tumbale et al. 2014], suggesting APTX architecturally parallels PNK in having a phosphoprotein targeting FHA domain linked flexibly to its DNA processing catalytic domain. For PNK, the interaction with XRCC1 also regulates substrate interactions, by promoting PNK dissociation from DNA products following either 5′-phosphorylation or 3′-dephosphorylation [Mani et al. 2007].
Overall an emerging view of the XRCC1 complex is that it enhances transitions between DNA repair enzymes through multivalent protein-protein interactions, both in SSBR and alternative-NHEJ [Della-Maria et al. 2011]. Connecting functionally intertwined activities provides a means for enhancing local concentration of repair factors, and for coordinating sequentially acting end processing activities that are needed in a variety of contexts that may arise with variable damage at DNA ends.
The XRCC4 scaffold in NHEJ
XRCC4 acts as a central hub in non-homologous end joining (NHEJ), and interacts with XLF, DNA Ligase IV, APTX, and PNK [Mahaney et al. 2013]. XRCC4 is comprised of an N-terminal head domain, made up of a 7-stranded anti-parallel beta sandwich, and a helix-turn-helix motif [Junop et al. 2000; Dore et al. 2006; Sibanda et al. 2001]. It also has an elongated, flexible α-helical coiled-coil region, and carboxyl termini tails which are phosphorylated by CK2 [Koch et al. 2004]. In addition to protein-protein interactions, XRCC4 efficiently binds long DNA substrates (>100bp), preferring nicks or broken ends in vitro [Modesti et al. 1999].
The XRCC4 head domain binds to the structurally related protein XLF [Hammel et al. 2011; Ropars et al. 2011; Wu et al. 2011; Andres et al. 2012]. X-ray structures, transmission electron microscopy (TEM), and SAXS indicate that alternating XRCC4 and XLF interact to form helical filaments. Crystal lattice packing and atomic force microscopy (AFM) imaging of XRCC4/XLF revealed large superhelical filaments (Figure 7A]. DNA binding by the superhelical filament may occur in grooves outside the filament or through a central pore that has been calculated to be large enough to enclose dsDNA (Figure 7A), or even an entire nucleosome. The filament has the potential to align mismatched broken DNA ends, providing a molecular basis for XRCC4/XLF mediated enhancement of ligation efficiency in NHEJ [Andres et al 2012; Ropars et al. 2011; Wu et al. 2011; Hammel et al. 2011].
C-terminal to the head domain, the XRCC4 homodimeric coil binds, stimulates, and controls nuclear import of DNA Ligase IV [Junop et al. 2000; Critchlow et al. 1997; Grawunder et al. 1997; Berg et al. 2011]. The XRCC4 coiled-coil region is encircled and bent by the Ligase IV helix-loop-helix clamp region between the Ligase IV tandem BRCT domains [Wu et al. 2009] (Figure 7B). At the XRCC4 unstructured C-terminus, CK2-phosphorylation sites regulate protein-protein interactions and binding of the FHA domains of PNK, APTX and the Aprataxin and PNK-like factor (APLF) [Clements et al. 2004; Koch et al. 2004; Iles et al. 2007; Macrae et al. 2008]. These interactions are hypothesized to coordinate requisite end processing reactions with the DNA ligation step in NHEJ, which is catalyzed by DNA Ligase IV [Koch et al. 2004].
Structural scaffolding by XRCC1 and XRCC4 may act in limiting release of mutagenic DNA-strand break intermediates, and provide a physical mechanism to efficiently orchestrate molecular hand-offs in DNA damage repair to avoid disrupting transcription and replication processes, as originally proposed for BER proteins [Mol et al. 2000; Wilson and Kunkel 2000]. Aside from logical inferences from structural characterizations, experimental evidence for substrate channeling between enzymatic activities remains limited [Prasad et al. 2010]. A significant challenge in the future requiring multi-pronged biochemical, structural and cell biological approaches will be to establish detailed mechanistic understanding of how these protein scaffolds and flexible multi-enzyme processing assemblies achieve high specificity, efficiency, and coordination in DNA strand break repair.
SUMMARY AND PERSPECTIVES
Ultimately, the mechanistic study of the structural chemistry of DNA damage response pathways has broad ranging implications for understanding cellular mechanisms of DNA strand break repair. This work is providing insights into individual genetic vulnerabilities to DNA damage, and an intimate understanding of the molecular principles of susceptibility and resistance to commonly employed cancer chemotherapeutics.
The importance of DNA end-processing factors in preserving genomic integrity is underscored by the fact that deficiencies in protein factors discussed here contribute to a variety of neurological diseases and cancer susceptibility [Rulten and Caldecott 2013]. For example, heritable mutations in the human APTX gene are linked to the autosomal recessive neurological disorders Ataxia with Oculomotor Apraxia 1 (AOA1) [Date et al. 2001; Moreira et al. 2001], a Parkinsons-like multiple system atrophy (MSA) [Baba et al. 2007], and Ataxia with coenzyme Q10 (coQ10) deficiency [Quinzii et al. 2005]. Germline hypomorphic NBS1 and MRE11 mutations cause radiosensitivity and chromosome instability syndromes, Nijmegen breakage syndrome (NBS) and ataxia-telangiectasia-like disorder (ATLD), respectively [Carney et al. 1998; Stewart et al. 1999]. Human CtIP mutations are linked to the microcephaly and dwarfism Seckel syndrome [Qvist et al 2011]. Mutations in PNK are associated with microcephaly with early onset seizures (MCSZ) [Shen et al. 2010; Reynolds et al. 2012], and TDP1 mutation is associated with SCAN1 [El-Khamisy et al. 2005; Takashima et al. 2002]. The emerging detailed understanding of DNA end processing mechanisms derived from structural biology is shedding light onto precisely how heritable DNA repair defects in these factors contributes to neurodegeneration, as well as heritable and somatic carcinogenesis.
Tumor cells are oftentimes deficient in repair and/or signaling of DNA damage, such that therapeutic targeting of backup DNA repair pathways that compensate for such deficits can be a winning strategy to selectively kill cancer cells [Martin et al. 2008; Curtin 2012]. Thus, the systematic definition of the molecular mechanisms of genome repair by DNA end processing factors may open new doors to these “synthetic lethal” treatment regimens, and also facilitate discovery and development of novel targeted DNA repair inhibitors for treatment of cancers that have acquired resistance to DNA crosslinkers, alkylating agents, topoisomerase poisons, and other DNA-targeted chemotherapeutics.
Finally, we note the recent advances in the field that indicate the abundance of RNA contamination in the genome outnumbers all other known potential threats to genomic integrity combined [Nick McElhinny et al. 2010; Reijns et al. 2012; Williams et al. 2013]. This raises important questions: 1) How does ribose chemistry influence DNA transactions carried out by known repair factors, and 2) what additional factors operate in the RNA-DNA realm to preserve integrity of the genome? Further, the identification of an RNA ligase that can function to join 3′-phosphorylated or 2′-3′ cyclic phosphate ends to 5′-OH termini in the context of DNA [Das et al. 2013] suggests that our de facto views of DNA end joining may require revision in the context of RNA-DNA (Huang and Pommier 2013]. Given the abundance of RNA incorporation in the genome it seems likely that other known and yet to be discovered factors may play roles in detection and resolution of RNA-derived lesions and act in RNA-DNA damage responses (RDDRs) similar to that recently documented for APTX [Tumbale et al. 2014].
Acknowledgements
This work was supported by the intramural research program of the US National Institutes of Health (NIH), National Institute of Environmental Health Sciences (NIEHS) projects 1Z01ES102765 to R.S.W. We thank J. Williams, D. Appel, R London, and L. Pedersen for comments on the manuscript.
Footnotes
Author contributions
All authors contributed to writing and editing the text.
