Background: Arginase and iNOS induction are key to macrophage polarization and function.
Results: ERK activation is needed for arginase induction in activated macrophages and the M2 phenotype.
Conclusion: MAP kinase family members have distinct and opposing roles in activating iNOS and arginase.
Significance: ERK and p38 may represent novel therapeutic targets for regulating macrophage phenotype in inflammatory diseases.
Keywords: c-Jun N-terminal Kinase (JNK), cyclic AMP (cAMP), Mitogen-activated Protein Kinase (MAPK), p38 MAPK, Toll-like Receptor 4 (TLR4), Macrophage Phenotype
Abstract
The mitogen-activated protein kinases (MAPK) have been shown to participate in iNOS induction following lipopolysaccharide (LPS) stimulation, while the role of MAPKs in the regulation of arginase remains unclear. We hypothesized that different MAPK family members are involved in iNOS and arginase expression following LPS stimulation. LPS-stimulated RAW 264.7 cells exhibited increased protein and mRNA levels for iNOS, arginase I, and arginase II; although the induction of arginase II was more robust than that for arginase I. A p38 inhibitor completely prevented iNOS expression while it only attenuated arginase II induction. In contrast, a MEK1/2 inhibitor (ERK pathway) completely abolished arginase II expression while actually enhancing iNOS induction in LPS-stimulated cells. Arginase II promoter activity was increased by ∼4-fold following LPS-stimulation, which was prevented by the ERK pathway inhibitor. Arginase II promoter activity was unaffected by a p38 inhibitor or JNK pathway interference. Transfection with a construct expressing a constitutively active RAS mutant increased LPS-induced arginase II promoter activity, while transfection with a vector expressing a dominant negative ERK2 mutant or a vector expressing MKP-3 inhibited the arginase II promoter activity. LPS-stimulated nitric oxide (NO) production was increased following siRNA-mediated knockdown of arginase II and decreased when arginase II was overexpressed. Our results demonstrate that while both the ERK and p38 pathways regulate arginase II induction in LPS-stimulated macrophages, iNOS induction by LPS is dependent on p38 activation. These results suggest that differential inhibition of the MAPK pathway may be a potential therapeutic strategy to regulate macrophage phenotype.
Introduction
Arginase I, arginase II, and inducible nitric-oxide synthase (iNOS)2 can be induced simultaneously by lipopolysaccharide (LPS) in macrophages (1). Classical activation of macrophages by IFN-γ, LPS, and/or TNF-α induces M1 polarization and the resultant up- regulation of pro-inflammatory cytokines, production of reactive oxygen species (ROS), and induction of iNOS (2, 3). On the other hand, exposure to Th2 cytokines leads to M2 macrophage polarization and the resultant up-regulation of a different set of chemokines and cytokines, as well as arginase (4, 5). Since arginase and iNOS share a common substrate, l- arginine, it has been suggested that arginase induction may regulate NO production by activated macrophages and thereby modulate the immune response (6, 7, 8). In addition to substrate competition, a recent study has also suggested that translational control of iNOS mRNA by arginase might be involved in regulating iNOS expression (9).
Arginase II is an extra-hepatic isoform of arginase that can be induced by LPS and/or 8-bromo-cAMP in murine macrophages (7). However, the biological importance of induction of arginase II in macrophages remains unclear. Induction of arginase II has been implicated in the attenuation of LPS/IFN-γ induced apoptosis, probably via competing for l-arginine with iNOS and thereby acting to suppress NO production (7). It has been reported that apoptotic cells may release a factor that induces arginase II to reduce NO production in macrophages (8). While the role of arginase induction in macrophages is not well understood, studies have suggested that arginase may be involved in switching the macrophage phenotype to M2 to facilitate healing (5, 10, 11).
The mitogen-activated protein kinases (MAPK) have been shown to participate in iNOS induction (12), while the role of MAPKs in the regulation of arginase expression in LPS-stimulated macrophages remains unclear. Previously we have shown that MAPK phosphatase-1 (MKP-1) modulates LPS-induced iNOS expression and switches arginine metabolism from NO production to urea production, at least partly through inactivating p38 and JNK (11). It has been suggested that activation of p38, rather than ERK, mediated LPS-induced iNOS expression in RAW 264.7 macrophages (12). Furthermore, it has been reported that IL-13 induced arginase I expression in the macrophage cell line J774A.1 is regulated by tyrosine kinases, cAMP/PKA, and p38 (4). In the present study, we have investigated the signal transduction pathways mediating arginase II induction in response to LPS in RAW264.7 macrophages. We found that ERK activation was pivotal for the induction of arginase II, while p38 is crucial for the induction of iNOS.
EXPERIMENTAL PROCEDURES
Cell Culture and Treatments
The murine macrophage cell line RAW 264.7 (American Type Culture Collection, Manassas, VA) was cultured in Dulbecco's modified Eagle's medium (DMEM; Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (FBS; Mediatech), 100 IU/ml penicillin and 100 μg/ml streptomycin (Mediatech) at 37 °C with 5% CO2.
