SUMMARY
Screening mammography is recognized as an imperfect imaging tool that performs poorly in women with dense breast tissue – a limitation which has driven demand for supplemental screening techniques. One potential supplemental technique is molecular breast imaging (MBI). Significant improvements in gamma camera technology allow MBI to be performed at low radiation doses, comparable with those of tomosynthesis and mammography. A recent screening trial in women with dense breast tissue yielded a cancer detection rate of 3.2 per 1000 for mammography alone and 12.0 per 1000 for the combination of mammography and MBI. MBI also demonstrated a lower recall rate than that of mammography. MBI is a promising supplemental screening technique in women with dense breast tissue.
Keywords: dense breast, mammography, molecular breast imaging, nuclear medicine, radiation dose, screening, Tc-99m sestamibi
The need for supplemental screening techniques
Breast cancer screening programs were established in the USA and some European countries in the early 1980s, and evidence from both randomized trials and observational studies has shown that screening mammography in women aged 40–70 years has contributed to a substantial reduction in breast cancer mortality [1–3]. Despite the success of screening mammography, it is recognized as an imperfect imaging tool that has come under strong criticism in recent years for a variety of reasons discussed below [4,5].
Mammography is known to underperform in some women, notable those with dense breast tissue [6]. The transition from analog-to-digital mammography and the addition of computer aided diagnosis has only marginally improved the performance of mammography in this population, although it has led to a significant increase in the overall cost of breast cancer screening [7]. The limitations of mammography in the dense breast population are the primary driver for much of the legislation that has already passed in 19 states and are the focus of a federal bill mandating that women with dense breast tissue be informed that mammography has a limited ability to detect breast lesions in that population [8].
While the incidence of early-stage breast cancer has increased since the advent of screening mammography, there has been no decrease in the incidence of regional and metastatic disease at diagnosis, suggesting that much of the increased detection represents overdiagnosis [4]. The implication being that mammography results in the detection of indolent cancers that will never progress to become a lethal disease if left untreated, and yet has failed to detect cancers that impact survival. However in order to save lives, cancers need to be found at an earlier, more treatable stage, so early detection of small cancers is very important. To solve this conundrum, we need to focus on screening technologies that can detect cancers with the biological and functional signatures that indicate likelihood to progress to aggressive disease [7].
Currently, the focus is on tomosynthesis as the next step to resolving the problems described above. Recent studies indicate that it will provide a measurable but small improvement in sensitivity and specificity [9] but is unlikely to solve the problems associated with breast density. There is also the issue of cost – mammography went from analog to digital with only a nominal increase in sensitivity (for certain subgroups), but with a to complete factor of 4–5 increase in the cost of the technology and a 50% increase in Medicare costs [10,11]. Tomosynthesis has recently been introduced at an additional equipment cost approximately 50% that of digital mammography, and with a strong recommendation from the American College of Radiology that it be reimbursed [12], yet there is minimal comparative effectiveness evidence that the benefits of tomosynthesis outperform digital mammography in a clinically important way [13].
Mammography remains the primary screening tool for breast cancer despite the fact that there are supplemental modalities that appear to perform significantly better [13]. By definition a supplemental technique is something added to complete the primary technique and should offer a small incremental improvement in either sensitivity/specificity or both. If a ‘supplemental’ technique is considerably better than the primary technique then it should no longer be considered supplemental, but rather as a replacement technique. In this article, we discuss one supplemental technique that appears to offer a significant improvement in tumor detection compared with mammography. Is it the ‘best’ technique? – probably not, but it is one of several technologies that may rival or exceed mammography for cancer detection. We have moved from analog mammography through digital mammography to tomosynthesis with only small percentage gains – it may be time to seriously consider alternative technologies as the primary tools for early detection of breast cancer, particularly in women with dense breast tissue.
Molecular breast imaging (MBI) is one of several promising supplemental techniques, along with other technologies such as positron emission mammography [14,15], ultra-fast MRI [16] and contrast enhanced CT [17] that may be viable alternatives to mammography. In considering what qualifies as a suitable supplemental technique, there are a number of criteria that are highly desirable. It needs to be easy to disseminate widely, it must allow for rapid interpretation by the radiologist, it must have high patient acceptance, and it must complement mammography by performing well in areas where mammography is known to perform poorly. MBI is one technique that appears to meet the above criteria and in the review below we discuss the strengths and limitations of this technology and the reasons why it merits consideration as an important imaging tool for women with dense breast tissue.