REFERENCES
- Ahel I, Rass U, El-Khamisy SF, Katyal S, Clements PM, McKinnon PJ, Caldecott KW, West SC. The neurodegenerative disease protein aprataxin resolves abortive DNA ligation intermediates. Nature. 2006;443:713–716. doi: 10.1038/nature05164. [DOI] [PubMed] [Google Scholar]
- Akopiants K, Mohapatra S, Menon V, Zhou T, Valerie K, Povirk LF. Tracking the processing of damaged DNA double-strand break ends by ligation-mediated PCR: increased persistence of 3'-phosphoglycolate termini in SCAN1 cells. Nucleic acids research. 2013:3125–3137. doi: 10.1093/nar/gkt1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AA, Jukes RM, Pearl LH, Oliver AW. Specific recognition of a multiply phosphorylated motif in the DNA repair scaffold XRCC1 by the FHA domain of human PNK. Nucleic Acids Res. 2009;37:1701–1712. doi: 10.1093/nar/gkn1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alchanati I, Teicher C, Cohen G, Shemesh V, Barr HM, Nakache P, Ben-Avraham D, Idelevich A, Angel I, Livnah N, et al. The E3 ubiquitin-ligase Bmi1/Ring1A controls the proteasomal degradation of Top2alpha cleavage complex - a potentially new drug target. PLoS One. 2009;4:e8104. doi: 10.1371/journal.pone.0008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres SN, Vergnes A, Ristic D, Wyman C, Modesti M, Junop M. A human XRCC4-XLF complex bridges DNA. Nucleic acids research. 2012;40:1868–1878. doi: 10.1093/nar/gks022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba Y, Uitti RJ, Boylan KB, Uehara Y, Yamada T, Farrer MJ, Couchon E, Batish SD, Wszolek ZK. Aprataxin (APTX) gene mutations resembling multiple system atrophy. Parkinsonism Relat Disord. 2007;13:139–142. doi: 10.1016/j.parkreldis.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Balakrishnan L, Bambara RA. Flap endonuclease 1. Annual review of biochemistry. 2013;82:119–138. doi: 10.1146/annurev-biochem-072511-122603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin EL, Osheroff N. Etoposide, topoisomerase II and cancer. Curr Med Chem Anticancer Agents. 2005;5:363–372. doi: 10.2174/1568011054222364. [DOI] [PubMed] [Google Scholar]
- Ban C, Ramakrishnan B, Sundaralingam M. A single 2'-hydroxyl group converts B-DNA to A-DNA. Crystal structure of the DNA-RNA chimeric decamer duplex d(CCGGC)r(G)d(CCGG) with a novel intermolecular G-C base-paired quadruplet. Journal of molecular biology. 1994;236:275–285. doi: 10.1006/jmbi.1994.1134. [DOI] [PubMed] [Google Scholar]
- Ban Y, Ho CW, Lin RK, Lyu YL, Liu LF. Activation of a Novel Ubiquitin-Independent Proteasome Pathway when RNA Polymerase II Encounters a Protein Roadblock. Molecular and cellular biology. 2013;33:4008–4016. doi: 10.1128/MCB.00403-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandele OJ, Osheroff N. (−)-Epigallocatechin gallate, a major constituent of green tea, poisons human type II topoisomerases. Chem Res Toxicol. 2008;21:936–943. doi: 10.1021/tx700434v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes T, Kim WC, Mantha AK, Kim SE, Izumi T, Mitra S, Lee CH. Identification of Apurinic/apyrimidinic endonuclease 1 (APE1) as the endoribonuclease that cleaves c-myc mRNA. Nucleic acids research. 2009;37:3946–3958. doi: 10.1093/nar/gkp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilay G, Mol CD, Robson CN, Walker LJ, Cunningham RP, Tainer JA, Hickson ID. Identification of critical active-site residues in the multifunctional human DNA repair enzyme HAP1. Nature structural biology. 1995;2:561–568. doi: 10.1038/nsb0795-561. [DOI] [PubMed] [Google Scholar]
- Becherel OJ, Jakob B, Cherry AL, Gueven N, Fusser M, Kijas AW, Peng C, Katyal S, McKinnon PJ, Chen J, et al. CK2 phosphorylation-dependent interaction between aprataxin and MDC1 in the DNA damage response. Nucleic Acids Res. 2010;38:1489–1503. doi: 10.1093/nar/gkp1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender RP, Ham AJ, Osheroff N. Quinone-induced enhancement of DNA cleavage by human topoisomerase IIalpha: adduction of cysteine residues 392 and 405. Biochemistry. 2007;46:2856–2864. doi: 10.1021/bi062017l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender RP, Lehmler HJ, Robertson LW, Ludewig G, Osheroff N. Polychlorinated biphenyl quinone metabolites poison human topoisomerase IIalpha: altering enzyme function by blocking the N-terminal protein gate. Biochemistry. 2006;45:10140–10152. doi: 10.1021/bi0524666. [DOI] [PubMed] [Google Scholar]
- Bender RP, Lindsey RH, Jr, Burden DA, Osheroff N. N-acetyl-p-benzoquinone imine, the toxic metabolite of acetaminophen, is a topoisomerase II poison. Biochemistry. 2004;43:3731–3739. doi: 10.1021/bi036107r. [DOI] [PubMed] [Google Scholar]
- Bennardo N, Cheng A, Huang N, Stark JM. Alternative-NHEJ is a mechanistically distinct pathway of mammalian chromosome break repair. PLoS Genet. 2008;27:e1000110. doi: 10.1371/journal.pgen.1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RA, Wilson DM, 3rd, Wong D, Demple B. Interaction of human apurinic endonuclease and DNA polymerase beta in the base excision repair pathway. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:7166–7169. doi: 10.1073/pnas.94.14.7166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg E, Christensen MO, Dalla Rosa I, Wannagat E, Janicke RU, Rosner LM, Dirks WG, Boege F, Mielke C. XRCC4 controls nuclear import and distribution of Ligase IV and exchanges faster at damaged DNA in complex with Ligase IV. DNA repair. 2011;10:1232–1242. doi: 10.1016/j.dnarep.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Bernstein NK, Hammel M, Mani RS, Weinfeld M, Pelikan M, Tainer JA, Glover JN. Mechanism of DNA substrate recognition by the mammalian DNA repair enzyme, Polynucleotide Kinase. Nucleic Acids Res. 2009;37:6161–6173. doi: 10.1093/nar/gkp597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein NK, Williams RS, Rakovszky ML, Cui D, Green R, Karimi-Busheri F, Mani RS, Galicia S, Koch CA, Cass CE, et al. The molecular architecture of the mammalian DNA repair enzyme, polynucleotide kinase. Molecular Cell. 2005;17:657–670. doi: 10.1016/j.molcel.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Buchko GW, Weinfeld M. Influence of nitrogen, oxygen, and nitroimidazole radiosensitizers on DNA damage induced by ionizing radiation. Biochemistry. 1993;32:2186–2193. doi: 10.1021/bi00060a009. [DOI] [PubMed] [Google Scholar]
- Burkovics P, Szukacsov V, Unk I, Haracska L. Human Ape2 protein has a 3'-5' exonuclease activity that acts preferentially on mismatched base pairs. Nucleic acids research. 2006;34:2508–2515. doi: 10.1093/nar/gkl259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldecott KW. Mammalian single-strand break repair: mechanisms and links with chromatin. DNA Repair (Amst) 2007;6:443–453. doi: 10.1016/j.dnarep.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Caldecott KW. Single-strand break repair and genetic disease. Nat Rev Genet. 2008;9:619–631. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- Caldecott KW. Molecular biology. Ribose--an internal threat to DNA. Science. 2014;343:260–261. doi: 10.1126/science.1248234. [DOI] [PubMed] [Google Scholar]
- Caldecott KW, McKeown CK, Tucker JD, Ljungquist S, Thompson LH. An interaction between the mammalian DNA repair protein XRCC1 and DNA ligase III. Molecular and Cellular Biology. 1994;14:68–76. doi: 10.1128/mcb.14.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney JP, Maser RS, Olivares H, Davis EM, Le Beau M, Yates JR, 3rd, Hays L, Morgan WF, Petrini JH. The hMre11/hRad50 protein complex and Nijmegen breakage syndrome: linkage of double-strand break repair to the cellular DNA damage response. Cell. 1998;93:477–486. doi: 10.1016/s0092-8674(00)81175-7. [DOI] [PubMed] [Google Scholar]
- Chabes A, Georgieva B, Domkin V, Zhao X, Rothstein R, Thelander L. Survival of DNA damage in yeast directly depends on increased dNTP levels allowed by relaxed feedback inhibition of ribonucleotide reductase. Cell. 2003;112:391–401. doi: 10.1016/s0092-8674(03)00075-8. [DOI] [PubMed] [Google Scholar]
- Chapados BR, Hosfield DJ, Han S, Qiu J, Yelent B, Shen B, Tainer JA. Structural basis for FEN-1 substrate specificity and PCNA-mediated activation in DNA replication and repair. Cell. 2004;116:39–50. doi: 10.1016/s0092-8674(03)01036-5. [DOI] [PubMed] [Google Scholar]
