Abstract
Cyanobacteria are globally important primary producers that have an exceptionally large iron requirement for photosynthesis. In many aquatic ecosystems, the levels of dissolved iron are so low and some of the chemical species so unreactive that growth of cyanobacteria is impaired. Pathways of iron uptake through cyanobacterial membranes are now being elucidated, but the molecular details are still largely unknown. Here we report that the non-siderophore-producing cyanobacterium Synechocystis sp. PCC 6803 contains three exbB-exbD gene clusters that are obligatorily required for growth and are involved in iron acquisition. The three exbB-exbDs are redundant, but single and double mutants have reduced rates of iron uptake compared with wild-type cells, and the triple mutant appeared to be lethal. Short-term measurements in chemically well-defined medium show that iron uptake by Synechocystis depends on inorganic iron (Fe′) concentration and ExbB-ExbD complexes are essentially required for the Fe′ transport process. Although transport of iron bound to a model siderophore, ferrioxamine B, is also reduced in the exbB-exbD mutants, the rate of uptake at similar total [Fe] is about 800-fold slower than Fe′, suggesting that hydroxamate siderophore iron uptake may be less ecologically relevant than free iron. These results provide the first evidence that ExbB-ExbD is involved in inorganic iron uptake and is an essential part of the iron acquisition pathway in cyanobacteria. The involvement of an ExbB-ExbD system for inorganic iron uptake may allow cyanobacteria to more tightly maintain iron homeostasis, particularly in variable environments where iron concentrations range from limiting to sufficient.
Introduction
Low concentrations of iron in surface waters of the open sea are maintained by efficient uptake systems of planktonic biota and limited solubility of the inorganic forms (Morel and Price, 2003). Complexation by siderophores and other uncharacterized organic ligands increases the solubility of the dissolved iron (Mawji et al., 2008) but reduces its availability to many taxa. At the extreme, such reduced iron bioavailability can impair phytoplankton growth (Boyd et al., 2007; Falkowski and Raven, 2007; Chappell et al., 2012; Wilhelm et al., 2013) and is thought to limit the primary productivity in as much as 40% of global ocean (Martin et al., 1994; Falkowski et al., 1998; Achilles et al., 2003) and some freshwater habitats (North et al., 2007; Havens et al., 2012).
Siderophores are strong iron chelators, secreted by many organisms, including bacteria, fungi, yeast and monocotyledonous plants to solubilize, bind and make available iron in the environment. Generally, organisms synthesize and secrete these low molecular weight chelators to bind Fe(III) and then transport the ferri-siderophore complex through the cell membrane. Unlike other organisms, Gram-negative bacteria possess an outer membrane (OM) as well as a cytoplasmic membrane (CM), which presents an additional barrier to the exchange of solutes. As ferri-siderophores are too large to passively diffuse through the OM porins, they must be actively transported across the membrane by specific receptor proteins (Miethke and Marahiel, 2007; Noinaj et al., 2010). The OM receptors/transporters bind the ferri-siderophore complexes and directly interact with the energizing TonB-ExbB-ExbD complex in the inner membrane to allow the iron complex to be transported into the periplasmic space. This transport process involves three components: (i) OM localized transporters; (ii) a CM-localized TonB-ExbB-ExbD complex, and (iii) ion electrochemical potential (Noinaj et al., 2010). Over the past three decades, many aspects of this TonB-ExbB-ExbD-dependent transport system have been revealed. The crystal structures of several OM transporters and their complexes with TonB are now known (Ferguson et al., 1998, 2002; Buchanan et al., 1999; Ferguson and Deisenhofer, 2004; Pawelek et al., 2006; Shultis et al., 2006; Krieg et al., 2009), the signal transduction of OM transporters by interaction with TonB has been elucidated (Ferguson et al., 2007; Kim et al., 2007) and the rotational mechanism of TonB motion has been reported (Jordan et al., 2013). However, with regard to the substrates of the transport system, we are probably only seeing the ‘tip of the iceberg' (Schauer et al., 2008). Originally, iron complexes and vitamin B12 were thought to be the main substrates of the TonB-ExbB-ExbD system, but more and more new substrates have been found to be transported, including citrate, transferrin, hemoproteins, heme, phages, colicins, maltodextrins, nickel chelators and sucrose (Schauer et al., 2008; Noinaj et al., 2010).
Cyanobacteria are globally important primary producers and dominate some iron-limited marine environments, such as the equatorial Pacific Ocean (Kolber et al., 1994). However, the siderophore-mediated iron uptake pathway described in non-photosynthetic bacteria has not been fully confirmed in cyanobacterial species (Stevanovic et al., 2012). The following observations are pertinent: (i) although cyanobacteria possess an OM and are commonly considered as Gram-negative bacteria, their cell envelopes are partly characteristic of Gram-positive bacteria, which do not possess the TonB-ExbB-ExbD system (Hoiczyk and Hansel, 2000); (ii) while some species produce strong siderophores, most cyanobacteria do not (Ito and Butler, 2005; Hopkinson and Morel, 2009; Mirus et al., 2009); and (iii) some cyanobacteria can use the iron bound to siderophores of other organisms (Kranzler et al., 2011). Hopkinson and Morel (2009) suggested that the role of siderophores in marine environments is probably overestimated but could reach no definitive conclusion, because the iron acquisition mechanisms of cyanobacteria are poorly understood. Kranzler et al. (2011, 2014) proposed an alternative reduction-based uptake strategy by which cyanobacteria can sequester iron from multiple complexes in dilute aquatic environments.
Overall, the iron uptake mechanism of cyanobacteria remains largely unclear, especially how iron crosses the cyanobacterial OM as well as the role of TonB-ExbB-ExbD system in cyanobacterial OM transport. In a siderophore-producing strain of Anabaena sp. PCC 7120, putative tonB, exbB and exbD genes have been identified, and their inactivation induces an iron starvation phenotype (Stevanovic et al., 2012), but direct evidence of their participation in iron transport is lacking. As most cyanobacteria do not produce siderophores, generalizing the results from Anabaena to other species may be inappropriate. Here we identify and characterize the ExbB-ExbD complexes in a non-siderophore-producing cyanobacterium, Synechocystis sp. PCC 6803, and find that they are required for inorganic iron (Fe′) uptake. Although substrates for the TonB-ExbB-ExbD-mediated transport pathway in non-photosynthetic bacteria are exclusively organic, our results suggest that cyanobacteria use the ExbB-ExbD complexes to activate a different class of OM transporter involved in inorganic iron uptake. These finding may be helpful in understanding how cyanobacteria acquire iron in nature and survive in iron-limited environments.
Materials and methods
Strains, culture conditions and general methods
A glucose-tolerant, axenic strain of Synechocystis sp. (PCC 6803) was cultured in BG11 medium at 30 °C under continuous illumination (40 μmol photons m−2 s−1). All growth media, buffers and solutions used during the experiments were autoclave sterilized. To analyze growth in iron-starved medium, ammonium ferric citrate was omitted from the BG11 medium and replaced by the same concentration of ammonium citrate. Glassware used for iron-starved conditions was soaked in 6 M HCl for about 12 h and rinsed seven times with Milli-Q water to remove residual iron. Before the experiment, exponential cells were harvested by centrifugation at 6000 g and 30 °C for 5 min and washed three times with BG11–Fe medium to remove extracellular iron from the cell surfaces. The specific growth rate and chlorophyll a content were assayed as described in previous studies (Jiang et al., 2010, 2012).
Construction of mutants, complementation and overexpression strains of Synechocystis 6803
The single mutants of the three exbB-exbD gene clusters were generated by introducing a single C.K2 (Kmr), C.CE2 (Emr) or Omega (Spr) resistance cassette into the open-reading frame of sll1404-sll1405, slr0677-slr0678 or sll0477-sll0478-sll0479, respectively, by the homologous recombination method (Jiang et al., 2010, 2012). The double mutants were generated by transforming two of the resistance cassettes into the genome. The triple mutant was generated by sequentially transforming all three cassettes into wild-type cells in different order. In the complementation and overexpression strains, the genes or gene clusters were expressed by a PpsbAII expression vector (Jiang et al., 2012). The resulting plasmids were used to transform the gene fragments into Synechocystis 6803 wild-type or mutant strains. The plasmid construction and primers used are listed in Supplementary Table S1.