For measurement of arginase I, arginase II, iNOS, nitrite, and luciferase assay, cells were incubated in complete medium or medium, containing 0.1 μg/ml LPS (serotype O127:B8, Sigma-Aldrich Inc.) or 0.5 mm 8-bromo-cyclic AMP (8-Br-cAMP, Enzo Life Sciences, Plymouth Meeting, PA) for 24 h before assay. For measurement of activation of the MAPK, cells were treated with vehicle, 0.1 μg/ml LPS, or 0.5 mm 8-bromo-cyclic AMP for different periods of time (0, 15, 30, 60, 120, 240, 360, or 480 min) before harvest. To investigate the effects of MAPK pathway inhibitors, cells were treated with U0126 (putative MEK1/2 inhibitor (ERK pathway), Promega, Madison, WI), SB203580 (putative p38 inhibitor, Enzo Life Sciences), SB600125 (putative JNK inhibitor, EMD Biosciences, San Diego, CA), or DMSO (as a negative control, Fisher Scientific) for 30 min, followed by the addition of vehicle, 0.1 μg/ml LPS, or 0.5 mm 8-bromo-cyclic AMP for 24 h.
Vectors
The 2113-bp fragment of the mouse arginase II promoter was obtained using PCR with murine genomic DNA. The PCR fragment was subcloned into pGL3 enhancer vector (Promega) to construct the mouse arginase II luciferase reporter vector (pGL3e-Arg2P), and the correct orientation was verified by sequencing. The following vectors were used in this study: vector pSRa-3xFlag-SrfI (13), vector pEBG-SrfI (14), vectors that constitutively express a constitutively active Ras mutant (pSRa-Ha-p21RasV12) (15), MKP3 (pSRa-3xFlag-MKP3), kinase-dead dominant negative ERK2 mutant (pcDNA-HA-ERK2K52R (16), a constitutively active SEK1 mutant pEBG-SEK1ED (17), and a kinase-dead SEK1 mutant (pEBG-SEK1KR) (17). The vector pSRa-3xFlag-MKP3 was constructed by cloning a PCR-amplified human MKP3 open reading frame into the SrfI cloning site as previously described (13). A vector expressing Renilla luciferase under the control of SV40 promoter pRL-SV40 was purchased from Promega.
Recombinant Adenovirus AdArgII Construction and Adenovirus Infection
The recombinant adenoviral vectors carrying the human arginase II gene (AdArgII) under the control of a CMV promoter were constructed using the AdEasy Adenoviral Vector System (Agilent Technologies, La Jolla, VA). The human arginase II cDNA clone, which contains the open reading frame and a long 3′-untranslated region, was purchased from Open Biosystems (Huntsville, AL). Briefly, the arginase II cDNA was cloned into a pShuttle-CMV vector, which was linearized, dephosphorylated, and then co-transformed with pAdEasy-1 vector into BJ5183 cells. The transformants containing the desired recombinant Ad plasmids were identified, and the authenticity of the clones were confirmed by sequencing. The recombinants were transfected into AD-293 cells to produce the primary viral stocks and this viral stock was used for further virus amplification in AD-293 cells. Viral stocks had titers ranging from 1–5 × 1010 plaque forming unites per ml (pfu/ml) and were stored at −80 °C.
For virus infection, RAW 264.7 cells were incubated in medium containing 80% DMEM, 10% FBS, and 10% L929 conditioned medium as a source for macrophage colony stimulating factor (M-CSF) at 37 °C with 5% CO2 for 48 h. RAW 264.7 cells were then infected with virus at MOI of 100 in DMEM for 2 h, followed by washing with PBS and then were incubated in complete DMEM medium for 24 h. Cells were then treated with or without 0.1 μg/ml LPS for 24 h. Medium was collected for nitrite assay, and cells were harvested for Western blotting.
siRNA Treatments
Mouse arginase II pre-designed siRNA (Intergrated DNA Technologies, Coralville, IA) was transfected into RAW 264.7 cells using Lipofectamine 2000 transfection reagent (Invitrogen) according to the manufacturer's instructions. Briefly, RAW 264.7 cells were seeded in 6-well plates 24 h before transfection to obtain 30–50% confluence at the time of transfection. Then the medium was replaced by complete medium and incubated for another 24 h. The cells were treated with the appropriate stimulation for an additional 24 h before harvest. Arginase II knockdown was verified by real-time PCR and Western blot analysis. A scramble Duplex siRNA (Integrated DNA Technologies) was used as a negative control.
Western Blot Analysis
RAW 264.7 cells were harvested in ice-cold lysis buffer containing 20 mm HEPES (pH 7.4), 50 mm β-glycerophosphate, 2 mm EGTA, 1 mm DTT, 10 mm NaF, 1 mm Na3VO4, 1% Triton X-100, 10% glycerol, 2 μm leupeptin, 2 μm aprotinin, and 1 mm phenolmethylsulfonyl fluoride (PMSF). Samples were centrifuged at 12,000 × g for 15 min, and the supernatants were collected and analyzed for total protein contents using the Bradford assay (Bio-Rad). The supernatants were stored at −80 °C for further study.