- Chappell C, Hanakahi LA, Karimi-Busheri F, Weinfeld M, West SC. Involvement of human polynucleotide kinase in double-strand break repair by non-homologous end joining. The EMBO journal. 2002;21:2827–2832. doi: 10.1093/emboj/21.11.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho JE, Kim N, Li YC, Jinks-Robertson S. Two distinct mechanisms of Topoisomerase 1-dependent mutagenesis in yeast. DNA repair. 2013;12:205–211. doi: 10.1016/j.dnarep.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou KM, Cheng YC. An exonucleolytic activity of human apurinic/apyrimidinic endonuclease on 3' mispaired DNA. Nature. 2002;415:655–659. doi: 10.1038/415655a. [DOI] [PubMed] [Google Scholar]
- Ciccia A, Elledge SJ. The DNA damage response: making it safe to play with knives. Molecular cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements PM, Breslin C, Deeks ED, Byrd PJ, Ju L, Bieganowski P, Brenner C, Moreira MC, Taylor AM, Caldecott KW. The ataxia-oculomotor apraxia 1 gene product has a role distinct from ATM and interacts with the DNA strand break repair proteins XRCC1 and XRCC4. DNA Repair (Amst) 2004;3:1493–1502. doi: 10.1016/j.dnarep.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Coquelle N, Havali-Shahriari Z, Bernstein N, Green R, Glover JNM. Structural basis for the phosphatase activity of PNKP on single- and double-stranded DNA substrates. Proc. Natl. Acad. Sci. USA. 2011 doi: 10.1073/pnas.1112036108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes Ledesma F, El Khamisy SF, Zuma MC, Osborn K, Caldecott KW. A human 5'-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature. 2009;461:674–678. doi: 10.1038/nature08444. [DOI] [PubMed] [Google Scholar]
- Cotner-Gohara E, Kim IK, Hammel M, Tainer JA, Tomkinson AE, Ellenberger T. Human DNA ligase III recognizes DNA ends by dynamic switching between two DNA-bound states. Biochemistry. 2010;49:6165–6176. doi: 10.1021/bi100503w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchlow SE, Bowater RP, Jackson SP. Mammalian DNA double-strand break repair protein XRCC4 interacts with DNA ligase IV. Current biology : CB. 1997;7:588–598. doi: 10.1016/s0960-9822(06)00258-2. [DOI] [PubMed] [Google Scholar]
- Cuneo MJ, Gabel SA, Krahn JM, Ricker MA, London RE. The structural basis for partitioning of the XRCC1/DNA ligase III-alpha BRCT-mediated dimer complexes. Nucleic acids research. 2011;39:7816–7827. doi: 10.1093/nar/gkr419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuneo MJ, London RE. Oxidation state of the XRCC1 N-terminal domain regulates DNA polymerase beta binding affinity. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6805–6810. doi: 10.1073/pnas.0914077107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin NJ. DNA repair dysregulation from cancer driver to therapeutic target. Nature reviews. Cancer. 2012;12:801–817. doi: 10.1038/nrc3399. [DOI] [PubMed] [Google Scholar]
- Daley JM, Wilson TE, Ramotar D. Genetic interactions between HNT3/Aprataxin and RAD27/FEN1 suggest parallel pathways for 5' end processing during base excision repair. DNA Repair (Amst) 2010;9:690–699. doi: 10.1016/j.dnarep.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das U, Chakravarty AK, Remus BS, Shuman S. Rewriting the rules for end joining via enzymatic splicing of DNA 3'-PO4 and 5'-OH ends. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20437–20442. doi: 10.1073/pnas.1314289110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Date H, Onodera O, Tanaka H, Iwabuchi K, Uekawa K, Igarashi S, Koike R, Hiroi T, Yuasa T, Awaya Y, et al. Early-onset ataxia with ocular motor apraxia and hypoalbuminemia is caused by mutations in a new HIT superfamily gene. Nat Genet. 2001;29:184–188. doi: 10.1038/ng1001-184. [DOI] [PubMed] [Google Scholar]
- Davies DR, Interthal H, Champoux JJ, Hol WG. The crystal structure of human tyrosyl-DNA phosphodiesterase, Tdp1. Structure. 2002;10:237–248. doi: 10.1016/s0969-2126(02)00707-4. [DOI] [PubMed] [Google Scholar]
- Davies DR, Interthal H, Champoux JJ, Hol WG. Crystal structure of a transition state mimic for Tdp1 assembled from vanadate, DNA, and a topoisomerase I-derived peptide. Chemistry & biology. 2003;10:139–147. doi: 10.1016/s1074-5521(03)00021-8. [DOI] [PubMed] [Google Scholar]
- Davies DR, Interthal H, Champoux JJ, Hol WG. Explorations of peptide and oligonucleotide binding sites of tyrosyl-DNA phosphodiesterase using vanadate complexes. Journal of medicinal chemistry. 2004;47:829–837. doi: 10.1021/jm030487x. [DOI] [PubMed] [Google Scholar]
- Dedon PC. The chemical toxicology of 2-deoxyribose oxidation in DNA. Chemical research in toxicology. 2008;21:206–219. doi: 10.1021/tx700283c. [DOI] [PubMed] [Google Scholar]
- Della-Maria J, Zhou Y, Tsai MS, Kuhnlein J, Carney P, Paull TT, Tomkinson AE. Human Mre11/human Rad50/Nbs1 and DNA ligase IIIalpha/XRCC1 protein complexes act together in an alternative nonhomologous end joining pathway. J Biol Chem. 2011;286:33845–33853. doi: 10.1074/jbc.M111.274159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demple B, Sung JS. Molecular and biological roles of Ape1 protein in mammalian base excision repair. DNA repair. 2005;4:1442–1449. doi: 10.1016/j.dnarep.2005.09.004. [DOI] [PubMed] [Google Scholar]
- DeRose EF, Perera L, Murray MS, Kunkel TA, London RE. Solution structure of the Dickerson DNA dodecamer containing a single ribonucleotide. Biochemistry. 2012;51:2407–2416. doi: 10.1021/bi201710q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai SD, Liu LF, Vazquez-Abad D, D'Arpa P. Ubiquitin-dependent destruction of topoisomerase I is stimulated by the antitumor drug camptothecin. The Journal of biological chemistry. 1997;272:24159–24164. doi: 10.1074/jbc.272.39.24159. [DOI] [PubMed] [Google Scholar]
- Deshpande RA, Williams GJ, Limbo O, Williams RS, Kuhnlein J, Lee JH, Classen S, Guenther G, Russell P, Tainer JA, et al. ATP-driven Rad50 conformations regulate DNA tethering, end resection, and ATM checkpoint signaling. The EMBO journal. 2014 doi: 10.1002/embj.201386100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deweese JE, Osheroff N. The DNA cleavage reaction of topoisomerase II: wolf in sheep's clothing. Nucleic Acids Res. 2009;37:738–748. doi: 10.1093/nar/gkn937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dore AS, Furnham N, Davies OR, Sibanda BL, Chirgadze DY, Jackson SP, Pellegrini L, Blundell TL. Structure of an Xrcc4-DNA ligase IV yeast ortholog complex reveals a novel BRCT interaction mode. DNA repair. 2006;5:362–368. doi: 10.1016/j.dnarep.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Drinkwater NR, Miller EC, Miller JA. Estimation of apurinic/apyrimidinic sites and phosphotriesters in deoxyribonucleic acid treated with electrophilic carcinogens and mutagens. Biochemistry. 1980;19:5087–5092. doi: 10.1021/bi00563a023. [DOI] [PubMed] [Google Scholar]
- Egli M, Usman N, Rich A. Conformational influence of the ribose 2'-hydroxyl group: crystal structures of DNA-RNA chimeric duplexes. Biochemistry. 1993;32:3221–3237. [PubMed] [Google Scholar]
- El-Khamisy SF, Saifi GM, Weinfeld M, Johansson F, Helleday T, Lupski JR, Caldecott KW. Defective DNA single-strand break repair in spinocerebellar ataxia with axonal neuropathy-1. Nature. 2005;434:108–113. doi: 10.1038/nature03314. [DOI] [PubMed] [Google Scholar]
- Fan JR, Peng AL, Chen HC, Lo SC, Huang TH, Li TK. Cellular processing pathways contribute to the activation of etoposide-induced DNA damage responses. DNA repair. 2008;7:452–463. doi: 10.1016/j.dnarep.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Fan Z, Beresford PJ, Zhang D, Xu Z, Novina CD, Yoshida A, Pommier Y, Lieberman J. Cleaving the oxidative repair protein Ape1 enhances cell death mediated by granzyme A. Nature immunology. 2003;4:145–153. doi: 10.1038/ni885. [DOI] [PubMed] [Google Scholar]