Extraction of RNA and reverse transcriptase–PCR
The cells of Synechocystis strains grown in standard BG11 medium or 24 h after transfer into iron-deficient BG11 medium were collected and quickly frozen in liquid nitrogen. The RNA extraction and reverse transcriptase–PCR methods are described previously (Jiang et al., 2012).
Determination of cellular iron contents and measurement of iron uptake rates
Cells were grown from OD730 (optical density at 730 nm) 0.2 to 2.0 in standard BG11 medium. Iron contents were measured according to Nicolaisen et al. (2008). Briefly, the cells were collected and washed three times in 20 mM EDTA, then dried and digested for element determination by an atomic absorption spectrometer (AA240FS, Varian, Palo Alto, CA, USA). The 55Fe3+ uptake rates shown in Figure 1f were measured as described previously (Jiang et al., 2012) in the presence of 1 mM Ferrozine. To determine the inorganic iron or desferrioxamine (DFB)-Fe uptake rates shown in Figures 4a and b, cells were resuspended in iron-free BG11 medium lacking citric acid and ferric ammonium citrate (the medium contained 2.68 μM EDTA). For measuring the inorganic iron uptake rate, 100 nM (3.7 kBq ml−1) 55FeCl3 was added to the cell solutions. For measuring the Fe-siderophores uptake rate, 100 nM 55FeCl3 complexed with 133 nM DFB was added to the cell solutions. To clarify the role of ExbB-ExbD in Fe′ uptake, more rigorous experiments were carried out using trace metal clean techniques and precomplexed FeEDTA solution, as described by Kranzler et al. (2011) with minor modification. Cells were grown to logarithmic phase in BG11 medium, harvested and washed three times in a modified BG11 medium lacking EDTA, citric acid, ferric ammonium citrate and trace metals (this medium contained no organic ligands). The samples were then resuspended in different concentrations of precomplexed EDTA-Fe buffered with the modified BG11 medium. Samples (0.2 ml) were collected at the indicated times, filtered on 0.22-μm pore size polycarbonate filters (Poretics, Livermore, CA, USA) and washed with 3 ml oxalate–EDTA washing solution (Tang and Morel, 2006). Fe′ concentrations were calculated using the Mineql speciation software (Westall et al., 1976; http://www.mineql.com/). The uptake experiment of Figure 4e was prepared by the same methods as Figures 4c and d. To obtain two higher Fe′ concentrations, 1 μM FeCl3 was buffered with insufficient 1.6 μM EDTA (yielding 12.8 nM Fe′), and 0.1 μM FeCl3 was added to the modified BG11 medium lacking organic ligands (yielding 99.9 nM Fe′) before measurement.
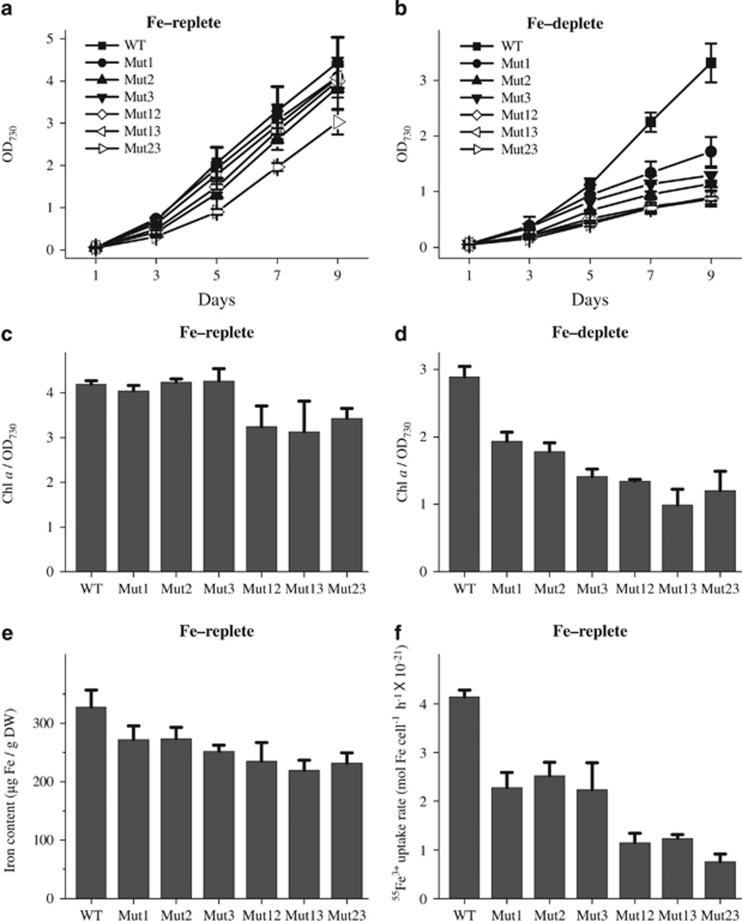
Figure 1.
Physiological phenotypes of Synechocystis exbBD mutants. Cells were grown in BG11 medium under standard iron concentrations (Fe-replete) (a, c, e and f) or iron-deficient conditions, in which ammonium ferric citrate was replaced with ammonium citrate (Fe-deplete) (b and d). The growth characteristics (a and b), pigment content (c and d), cellular iron content (e) and short-term 55Fe3+ uptake rate (f) were determined as previously described (Jiang et al., 2012).
Yeast two-hybrid assays
In the yeast two-hybrid assays, the protein–protein interaction of Sll1404 and Sll1405 was detected by a Matchmaker GAL4 Two-Hybrid System 3 (Clontech, Palo Alto, CA, USA). The sll1404 and sll1405 gene fragments were cloned into pGBKT7 and pGADT7, respectively. The resulting plasmids were co-transformed into Saccharomyces cerevisiae AH109 and selected on SD/-Trp-Leu-His agar plates. The transformants were then grown on SD/-Trp-Leu-His-Ade plates for 3 days.
Localization of ExbB and ExbD proteins in Synechocystis 6803
Total protein was extracted from Synechocystis 6803 cells and centrifuged at 50 000 g for 1 h to isolate soluble and membrane proteins. Cytoplasmic and thylakoid membranes were isolated by the 2D-separation method combined with sucrose density centrifugation (Norling et al., 1998; Huang et al., 2006). Samples were resolved on 12% sodium dodecyl sulfate polyacrylamide gel and immunoblotted with corresponding antibodies. The marker proteins of cytoplasmic and thylakoid membranes were NrtA and CP47, respectively.
Results
Three exbB-exbD gene clusters in the Synechocystis 6803 genome were induced by iron starvation, and their inactivation stimulated known iron uptake genes
Previously, we identified a Synechocystis 6803 strain with a mutant putative exbB-exbD gene cluster (Δsll1404-sll1405) that grew more slowly than the wild type under iron-deficient conditions (Jiang et al., 2012). At toxic iron concentrations (1 mM), the same mutant grew more rapidly than the wild type (Supplementary Figure S1A), indicating a positive role of the gene cluster in iron homeostasis. Bioinformatic analysis revealed a further two putative exbB-exbD gene clusters (slr0677-slr0678 and sll0477-sll0478-sll0479) in the genome of Synechocystis 6803 (Supplementary Figure S2A). The three clusters, respectively, encoded putative ExbB proteins Sll1404, Slr0677 and Sll0477, with 57–61% similarity in their amino-acid sequences, and putative ExbD proteins Sll1405, Slr0678, Sll0478 and Sll0479, with 46–58% amino-acid similarity (Supplementary Figure S2B). Genes encoding TonB-resembling proteins, slr1484, and a putative OM transporter, sll1406, were located close to the sll1404-sll1405 gene cluster (Supplementary Figure S2A). It should be noted that, in Synechocystis 6803, the three clusters that encode putative ExbB-ExbD also show similarity with Escherichia coli TolQ-TolR (data not shown). Although the TolA-TolQ-TolR and TonB-ExbB-ExbD energy-coupled import systems show strong sequence homology and mutual functional substitution, no uptake functions for TolA-TolQ-TolR have been described other than for certain bacteriophages and colicins (Braun and Herrmann, 1993).