Historical background to breast imaging in nuclear medicine
While a number of different radiopharmaceuticals were known to demonstrate uptake in breast cancer [18], it was not until the availability of Tc-99m sestamibi in the early 1980s that breast imaging in nuclear medicine received serious attention. Tc-99m sestamibi was developed as a cardiac imaging agent and very rapidly replaced Tl-201 in nuclear cardiology [19]. Shortly after its introduction, it was noted that sestamibi also localized in several types of tumors, primarily parathyroid adenomas, lung cancer and breast cancer. The first report of avid Tc-99m sestamibi uptake in breast cancer was by Aktolun et al. in 1992 [20]. Another new cardiac imaging agent, Tc-99m tetrofosmin, with properties very similar to Tc-99m sestamibi, was also reported to have avid uptake in breast tumors [21]. These serendipitous discoveries of radiopharmaceutical uptake in breast cancer led to the development of scintimammography, the name given to nuclear medicine breast imaging performed with conventional scintillating gamma cameras. Several large multi-center trials, along with a meta-analysis of scintimammography literature performed prior to 1999 reported an overall sensitivity between 71 and 93%. However this dropped to 40–61% when only nonpalpable masses were considered [22,23], leading to the overall conclusion that scintimammography was not able to provide reliable detection of nonpalpable, small breast tumors [24,25]. This decreased sensitivity for small breast tumors was attributed to the limited resolving power of conventional gamma cameras [26]. At the time, investigators also suspected that Tc-99m sestamibi had poor uptake in some breast cancers [26,27]. As a result, breast imaging with nuclear medicine was largely abandoned by the late 1990s. At the same time, however, researchers were just beginning to explore the potential of new dedicated nuclear systems that offered significantly improved detection of small breast lesions [28].
About 15 years ago, the first compact gamma camera system optimized for breast imaging became commercially available. Known as breast-specific gamma imaging (BSGI), this technology achieved a substantial improvement in spatial resolution by enabling the gamma camera to be positioned close to the breast in a manner analogous to mammography. Technical factors limited pixel size in these systems to greater than 3 mm and these systems had poorer energy resolution than conventional gamma cameras [29–31] which degraded image contrast. However, because of the greatly reduced lesion-to-detector distance, these systems achieved a substantial improvement in the sensitivity for the detection of small breast cancers (<10 mm), with reported sensitivities ranging from 67 to 87% [26,32].
Over the last 10 years, a new generation of dedicated breast imaging systems has emerged based on solid-state detectors that utilize materials such as cadmium zinc telluride. These detectors provide better energy resolution and smaller pixel sizes than the earlier BSGI systems [33,34]. Usually referred to as MBI systems, these employ two opposing small cadmium zinc telluride detectors in a dual-head configuration. This allows the breast to be lightly compressed between the two detectors as with BSGI. MBI systems can achieve an intrinsic resolution down to 1.6 mm, with the possibility of even finer resolution in the future [35]. While a dual-head configuration is more expensive than a single-head system, this arrangement has the advantage of ensuring that a breast lesion can never be more than half of the breast thickness from either detector. This results in improved sensitivity for the detection of small breast tumors, particularly those located in the upper inner quadrant of the breast [36], with reported sensitivities of 82–91% for tumors less than 10 mm in size [36,37].
Radiation dose concerns
Concern about the radiation risk associated with nuclear medicine breast imaging is one of the primary factors that have limited its clinical adoption. The breast is considered one of the most radiosensitive organs in the body [38]. Hence there will always be considerable scrutiny of any breast imaging procedure that involves ionizing radiation. Numerous reports have documented the very low risk of any harmful effects associated with radiation received from mammography, and that risk is even lower with today’s digital mammography detectors [39]. Hence, in order to compete against mammography with respect to radiation dose, various technological enhancements and dose reduction strategies have been implemented that allow MBI to be performed at radiation doses comparable with mammography.
Scintimammography initially employed doses of Tc-99m sestamibi in the range of 740–1110 MBq resulting in an estimated absorbed dose to breast tissue of up to 2 mGy. While this is less than the mean glandular dose to the breast with digital mammography (~4 mGy), most of the radiation burden from Tc-99m sestamibi is to organs other than the breast, mainly the upper and lower intestines. Hence it is more appropriate to utilize effective dose when comparing the two modalities. Using an administered dose of 1110 MBq, the comparable effective dose to a patient from Tc-99m sestamibi is 8.1 mSv [40]. By comparison, the effective dose from digital mammography is 0.5 mSv and that from mammography combined with digital breast tomosynthesis is 1.2 mSv – a factor of approximately seven-times less [41,42].
With the development of BSGI systems, image quality was improved but the administered dose of Tc-99m sestamibi remained comparable to that employed by conventional scintimammography [26]. Considerable work has been done over the last few years to improve the sensitivity of MBI through innovations in the technology and through optimization of patient preparation [43–45]. This has allowed the administered dose of Tc-99m sestamibi to be reduced to approximately 150 MBq, and promising work with some of the newer radiopharmaceuticals may yield an additional factor of 2–3 reduction [46]. This would bring the effective dose down below that of digital mammography, which is generally considered to deliver an effective dose of approximately 0.5 mSv [41]. Administered doses of approximately 150 MBq Tc-99m sestamibi result in a breast dose of 0.25 mGy and an effective dose of approximately 1.1 mSv to the body. At these dose levels, it is now reasonable to consider the incorporation of MBI into a screening regime. Note: the US FDA Mammography Quality Standards Act and Program requires that a single mammographic exposure not exceed 3 mGy, equivalent to an effective dose of 1.4 mSv for a standard two-view mammogram [47].