- Ferraro P, Franzolin E, Pontarin G, Reichard P, Bianchi V. Quantitation of cellular deoxynucleoside triphosphates. Nucleic acids research. 2010;38:e85. doi: 10.1093/nar/gkp1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freudenthal BD, Beard WA, Shock DD, Wilson SH. Observing a DNA polymerase choose right from wrong. Cell. 2013;154:157–168. doi: 10.1016/j.cell.2013.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg EC, Aguilera A, Gellert M, Hanawalt PC, Hays JB, Lehmann AR, Lindahl T, Lowndes N, Sarasin A, Wood RD. DNA repair: from molecular mechanism to human disease. DNA repair. 2006;5:986–996. doi: 10.1016/j.dnarep.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Garces F, Pearl LH, Oliver AW. The structural basis for substrate recognition by mammalian polynucleotide kinase 3' phosphatase. Molecular Cell. 2011;44:385–396. doi: 10.1016/j.molcel.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia V, Phelps SE, Gray S, Neale MJ. Bidirectional resection of DNA double-strand breaks by Mre11 and Exo1. Nature. 2011;479:241–244. doi: 10.1038/nature10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gell D, Jackson SP. Mapping of protein-protein interactions within the DNA-dependent protein kinase complex. Nucleic Acids Res. 1999;27:3494–3502. doi: 10.1093/nar/27.17.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Herreros F, Romero-Granados R, Zeng Z, Alvarez-Quilon A, Quintero C, Ju L, Umans L, Vermeire L, Huylebroeck D, Caldecott KW, et al. TDP2-dependent non-homologous end-joining protects against topoisomerase II-induced DNA breaks and genome instability in cells and in vivo. PLoS genetics. 2013;9:e1003226. doi: 10.1371/journal.pgen.1003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grawunder U, Wilm M, Wu X, Kulesza P, Wilson TE, Mann M, Lieber MR. Activity of DNA ligase IV stimulated by complex formation with XRCC4 protein in mammalian cells. Nature. 1997;388:492–495. doi: 10.1038/41358. [DOI] [PubMed] [Google Scholar]
- Hadi MZ, Ginalski K, Nguyen LH, Wilson DM., 3rd Determinants in nuclease specificity of Ape1 and Ape2, human homologues of Escherichia coli exonuclease III. Journal of molecular biology. 2002;316:853–866. doi: 10.1006/jmbi.2001.5382. [DOI] [PubMed] [Google Scholar]
- Hammel M, Rey M, Yu Y, Mani RS, Classen S, Liu M, Pique ME, Fang S, Mahaney BL, Weinfeld M, et al. XRCC4 protein interactions with XRCC4-like factor (XLF) create an extended grooved scaffold for DNA ligation and double strand break repair. The Journal of biological chemistry. 2011;286:32638–32650. doi: 10.1074/jbc.M111.272641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel M, Yu Y, Fang S, Lees-Miller SP, Tainer JA. XLF regulates filament architecture of the XRCC4.ligase IV complex. Structure. 2010;18:1431–1442. doi: 10.1016/j.str.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammel M, Yu Y, Mahaney BL, Cai B, Ye R, Phipps BM, Rambo RP, Hura GL, Pelikan M, So S, et al. Ku and DNA-dependent protein kinase dynamic conformations and assembly regulate DNA binding and the initial non-homologous end joining complex. The Journal of biological chemistry. 2010;285:1414–1423. doi: 10.1074/jbc.M109.065615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hande KR. Etoposide: four decades of development of a topoisomerase II inhibitor. Eur J Cancer. 1998;34:1514–1521. doi: 10.1016/s0959-8049(98)00228-7. [DOI] [PubMed] [Google Scholar]
- Harris JL, Jakob B, Taucher-Scholz G, Dianov GL, Becherel OJ, Lavin MF. Aprataxin, poly-ADP ribose polymerase 1 (PARP-1) and apurinic endonuclease 1 (APE1) function together to protect the genome against oxidative damage. Hum Mol Genet. 2009;18:4102–4117. doi: 10.1093/hmg/ddp359. [DOI] [PubMed] [Google Scholar]
- Harris R, Esposito D, Sankar A, Maman JD, Hinks JA, Pearl LH, Driscoll PC. The 3D solution structure of the C-terminal region of Ku86 (Ku86CTR) J Mol Biol. 2004;335:573–582. doi: 10.1016/j.jmb.2003.10.047. [DOI] [PubMed] [Google Scholar]
- Hartley KO, Gell D, Smith GC, Zhang H, Divecha N, Connelly MA, Admon A, Lees-Miller SP, Anderson CW, Jackson SP. DNA-dependent protein kinase catalytic subunit: a relative of phosphatidylinositol 3-kinase and the ataxia telangiectasia gene product. Cell. 1995;82:849–856. doi: 10.1016/0092-8674(95)90482-4. [DOI] [PubMed] [Google Scholar]
- Hartsuiker E, Neale MJ, Carr AM. Distinct requirements for the Rad32(Mre11) nuclease and Ctp1(CtIP) in the removal of covalently bound topoisomerase I and II from DNA. Mol Cell. 2009;33:117–123. doi: 10.1016/j.molcel.2008.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller B, Achleitner M, Glage S, Naumann R, Behrendt R, Roers A. Mammalian RNase H2 removes ribonucleotides from DNA to maintain genome integrity. The Journal of experimental medicine. 2012;209:1419–1426. doi: 10.1084/jem.20120876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano M, Yamamoto A, Mori T, Lan L, Iwamoto TA, Aoki M, Shimada K, Furiya Y, Kariya S, Asai H, et al. DNA single-strand break repair is impaired in aprataxin-related ataxia. Ann Neurol. 2007;61:162–174. doi: 10.1002/ana.21078. [DOI] [PubMed] [Google Scholar]
- Hopfner KP, Karcher A, Craig L, Woo TT, Carney JP, Tainer JA. Structural biochemistry and interaction architecture of the DNA double-strand break repair Mre11 nuclease and Rad50-ATPase. Cell. 2001;105:473–485. doi: 10.1016/s0092-8674(01)00335-x. [DOI] [PubMed] [Google Scholar]
- Huang SY, Pommier Y. Cross-over of RNA 3'-phosphate ligase into the DNA world. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:20354–20355. doi: 10.1073/pnas.1320033110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles N, Rulten S, El-Khamisy SF, Caldecott KW. APLF (C2orf13) is a novel human protein involved in the cellular response to chromosomal DNA strand breaks. Mol Cell Biol. 2007;27:3793–3803. doi: 10.1128/MCB.02269-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamdar KV, Pouliot JJ, Zhou T, Lees-Miller SP, Rasouli-Nia A, Povirk LF. Conversion of phosphoglycolate to phosphate termini on 3' overhangs of DNA double strand breaks by the human tyrosyl-DNA phosphodiesterase hTdp1. The Journal of biological chemistry. 2002;277:27162–27168. doi: 10.1074/jbc.M204688200. [DOI] [PubMed] [Google Scholar]
- Interthal H, Chen HJ, Champoux JJ. Human Tdp1 cleaves a broad spectrum of substrates, including phosphoamide linkages. The Journal of biological chemistry. 2005;280:36518–36528. doi: 10.1074/jbc.M508898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interthal H, Chen HJ, Kehl-Fie TE, Zotzmann J, Leppard JB, Champoux JJ. SCAN1 mutant Tdp1 accumulates the enzyme--DNA intermediate and causes camptothecin hypersensitivity. The EMBO journal. 2005;24:2224–2233. doi: 10.1038/sj.emboj.7600694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs AC, Kreller CR, Greenberg MM. Long patch base excision repair compensates for DNA polymerase beta inactivation by the C4'-oxidized abasic site. Biochemistry. 2011;50:136–143. doi: 10.1021/bi1017667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilani A, Ramotar D, Slack C, Ong C, Yang XM, Scherer SW, Lasko DD. Molecular cloning of the human gene, PNKP, encoding a polynucleotide kinase 3'-phosphatase and evidence for its role in repair of DNA strand breaks caused by oxidative damage. The Journal of biological chemistry. 1999;274:24176–24186. doi: 10.1074/jbc.274.34.24176. [DOI] [PubMed] [Google Scholar]
- Johnson RE, Torres-Ramos CA, Izumi T, Mitra S, Prakash S, Prakash L. Identification of APN2, the Saccharomyces cerevisiae homolog of the major human AP endonuclease HAP1, and its role in the repair of abasic sites. Genes Dev. 1998;12:3137–3143. doi: 10.1101/gad.12.19.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KY, Kodama T, Greenberg MM. Repair of the major lesion resulting from C5'-oxidation of DNA. Biochemistry. 2011;50:6273–6279. doi: 10.1021/bi200787e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junop MS, Modesti M, Guarne A, Ghirlando R, Gellert M, Yang W. Crystal structure of the Xrcc4 DNA repair protein and implications for end joining. The EMBO journal. 2000;19:5962–5970. doi: 10.1093/emboj/19.22.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasiviswanathan R, Copeland WC. Ribonucleotide discrimination and reverse transcription by the human mitochondrial DNA polymerase. The Journal of biological chemistry. 2011;286:31490–31500. doi: 10.1074/jbc.M111.252460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Huang SN, Williams JS, Li YC, Clark AB, Cho JE, Kunkel TA, Pommier Y, Jinks-Robertson S. Mutagenic processing of ribonucleotides in DNA by yeast topoisomerase I. Science. 2011;332:1561–1564. doi: 10.1126/science.1205016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WC, Berquist BR, Chohan M, Uy C, Wilson DM, 3rd, Lee CH. Characterization of the endoribonuclease active site of human apurinic/apyrimidinic endonuclease 1. Journal of molecular biology. 2011;411:960–971. doi: 10.1016/j.jmb.2011.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WC, King D, Lee CH. RNA-cleaving properties of human apurinic/apyrimidinic endonuclease 1 (APE1) International journal of biochemistry and molecular biology. 2010;1:12–25. [PMC free article] [PubMed] [Google Scholar]