Synechocystis grown in iron-replete (21 μM Fe) BG11 medium (standard conditions) showed low expression of sll1404, sll0477 and slr0677, but the transcript abundance of all genes dramatically increased following 24 h iron starvation (Supplementary Figure S2C). The three putative exbB genes were highly expressed in a cation diffusion facilitator iron transport mutant (sll1263::C.K2) (Jiang et al., 2012) in the same iron-enriched medium (21 μM Fe) and were further induced after 24 h iron starvation (Supplementary Figure S2C). As genes in the putative exbB-exbD clusters were highly expressed in iron-starved cells, their products are likely involved in iron uptake. Indeed, the expression of several known iron uptake genes (fut and feo genes) (Katoh et al., 2001) is elevated in exbB-exbD mutants (see Supplementary Figure S3 for mutant constructions), even during growth in standard iron-replete conditions (Supplementary Figure S2D). These genes are further expressed in iron-deficient medium (Supplementary Figure S2D), and each exbB gene is enhanced when other homologs are knocked out (Supplementary Figure S2E). This result suggests that the three exbB-exbD gene clusters are redundant and perform a similar function.
Mutation of any one of the exbB-exbD gene clusters resulted in a low-iron-sensitive phenotype, which was reinforced in double mutants
Three single mutants and three double mutants of the putative exbB-exbD gene clusters were constructed (Supplementary Figure S3). For convenience, we refer to gene clusters sll1404-sll1405, slr0677-slr0678 and sll0477-sll0478-sll0479 as exbBD1, exbBD2 and exbBD3, respectively, and their corresponding single mutants are denoted Mut1, Mut2 and Mut3, respectively. Double mutants were deficient in exbBD1 and exbBD2 (Mut12), exbBD1 and exbBD3 (Mut13) and exbBD2 and exbBD3 (Mut23). As shown in Figures 1a and c, the growth and chlorophyll a concentration of all three single mutants grown in iron-replete BG11 medium was similar to that of wild-type cells, while that of the double mutants was slightly decreased. In iron-deficient medium, however, growth and chlorophyll a concentration of the mutants was significantly lower than wild-type levels (Figures 1b and d). The iron-deficient phenotype was more severe in double mutants than in single mutants (Figures 1b and d).
Although their growth characteristics were indistinguishable in iron-replete medium (see Figure 1a), the mutants contained about 17–33% less cellular iron than the wild type (Figure 1e), with double mutants containing the lesser iron quotas (Figure 1e). These reduced cellular iron levels were consistent with the upregulation of iron uptake-related genes in the mutants growing in standard BG11 medium (see Supplementary Figure S2D). To evaluate whether the reduced iron content resulted from slower rates of iron transport and/or reduced iron storage capacity, the short term 55Fe3+ uptake rates were measured in mutant and wild-type strains. Cells were grown in standard BG11 medium (21 μM iron) and the Fe2+ produced by photochemical or cellular reduction was trapped by the iron reagent ferrozine. Fe3+ uptake rates were found to be significantly slower in the single and double mutants than in wild-type cells (39–46% and 70–82% reduction, respectively; see Figure 1f).
The three exbB-exbDs gene clusters were functionally redundant, and the triple-mutant appeared to be lethal
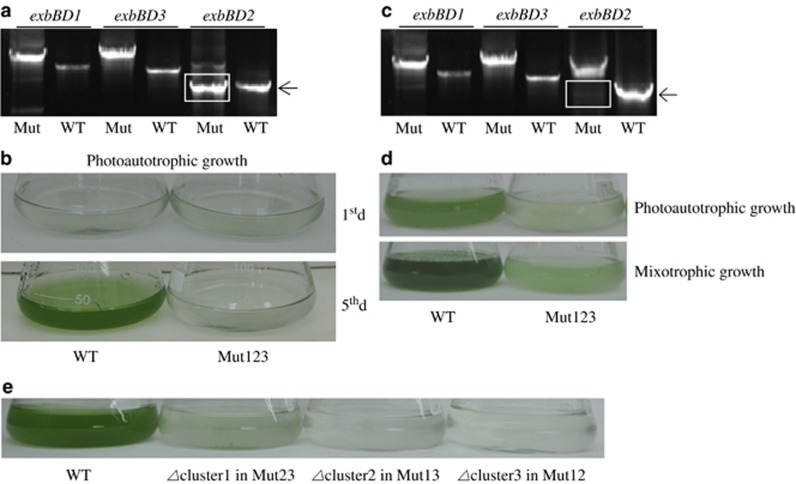
To elucidate whether the iron-deficient phenotypes resulted from inactivation of the exbB-exbD gene clusters, we constructed several complementation and overexpression strains. Growth assays of the strains in iron-deficient medium (Table 1) showed that the iron-deficient phenotype of each mutant was complemented by the gene cluster itself, ruling out possible second point mutations and polar effects. Moreover, each gene cluster corrected the low-iron-sensitive phenotypes of mutants lacking both alternative gene clusters (Table 1). This result showed that the three gene clusters are functionally interchangeable and probably undertake a similar function. The mutant lacking all three exbB-exbD gene clusters was never completely segregated, even after 6 months culturing of the transformants in BG11 medium supplemented with the corresponding antibiotics (in this test, cells were alternately streaked on BG11 solid medium and cultured in liquid BG11 medium). As shown in Figure 2a, PCR amplification of the triple mutant genome yielded wild-type gene copies of exbB-exbD. Nonetheless, this triple mutant knockdown line scarcely survived in standard BG11 medium (21 μM iron) (Figure 2b). A triple mutant was alternatively acquired by supplementing the medium with 5 mM glucose to allow mixotrophy, thereby reducing the growth requirement of cellular iron. Although levels of wild-type copies were lower in the mixotrophic than in photoautotrophic cells, the fragments remained incompletely segregated on the gel (Figure 2c). This ‘approximate' triple-mutant scarcely survived under photoautotrophic conditions but performed moderately well in glucose-supplemented medium (Figure 2d), presumably because of the reduced iron growth costs in this medium. To exclude possible polar effects while obtaining a triple mutant from Mut13, we attempted to knockout exbBD1 in Mut23 and exbBD3 in Mut12 but to no avail (Figure 2e). Collectively, the results confirmed that the three exbB-exbD gene clusters have similar functions and are essential to the survival of Synechocystis 6803.
Table 1. Specific growth rates of the wild-type, mutants, overexpression and complementation strains of Synechocystis 6803 cultured in iron-deficient medium.
| Strains (Synechocystis PCC 6803) | Specific growth ratesa |
|---|---|
| WT | 0.748±0.025 |
| Mut1 | 0.605±0.024 |
| exbBD1-OE | 0.688±0.018 |
| Mut1 complemented by exbBD1 | 0.667±0.026 |
| Mut1 complemented by exbBD2 | 0.688±0.015 |
| Mut1 complemented by exbBD3 | 0.761±0.005 |
| Mut2 | 0.556±0.010 |
| exbBD2-OE | 0.674±0.012 |
| Mut2 complemented by exbBD1 | 0.725±0.002 |
| Mut2 complemented by exbBD2 | 0.692±0.013 |
| Mut2 complemented by exbBD3 | 0.749±0.020 |
Abbreviation: WT, wild type. The values in bold show the significantly reduced growth rates of the mutants.
The values are the means±s.ds. of triplicate cultures.
Figure 2.
Synechocystis 6803 triple mutant lacking all three functional exbBD gene clusters (Mut123) cannot be fully segregated. (a) The knockdown line of Mut123 is not completely segregated on the PCR gel after several months' incubations and transfers in standard BG11 medium supplemented with antibiotics. The arrow indicates the presence of the wild-type exbBD gene cluster. (b) Photograph of the knockdown line Mut123 strain grown in standard BG11 medium. Labels 1st d and 5th d represent the first and fifth days of growth, respectively. (c) PCR result of a putative triple mutant Mut123 obtained from mixotrophic growth conditions. (d) Photographs of the wild-type and the putative triple mutant on the fifth day of growth in photoautotrophic and mixotrophic growth conditions. (e) Photographs of the transformants of triple mutants grown in standard BG11 medium for 5 days. The triple mutants (left to right) were obtained by knocking out exbBD1 in Mut23, exbBD2 in Mut13 or exbBD3 in Mut12, respectively.