The cell extracts were assayed for arginase I, arginase II, iNOS, phospho-ERK (pERK), ERK, phospho-JNK (pJNK), JNK, phospho-p38 (pp38), p38, or β-actin protein by Western blot analysis. Briefly, aliquots of cell extracts containing equal amounts of protein were mixed with NuPAGE LDS sample buffer (Invitrogen), heated to 95 °C for 5 min, and then centrifuged at 10,000 × g at room temperature for 2 min. The aliquots of the supernatant were used for NuPAGE gel electrophoresis. The proteins were transferred to polyvinylidene difluoride membranes and blocked in Tris-buffered saline with 0.1% Tween (TBST, pH 7.6) containing 5% nonfat dried milk at room temperature for 1 h. The membranes were then incubated with the primary antibody, arginase I (1:500; Santa Cruz Biotechnology, Santa Cruz, CA), arginase II (1:500, Santa Cruz Biotechnology), iNOS (1:1,000; BD Transduction, San Jose, CA), pERK (1:1000, Cell Signaling Technology, Danvers, MA), ERK (1:500, BD Transduction), pJNK (1:1000, Cell Signaling Technology), JNK (1:1000, Cell Signaling Technology), pp38 (1:1000, Cell Signaling Technology), or p38 (1:1000, Cell Signaling Technology) overnight at 4 °C and then washed three times with TBST. The membranes were then incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (1:15,000; Bio-Rad) or HRP-conjugated goat anti-mouse IgG (1:10,000; Bio-Rad) for 1 h and washed three times with TBST. The protein bands were visualized using chemiluminescence (ECL reagent; GE Healthcare Biosciences, Piscataway, NJ). To control for protein loading, the blots were stripped using a Western ReProbe stripping buffer (G-Biosciences, St Louis, MO), and then were reprobed for β-actin (1:5,000; Sigma-Aldrich) as described above. The densities of the respective pMAPKs were normalized to their respective total MAPK, all other proteins were normalized to β-actin.
mRNA Isolation and Quantitative Real-time PCR
RNA was isolated from RAW 264.7 cells using Trizol (Invitrogen) following the manufacturer's protocol. Total RNA was treated with RQ1 RNase-free DNase (Promega) to eliminate any genomic DNA contamination and the mRNA was reverse-transcripted into cDNA using GoScript Reverse Transcriptase (Promega). Real-time PCR was performed on Chromo4 Real-time PCR System (Bio-Rad) using the Absolute Blue QPCR SYBR Green Mix + ROX (ThermoFisher Scientific, Pittsburgh, PA) according to the manufacturer's instruction. No template control and no reverse transcription control were used as negative controls. 18S was used as the reference gene. Forward and reverse primer sequences were as follows: murine arginase I, forward: 5′-TGG CTT TAA CCT TGG CTT GCT TCG-3′, reverse: 5′-AAA GAA CAA GCC CTT GGG AGG AGA-3′; murine arginase II, forward: 5′-ATA TGG TCC AGC TGC CAT TCG AGA-3′, reverse: 5′-TAA CCA CTT CAG CCA GTT CCT GGT-3′; murine iNOS, forward: 5′-CTG CTG GTG GTG ACA AGC ACA TTT-3′, reverse: 5′-ATG TCA TGA GCA AAG GCG CAG AAC-3′; murine 18S, forward: 5′-CCA GAG CGA AAG CAT TTG CCA AGA-3′, reverse: 5′-TCG GCA TCG TTT ATG GTC GGA ACT-3′. ΔΔCt method was used to calculate the fold-change in mRNA expression: ΔCt = Ct (target gene) − Ct (housekeeping gene); ΔΔCt = ΔCt (treatment) − ΔCt (control); fold change = 2(−ΔΔCt).
Nitrite Assay
The medium samples were measured in duplicate for nitrite using a chemiluminescence nitric oxide (NO) analyzer (model 280i, GE Analytical Instruments, Boulder, CO) as previously described (11). Briefly, 50 μl of sample was injected into a reaction chamber containing NaI in glacial acetic acid to reduce nitrite to NO. The NO gas was carried into the NO analyzer with a constant flow of helium gas. The analyzer was calibrated using a NaNO2 standard curve.
Dual-Luciferase Activity Assay
To measure reporter activity, the pGL3e-Arg2P reporter plasmid containing the mouse arginase II promoter linked to a Firefly luciferase gene was used. RAW 264.7 cells were co-transfected with pGL3e-Arg2P, pRL-SV40, and with or without pSRa-3xFlag-SrfI, pEBG-SrfI, pSRa-Ha-p21RasV12, pSRa-3xFlag-MKP3, pSRa-HA-ERK2K52R, pEBG-SEK1ED, or pEBG-SEK1KR using Lipofectamine 2000 transfection reagent according to the manufacturer's protocol. Briefly, cells were seeded in 24-well plates for 24 h to reach 90% confluent at the time of transfection. After transfection, cells were incubated for 24 h, followed by appropriate stimulation for 24 h. Cells were harvested and assayed for luciferase activity using the Dual-Luciferase Reporter Assay System (Promega) according to the manufacturer's instruction. Firefly luciferase was normalized to Renilla luciferase, and the normalized activities of the treatment groups were normalized to that of control to yield fold-change.