It is helpful to note that despite radiation scare articles that occasionally appear in the scientific press [48–50], the major scientific organizations that oversee radiation protection have provided a consistent message that speculative estimates of radiation induced mortality at doses at or below worldwide background levels (2–10 mSv) using hypothetical models with extrapolation from high dose studies is both unsound science and inappropriate use of these risk models [51–54]. The risk of breast cancer is real (one in eight women will develop breast cancer over their lives) and the radiation doses associated with mammography and tomosynthesis have been determined to be of very low risk compared with the expected mortality reductions achievable through mammographic screening. The hypothetical deaths from background radiation are more than 50-times greater than those from the cumulative effects of 40 years of screening mammography [42]. With improvements in instrumentation and better radiopharmaceuticals, we believe that nuclear medicine procedures are capable of achieving comparable or lower effective doses than that from mammography, thereby minimizing the impact of radiation concerns as a barrier to widespread application of nuclear medicine technologies in breast imaging.
Evaluation of MBI as a supplemental screening technique
The first screening trial evaluating MBI in women with dense breast tissue was reported by Rhodes et al. in 2011 [55]. This single-center trial compared the efficacy of MBI and screening mammography in 936 asymptomatic women with dense breasts and additional risk factors for breast cancer. Results showed that the addition of a one-time or prevalence screen MBI, performed using 740 MBq Tc-99m sestamibi, to incident screening mammography significantly increased diagnostic yield from 3.2 per 1000 with mammography alone to 10.7 per 1000 with the combination of tests (p = 0.016). Although the specificity of incident mammography and prevalent MBI used independently was similar (91 vs 93%, respectively), the specificity of the combination of techniques was reduced to 85% (p < 0.001 when compared with mammography alone).
The major limitation of this pilot study was the high administered dose of Tc-99m sestamibi, and it raised the valid question as to whether or not the technology would perform in a similar manner at lower doses. Following implementation of many of the technical improvements to MBI mentioned above, a second large screening trial has now been completed using a lower administered dose of approximately 240 MBq Tc-99m sestamibi [56]. This trial again recruited an asymptomatic population of women with dense breast tissue, although it differed slightly from the first trial in that no other risk factors were considered for entry into the trial. A total of 1587 women were successfully screened with MBI and followed up for 1 year in order to determine a reference standard comparable to that used in similar trials such as ACRIN 6666 [57]. Again the cancer detection rate per 1000 screened was 3.2 (95% CI: 1.3–7.4) for mammography alone and 12.0 (95% CI: 7.7–18.6; p < 0.001) for the combination of mammography and MBI, with a supplemental yield of 8.8 (95% CI: 4.3–13.3) – results that were almost identical to those reported in the first trial [50]. The specificity of incident mammography and prevalent MBI used independently was again very similar (89 vs 94%, respectively). The specificity of mammography with adjunct MBI was 83.4% (p < 0.001 when compared with mammography alone). We would anticipate that with repeated MBI screening, the specificity would improve, as some of the more common false positives seen with MBI, such as uptake in fibroadenomas or intramammary lymph nodes, can be ruled out when a prior MBI can be referenced.
Comparison between the results of these two trials and those with other supplemental screening techniques is difficult as the reference population differs in terms of risk factors, breast density, and so on. Nevertheless, with this caveat, Table 1 shows the supplemental yield from the MBI study described above and recent studies on handheld ultrasound [57], MRI [58], tomosynthesis [9,58], and most recently, automated ultrasound [59]. Supplemental yield with MBI is higher than that with either ultrasound or tomosynthesis and is approaching the level seen with contrast-enhanced breast MRI. MBI offers a significant advantage over ultrasound as it can be interpreted rapidly and requires minimal radiologist training to achieve substantial interobserver agreement for BI-RADS final assessment category [60,61]. Figure 1 shows examples of the complete image sets in a normal study and in two cases of invasive carcinoma. The small number of images (typically eight) and compact datasets, should allow this technology to be rapidly disseminated into community practices without requiring the investment in data storage and network band-width required of competing technologies such as tomosynthesis and contrast-enhanced breast MRI. The lower cost of the MBI technology and its small footprint provide additional advantages of MBI over contrast-enhanced breast MRI in this type of practice.
Table 1.