E. coli homologs can complement the phenotype of cyanobacterial ExbB-ExbD mutant
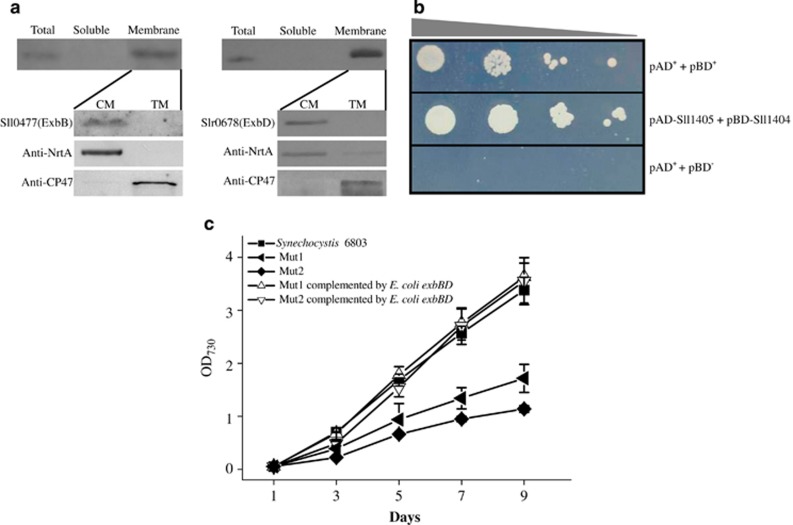
Although the encoding products of the exbB-exbD gene clusters possess similar amino-acid sequences to known ExbB and ExbD protein homologs, whether they are functional homologs requires further genetic or biochemical evidence. Thus, Synechocystis 6803 Mut1 and Mut2 were transformed with the expression plasmid containing exbB-exbD from E. coli K-12. Growth assays confirmed that E. coli ExbB-ExbD restored the iron-deficient phenotypes of Synechocystis 6803 (Figure 3c), providing direct genetic evidence that the putative gene clusters in Synechocystis 6803 are indeed ExbB-ExbD-encoding genes. To further elucidate the role of these ExbB-ExbD proteins in Synechocystis 6803, we investigated their sub-cellular location and interactions. Analysis of soluble and membrane protein fractions revealed that both ExbB (Sll0477) and ExbD (Slr0678) were localized at the CM (Figure 3a). Yeast two-hybrid assay showed that the yeast Saccharomyces AH109 transformed with plasmids pAD-Sll1405 and pBD-Sll1404 could grow on synthetic defined (SD) plates lacking tryptophan, leucine, histidine and adenine (SD/-Trp-Leu-His-Ade). This suggests a protein–protein interaction between Sll1404 (ExbB) and Sll1405 (ExbD) (Figure 3b). Given the high degree of similarity among the ExbB-ExbD homologs (Supplementary Figure S2B), we propose that the three exbB-exbD gene clusters encode three CM-located ExbB-ExbD complexes with overlapping functions in Synechocystis 6803.
Figure 3.
Localization and protein–protein interactions of ExbB and ExbD in Synechocystis 6803 and their functional comparison with Escherichia coli homologs. (a) Localization of ExbB and ExbD in Synechocystis 6803. NrtA and CP47 were selected as cytoplasmic membrane (CM) and thylakoid membrane (TM) marker proteins, respectively. (b) Protein–protein interaction analysis of ExbB and ExbD in Synechocystis 6803. The yeast transformants (20 μl each of cell suspensions diluted from optical density at 600 nm (OD600) 0.1 to 0.0001) expressing positive control plasmids (pAD+ +pBD+), negative control plasmids (pAD+ +pBD−) and detected plasmids (pAD-Sll1405+pBD-Sll1404) were grown on SD/-Trp-Leu-His-Ade agar plate for 3 days. (c) The growth curves of Synechocystis wild-type, Mut1, Mut2, Mut1 complemented by E. coli exbBD and Mut2 complemented by E. coli exbBD cultured under iron-deficient conditions. A full color version of this figure is available at The ISME Journal online.
ExbB-ExbD complexes are required for Fe′ uptake in Synechocystis 6803
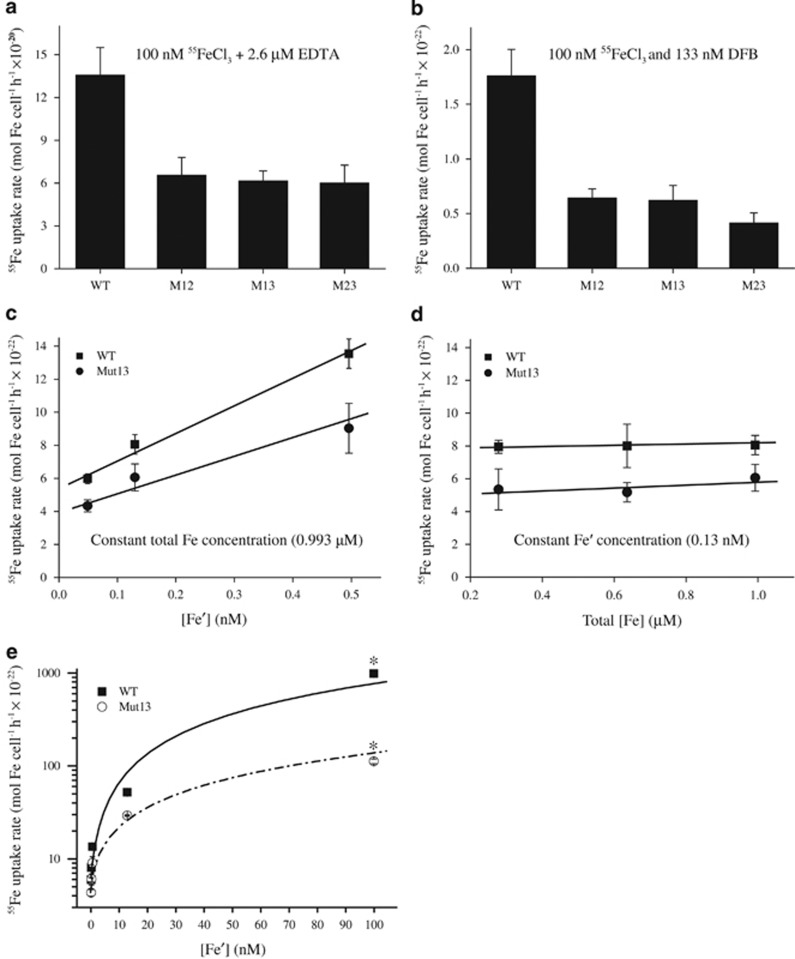
Hopkinson and Morel (2009) have reported that Synechocystis 6803 possesses neither siderophore biosynthesis nor siderophore transport genes within its genome. Kranzler et al. (2011) have proved direct evidence that Synechocystis 6803 does not produce siderophore during iron starvation. Interestingly, the species grows well in minimal medium without organic substrates (Supplementary Figure S1B). Like other photosynthetic microbes, Synechocystis 6803 is unable to transport Fe-EDTA complexes directly (Kranzler et al., 2011). Short-term measurements revealed that iron uptake rates were strongly affected by complexation. For example, at the same total iron concentration (100 nM), iron uptake was roughly 800-fold faster when added as an FeEDTA than when added as a DFB complex (Figures 4a and b). Unexpectedly, iron uptake rates under both conditions were two to three times slower in the mutant than in the wild-type cells. As FeEDTA is unavailable for transport, the substrates sequestered by the cells are probably the inorganic complexes and free Fe3+ (collectively Fe′) in equilibrium with FeEDTA or produced by cellular reduction of FeEDTA species. Thus the ExbBs and ExbDs of Synechocystis are required for iron uptake from both inorganic and organic (ferrioxamine) complexes. These results also indicated that Fe′ is a more available iron resource for Synechocystis 6803 than Fe-DFB.
Figure 4.