Statistical Analysis
Values are expressed as the mean ± S.E. of at least three independent experiments. A t test was used for experiments wherein only two groups were compared. For experiments with more than 2 groups a one-way ANOVA was used, and significant differences were identified using a Neuman-Keuls post hoc test. Differences were considered significant when p < 0.05.
RESULTS
Both LPS and cAMP Induced Arginase Expression While Only LPS Induced iNOS Expression
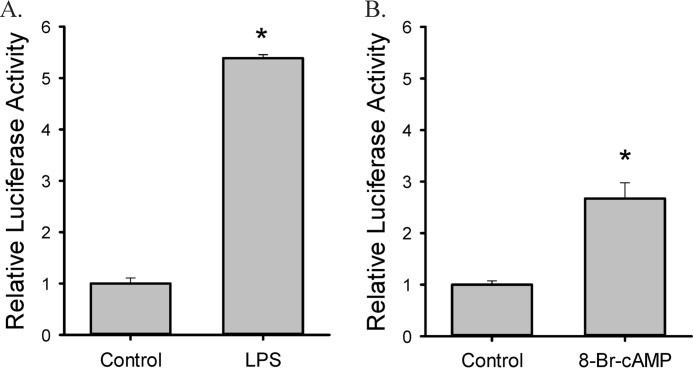
Treatment with 0.1 μg/ml LPS increased both mRNA and protein expression of arginase II, arginase I, and iNOS in RAW264.7 cells (Fig. 1, A and B). Arginase II expression was more robustly induced by LPS compared with arginase I expression. On the other hand, 0.5 mm 8-Br-cAMP stimulated arginase I and arginase II expression, but had little effect on iNOS expression (Fig. 1C&D). Both mRNA and protein expression of arginase II were more robustly induced by LPS than by 8-Br-cAMP, while 8-Br-cAMP had a greater effect on arginase I than did LPS (comparing Fig. 1, B to D). In arginase II promoter-luciferase reporter assays, both LPS and 8-Br-cAMP up-regulated arginase II promoter activity and the effect of LPS was more robust compared with that of 8-Br-cAMP (Fig. 2).
FIGURE 1.
Both LPS and 8-Br-cAMP treatment induce expression of arginase I and arginase II, however only LPS treatment induces iNOS expression, in RAW 264.7 macrophages. A, RAW 264.7 cells were treated with 0.1 μg/ml LPS and arginase I, arginase II, and iNOS protein expression were examined by Western blot. The bar graphs show densitometric quantification of protein levels, *, p < 0.01 LPS treated different from control. B, expression levels of mRNA for arginase I, arginase II, and iNOS from LPS-treated RAW 264.7 cells were examined by real-time PCR, please note different y axis scales. *, LPS different from control, p < 0.01 C, RAW 264.7 cells were treated with 0.5 mm 8-Br-cAMP and arginase I, arginase II, and iNOS protein expression were examined by Western blot. In these conditions iNOS protein levels were undetectable on Western blot. The bar graphs show densitometric quantification of arginase II and arginase I protein levels, *, p < 0.01 LPS treated different from control. D, mRNA expression of arginase I, arginase II, and iNOS from 8-Br-cAMP-treated RAW 264.7 cells by real-time PCR, please note different y axis scales. mRNA expression was expressed as fold relative to the control. *, 8-Br-cAMP different from control, p < 0.05.
FIGURE 2.
Both LPS and 8-Br-cAMP treatment increase ArgII promoter activity in RAW 264.7 macrophages. Cells were transfected with pGL3e-Arg2P (the arginase II promoter-Firefly luciferase reporter) and pRL-SV40 (SV40-Renilla luciferase reporter). Data represent three independent experiments. *, significantly different from control, p < 0.05.
MAPKs Are Involved in LPS-stimulated Arginase Expression
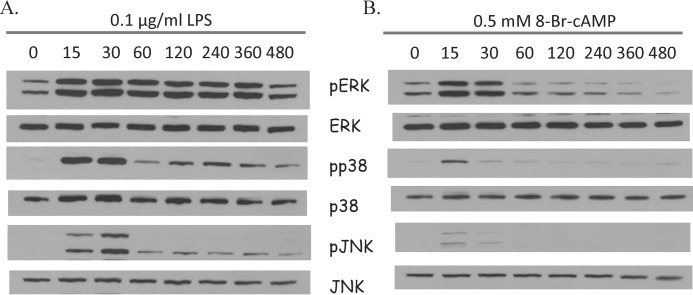
To investigate the effects of LPS or cAMP on the activation of MAPK, RAW 264.7 cells were treated with LPS or 8-Br-cAMP for different times up to 8 h (480 min). Western blot results showed that ERK, p38, and JNK were activated (phosphorylated) to maximum levels 15–30 min after LPS stimulation or 8-Br-cAMP treatment (Fig. 3). LPS stimulation resulted in a more robust and longer lasting MAPK activation than did 8-Br-cAMP (Fig. 3).