Comparison of the cancer yield (cancers detected/1000 women screened) for mammography and various adjunct screening techniques (ultrasound, MRI, tomosynthesis and molecular breast imaging).
| Supplemental screening modality | Cancers detected (n) per 1000 women screened |
Cancers detected (n) per 1000 women screened + adjunct |
Supplemental yield† |
Increase in cancers detected (%) |
Ref. |
|---|---|---|---|---|---|
| Ultrasound ACRIN 6666 year 1 dense breast + additional risk |
7.5 | 12.8 | 5.3 | 71 | [57] |
| Ultrasound ACRIN 6666 year 2, 3 dense breast + additional risk |
8.1 | 11.8 | 3.7 | 46 | [62] |
| MRI ACRIN 6666 year 3 dense breast + additional risk |
8.2 | 26.1 | 17.9 | 220 | [62] |
| Tomosynthesis (Skaane) age 50–69 years all densities |
6.1 | 8.0 | 1.9 | 31 | [9] |
| Tomosynthesis (Ciatto) age ≥48 dense subset |
4.1 | 6.6 | 2.5 | 61 | [58] |
| Automated ultrasound (Brem) dense breasts |
5.4 | 7.3 | 1.9 | 35 | [59] |
| Standard dose MBI dense breasts | 3.2 | 10.7 | 7.4 | 231 | [55] |
| Low-dose MBI dense breast | 3.2 | 12.0 | 8.8 | 275 | [56] |
Supplemental yield is given as (number of cancers detected per 1000 women screened + adjunct) − (number of cancers detected per 1000 women screened). MBI: Molecular breast imaging.
Figure 1.
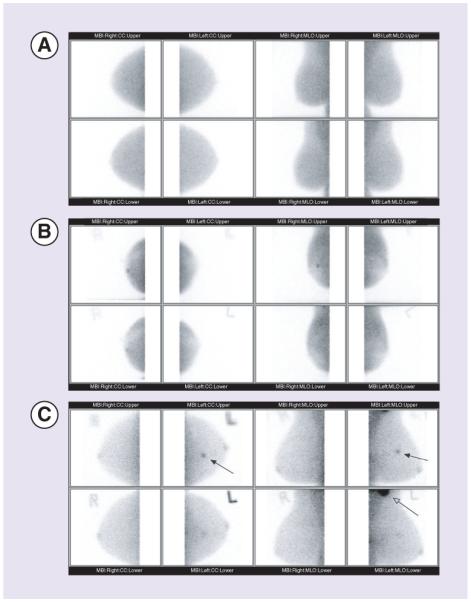
(A) Example of a complete molecular breast imaging (MBI) image set in a patient with no known breast disease. Images are presented in the standard mammographic orientation - cranio-caudal (CC) and mediolateral oblique (MLO). Each column shows the images acquired from the two opposing detectors. (B) Complete MBI image set in a patient with invasive ductal carcinoma. Patient presented with a small palpable mass in the right breast that was negative on diagnostic mammogram. MBI showed a small focal area of increased uptake (arrows) best seen on the upper detector CC and MLO views. Target ultrasound showed a 6 mm × 7 mm × 5 mm hypoechoic nodule. Pathology indicated an infiltrating ductal carcinoma, Nottingham grade II (of III), measuring 0.7 cm in greatest linear extent. (C) MBI study in a patient with invasive mammary carcinoma. Patient had heterogeneously dense breast tissue on a recent mammogram with no evidence of malignancy. A mass was noted in the left breast as an incidental finding on a CT scan of the chest. Targeted ultrasound showed a 14 mm × 9 mm hypoechoic mass in the left breast at 11 o’clock middle depth 5 cm from the nipple. MBI showed a 14-mm lesion in the upper inner left breast (solid arrows), as well as intense uptake in the left axilla corresponding to axillary lymph node metastases (open arrow). Pathology indicated mixed infiltrating ductal/lobular carcinoma, Nottingham grade III (of III), measuring 1.4 cm in diameter.
Relative to the issues discussed above with screening mammography, consider that approximately 80% of cancers seen only on MBI were invasive, suggesting that MBI is not selectively detecting clinically unimportant cancers (over-diagnosis) [56]. Four-fifths (9/11) of the invasive cancers seen only on MBI were node negative, suggesting that MBI contributes to early detection of clinically important cancers [56]. MBI also detected larger and node-positive invasive cancers that were mammographically occult, suggesting a role for MBI in identifying cancers masked on repeated mammographic screenings by dense breast parenchyma.
Like every technology, MBI has its limitations. False-positive findings can occasionally be observed in intramammary lymph nodes and in a number of benign conditions such as fibroadenomas, papillomas, fat necrosis etc. [63]. Lesions less than 4–5 mm in size are usually poorly seen [36] and any lesion close to the chest wall may be missed as it is difficult to position the breast in the detector such that tissue adjacent to the chest wall is well seen. Similar to background parenchymal enhancement seen on MRI, physiologic background uptake of Tc-99m sestamibi can occur in normal breast glandular tissue, and is typically observed in 10–20% of studies. Scheduling the MBI study at a favorable time in the patient’s menstrual cycle can help minimize this effect [64].