55Iron uptake rate of the Synechocystis 6803 exbBD double-mutant strains cultured in standard BG11 medium. (a, b) Cells were grown in BG11 medium to logarithmic phase, harvested, washed and re-suspended in iron-free BG11 medium lacking citric acid and ferric ammonium citrate. For free iron uptake rate measurement, 55FeCl3 was added to the cell solutions at 100 nM (3.7 kBq ml−1) complexed with 2.6 μM EDTA (a). For measurement of iron-siderophores uptake rate, 100 nM 55FeCl3 and 133 nM DFB were added (b). (c, d) Cells were grown in BG11 medium to logarithmic phase, and iron uptake experiments were conducted using the trace metal clean technique. The 55iron was precomplexed with EDTA over 24 h, as described in Kranzler et al. (2011). (c) Uptake rates of Synechocystis wild-type and Mut13 supplied with the same total iron concentration (0.993 μM) but varying free Fe′ concentrations. (d) Uptake rates of cells supplied with the same free Fe′ concentrations (0.13 nM) but different total iron concentration. (e) Uptake rates of Synechocystis wild-type and Mut13 supplied with different iron concentrations. Note the two additional measurements. The data marked with asterisk ‘*' were obtained using the same experimental procedure as the data of panels (c) and (d), but the medium lacked EDTA and all organic compounds.
TonB-ExbB-ExbD-dependent OM transporters are thought to be involved in uptake of organically chelated iron, such as siderophore-iron (Noinaj et al., 2010). To our knowledge, the involvement of this transport system in Fe′ uptake has not been experimentally verified. Therefore we evaluated the relationship between iron uptake and [Fe′] in exbB-exbD mutant and wild-type cells, using an EDTA-buffered system (Maldonado and Price, 1999). Cultures were grown to exponential phase in standard BG11 medium, and 55Fe was added (as FeCl3) at a total concentration of 0.993 μM preequilibrated with EDTA to achieve [Fe′] of 0.049, 0.130 and 0.496 nM (Figure 4c). Under these conditions, we calculated that >99% of the added iron was bound to EDTA. As shown in Figure 4c, the iron uptake rate increased with increasing [Fe′] in both the wild type and Mut13 but was suppressed in the mutants, being 67–75% that of the wild type under all conditions. Iron uptake rate was independent of FeEDTA concentration, confirming that this substrate is not transported (Figure 4d). These results strongly suggest that iron uptake by Synechocystis 6803 depends on [Fe′] rather than on total iron concentration and that ExbB-ExbD complexes are involved in Fe′ transport.
Iron uptake rates were also measured at much higher Fe′ concentrations: 1 μM FeCl3 buffered with 1.6 μM EDTA (12.8 nM [Fe′]); and 0.1 μM FeCl3 without EDTA (99.9 nM [Fe′]). Under these conditions, iron speciation was less well controlled than at high EDTA levels, but the transport rates showed the same pattern of dependence on ExbB-ExbD (Figure 4e). At the highest [Fe′] tested, the uptake rate by the mutant was reduced to 11% that of the wild type (see the points marked with an asterisk ‘*' in Figure 4e).
The distribution of ExbB-ExbD complexes shows their functional universality in diverse cyanobacterial species
Putative homologs of ExbB-ExbD proteins are found in almost all the cyanobacterial species whose genomes have been fully sequenced. The exceptions are some Prochlorococcus marinus and marine Synechococcus strains (Cyanobase, http://genome.kazusa.or.jp/cyanobase) (Table 2). The ExbB proteins show high amino-acid sequence similarity with the known ExbB protein Slr1404 (E-value <2 × e−8). The genes encoding ExbB and ExbD are closely located in the genomes of diverse cyanobacteria and range in copy number from 1 to 5 (Table 2). To determine whether cyanobacterial exbB-exbD genes perform the same function as in Synechocystis 6803, we complemented the mutant strains with homologs from a filamentous, multi-cellular, heterocyst-forming strain Anabaena sp. PCC 7120 and a unicellular marine strain Synechococcus sp. PCC 7002. Growth bioassays show that both of the ExbB-ExbD homologs rescued the iron-deficient phenotype of Synechocystis 6803 ExbB-ExbD mutant (Mut1) (Supplementary Figure S1C). Thus ExbB-ExbD protein complexes likely perform a similar function in Fe′ uptake of most cyanobacteria.
Table 2. Putative exbB and exbD genes in selected cyanobacterial species/strains.
| Species/strains |
Number of genes |
Accession number |
E values |
|||
|---|---|---|---|---|---|---|
| exbB | exbD | exbB | exbD | exbB | exbD | |
| Freshwater cyanobacteria | ||||||
| Anabaena sp. PCC 7120 | 3 | 2 | all5047 | all5046 | 4e–65 | 4e–27 |
| alr0643 | alr0644 | 6e–33 | 6e–13 | |||
| alr4587 | — | 1e–60 | — | |||
| Anabaena variabilis ATCC 29413 | 3 | 2 | Ava_2306 | Ava_2305 | 3e–65 | 5e–27 |
| Ava_4574 | Ava_4575 | 3e–33 | 7e–13 | |||
| Ava_2465 | — | 1e–61 | — | |||
| Arthrospira platensis NIES-39 | 1 | 1 | NIES39_E01480 | NIES39_E01470 | 2e–34 | 2e–17 |
| Chlorobium tepidum TLS | 2 | 2 | CT0633 | CT0634 | 1e–09 | 2e–05 |
| CT1586 | CT1584 | 8e–14 | 7e–04 | |||
| Cyanothece sp. PCC 8801 | 2 | 2 | PCC8801_3017 | PCC8801_3016 | 7e–77 | 3e–33 |
| PCC8801_3262 | PCC8801_3261 | 7e–36 | 8e–16 | |||
| Cyanothece sp. PCC 7424 | 2 | 2 | PCC7424_2391 | PCC7424_2392 | 3e–78 | 9e–35 |
| PCC7424_2011 | PCC7424_2012 | 9e–34 | 6e–16 | |||
| Cyanothece sp. PCC 7425 | 2 | 2 | Cyan7425_2418 | Cyan7425_2417 | 2e–67 | 1e–33 |
| Cyan7425_0843 | Cyan7425_0844 | 4e–35 | 1e–16 | |||
| Gloeobacter violaceus PCC 7421 | 3 | 3 | glr2402 | glr2403 | 1e–41 | 8e–18 |
| glr1387 | glr1388 | 1e–41 | 1e–21 | |||
| gll1141 | gll1140 | 4e–13 | 6e–11 | |||
| Microcystis aeruginosa NIES-843 | 2 | 1 | MAE08540 | MAE08550 | 8e–36 | 5e–16 |
| MAE43740 | — | 7e–16 | — | |||
| Nostoc punctiforme ATCC 29133 | 3 | 2 | Npun_R0782 | Npun_R0781 | 4e–65 | 5e–28 |
| Npun_R5174 | Npun_R5173 | 2e–31 | 1e–13 | |||
| Npun_R4966 | — | 1e–63 | — | |||
| Synechococcus elongates PCC 6301 | 1 | 1 | syc_1677_d | syc_1678_d | 1e–33 | 9e–37 |
| Synechococcus elongates PCC 7942 | 1 | 1 | Synpcc7942_2429 | Synpcc7942_2428 | 1e–33 | 1e–16 |
| Synechococcus sp. JA-3-3Ab | 1 | 1 | CYA_2815 | CYA_2816 | 6e–54 | 2e–26 |
| Synechococcus sp. JA -2-3B′a (2-13) | 1 | 1 | CYB_0819 | CYB_0820 | 3e–53 | 2e–25 |
| Thermosynechococcus elongatus BP-1 | 1 | 1 | tll0316 | tlr0055 | 5e–32 | 2e–19 |
| Marine cyanobacteria | ||||||
| Acaryochloris marina MBIC11017 | 5 | 6 | AM1_B0122 | AM1_B0121 | 5e–60 | 1e–29 |
| AM1_3411 | AM1_3410 | 9e–60 | 3e–31 | |||
| AM1_0171 | AM1_1070 | 9e–33 | 7e–10 | |||
| AM1_4903 | AM1_4902 | 2e–29 | 8e–15 | |||
| AM1_A0165 | AM1_A0164 | 1e–59 | 2e–28 | |||
| — | AM1_0169 | — | 3e–09 | |||
| Cyanothece sp. ATCC 51142 | 3 | 3 | cce_3054 | cce_3053 | 5e–79 | 2e–34 |
| cce_1116 | cce_1115 | 6e–36 | 4e–15 | |||
| cce_1159 | cce_1160 | 7e–32 | 4e–11 | |||
| Prochlorococcus marinus MIT9202 | 1 | 1 | P9202_71 (gb: EEE39300.1) | Unnamed (gb: EEE41044.1) | 3e-27 | 1e-15 |
| Synechococcus sp. PCC 7002 | 2 | 2 | SYNPCC7002_G0137 | SYNPCC7002_G0136 | 6e–70 | 2e–32 |
| SYNPCC7002_A1318 | SYNPCC7002_A1317 | 4e–29 | 2e–14 | |||
| Synechococcus sp. RCC307 | 1 | — | SynRCC307_1279 | — | 2e–14 | — |
| Synechococcus sp. WH7803 | 1 | — | SynWH7803_0707 | — | 2e–09 | — |
| Trichodesmium erythraeum IMS101 | 1 | 1 | Tery_4448 | Tery_4449 | 6e–35 | 1e–17 |
Discussion