- Koch CA, Agyei R, Galicia S, Metalnikov P, O'Donnell P, Starostine A, Weinfeld M, Durocher D. Xrcc4 physically links DNA end processing by polynucleotide kinase to DNA ligation by DNA ligase IV. EMBO J. 2004;23:3874–3885. doi: 10.1038/sj.emboj.7600375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Nash RA, Klungland A, Schar P, Barnes DE, Lindahl T. Reconstitution of DNA base excision-repair with purified human proteins: interaction between DNA polymerase beta and the XRCC1 protein. The EMBO journal. 1996;15:6662–6670. [PMC free article] [PubMed] [Google Scholar]
- Kuninger DT, Izumi T, Papaconstantinou J, Mitra S. Human AP-endonuclease 1 and hnRNP-L interact with a nCaRE-like repressor element in the AP-endonuclease 1 promoter. Nucleic acids research. 2002;30:823–829. doi: 10.1093/nar/30.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebedeva N, Auffret Vander Kemp P, Bjornsti MA, Lavrik O, Boiteux S. Trapping of DNA topoisomerase I on nick-containing DNA in cell free extracts of Saccharomyces cerevisiae. DNA repair. 2006;5:799–809. doi: 10.1016/j.dnarep.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- Lee-Theilen M, Matthews AJ, Kelly D, Zheng S, Chaudhuri J. CtIP promotes michrohomology-mediated alternative end joining during class-switch recombination. Nat Struct Mol Biol. 2011;18:75–79. doi: 10.1038/nsmb.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Wilson DM., 3rd Human Apurinic/Apyrimidinic Endonuclease 1. Antioxidants & redox signaling. 2013 doi: 10.1089/ars.2013.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim HS, Kim JS, Park YB, Gwon GH, Cho Y. Crystal structure of the Mre11-Rad50-ATPgammaS complex: understanding the interplay between Mre11 and Rad50. Genes & development. 2011;25:1091–1104. doi: 10.1101/gad.2037811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima CD, D'Amico KL, Naday I, Rosenbaum G, Westbrook EM, Hendrickson WA. MAD analysis of FHIT, a putative human tumor suppressor from the HIT protein family. Structure. 1997a;5:763–774. doi: 10.1016/s0969-2126(97)00231-1. [DOI] [PubMed] [Google Scholar]
- Lima CD, Klein MG, Hendrickson WA. Structure-based analysis of catalysis and substrate definition in the HIT protein family. Science. 1997b;278:286–290. doi: 10.1126/science.278.5336.286. [DOI] [PubMed] [Google Scholar]
- Lin CP, Ban Y, Lyu YL, Desai SD, Liu LF. A ubiquitin-proteasome pathway for the repair of topoisomerase I-DNA covalent complexes. The Journal of biological chemistry. 2008;283:21074–21083. doi: 10.1074/jbc.M803493200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T, Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972;11:3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- Liu LF, Rowe TC, Yang L, Tewey KM, Chen GL. Cleavage of DNA by mammalian DNA topoisomerase II. The Journal of biological chemistry. 1983;258:15365–15370. [PubMed] [Google Scholar]
- Liu Y, Wilson SH. DNA base excision repair: a mechanism of trinucleotide repeat expansion. Trends in biochemical sciences. 2012;37:162–172. doi: 10.1016/j.tibs.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizou JI, El-Khamisy SF, Zlatanou A, Moore DJ, Chan DW, Qin J, Sarno S, Meggio F, Pinna LA, Caldecott KW. The protein kinase CK2 facilitates repair of chromosomal DNA single-strand breaks. Cell. 2004;117:17–28. doi: 10.1016/s0092-8674(04)00206-5. [DOI] [PubMed] [Google Scholar]
- Lu D, Silhan J, MacDonald JT, Carpenter EP, Jensen K, Tang CM, Baldwin GS, Freemont PS. Structural basis for the recognition and cleavage of abasic DNA in Neisseria meningitidis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:16852–16857. doi: 10.1073/pnas.1206563109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Chan DW, Yang T, Rodriguez M, Chen BP, Leng M, Mu JJ, Chen D, Songyang Z, Wang Y, et al. A new XRCC1-containing complex and its role in cellular survival of methyl methanesulfonate treatment. Mol Cell Biol. 2004;24:8356–8365. doi: 10.1128/MCB.24.19.8356-8365.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae CJ, McCulloch RD, Ylanko J, Durocher D, Koch CA. APLF (C2orf13) facilitates nonhomologous end-joining and undergoes ATM-dependent hyperphosphorylation following ionizing radiation. DNA Repair (Amst) 2008;7:292–302. doi: 10.1016/j.dnarep.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Mahaney BL, Hammel M, Meek K, Tainer JA, Lees-Miller SP. XRCC4 and XLF form long helical protein filaments suitable for DNA end protection and alignment to facilitate DNA double strand break repair. Biochemistry and cell biology = Biochimie et biologie cellulaire. 2013;91:31–41. doi: 10.1139/bcb-2012-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani R, Fanta M, Karimi-Busheri F, Silver E, Virgen CA, Caldecott KW, Cass CE, Weinfeld M. XRCC1 stimulates polynucleotide kinase by enhancing its damage discrimination and displacement from DNA repair intermediates. J. Biol. Chem. 2007;282:28004–28013. doi: 10.1074/jbc.M704867200. [DOI] [PubMed] [Google Scholar]
- Martin SA, Lord CJ, Ashworth A. DNA repair deficiency as a therapeutic target in cancer. Current opinion in genetics & development. 2008;18:80–86. doi: 10.1016/j.gde.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Kim K. Excision of deoxyribose phosphate residues by DNA polymerase beta during DNA repair. Science. 1995;269:699–702. doi: 10.1126/science.7624801. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Kim K, Katz DS, Feng JA. Catalytic center of DNA polymerase beta for excision of deoxyribose phosphate groups. Biochemistry. 1998;37:6456–6464. doi: 10.1021/bi9727545. [DOI] [PubMed] [Google Scholar]
- Mimitou EP, Symington LS. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature. 2008;455:770–774. doi: 10.1038/nature07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimitou EP, Symington DNA end resection-unraveling the tail. DNA Repair. 2011;10:344–348. doi: 10.1016/j.dnarep.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mockel C, Lammens K, Schele A, Hopfner KP. ATP driven structural changes of the bacterial Mre11:Rad50 catalytic head complex. Nucleic acids research. 2012;40:914–927. doi: 10.1093/nar/gkr749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modesti M, Hesse JE, Gellert M. DNA binding of Xrcc4 protein is associated with V(D)J recombination but not with stimulation of DNA ligase IV activity. The EMBO journal. 1999;18:2008–2018. doi: 10.1093/emboj/18.7.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol CD, Izumi T, Mitra S, Tainer JA. DNA-bound structures and mutants reveal abasic DNA binding by APE1 and DNA repair coordination. Nature. 2000;403:451–456. doi: 10.1038/35000249. [DOI] [PubMed] [Google Scholar]
- Moreira MC, Barbot C, Tachi N, Kozuka N, Uchida E, Gibson T, Mendonca P, Costa M, Barros J, Yanagisawa T, et al. The gene mutated in ataxia-ocular apraxia 1 encodes the new HIT/Zn-finger protein aprataxin. Nat Genet. 2001;29:189–193. doi: 10.1038/ng1001-189. [DOI] [PubMed] [Google Scholar]
- Moreno-Herrero F, de Jager M, Dekker NH, Kanaar R, Wyman C, Dekker C. Mesoscale conformational changes in the DNA-repair complex Rad50/Mre11/Nbs1 upon binding DNA. Nature. 2005;437:440–443. doi: 10.1038/nature03927. [DOI] [PubMed] [Google Scholar]
- Mosesso P, Piane M, Palitti F, Pepe G, Penna S, Chessa L. The novel human gene aprataxin is directly involved in DNA single-strand-break repair. Cell Mol Life Sci. 2005;62:485–491. doi: 10.1007/s00018-004-4441-0. [DOI] [PubMed] [Google Scholar]
- Nakamura J, Swenberg JA. Endogenous apurinic/apyrimidinic sites in genomic DNA of mammalian tissues. Cancer research. 1999;59:2522–2526. [PubMed] [Google Scholar]