Conclusion & future perspective
US Preventative Services Task Force’s last recommendation on breast cancer screening, released in 2009, sparked immediate controversy after it rescinded the group’s recommendation that women in their 40s receive routine mammography screening [65]. As 5 years have now passed, their policy is again due for review. With the controversy on mammography continuing unabated, the next set of recommendations from this task force are unlikely to quell this debate and may further downgrade the reliance on mammography as the primary screening modality. Coupled with the expanding legislation on breast density [8], this is likely to increase the demand from patients and clinicians for supplemental screening techniques. This then poses the question, which supplemental screening technique has the best balance of increased cancer detection to false-positive findings and which will best be accepted by patients and providers?
While two clinical trials have demonstrated the value of MBI, it is likely that a head-to-head comparison of MBI, ultrasound and tomosynthesis in a multi-trial setting will be required to confirm the findings of these two single-center trials. The key outcomes that will be scrutinized from such a trial are the invasiveness and node negativity of the cancers detected by each modality, as these outcomes will begin to directly address issues related to overdiagnosis and failure to reduce the rate at which women present with advanced disease. We suspect that of all the modalities tested, mammography will be the poorest performer, which may ultimately raise the question of why this should remain the primary screening modality. Only an appropriately powered multicenter screening trial set in both academic and community practice setting will eventually resolve this question.
Practice points.
Improvements in x-ray mammography (from analog to digital to tomosynthesis) have only marginally reduced the rates of cancer detection.
Nuclear medicine techniques in breast imaging have undergone a quantum leap in image quality and sensitivity over the last 5–10 years.
The latest nuclear medicine technique, molecular breast imaging (MBI) can be performed at radiation doses acceptable for routine breast cancer screening.
Addition of MBI to screening mammography in women with mammographically dense breasts increased the cancer detection rate from three to 12 cancers per 1000 women screened in two large screening studies.
Supplemental screening MBI appears to have a comparable or lower recall rate than screening mammography.
MBI offers a supplemental screening option with a favorable balance of increased diagnostic yield to false-positive findings which is well-suited to women with mammographically dense breasts.
Acknowledgments
Financial & competing interests disclosure
This work was funded in part by grants from the Susan G Komen Foundation, NIH (CA 128407) and the Mayo Foundation. The author receives royalties per licensing agreement between Mayo Clinic and Gamma Medica, a manufacturer of MBI systems.
Footnotes
The author has no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
- 1.Independent UK panel on Breast Cancer Screening The benefits and harms of breast cancer screening: an independent review. Lancet. 2012;380:1778–1786. doi: 10.1016/S0140-6736(12)61611-0. [DOI] [PubMed] [Google Scholar]
- 2.Duffy SW, Yen AM, Chen TH, et al. Long-term benefits of breast screening. Breast Cancer Manag. 2012;1:31–38. [Google Scholar]
- 3.Nelson HD, Tyne K, Naik A, et al. Screening for breast cancer: an update for the U.S. Preventative Task Force. Ann. Intern. Med. 2009;151:727–73. doi: 10.1059/0003-4819-151-10-200911170-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bleyer A, Gilbert-Welch H. Effect of three decades of screening mammography on breast cancer incidence. N. Engl. J. Med. 2012;367:1998–2005. doi: 10.1056/NEJMoa1206809. [DOI] [PubMed] [Google Scholar]
- • Interesting article that sparked much of the debate on the value of screening mammography.
- 5.Kaplan HG, Malmgren JA. The breast cancer overdiagnosis conundrum: an oncologist’s viewpoint. Ann. Intern. Med. 2013;158:60–62. doi: 10.7326/0003-4819-158-1-201301010-00011. [DOI] [PubMed] [Google Scholar]
- • Similar to the article above, this reviews the issue of overdiagnosis in screening mammography.
- 6.Mandelson MT, Oestreicher N, Porter PL, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J. Natl Cancer Inst. 2000;15:1081–1087. doi: 10.1093/jnci/92.13.1081. [DOI] [PubMed] [Google Scholar]
- 7.Esserman LJ, Thompson IM, Reid B. Overdiagnosis and overtreatment in cancer. an opportunity for improvement. JAMA. 2013;310:797–798. doi: 10.1001/jama.2013.108415. [DOI] [PubMed] [Google Scholar]
- 8.National Cancer groups join Are You Dense Advocacy, Inc. Supporting a National Standard to Report Density. www.areyoudenseadvocacy.org/worxcms.
- 9.Skaane P, Bandos AI, Gullien R, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology. 2013;267:47–56. doi: 10.1148/radiol.12121373. [DOI] [PubMed] [Google Scholar]
- 10.Killelea BK, Long JB, Chagpar AB. Evolution of breast cancer screening in the medicare population: clinical and economic implications. J. Natl Cancer Inst. 2014;106 doi: 10.1093/jnci/dju159. doi:10.1093/jnci/dju159. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- • Very informative article reviewing the economic consequences of shifting from analog to digital mammography.