FIGURE 3.
Time-dependent effects of LPS and 8-Br-cAMP on MAPK expression in RAW 264.7 macrophages. A, RAW 264.7 cells were treated with 0.1 μg/ml LPS for 0, 15, 30, 60, 120, 240, 360, or 480 min and protein expression of pERK, ERK, pp38, p38, pJNK, and JNK were determined by Western blot. B, RAW 264.7 cells were treated with 0.5 mm 8-Br-cAMP for 0, 15, 30, 60, 120, 240, 360, or 480 min and expression of pERK, ERK, pp38, p38, pJNK, and JNK protein were examined by Western blot. Representative Westerns shown from three independent experiments.
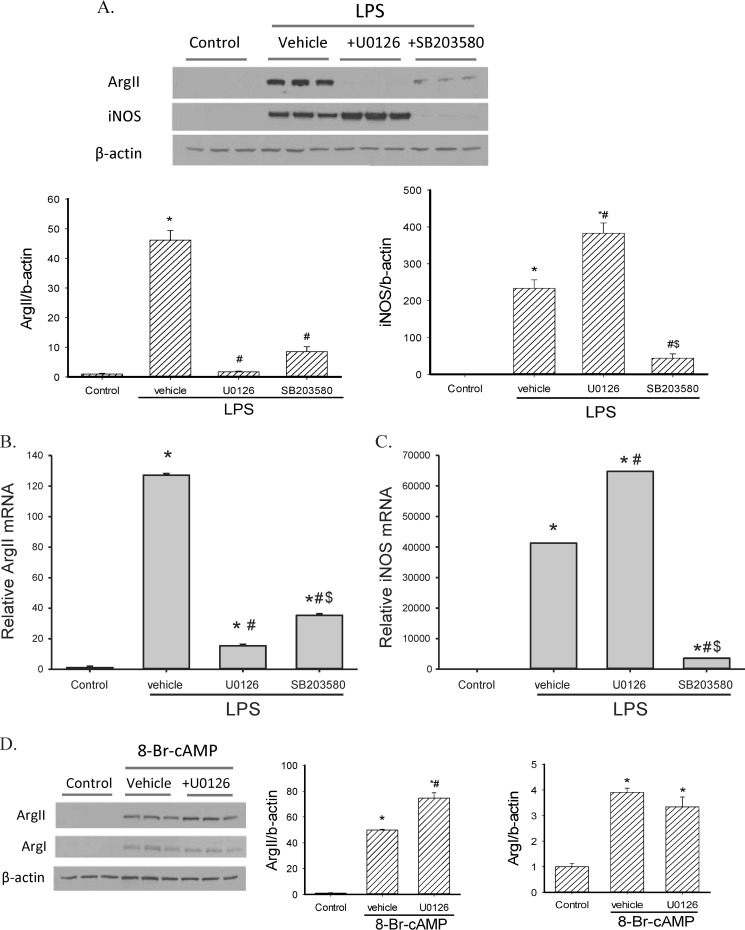
To investigate the role of MAPKs in the induction of arginase and iNOS following LPS- stimulation, RAW 264.7 cells were pretreated with an ERK pathway inhibitor U0126, or a p38 inhibitor SB203580, 30 min before LPS stimulation. Both Western blot and real-time PCR results showed that U0126 inhibited LPS-induced arginase II expression (Fig. 4, A and B), while having no inhibitory effect on iNOS induction following LPS stimulation. In fact, U0126 actually increased iNOS mRNA and protein levels (Fig. 4, A and C). Conversely, SB203580 prevented LPS-induced iNOS expression but had less of an effect on the LPS-induced expression of arginase II than did U0126 (Fig. 4). We also added both U0126 and SB203580 to some RAW 264.7 cells stimulated with LPS. The effect on iNOS protein and mRNA levels were similar to that seen with SB203850 alone, and the effect on arginase II protein and mRNA levels were similar to that seen with U0126 alone (data not shown). These results suggest that ERK is necessary for LPS-induced arginase II expression while p38 is required for LPS-induced iNOS induction. In 8-Br-cAMP-stimulated RAW 264.7 cells, U0126 slightly enhanced aginase II expression but had little effect on arginase I expression (Fig. 4D), indicating that cAMP and LPS engage different pathways to induce arginase expression.
FIGURE 4.