- Nick McElhinny SA, Kumar D, Clark AB, Watt DL, Watts BE, Lundstrom EB, Johansson E, Chabes A, Kunkel TA. Genome instability due to ribonucleotide incorporation into DNA. Nature chemical biology. 2010;6:774–781. doi: 10.1038/nchembio.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick McElhinny SA, Ramsden DA. Polymerase mu is a DNA-directed DNA/RNA polymerase. Molecular and cellular biology. 2003;23:2309–2315. doi: 10.1128/MCB.23.7.2309-2315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick McElhinny SA, Watts BE, Kumar D, Watt DL, Lundstrom EB, Burgers PM, Johansson E, Chabes A, Kunkel TA. Abundant ribonucleotide incorporation into DNA by yeast replicative polymerases. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4949–4954. doi: 10.1073/pnas.0914857107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolette ML, Lee K, Guo Z, Rani M, Chow JM, Lee SE, Paull TT. Mre11-Rad50-Xrs2 and Sae2 promote 5' strand resection of DNA double-strand breaks. Nature structural & molecular biology. 2010;17:1478–1485. doi: 10.1038/nsmb.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H, Chung WH, Zhu Z, Kwon Y, Zhao W, Chi P, Prakash R, Seong C, Liu D, Lu L, et al. Mechanism of the ATP-dependent DNA end-resection machinery from Saccharomyces cerevisiae. Nature. 2010;467:108–111. doi: 10.1038/nature09318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitiss JL. DNA topoisomerase II and its growing repertoire of biological functions. Nat Rev Cancer. 2009a;9:327–337. doi: 10.1038/nrc2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitiss JL. Targeting DNA topoisomerase II in cancer chemotherapy. Nat Rev Cancer. 2009b;9:338–350. doi: 10.1038/nrc2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki T, Chung U, Nishishita T, Ebisu S, Usuda S, Mishiro S, Xanthoudakis S, Igarashi T, Ogata E. A redox factor protein, ref1, is involved in negative gene regulation by extracellular calcium. The Journal of biological chemistry. 1994;269:27855–27862. [PubMed] [Google Scholar]
- Okura A, Arakawa H, Oka H, Yoshinari T, Monden Y. Effect of genistein on topoisomerase activity and on the growth of (Val 12)Ha-ras-transformed NIH 3T3 cells. Biochem Biophys Res Commun. 1988;157:183–189. doi: 10.1016/s0006-291x(88)80030-5. [DOI] [PubMed] [Google Scholar]
- Orans J, McSweeney EA, Iyer RR, Hast MA, Hellinga HW, Modrich P, Beese LS. Structures of human exonuclease 1 DNA complexes suggest a unified mechanism for nuclease family. Cell. 2011;145:212–223. doi: 10.1016/j.cell.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JL, Dianova II, Finch D, Tait PS, Strom CE, Helleday T, Dianov GL. XRCC1 phosphorylation by CK2 is required for its stability and efficient DNA repair. DNA repair. 2010;9:835–841. doi: 10.1016/j.dnarep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Pascal JM, O'Brien PJ, Tomkinson AE, Ellenberger T. Human DNA ligase I completely encircles and partially unwinds nicked DNA. Nature. 2004;432:473–478. doi: 10.1038/nature03082. [DOI] [PubMed] [Google Scholar]
- Patel N, Atack JM, Finger LD, Exell JC, Thompson P, Tsutakawa S, Tainer JA, Williams DM, Grasby JA. Flap endonucleases pass 5'-flaps through a flexible arch using a disorder-thread-order mechanism to confer specificity for free 5'-ends. Nucleic acids research. 2012;40:4507–4519. doi: 10.1093/nar/gks051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull TT, Gellert M. The 3' to 5' exonuclease activity of Mre11 facilitates repair of DNA double-strand breaks. Mol Cell. 1998;1:969–979. doi: 10.1016/s1097-2765(00)80097-0. [DOI] [PubMed] [Google Scholar]
- Pelletier H, Sawaya MR, Kumar A, Wilson SH, Kraut J. Structures of ternary complexes of rat DNA polymerase beta, a DNA template-primer, and ddCTP. Science. 1994;264:1891–1903. [PubMed] [Google Scholar]
- Pelletier H, Sawaya MR, Wolfle W, Wilson SH, Kraut J. Crystal structures of human DNA polymerase beta complexed with DNA: implications for catalytic mechanism, processivity, and fidelity. Biochemistry. 1996;35:12742–12761. doi: 10.1021/bi952955d. [DOI] [PubMed] [Google Scholar]
- Pheiffer BH, Zimmerman SB. 3'-Phosphatase activity of the DNA kinase from rat liver. Biochem Biophys Res Commun. 1982;109:1297–1302. doi: 10.1016/0006-291x(82)91918-0. [DOI] [PubMed] [Google Scholar]
- Piersen CE, Prasad R, Wilson SH, Lloyd RS. Evidence for an imino intermediate in the DNA polymerase beta deoxyribose phosphate excision reaction. The Journal of biological chemistry. 1996;271:17811–17815. doi: 10.1074/jbc.271.30.17811. [DOI] [PubMed] [Google Scholar]
- Plo I, Liao ZY, Barcelo JM, Kohlhagen G, Caldecott KW, Weinfeld M, Pommier Y. Association of XRCC1 and tyrosyl DNA phosphodiesterase (Tdp1) for the repair of topoisomerase I-mediated DNA lesions. DNA repair. 2003;2:1087–1100. doi: 10.1016/s1568-7864(03)00116-2. [DOI] [PubMed] [Google Scholar]
- Pommier Y. DNA topoisomerase I inhibitors: chemistry, biology, and interfacial inhibition. Chemical reviews. 2009;109:2894–2902. doi: 10.1021/cr900097c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier Y. Drugging topoisomerases: lessons and challenges. ACS chemical biology. 2013;8:82–95. doi: 10.1021/cb300648v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourquier P, Pilon AA, Kohlhagen G, Mazumder A, Sharma A, Pommier Y. Trapping of mammalian topoisomerase I and recombinations induced by damaged DNA containing nicks or gaps. Importance of DNA end phosphorylation and camptothecin effects. The Journal of biological chemistry. 1997;272:26441–26447. doi: 10.1074/jbc.272.42.26441. [DOI] [PubMed] [Google Scholar]
- Pourquier P, Ueng LM, Fertala J, Wang D, Park HJ, Essigmann JM, Bjornsti MA, Pommier Y. Induction of reversible complexes between eukaryotic DNA topoisomerase I and DNA-containing oxidative base damages. 7, 8-dihydro-8-oxoguanine and 5-hydroxycytosine. The Journal of biological chemistry. 1999;274:8516–8523. doi: 10.1074/jbc.274.13.8516. [DOI] [PubMed] [Google Scholar]
- Pourquier P, Ueng LM, Kohlhagen G, Mazumder A, Gupta M, Kohn KW, Pommier Y. Effects of uracil incorporation, DNA mismatches, and abasic sites on cleavage and religation activities of mammalian topoisomerase I. The Journal of biological chemistry. 1997;272:7792–7796. doi: 10.1074/jbc.272.12.7792. [DOI] [PubMed] [Google Scholar]
- Povirk LF. Processing of damaged DNA ends for double-strand break repair in mammalian cells. ISRN molecular biology 2012. 2012 doi: 10.5402/2012/345805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad R, Batra VK, Yang XP, Krahn JM, Pedersen LC, Beard WA, Wilson SH. Structural insight into the DNA polymerase beta deoxyribose phosphate lyase mechanism. DNA repair. 2005;4:1347–1357. doi: 10.1016/j.dnarep.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Prasad R, Beard WA, Chyan JY, Maciejewski MW, Mullen GP, Wilson SH. Functional analysis of the amino-terminal 8-kDa domain of DNA polymerase beta as revealed by site-directed mutagenesis. DNA binding and 5'-deoxyribose phosphate lyase activities. The Journal of biological chemistry. 1998;273:11121–11126. doi: 10.1074/jbc.273.18.11121. [DOI] [PubMed] [Google Scholar]
- Prasad R, Beard WA, Strauss PR, Wilson SH. Human DNA polymerase beta deoxyribose phosphate lyase. Substrate specificity and catalytic mechanism. The Journal of biological chemistry. 1998;273:15263–15270. doi: 10.1074/jbc.273.24.15263. [DOI] [PubMed] [Google Scholar]
- Prasad R, Beard WA, Wilson SH. Studies of gapped DNA substrate binding by mammalian DNA polymerase beta. Dependence on 5'-phosphate group. The Journal of biological chemistry. 1994;269:18096–18101. [PubMed] [Google Scholar]
- Prasad R, Shock DD, Beard WA, Wilson SH. Substrate channeling in mammalian base excision repair pathways: passing the baton. The Journal of biological chemistry. 2010;285:40479–40488. doi: 10.1074/jbc.M110.155267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quennet V, Beucher A, Barton O, Takeda S, Lobrich M. CtIP and MRN promote non-homologous end-joining of etoposide-induced DNA double-strand breaks in G1. Nucleic acids research. 2011;39:2144–2152. doi: 10.1093/nar/gkq1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinzii CM, Kattah AG, Naini A, Akman HO, Mootha VK, DiMauro S, Hirano M. Coenzyme Q deficiency and cerebellar ataxia associated with an aprataxin mutation. Neurology. 2005;64:539–541. doi: 10.1212/01.WNL.0000150588.75281.58. [DOI] [PubMed] [Google Scholar]
- Qvist P, Huertas P, Jimeno S, Nyegaard M, Hassan MJ, Jackson SP, Borglum AD. CtIP Mutations Cause Seckel and Jawad Syndromes. PLoS Genet. 2011;7:e1002310. doi: 10.1371/journal.pgen.1002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rass U, Ahel I, West SC. Actions of aprataxin in multiple DNA repair pathways. J Biol Chem. 2007;282:9469–9474. doi: 10.1074/jbc.M611489200. [DOI] [PubMed] [Google Scholar]