- 11.Kerlikowske K, Hubbard R, Tosteson ANA. Editorial: higher mammography screening costs without appreciable clinical benefit: the case of digital mammography. J. Natl Cancer Inst. 2014;107:1–2. doi: 10.1093/jnci/dju191. [DOI] [PubMed] [Google Scholar]
- • Accompanying editorial on reference 10 that discusses the implications for digital mammography and tomosynthesis.
- 12.ACR urges CMS to cover breast tomosynthesis. www.auntminnie.com/index.aspx.
- 13.Gotzsche PC, Olsen O. Is screening for breast cancer with mammography justifiable? Lancet. 2000;355:129–134. doi: 10.1016/S0140-6736(99)06065-1. [DOI] [PubMed] [Google Scholar]
- 14.Weinberg IN. Applications for positron emission mammography. Phys. Med. 2006;21(Suppl. 1):132–137. doi: 10.1016/S1120-1797(06)80045-1. [DOI] [PubMed] [Google Scholar]
- 15.Moliner L, Benlloch JM, Soriano A, et al. Design and evaluation of the MAMMI dedicated breast PET. Med. Phys. 2012;39:5393–5404. doi: 10.1118/1.4742850. [DOI] [PubMed] [Google Scholar]
- 16.Morris EA. Rethinking breast cancer screening: Ultra FAST breast magnetic resonance imaging. J. Clin. Oncol. 2014;32:2281–2283. doi: 10.1200/JCO.2014.56.1514. [DOI] [PubMed] [Google Scholar]
- 17.Prionas ND, Lindfors KK, Ray S, et al. Contrast-enhanced dedicated breast CT: initial clinical experience. Radiology. 2010;256:714–723. doi: 10.1148/radiol.10092311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taillefer R. The role of 99mTc-sestamibi and other conventional radiopharmaceuticals in breast cancer diagnosis. Semin. Nucl. Med. 1999;29:16–40. doi: 10.1016/s0001-2998(99)80027-0. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh PR. FDA approves two new technetium-labeled cardiac agents and a pharmacologic alternative to exercise in stress-thallium studies. J. Nucl. Med. 1991;32:11N–19N. [PubMed] [Google Scholar]
- 20.Aktolun C, Bayhan H, Kir M. Clinical experience with Tc-99m MIBI imaging in patients with malignant tumors. Preliminary results and comparison with Tl-201. Clin. Nucl. Med. 1992;17:171–176. doi: 10.1097/00003072-199203000-00003. [DOI] [PubMed] [Google Scholar]
- 21.Rambaldi PF, Mansi L, Procaccini E, Di Gregorio F, Del Vecchio E. Breast cancer detection with Tc-99m tetrofosmin. Clin. Nucl. Med. 1995;20:703–705. doi: 10.1097/00003072-199508000-00009. [DOI] [PubMed] [Google Scholar]
- 22.Liberman M, Sampalis F, Mulder DS, Sampalis JS. Breast cancer diagnosis by scintimammography: a meta-analysis and review of the literature. Breast Cancer Res. Treat. 2003;80:115–126. doi: 10.1023/A:1024417331304. [DOI] [PubMed] [Google Scholar]
- 23.Khalkhali I, Villanueva-Meyer J, Edell SL, et al. Diagnostic accuracy of 99mTc-sestamibi breast imaging: multicenter trial results. J. Nucl. Med. 2000;41:1973–1979. [PubMed] [Google Scholar]
- 24.Palmedo H, Biersack HJ, Lastoria S. Scintimammography with technetium-99m methoxyisobutylisonitrile: results of a prospective European multicentre trial. Eur. J. Nucl. Med. 1998;25:375–385. doi: 10.1007/s002590050235. [DOI] [PubMed] [Google Scholar]
- 25.Waxman AD. The role of (99m)Tc methoxyisobutylisonitrile in imaging breast cancer. Semin. Nucl. Med. 1997;27:40–54. doi: 10.1016/s0001-2998(97)80035-9. [DOI] [PubMed] [Google Scholar]
- 26.Brem RF, Schoonjans JM, Kieper DA, Majewski S, Goodman S, Civelek C. High-resolution scintimammography: a pilot study. J. Nucl. Med. 2002;43:909–915. [PubMed] [Google Scholar]
- 27.Papantoniou V, Christodoulidou J, Papadaki E, et al. 99mTc-(V)DMSA scintimammography in the assessment of breast lesions: comparative study with 99mTc-MIBI. Eur. J. Nucl. Med. 2001;28:923–928. doi: 10.1007/s002590100545. [DOI] [PubMed] [Google Scholar]
- 28.Weinberg IN, Pani R, Pellegrini R, et al. Small lesion visualization in scintimammography. IEEE Trans. Nucl. Sci. 1997;44:1398–1402. [Google Scholar]
- 29.Pani R, De Vincentis G, Scopinaro F, et al. Dedicated gamma camera for single photon emission mammography (SPEM) IEEE Trans. Nucl. Sci. 1998;45:3127–3133. [Google Scholar]