Inhibition of ERK prevented LPS-induced arginase II expression, while augmenting LPS-induced iNOS expression in RAW 264.7 macrophages. A, RAW 264.7 cells were pretreated with either DMSO (as a negative control), 10 μm U0126 (ERK pathway inhibitor) or 10 μm SB203580 (p38 inhibitor) for 30 min, followed by 0.1 μg/ml LPS for 24 h. Western blot showed that U0126 prevented LPS-induced arginase II expression while SB203580 prevented LPS-induced iNOS expression. Representative Western blot from three independent experiments. The bar graphs below the representative Western blot show densitometric quantification of protein levels, *, LPS treated different from control, p < 0.01; #, LPS-treated inhibitor different from LPS-treated vehicle, p < 0.01; $, SB203580 different from U0126, p < 0.01. B and C, mRNA expression of arginase II (B) and iNOS (C) in MAPK inhibitor-pretreated LPS-stimulated RAW 264.7 cells by real-time PCR. Arginase II mRNA expression was suppressed by U0126 and iNOS expression was inhibited by SB203580 in LPS-stimulated cells. *, LPS treatment different from control, p < 0.001. #, U0126 different from vehicle, p < 0.001. $, SB203580 different from U0126, p < 0.001. There was little effect of either U0126 or SB203580 on arginase I expression (data not shown). D, RAW 264.7 cells were pretreated with 10 μm U0126 or DMSO, followed by 0.5 mm 8-Br-cAMP for 24 h. Protein expression of arginase I, arginase II, and iNOS were examined by Western blot. Arginase II and Arginase I expression were induced by 8-Br-cAMP while iNOS protein was undetectable (data not shown). Representative Western blot from three independent experiments. The bar graphs to the right of the representative Western blot show densitometric quantification of protein levels, *, 8-Br-cAMP treated different from control, p < 0.01; #, U0126 different from vehicle, p < 0.05.
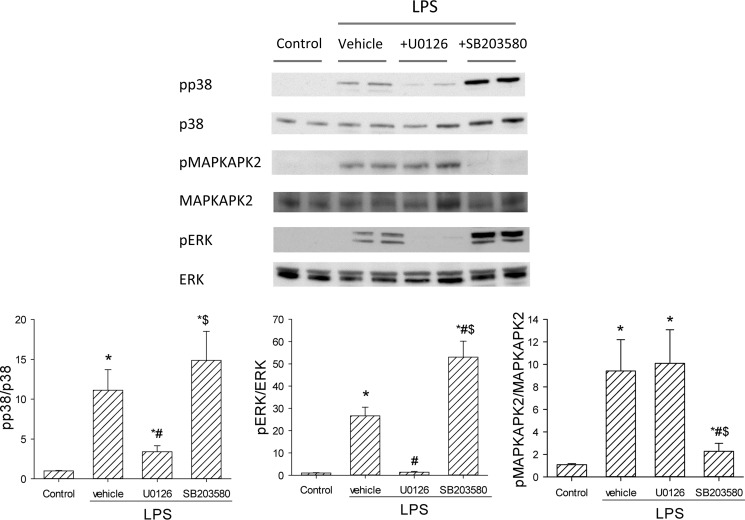
Given the differential effects of U0126 and SB203580 on arginase II and iNOS, we verified the inhibition of the inhibitors on the two MAPK pathways. We examined the phosphorylation of MAPK-activated protein kinase 2 (MAPKAPK2), the downstream target of p38, to confirm p38 inhibition by SB203580. Likewise, ERK phosphorylation was assessed to examine the inhibitory effect of U0126 on MEK1/2, the upstream activator of ERK1/2. RAW 264.7 cells were treated with vehicle (DMSO), U0126, or SB203580 for 30 min and then stimulated with LPS for 30 min. Protein was harvested for Western blotting for pMAPKAPK2 and total MAPKAPK2, and pERK and total ERK. As expected, the MEK1/2 inhibitor, U0126, prevented the appearance of pERK, but had little effect on pMAPKAPK2 levels (Fig. 5). While SB203580 increased pERK levels, it substantially attenuated pMAPKAPK2 levels (Fig. 5).
FIGURE 5.
The effects of U0126 and SB203580 on LPS-induced MAPK pathways. Cells were treated with vehicle, U0126, or SB203580 for 30 min and then stimulated with LPS for 30 min. Representative Western blots for pMAPKAPK2 and total MAPKAPK2, and pERK and total ERK are shown. Below the Western blots are the densitometric data, *, LPS different from control, p < 0.01; #, inhibitor different from vehicle, p < 0.05; $, SB203580 different from U0126, p < 0.05.