- Rass U, Ahel I, West SC. Molecular mechanism of DNA deadenylation by the neurological disease protein aprataxin. J Biol Chem. 2008;283:33994–34001. doi: 10.1074/jbc.M807124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rass U, Ahel I, West SC. Molecular mechanism of DNA deadenylation by the neurological disease protein aprataxin. J Biol Chem. 2008 doi: 10.1074/jbc.M807124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond AC, Rideout MC, Staker B, Hjerrild K, Burgin AB., Jr Analysis of human tyrosyl-DNA phosphodiesterase I catalytic residues. Journal of molecular biology. 2004;338:895–906. doi: 10.1016/j.jmb.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Reijns MA, Rabe B, Rigby RE, Mill P, Astell KR, Lettice LA, Boyle S, Leitch A, Keighren M, Kilanowski F, et al. Enzymatic removal of ribonucleotides from DNA is essential for mammalian genome integrity and development. Cell. 2012;149:1008–1022. doi: 10.1016/j.cell.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JJ, El-Khamisy SF, Katyal S, Clements P, McKinnon PJ, Caldecott KW. Defective DNA ligation during short-patch single-strand break repair in ataxia oculomotor apraxia 1. Mol Cell Biol. 2009;29:1354–1362. doi: 10.1128/MCB.01471-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JJ, Walker AK, Gilmore EC, Walsh CA, Caldecott KW. Impact of PNKP mutations associated with microcephaly, seizures and developmental delay on enzyme activity and DNA strand break repair. Nucleic acids research. 2012;40:6608–6619. doi: 10.1093/nar/gks318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts KP, Sobrino JA, Payton J, Mason LB, Turesky RJ. Determination of apurinic/apyrimidinic lesions in DNA with high-performance liquid chromatography and tandem mass spectrometry. Chemical research in toxicology. 2006;19:300–309. doi: 10.1021/tx0502589. [DOI] [PubMed] [Google Scholar]
- Roberts SA, Strande N, Burkhalter MD, Strom C, Havener JM, Hasty P, Ramsden DA. Ku is a 5'-dRP/AP lyase that excises nucleotide damage near broken ends. Nature. 2010;464:1214–1217. doi: 10.1038/nature08926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca J, Wang JC. DNA transport by a type II DNA topoisomerase: evidence in favor of a two-gate mechanism. Cell. 1994;77:609–616. doi: 10.1016/0092-8674(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Ropars V, Drevet P, Legrand P, Baconnais S, Amram J, Faure G, Marquez JA, Pietrement O, Guerois R, Callebaut I, et al. Structural characterization of filaments formed by human Xrcc4-Cernunnos/XLF complex involved in nonhomologous DNA end-joining. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:12663–12668. doi: 10.1073/pnas.1100758108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg M, Kohli J, Ludin K. Ctp1 and the MRN-complex are required for endonucleolytic Rec12 removal with release of a single class of oligonucleotides in fission yeast. PLoS genetics. 2009;5:e1000722. doi: 10.1371/journal.pgen.1000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe TC, Tewey KM, Liu LF. Identification of the breakage-reunion subunit of T4 DNA topoisomerase. The Journal of biological chemistry. 1984;259:9177–9181. [PubMed] [Google Scholar]
- Rulten SL, Caldecott KW. DNA strand break repair and neurodegeneration. DNA repair. 2013;12:558–567. doi: 10.1016/j.dnarep.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Sartori AA, Lukas C, Coates J, Mistrik M, Fu S, Bartek J, Baer R, Lukas J, Jackson SP. Human CtIP promotes DNA end resection. Nature. 2007;450:509–514. doi: 10.1038/nature06337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg MJ, Appel CD, Adhikari S, Robertson PD, Ramsden DA, Williams RS. Mechanism of repair of 5'-topoisomerase II-DNA adducts by mammalian tyrosyl-DNA phosphodiesterase 2. Nature structural & molecular biology. 2012;19:1363–1371. doi: 10.1038/nsmb.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg MJ, Williams RS. DNA end processing by polynucleotide kinase/phosphatase. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:20855–20856. doi: 10.1073/pnas.1118214109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi J, Shuman S. Site-specific ribonuclease activity of eukaryotic DNA topoisomerase I. Molecular cell. 1997;1:89–97. doi: 10.1016/s1097-2765(00)80010-6. [DOI] [PubMed] [Google Scholar]
- Shen J, Gilmore EC, Marshall CA, Haddadin M, Reynolds JJ, Eyaid W, Bodell A, Barry B, Gleason D, Allen K, et al. Mutations in PNKP cause microcephaly, seizures and defects in DNA repair. Nat Genet. 2010;42:245–249. doi: 10.1038/ng.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi K, Kurahashi K, Gao R, Tsutakawa SE, Tainer JA, Pommier Y, Aihara H. Structural basis for recognition of 5'-phosphotyrosine adducts by Tdp2. Nature structural & molecular biology. 2012;19:1372–1377. doi: 10.1038/nsmb.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller CB, Lammens K, Guerini I, Coordes B, Feldmann H, Schlauderer F, Mockel C, Schele A, Strasser K, Jackson SP, et al. Structure of Mre11-Nbs1 complex yields insights into ataxia-telangiectasia-like disease mutations and DNA damage signaling. Nature structural & molecular biology. 2012;19:693–700. doi: 10.1038/nsmb.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuman S. Polynucleotide ligase activity of eukaryotic topoisomerase I. Molecular cell. 1998;1:741–748. doi: 10.1016/s1097-2765(00)80073-8. [DOI] [PubMed] [Google Scholar]
- Sibanda BL, Critchlow SE, Begun J, Pei XY, Jackson SP, Blundell TL, Pellegrini L. Crystal structure of an Xrcc4-DNA ligase IV complex. Nature structural biology. 2001;8:1015–1019. doi: 10.1038/nsb725. [DOI] [PubMed] [Google Scholar]
- Sparks JL, Chon H, Cerritelli SM, Kunkel TA, Johansson E, Crouch RJ, Burgers PM. RNase H2-initiated ribonucleotide excision repair. Molecular cell. 2012;47:980–986. doi: 10.1016/j.molcel.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart GS, Maser RS, Stankovic T, Bressan DA, Kaplan MI, Jaspers NG, Raams A, Byrd PJ, Petrini JH, Taylor AM. The DNA double-strand break repair gene hMRE11 is mutated in individuals with an ataxia-telangiectasia-like disorder. Cell. 1999;99:577–587. doi: 10.1016/s0092-8674(00)81547-0. [DOI] [PubMed] [Google Scholar]
- Strande N, Roberts SA, Oh S, Hendrickson EA, Ramsden DA. Specificity of the dRP/AP lyase of Ku promotes nonhomologous end joining (NHEJ) fidelity at damaged ends. The Journal of biological chemistry. 2012;287:13686–13693. doi: 10.1074/jbc.M111.329730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykora P, Croteau DL, Bohr VA, Wilson DM., 3rd Aprataxin localizes to mitochondria and preserves mitochondrial function. Proc Natl Acad Sci U S A. 2011;108:7437–7442. doi: 10.1073/pnas.1100084108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykora P, Wilson DM, 3rd, Bohr VA. Base excision repair in the mammalian brain: implication for age related neurodegeneration. Mechanisms of ageing and development. 2013;134:440–448. doi: 10.1016/j.mad.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima H, Boerkoel CF, John J, Saifi GM, Salih MA, Armstrong D, Mao Y, Quiocho FA, Roa BB, Nakagawa M, et al. Mutation of TDP1, encoding a topoisomerase I-dependent DNA damage repair enzyme, in spinocerebellar ataxia with axonal neuropathy. Nature genetics. 2002;32:267–272. doi: 10.1038/ng987. [DOI] [PubMed] [Google Scholar]
- Tomkinson AE, Vijayakumar S, Pascal JM, Ellenberger T. DNA ligases: structure, reaction mechanism, and function. Chem Rev. 2006;106:687–699. doi: 10.1021/cr040498d. [DOI] [PubMed] [Google Scholar]
- Trujillo KM, Yuan SS, Lee EY, Sung P. Nuclease activities in a complex of human recombination and DNA repair factors Rad50, Mre11, and p95. J Biol Chem. 1998;273:21447–21450. doi: 10.1074/jbc.273.34.21447. [DOI] [PubMed] [Google Scholar]
- Tsutakawa SE, Classen S, Chapados BR, Arvai AS, Finger LD, Guenther G, Tomlinson CG, Thompson P, Sarker AH, Shen B, et al. Human flap endonuclease structures, DNA double-base flipping, and a unified understanding of the FEN1 superfamily. Cell. 2011;145:198–211. doi: 10.1016/j.cell.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbale P, Appel CD, Kraehenbuehl R, Robertson PD, Williams JS, Krahn J, Ahel I, Williams RS. Structure of an aprataxin-DNA complex with insights into AOA1 neurodegenerative disease. Nat Struct Mol Biol. 2011;18:1189–1195. doi: 10.1038/nsmb.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumbale P, Williams JS, Schellenberg MJ, Kunkel TA, Williams RS. Aprataxin resolves adenylated RNA-DNA junctions to maintain genome integrity. Nature. 2014;506:111–115. doi: 10.1038/nature12824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unk I, Haracska L, Johnson RE, Prakash S, Prakash L. Apurinic endonuclease activity of yeast Apn2 protein. The Journal of biological chemistry. 2000;275:22427–22434. doi: 10.1074/jbc.M002845200. [DOI] [PubMed] [Google Scholar]