The widespread distribution of ExbB-ExbD proteins among cyanobacteria and their functional complementarity suggests that inorganic iron maybe an important iron source for growth of many species. All the freshwater cyanobacterial strains whose genomes have been fully sequenced and many ecologically relevant marine cyanobacteria have this active transport system. For example, the coastal strain Synechococcus sp. PCC 7002 has two putative exbB-exbD gene clusters as does Trichodesmium erythraeum IMS101, an important nitrogen fixer. Prochlorococcus marinus MIT9202, isolated from low-iron waters near the equatorial Pacific Ocean, also has an ExbB-ExbD active transport system possibly reflecting the importance of inorganic iron in this habitat. Interestingly, most open ocean Synechococcus and Prochlorococcus strains do not have ExbB-ExbD homologs and so may rely on different mechanisms for inorganic iron uptake or on different chemical species of iron for nutrition. We hypothesize that the use of an ExbB-ExbD system for inorganic iron uptake may allow cyanobacteria to more tightly maintain iron homeostasis, particularly in variable environments where iron concentrations may range from limiting to sufficient. A regulated OM transporter may allow cyanobacteria to buffer fluctuating levels of iron in their environment and to more efficiently capture low levels of iron when it is limiting. Areas of the ocean experiencing upwelling, dust input and freshwater runoff as well as the epilimnia of lakes exposed to iron-rich hypolimnetic waters may be habitats where inorganic iron is variable and where such an inorganic iron transport system may be adaptive.
In this study, we experimentally identified CM-localized ExbB-ExbD complexes in the non-siderophore-producing cyanobacterium, Synechocystis 6803, and provided direct evidence that cyanobacteria sequester iron through the OM via an ExbB-ExbD-dependent transport system. In bacteria Ton transport systems, the ExbB-ExbD membrane protein is not a transporter, but a molecular complex that harvests energy from the ion electrochemical potential generated across the CM and transmits it to the TonB subunit to open a channel in the OM transporter. Our results suggest that ExbB-ExbD complexes from Synechocystis 6803 operate like those in bacteria, because E. coli homologs restore the Synechocystis mutants. Low sequence similarity between putative TonBs and OM transporters of Synechocystis and well-characterized models in Gram-negative bacteria have hampered our attempts to describe all the interacting protein partners of ExbB-ExbD complexes in the cyanobacteria. A mutant of the putative TonB protein, Slr1484, only showed moderate decline in iron uptake rate compared with the wild type (data not shown), suggesting that other unknown TonB proteins may exist in Synechocystis 6803. Identifying the OM transporters may be equally elusive, judging from the work of Katoh et al. (2001) who knocked out four putative OM candidates in the same cyanobacterial species but did not find an obvious phenotype compared with the wild type. Regardless of the complexity of TonB proteins and OM transporters, the interesting finding in the present study is that ExbB-ExbD complexes are essentially required by Synechocystis 6803 for Fe′ uptake. Our results also show that this ExbB-ExbD-mediated iron uptake strategy is probably prevalent among most cyanobacteria species. The Ton transport system has been investigated for over 30 years in non-photosynthetic bacteria, and it is generally thought to be involved in transport of organic compounds such as Fe chelators, as the compounds are too large to pass through porins (Noinaj et al., 2010). For the first time, we have shown that the ExbB-ExbD-dependent transport system has an essential role in inorganic iron uptake in cyanobacteria.
ExbB-ExbD complexes may be more essential for iron uptake in the photosynthetic cyanobacterium Synechocystis 6803 than in non-photosynthetic Gram-negative bacteria. In E. coli, for example, the mutant RA1051 (ΔexbBD::kan ΔtolQR), lacks the single ExbB-ExbD complex as well as another active transport system, TolQ-TolR, but nonetheless grows well in Luria–Bertani broth medium and only shows growth inhibition under low iron bioavailability (Brinkman and Larsen, 2008). Synechocystis 6803, on the other hand, has three essential exbB-exbD gene clusters that are functionally redundant and are regulated in a compensatory manne when one of the gene clusters is inactivated (Supplementary Figure S2E). Two factors may explain why ExbB-ExbD is essential in cyanobacteria but not in other Gram-negative bacteria. First, iron demand is much higher in photosynthetic cyanobacteria than in non-photosynthetic bacteria because of its use in the photosynthetic electron transport chain. Indeed, estimates are that the cyanobacteria require 10-fold more iron than bacteria of similar cell size to maintain photosynthetic activity (Raven et al., 1999; Shcolnick et al., 2009). Without TonB-ExbB-ExbD-dependent active uptake pathway, cyanobacterial cells might rely on passive transport of iron through porins, which may provide insufficient iron for photosynthesis. Mutant cells survived in glucose, albeit with reduced growth, indicating a relatively lower iron requirement under mixotrophic culture conditions (Figure 2d). Second, the substrate preference of the TonB-ExbB-ExbD system differs between cyanobacteria and non-photosynthetic bacteria. In E. coli, vitamin B12, nickel chelates and carbohydrates are also taken up by this active transport system as well as iron, and colicins and phages exploit the same transport system to gain entry to the cells (Miethke and Marahiel, 2007; Schauer et al., 2008). As autotrophic organisms, cyanobacteria require much more iron but few organic compounds for growth. The recipe of standard cyanobacterial BG11 medium usually excludes or contains very low concentration of cobalt, nickel and carbohydrates (Allen and Stanier, 1968; Stanier et al., 1971). In summary, cyanobacteria require ExbB-ExbD complexes for Fe′ transport for three reasons: they have a high iron demand for photosynthesis, they inhabit low-iron environments, and they have high substrate specificity for iron.
Several reports have suggested that cyanobacteria can extract free iron with high efficiency if free iron is available (Fujii et al., 2010, 2011; Kranzler et al., 2011), but until now, direct evidence that Fe′ uptake by cyanobacteria requires TonB-ExbB-ExbD-dependent transport system has been lacking. Although Fe-siderophores can be slowly sequestered by Synechocystis 6803 (Figure 4b), the role of siderophores in iron acquisition by cyanobacteria has probably been overrated in natural water systems (Hopkinson and Morel, 2009; Wirtz et al., 2010). As many cyanobacterial species other than Synechocystis 6803 do not excrete siderophores, the importance of siderophore-mediated iron uptake in cyanobacteria has been questioned. Hopkinson and Morel (2009) and Kranzler et al. (2011, 2014) suggested that cyanobacteria probably use uptake pathways involving a reduction step, rather than direct internalization of siderophores and other iron chelates. We hypothesize that organic chelators such as siderophores may provide iron pools, which maintain iron solubility and recruit iron in dilute water environments, while cyanobacteria prefer free iron and efficiently sequester it with high rate via TonB-ExbB-ExbD-dependent active transport system (see a hypothesized model shown in Figure 5). Thermal dissolution and photochemical reduction of siderophore-bound iron may provide a source of free Fe′ for uptake. Although both siderophore-mediated and reductive iron uptake pathway have been reported in cyanobacteria (Kranzler et al., 2012; Stevanovic et al., 2012), the latter seems to be the dominant pathway. OM transport of both siderophore-Fe (despite its low rate) and Fe′ are under the control of the TonB-ExbB-ExbD-dependent transport system (Figures 4a and b). Fe′ may be able to enter the cells by passive transport via OM porins at a very low rate, but the majority would be absorbed by energy-coupled active transport. As it crosses the OM, Fe′ maybe reduced in the periplasmic space before transport across the cyanobacterial plasma membrane (Kranzler et al., 2011, 2014). The findings in the present study provide new insights into the iron acquisition pathway of photosynthetic cyanobacteria in aqueous environments. The presence of unique OM receptors, TonBs and the mechanism of the TonB-ExbB-ExbD dependent Fe′ uptake system in cyanobacteria are currently under investigation in our lab.