ERK Modulates LPS-induced Arginase II Expression
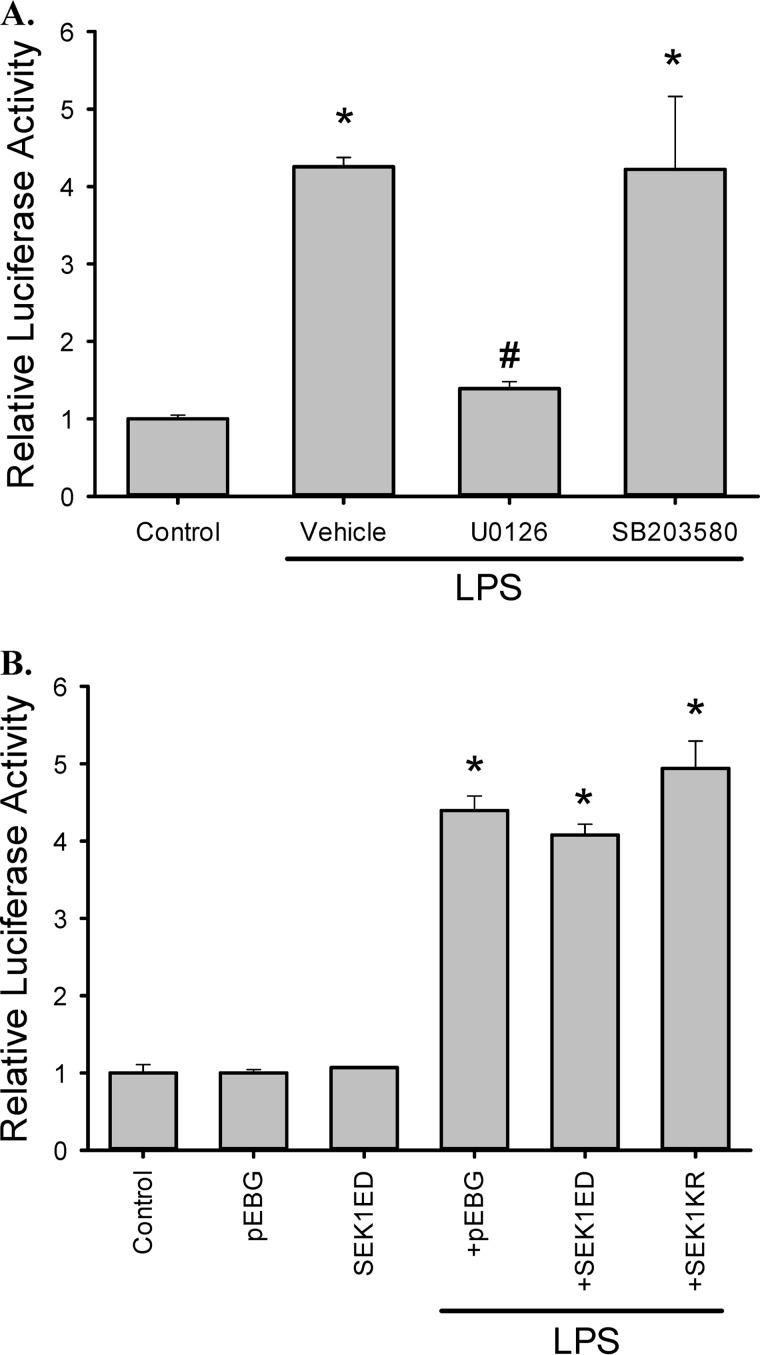
To further define the role of the ERK pathway in the regulation of arginase II expression in LPS-stimulated RAW 264.7 cells, we assessed the effects of MAPK pathway inhibition on the activity of the arginase II promoter. RAW264.7 cells were transfected with the arginase II promoter-luciferase reporter pGL3e-Arg2P together with the pRL-SV40 (an internal transfection control). These cells were pre-treated with U0126, or with SB203580, and then stimulated with LPS. As shown in Fig. 6A, LPS induced a ∼4-fold increase in arginase II promoter activity, which was prevented by U0126. These results are consistent with the Western blot and real-time PCR results of LPS-stimulated macrophages pre-treated with U0126. Interestingly, SB203580 had little effect on promoter activity, although p38 substantially decreased the levels of Arginase II protein and mRNA in LPS-stimulated cells (Fig. 4, A and B). We also investigated the role of the JNK pathway in regulating arginase II expression. Co-transfection experiments with either a dominant positive JNK activator (SEK1ED, a constitutively active SEK1 mutant) or a dominant negative JNK inhibitor (SEK1KR) showed that JNK had no significant effect on either basal or LPS-mediated luciferase activity (Fig. 6B), suggesting that JNK plays little role in LPS-mediated arginase II induction.
FIGURE 6.
ERK inhibition prevented LPS-induced arginase II promoter activity. A, RAW 264.7 cells transfected with pGL3e-Arg2P and pRL-SV40 were treated with DMSO, 10 μm U0126, or 10 μm SB20358 for 30 min, followed by 0.1 μg/ml LPS for 24 h. Dual-luciferase activity assay was used to examine reporter activity. *, different from control, p < 0.01. #, U0126 different from vehicle, p < 0.01. B, experiments were repeated using dominant positive or dominant negative JNK, and the results suggest that JNK has little effect on arginase II promoter activity in RAW 264.7 cells. Cells were transfected with pGL3e-Arg2P, pRL-SV40, and either an empty vector pEBG, a JNK dominant positive vector pEBG-SEK1ED, or a JNK dominant negative vector pEBG-SEK1KR. Subsequently, cells were treated with control or 0.1 μg/ml LPS for 24 h and then dual-luciferase activity assays were performed. *, different from control, p < 0.05.