- 30.Hruska CB, O’Connor MK, Collins DA. Comparison of small field of view gamma camera systems for scintimammography. Nucl. Med. Commun. 2005;26:441–445. doi: 10.1097/00006231-200505000-00008. [DOI] [PubMed] [Google Scholar]
- 31.More MJ, Goodale PJ, Majewski S, Williams MB. Evaluation of gamma cameras for use in dedicated breast imaging. IEEE Trans. Nucl. Sci. 2006;53:2675–2679. [Google Scholar]
- 32.Brem RF, Floerke AC, Rapelyea JA, et al. Breast-specific gamma imaging as an adjunct imaging modality for the diagnosis of breast cancer. Radiology. 2008;247:651–657. doi: 10.1148/radiol.2473061678. [DOI] [PubMed] [Google Scholar]
- 33.Butler JF, Lingren CL, Friesenhahn SJ. CdZnTe solid-state gamma camera. IEEE Trans. Nucl. Sci. 1998;45:359–363. [Google Scholar]
- 34.Mueller B, O’Connor MK, Blevis I, et al. Evaluation of a small cadmium zinc telluride detector for scintimammography. J. Nucl. Med. 2003;44:602–609. [PubMed] [Google Scholar]
- 35.Robert C, Montemont G, Rebuffel V, Buvat I, Guerin L, Verger L. Simulation-based evaluation and optimization of a new CdZnTe gamma-camera architecture (HiSens) Phys. Med. Biol. 2010;55:2709–2726. doi: 10.1088/0031-9155/55/9/019. [DOI] [PubMed] [Google Scholar]
- 36.Hruska CB, Phillips SW, Whaley DH, Rhodes DJ, O’Connor MK. Molecular breast imaging: use of a dual-head dedicated gamma camera to detect small breast tumors. Am. J. Roentgenol. 2008;191:1805–1815. doi: 10.2214/AJR.07.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spanu A, Chessa F, Meloni GB, et al. The role of planar scintimammography with high-resolution dedicated breast camera in the diagnosis of primary breast cancer. Clin. Nucl. Med. 2008;33:739–742. doi: 10.1097/RLU.0b013e318187ee75. [DOI] [PubMed] [Google Scholar]
- 38.National Research Council . Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. The National Academies Press; Washington, DC: 2006. Committee to assess health risks from exposure to low levels of ionizing radiation. [PubMed] [Google Scholar]
- 39.Yaffe MJ, Mainprize JG. Risk of radiation-induced breast cancer from mammographic screening. Radiology. 2011;258:98–105. doi: 10.1148/radiol.10100655. [DOI] [PubMed] [Google Scholar]
- 40.DePuey EG, Mahmarian JJ, Miller TD. ASNC preferred practice statement: Patient centered imaging. J. Nucl. Cardiol. 2012;19:185–215. doi: 10.1007/s12350-012-9523-z. [DOI] [PubMed] [Google Scholar]
- 41.Hendrick RE. Radiation doses and cancer risks from breast imaging studies. Radiology. 2010;257:246–253. doi: 10.1148/radiol.10100570. [DOI] [PubMed] [Google Scholar]
- 42.O’Connor MK, Li H, Rhodes DJ, Hruska CB, Clancy CB, Vetter RJ. Comparison of radiation exposure and associated radiation-induced cancer risks from mammography and molecular imaging of the breast. Med. Phys. 2010;37:6187–6198. doi: 10.1118/1.3512759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hruska CB, WeinmAnn AL, O’Connor MK. Proof of concept for low-dose molecular breast imaging with a dual-head CZT gamma camera. Part I. Evaluation in phantoms. Med. Phys. 2012;39:3466–3475. doi: 10.1118/1.4718665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hruska CB, WeinmAnn AL, Tello Skjerseth CM, et al. Proof of concept for low-dose molecular breast imaging with a dual-head CZT gamma camera. Part II. Evaluation in patients. Med. Phys. 2012;39:3476–3483. doi: 10.1118/1.4719959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swanson T, Troung T, Hruska C, et al. Evaluation of the effect of fasting and exercise on uptake of Tc-99m sestamibi in breast tissue. J. Nucl. Med. 2014;55(Suppl. 1):2504. [Google Scholar]
- 46.O’Connor MK, Conners AL, Jones K, et al. Relative uptake of the αvβ3 radiopharmaceutical Tc-99m maraciclatide and Tc-99m sestamibi in the breast tissue of patients with suspected breast cancer. J. Nucl. Med. 2014;55(Suppl. 1):1547. [Google Scholar]
- 47.Mammography Quality Standards Act and Program. FDA 2014. www.fda.gov/Radiation-EmittingProducts.