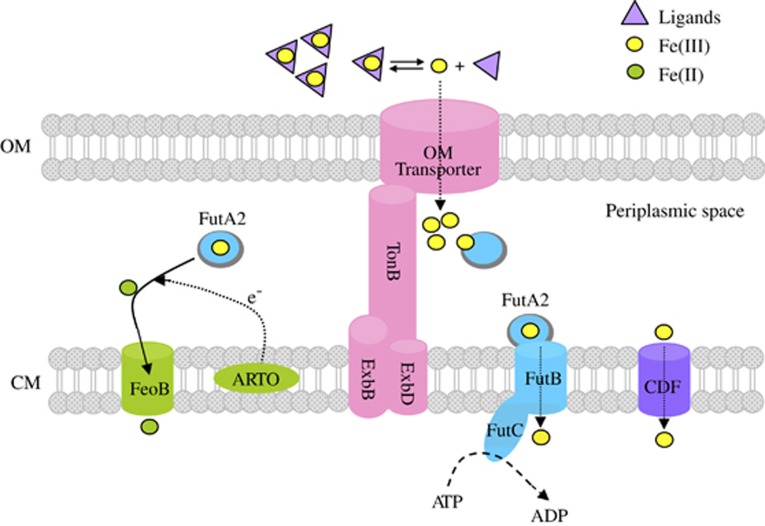
Figure 5.
Iron uptake pathway model for Synechocystis sp. PCC 6803. In oxygenic aquatic environments, iron exists primarily in organic complexes that increase the solubility of dissolved iron and buffer an extraordinarily low concentration of Fe′. Synechocystis cells efficiently sequester Fe(III) that in equilibrium with the organically complexed Fe pool through active transport via TonB-ExbB-ExbD-dependent transport system. Outer membrane (OM) transport of siderophore-Fe is also under control with the TonB-ExbB-ExbD-dependent transport system despite its low rate. Once Fe(III) crosses the OM, it is probably bound to periplasmic protein FutA2 and then reduced to Fe(II) by a reductive iron uptake pathway (Kranzler et al., 2014). Immediately, Fe(II) could be transported into CM by CM-located FeoB transporter. As the half-life of Fe(II) is very short, Fe(III) will be transported into CM by an ATP-binding cassette transporter FutB and FutC. The cation diffusion facilitator protein (CDF) will improve the Fe(III) transport across CM when cells were under iron deficiency (Jiang et al., 2012).
Acknowledgments
We are grateful to Professor Xudong Xu (Institute of Hydrobiology, Chinese Academy of Sciences) for kindly providing the antibody of Anti-CP47 and Anti-NrtA. This study was supported by the National Natural Science Foundation of China (No. 31100184) and Natural Sciences and Engineering Research Council of Canada.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Achilles KM, Church TM, Wilhelm SW, Luther GW, III, Hutchins DA. Bioavailability of iron to Trichodesmium colonies in the western subtropical Atlantic Ocean. Limnol Oceanogr. 2003;48:2250–2255. [Google Scholar]
- Allen MM, Stanier RY. Growth and division of some unicellular blue-green algae. J Gen Microbiol. 1968;51:199–202. doi: 10.1099/00221287-51-2-199. [DOI] [PubMed] [Google Scholar]
- Boyd P, Jickells T, Law CS, Blain S, Boyle EA, Buesseler KO, et al. Mesoscale iron enrichment experiments 1993–2005: synthesis and future directions. Science. 2007;315:612–617. doi: 10.1126/science.1131669. [DOI] [PubMed] [Google Scholar]
- Braun V, Herrmann C. Evolutionary relationship of uptake systems for biopolymers in Escherichia coli: cross-complementation between the TonB-ExbB-ExbD and the TolA-TolQ-TolR proteins. Mol Microbiol. 1993;8:261–268. doi: 10.1111/j.1365-2958.1993.tb01570.x. [DOI] [PubMed] [Google Scholar]
- Brinkman KK, Larsen RA. Interactions of the energy transducer TonB with noncognate energy-harvesting complexes. J Bacteriol. 2008;190:421–427. doi: 10.1128/JB.01093-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan SK, Smith BS, Venkatramani L, Xia D, Esser L, Palnitkar M, et al. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat Struct Biol. 1999;6:56–63. doi: 10.1038/4931. [DOI] [PubMed] [Google Scholar]
- Chappell PD, Moffett JW, Hynes AM, Webb EA. Molecular evidence of iron limitation and availability in the global diazotroph Trichodesmium. ISME J. 2012;6:1728–1739. doi: 10.1038/ismej.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowski PG, Barber RT, Smetacek VV. Biogeochemical controls and feedbacks on ocean primary production. Science. 1998;281:200–207. doi: 10.1126/science.281.5374.200. [DOI] [PubMed] [Google Scholar]
- Falkowski PG, Raven JA.2007Aquatic Photosynthesis2nd edn,Princeton University Press; ISBN 0-632-06139-1. [Google Scholar]
- Ferguson AD, Amezcua CA, Halabi NM, Chelliah Y, Rosen MK, Ranganathan R, Deisenhofer J. Signal transduction pathway of TonB-dependent transporters. Proc Natl Acad Sci USA. 2007;104:513–518. doi: 10.1073/pnas.0609887104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson AD, Chakraborty R, Smith BS, Esser L, van der Helm D, Deisenhofer J. Structural basis of gating by the outer membrane transporter FecA. Science. 2002;295:1715–1719. doi: 10.1126/science.1067313. [DOI] [PubMed] [Google Scholar]
- Ferguson AD, Deisenhofer J. Metal import through microbial membranes. Cell. 2004;116:15–24. doi: 10.1016/s0092-8674(03)01030-4. [DOI] [PubMed] [Google Scholar]
- Ferguson AD, Hofmann E, Coulton JW, Diederichs K, Welte W. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science. 1998;282:2215–2220. doi: 10.1126/science.282.5397.2215. [DOI] [PubMed] [Google Scholar]
- Fujii M, Dang TC, Rose AL, Omura T, Waite TD. Effect of light on iron uptake by the freshwater cyanobacterium Microcystis aeruginosa. Environ Sci Technol. 2011;45:1391–1398. doi: 10.1021/es103311h. [DOI] [PubMed] [Google Scholar]
- Fujii M, Rose AL, Omura T, Waite TD. Effect of Fe(II) and Fe(III) transformation kinetics on iron acquisition by a toxic strain of Microcystis aeruginosa. Environ Sci Technol. 2010;44:1980–1986. doi: 10.1021/es901315a. [DOI] [PubMed] [Google Scholar]
- Havens SM, Hassler CS, North RL, Guildford SJ, Silsbe G, Wilhelm SW, Twiss MR. Iron plays a role in nitrate drawdown by phytoplankton in Lake Erie surface waters as observed in lake-wide assessments. Can J Fish Aquat Sci. 2012;69:369–381. [Google Scholar]
- Hoiczyk E, Hansel A. Cyanobacterial cell walls: news from an unusual prokaryotic envelope. J Bacteriol. 2000;182:1191–1199. doi: 10.1128/jb.182.5.1191-1199.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkinson BM, Morel FMM. The role of siderophores in iron acquisition by photosynthetic marine microorganisms. Biometals. 2009;22:659–669. doi: 10.1007/s10534-009-9235-2. [DOI] [PubMed] [Google Scholar]
- Huang F, Fulda S, Hagemann M, Norling B. Proteomic screening of salt-stress-induced changes in plasma membranes of Synechocystis sp. strain PCC 6803. Proteomics. 2006;6:910–920. doi: 10.1002/pmic.200500114. [DOI] [PubMed] [Google Scholar]
- Ito Y, Butler A. Structure of synechobactins, new siderophores of the marine cyanobacterium Synechococcus sp. PCC 7002. Limnol Oceanogr. 2005;50:1918–1923. [Google Scholar]
- Jiang HB, Kong RQ, Xu XD. The N-acetylmuramic acid 6-phosphate etherase gene promotes the growth and cell differentiation in cyanobacteria under light-limiting conditions. J Bacteriol. 2010;192:2239–2245. doi: 10.1128/JB.01661-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HB, Lou WJ, Du HY, Price NM, Qiu BS. Sll1263, a unique cation diffusion facilitator protein that promotes iron uptake in the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Cell Physiol. 2012;53:1404–1417. doi: 10.1093/pcp/pcs086. [DOI] [PubMed] [Google Scholar]