- Unk I, Haracska L, Prakash S, Prakash L. 3'-phosphodiesterase and 3'-->5' exonuclease activities of yeast Apn2 protein and requirement of these activities for repair of oxidative DNA damage. Molecular and Cellular Biology. 2001;21:1656–1661. doi: 10.1128/MCB.21.5.1656-1661.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgen-Slane R, Rozovics JM, Fitzgerald KD, Ngo T, Chou W, van der Heden van Noort GJ, Filippov DV, Gershon PD, Semler BL. An RNA virus hijacks an incognito function of a DNA repair enzyme. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:14634–14639. doi: 10.1073/pnas.1208096109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JR, Corpina RA, Goldberg J. Structure of the Ku heterodimer bound to DNA and its implications for double-strand break repair. Nature. 2001;412:607–614. doi: 10.1038/35088000. [DOI] [PubMed] [Google Scholar]
- Wallace SS. DNA glycosylases search for and remove oxidized DNA bases. Environmental and molecular mutagenesis. 2013;54:691–704. doi: 10.1002/em.21820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinfeld M, Mani RS, Abdou I, Aceytuno RD, Glover JN. Tidying up loose ends: the role of polynucleotide kinase/phosphatase in DNA strand break repair. Trends Biochem Sci. 2011;36:262–271. doi: 10.1016/j.tibs.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmoreland JW, Resnick MA. Coincident resection at both ends of random, gamma-induced double-strand breaks requires MRX (MRN), Sae2 (Ctp1), and Mre11-nuclease. PLoS genetics. 2013;9:e1003420. doi: 10.1371/journal.pgen.1003420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse CJ, Taylor RM, Thistlethwaite A, Zhang H, Karimi-Busheri F, Lasko DD, Weinfeld M, Caldecott KW. XRCC1 stimulates human polynucleotide kinase activity at damaged DNA termini and accelerates DNA single-strand break repair. Cell. 2001;104:107–117. doi: 10.1016/s0092-8674(01)00195-7. [DOI] [PubMed] [Google Scholar]
- Wiederhold L, Leppard JB, Kedar P, Karimi-Busheri F, Rasouli-Nia A, Weinfeld M, Tomkinson AE, Izumi T, Prasad R, Wilson SH, et al. AP endonuclease-independent DNA base excision repair in human cells. Molecular Cell. 2004;15:209–220. doi: 10.1016/j.molcel.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Williams GJ, Williams RS, Williams JS, Moncalian G, Arvai AS, Limbo O, Guenther G, SilDas S, Hammel M, Russell P, et al. ABC ATPase signature helices in Rad50 link nucleotide state to Mre11 interface for DNA repair. Nature structural & molecular biology. 2011;18:423–431. doi: 10.1038/nsmb.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JS, Smith DJ, Marjavaara L, Lujan SA, Chabes A, Kunkel TA. Topoisomerase 1-mediated removal of ribonucleotides from nascent leading-strand DNA. Molecular cell. 2013;49:1010–1015. doi: 10.1016/j.molcel.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RS, Dodson GE, Limbo O, Yamada Y, Williams JS, Guenther G, Classen S, Glover JN, Iwasaki H, Russell P, et al. Nbs1 flexibly tethers Ctp1 and Mre11-Rad50 to coordinate DNA double-strand break processing and repair. Cell. 2009;139:87–99. doi: 10.1016/j.cell.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RS, Green R, Glover JN. Crystal structure of the BRCT repeat region from the breast cancer-associated protein BRCA1. Nat Struct Biol. 2001;8:838–842. doi: 10.1038/nsb1001-838. [DOI] [PubMed] [Google Scholar]
- Williams RS, Kunkel TA. FEN nucleases: bind, bend, fray, cut. Cell. 2011;145:171–172. doi: 10.1016/j.cell.2011.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RS, Lee MS, Hau DD, Glover JN. Structural basis of phosphopeptide recognition by the BRCT domain of BRCA1. Nat Struct Mol Biol. 2004;11:519–525. doi: 10.1038/nsmb776. [DOI] [PubMed] [Google Scholar]
- Williams RS, Moncalian G, Williams JS, Yamada Y, Limbo O, Shin DS, Groocock LM, Cahill D, Hitomi C, Guenther G, et al. Mre11 dimers coordinate DNA end bridging and nuclease processing in double-strand-break repair. Cell. 2008;135:97–109. doi: 10.1016/j.cell.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams RS, Tainer JA. A Nanomachine for Making Ends Meet: MRN Is a Flexing Scaffold for the Repair of DNA Double-Strand Breaks. Mol Cell. 2005;19:724–726. doi: 10.1016/j.molcel.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Williams RS, Williams JS, Tainer JA. Mre11-Rad50-Nbs1 is a keystone complex connecting DNA repair machinery, double-strand break signaling, and the chromatin template. Biochem Cell Biol. 2007;85:509–520. doi: 10.1139/O07-069. [DOI] [PubMed] [Google Scholar]
- Willis J, Patel Y, Lentz BL, Yan S. APE2 is required for ATR-Chk1 checkpoint activation in response to oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:10592–10597. doi: 10.1073/pnas.1301445110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DM., 3rd Properties of and substrate determinants for the exonuclease activity of human apurinic endonuclease Ape1. Journal of molecular biology. 2003;330:1027–1037. doi: 10.1016/s0022-2836(03)00712-5. [DOI] [PubMed] [Google Scholar]
- Wilson DM, 3rd, Barsky D. The major human abasic endonuclease: formation, consequences and repair of abasic lesions in DNA. Mutation research. 2001;485:283–307. doi: 10.1016/s0921-8777(01)00063-5. [DOI] [PubMed] [Google Scholar]
- Wilson SH, Kunkel TA. Passing the baton in base excision repair. Nat Struct Biol. 2000;7:176–178. doi: 10.1038/73260. [DOI] [PubMed] [Google Scholar]
- Wilstermann AM, Osheroff N. Base excision repair intermediates as topoisomerase II poisons. J Biol Chem. 2001;276:46290–46296. doi: 10.1074/jbc.M105733200. [DOI] [PubMed] [Google Scholar]
- Wong RS, Sczepanski JT, Greenberg MM. Excision of a lyase-resistant oxidized abasic lesion from DNA. Chemical research in toxicology. 2010;23:766–770. doi: 10.1021/tx9003984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PY, Frit P, Meesala S, Dauvillier S, Modesti M, Andres SN, Huang Y, Sekiguchi J, Calsou P, Salles B, et al. Structural and functional interaction between the human DNA repair proteins DNA ligase IV and XRCC4. Molecular and cellular biology. 2009;29:3163–3172. doi: 10.1128/MCB.01895-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Ochi T, Matak-Vinkovic D, Robinson CV, Chirgadze DY, Blundell TL. Non-homologous end-joining partners in a helical dance: structural studies of XLF-XRCC4 interactions. Biochemical Society transactions. 2011;39(suppl):1387–1392. doi: 10.1042/BST0391387. 1382 p following 1392. [DOI] [PubMed] [Google Scholar]
- Yang MY, Bowmaker M, Reyes A, Vergani L, Angeli P, Gringeri E, Jacobs HT, Holt IJ. Biased incorporation of ribonucleotides on the mitochondrial L-strand accounts for apparent strand-asymmetric DNA replication. Cell. 2002;111:495–505. doi: 10.1016/s0092-8674(02)01075-9. [DOI] [PubMed] [Google Scholar]
- Yang SW, Burgin AB, Jr, Huizenga BN, Robertson CA, Yao KC, Nash HA. A eukaryotic enzyme that can disjoin dead-end covalent complexes between DNA and type I topoisomerases. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:11534–11539. doi: 10.1073/pnas.93.21.11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Z, Cortes-Ledesma F, El Khamisy SF, Caldecott KW. TDP2/TTRAP is the major 5'-tyrosyl DNA phosphodiesterase activity in vertebrate cells and is critical for cellular resistance to topoisomerase II-induced DNA damage. J Biol Chem. 286:403–409. doi: 10.1074/jbc.M110.181016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Morera S, Bates PA, Whitehead PC, Coffer AI, Hainbucher K, Nash RA, Sternberg MJ, Lindahl T, Freemont PS. Structure of an XRCC1 BRCT domain: a new protein-protein interaction module. Embo J. 1998;17:6404–6411. doi: 10.1093/emboj/17.21.6404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Jasin M. An essential role for CtIP in chromosomal translocation formation through an alternative end-joining pathway. Nat Struct Mol Biol. 2011;18:80–84. doi: 10.1038/nsmb.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Chung WH, Shim EY, Lee SE, Ira G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134:981–994. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]