- 48.Berrington de González A, Reeves G. Mammographic screening before age 50 years in the UK: comparison of the radiation risks with the mortality benefits. Br. J. Cancer. 2005;93:590–596. doi: 10.1038/sj.bjc.6602683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feig SA, Hendrick RE. Radiation risk from screening mammography of women aged 40–49 Years. J. Natl Cancer Inst. Monogr. 1997;22:119–124. doi: 10.1093/jncimono/1997.22.119. [DOI] [PubMed] [Google Scholar]
- 50.Berrington de Gonzalez A, Berg CD, Visvanathan K, Robson M. Estimated risk of radiation-induced breast cancer from mammographic screening for young BRCA mutation carriers. J. Natl Cancer Inst. 2009;101:205–209. doi: 10.1093/jnci/djn440. [DOI] [PubMed] [Google Scholar]
- 51.The 2007 Recommendations of the International Commission on Radiological Protection. Ann. ICRP. 2012;37(2007) doi: 10.1016/j.icrp.2007.10.003. ICRP Publication 103. [DOI] [PubMed] [Google Scholar]
- 52.United Nations Scientific Committee on the Effects of Atomic Radiation Report of the United Nations Scientific Committee on the Effects of Atomic Radiation, 59th session (May 21–25, 2012) General Assembly Official Records. 67th session, Supplement No. 46. http://daccess-dds-ny.un.org/doc/UNDOC/
- • Chapter III, section (f) of this report contains the key statements addressing the issue of biological effects of low doses of ionizing radiation.
- 53.American Association of Physicists in Medicine Position statement of the American Association of Physicists in Medicine. Radiation risks from medical imaging procedures. www.aapm.org/org/policies/
- 54.Health Physics Society Position statement of the Health Physics Society: radiation risk in perspective. http://hps.org/documents/
- 55.Rhodes DJ, Hruska CB, Phillips SW, Whaley DW, O’Connor MK. Dedicated dual-head gamma imaging for breast cancer screening in women with mammographically dense breasts. Radiology. 2011;258:106–118. doi: 10.1148/radiol.10100625. [DOI] [PubMed] [Google Scholar]
- • First large screening trial comparing mammography and mammography with adjunct MBI.
- 56.Rhodes DJ, Hruska CB, Conners AL, et al. Molecular breast imaging at reduced radiation dose for supplemental screening in mammographically dense breasts. Amer. J. Roentgen. 2015;204 doi: 10.2214/AJR.14.13357. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- • Large low-dose screening trial comparing mammography and mammography with adjunct MBI. Confirmed results of earlier trial.
- 57.Berg WA, Blume JD, Cormack JB, et al. Combined screening with ultrasound and mammography vs mammography alone in women at elevated risk of breast cancer. JAMA. 2008;299:2151–2163. doi: 10.1001/jama.299.18.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ciatto S, Houssami N, Bernardi D, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol. 2013;14:583–589. doi: 10.1016/S1470-2045(13)70134-7. [DOI] [PubMed] [Google Scholar]
- 59.Brem RF, Tabar L, Duffy SW, et al. Assessing improvement in detection of breast cancer with three-dimensional automated breast US in women with dense breast tissue: the SomoInsight study. Radiology. 2014 doi: 10.1148/radiol.14132832. doi:10.1148/ radiol.14132832. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- • Large screening trial comparing mammography and automated ultrasound. Illustrates one of the main limitations of automated ultrasound – poor specificity leading to high recall rates.
- 60.Conners AL, Hruska CB, Tortorelli CL, et al. Lexicon for standardized interpretation of gamma camera molecular breast imaging: observer agreement and diagnostic accuracy. Eur. J. Nucl. Med. Mol. Imaging. 2012;39:971–982. doi: 10.1007/s00259-011-2054-z. [DOI] [PubMed] [Google Scholar]
- 61.Schillaci O. The importance of standardized interpretation of molecular breast imaging with dedicated gamma cameras. Eur. J. Nucl. Med. Mol. Imaging. 2012;39:967–70. doi: 10.1007/s00259-012-2095-y. [DOI] [PubMed] [Google Scholar]
- 62.Berg WA, Zhang Z, Lehrer D, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012;307:1394–404. doi: 10.1001/jama.2012.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Connor MK, Rhodes DB, Hruska C. Molecular Breast Imaging. Expert Rev. Anticancer Ther. 2009;9:1073–1080. doi: 10.1586/era.09.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Conners AL, Maxwell RW, Tortorelli CL, et al. Gamma Camera Breast Imaging Lexicon. Amer. J. Roenten. 2012;199:W767–W774. doi: 10.2214/AJR.11.8298. [DOI] [PubMed] [Google Scholar]
- 65.U.S. Preventative Services Task Force Screening for Breast Cancer. www.uspreventiveservicestaskforce.org/