- Jordan LD, Zhou Y, Smallwood CR, Lill Y, Ritchie K, Yip W, et al. Energy-dependent motion of TonB in the Gram-negative bacterial inner membrane. Proc Natl Acad Sci USA. 2013;110:11553–11558. doi: 10.1073/pnas.1304243110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh H, Hagino N, Grossman AR, Ogawa T. Genes essential to iron transport in the cyanobacterium Synechocystis sp. strain PCC 6803. J Bacteriol. 2001;183:2779–2784. doi: 10.1128/JB.183.9.2779-2784.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Fanucci GE, Cafiso DS. Substrate-dependent transmembrane signaling in TonB-dependent transporters is not conserved. Proc Natl Acad Sci USA. 2007;104:11975–11980. doi: 10.1073/pnas.0702172104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolber ZS, Barber RT, Coale KH, Fitzwater SE, Greene RM, Johnson KS, et al. Iron limitation of phytoplankton photosynthesis in the equatorial Pacific Ocean. Nature. 1994;371:145–149. [Google Scholar]
- Kranzler C, Lis H, Finkel OM, Schmetterer G, Shaked Y, Keren N. Coordinated transporter activity shapes high-affinity iron acquisition in cyanobacteria. ISME J. 2014;8:409–417. doi: 10.1038/ismej.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler C, Lis H, Shaked Y, Keren N. The role of reduction in iron uptake processes in a unicellular, planktonic cyanobacterium. Environ Microbiol. 2011;13:2990–2999. doi: 10.1111/j.1462-2920.2011.02572.x. [DOI] [PubMed] [Google Scholar]
- Kranzler C, Rudolf M, Keren N, Schleiff E. Chapter three—Iron in cyanobacteria. Adv Bot Res. 2012;65:57–105. [Google Scholar]
- Krieg S, Huché F, Diederichs K, Izadi-Pruneyre N, Lecroisey A, Wandersman C, et al. Heme uptake across the outer membrane as revealed by crystal structures of the receptor-hemophore complex. Proc Natl Acad Sci USA. 2009;106:1045–1050. doi: 10.1073/pnas.0809406106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado MT, Price NM. Utilization of iron bound to strong organic ligands by plankton communities in the subarctic Pacific Ocean. Deep Sea Res Part II. 1999;46:2447–2473. [Google Scholar]
- Martin JH, Coale KH, Johnson KS, Fitzwater SE, Gordon RM, Tanner SJ, et al. Testing the iron hypothesis in ecosystems of the equatorial Pacific Ocean. Nature. 1994;371:123–129. [Google Scholar]
- Mawji E, Gledhill M, Milton JA, Tarran GA, Ussher S, Thompson A, et al. Hydroxamate siderophores: occurrence and importance in the Atlantic Ocean. Environ Sci Technol. 2008;42:8675–8680. doi: 10.1021/es801884r. [DOI] [PubMed] [Google Scholar]
- Miethke M, Marahiel MA. Siderophore-based iron acquisition and pathogen control. Microb Mol Biol Rev. 2007;71:413–451. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirus O, Strauss S, Nicolaisen K, von Haeseler A, Schleiff E. TonB-dependent transporters and their occurrence in cyanobacteria. BMC Biol. 2009;7:68. doi: 10.1186/1741-7007-7-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel FMM, Price NM. The biogeochemical cycles of trace metals in the oceans. Science. 2003;300:944–947. doi: 10.1126/science.1083545. [DOI] [PubMed] [Google Scholar]
- Nicolaisen K, Moslavac S, Samborski A, Valdebenito M, Hantke K, Maldener L, et al. Alr0397 is an outer membrane transporter for the siderophore schizokinen in Anabaena sp. strain PCC 7120. J Bacteriol. 2008;190:7500–7507. doi: 10.1128/JB.01062-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noinaj N, Guillier M, Barnard TJ, Buchanan SK. TonB-dependent transporters: regulation, structure, and function. Annu Rev Microbiol. 2010;64:43–60. doi: 10.1146/annurev.micro.112408.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norling B, Zak E, Bl Andersson, Pakrasi HB. 2D-isolation of pure plasma and thylakoid membranes from the cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett. 1998;436:189–192. doi: 10.1016/s0014-5793(98)01123-5. [DOI] [PubMed] [Google Scholar]
- North RL, Guildford SJ, Smith REH, Havens SM, Twiss MR. Evidence for phosphorus, nitrogen, and iron colimitation of phytoplankton communities in Lake Erie. Limnol Oceanogr. 2007;52:315–328. [Google Scholar]
- Pawelek PD, Croteau N, Ng-Thow-Hing C, Khursigara CM, Moiseeva N, Allaire M, Coulton JW. Structure of TonB in complex with FhuA, E. coli outer membrane receptor. Science. 2006;312:1399–1402. doi: 10.1126/science.1128057. [DOI] [PubMed] [Google Scholar]
- Raven JA, Evans MCW, Korb RE. The role of trace metals in photosynthetic electron transport in O2-evolving organisms. Photosynth Res. 1999;60:111–149. [Google Scholar]
- Schauer K, Rodionov DA, de Reuse H. New substrates for TonB-dependent transport: do we only see the ‘tip of the iceberg'. Trends Biochem Sci. 2008;33:330–338. doi: 10.1016/j.tibs.2008.04.012. [DOI] [PubMed] [Google Scholar]
- Shcolnick S, Summerfield TC, Reytman L, Sherman LA, Keren N. The mechanism of iron homeostasis in the unicellular cyanobacterium Synechocystis sp. PCC 6803 and its relationship to oxidative stress. Plant Physiol. 2009;150:2045–2056. doi: 10.1104/pp.109.141853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultis DD, Purdy MD, Banchs CN, Wiener MC. Outer membrane active transport: structure of the BtuB:TonB complex. Science. 2006;312:1396–1399. doi: 10.1126/science.1127694. [DOI] [PubMed] [Google Scholar]
- Stanier RY, Kunisawa MM, Cohen-Bazire G. Purification and properties of unicellular blue-green algae (order Chroococcales) Bacteriol Rev. 1971;35:171–201. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevanovic M, Hahn A, Nicolaisen K, Mirus O, Schleiff E. The components of the putative iron transport system in the cyanobacterium Anabaena sp. PCC 7120. Environ Microbiol. 2012;14:1655–1670. doi: 10.1111/j.1462-2920.2011.02619.x. [DOI] [PubMed] [Google Scholar]
- Tang D, Morel FMM. Distinguishing between cellular and Fe-oxide associated trace elements in phytoplankton. Mar Chem. 2006;98:18–30. [Google Scholar]
- Westall J, Zachary JL, Morel FMM.1976MINEQL, a Computer Program for the Calculation of Chemical Equilibrium Composition of Aqueous SystemsTechnical Note no. 18Ralph M. Parsons Lab., MIT: Cambridge, MA, USA [Google Scholar]
- Wilhelm SW, King AL, Twining BS, LeCleir GR, DeBruyn JM, Strzepek RF, et al. Elemental quotas and physiology of a southwestern Pacific Ocean plankton community as a function of iron availability. Aquat Microb Ecol. 2013;68:185–194. [Google Scholar]
- Wirtz NL, Treble RG, Weger HG. Siderophore-independent iron uptake by iron-limited cells of the cyanobacterium Anabaena flos-aquae. J Phycol. 2010;46:947–957. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.