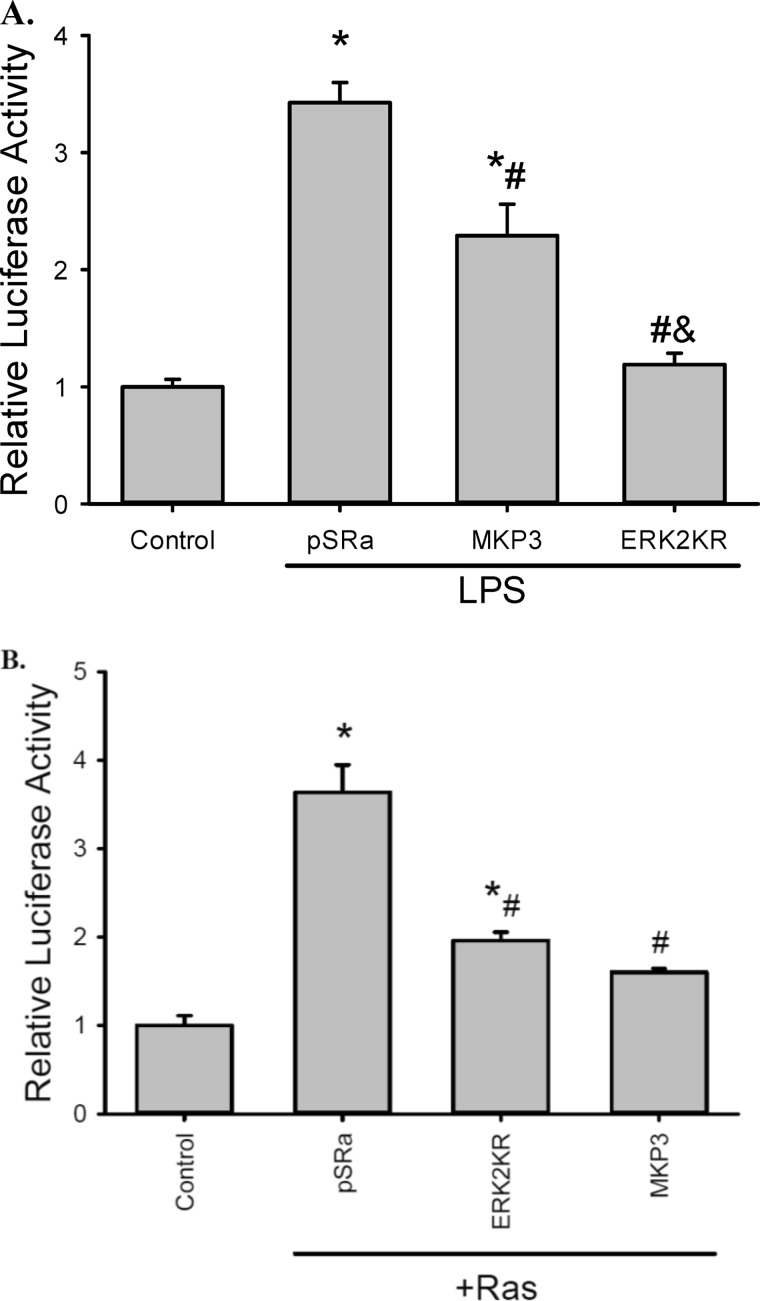
To further investigate the importance of ERK in arginase induction by LPS, the arginase II promoter reporter was transfected into RAW264.7 cells with a vector expressing a dominant negative ERK2 (ERK2K52R), a construct expressing MKP3 (an ERK-specific MAPK phosphatase), or an empty vector (pSRa). ERK2K52R is a kinase dead mutant, which inhibits the ERK pathway by competing with the endogenous ERK. MKP3 inactivates ERK by catalyzing the dephosphorylation of the tyrosine and threonine residues of ERK. Expression of MKP3 resulted in lower arginase II promoter activity following LPS stimulation (Fig. 7A). The ERK2K52R mutant completely prevented LPS-induced arginase II promoter activation (Fig. 7A). These results are consistent with the pharmacological inhibition studies.
FIGURE 7.
ERK signaling is required for LPS-induced arginase II promoter activity. A, RAW 264.7 cells were transfected with pGL3e-Arg2P, pRL-SV40, and either an empty vector pSRa-3xFlag-SrfI (pSRa), a MKP3 expression vector pSRa-3xFlag-MKP3 (MKP3, MKP3 preferentially de-phosphorylates ERK), or an ERK2 dominant negative vector pcDNA-HA-ERK2K52R (ERK2KR). Cells were then incubated in control media or media containing 0.1 μg/ml LPS for 24 h before the luciferase assay. *, different from control, p < 0.005; #, different from empty vector, p < 0.005; and ERK2KR different from MKP3, p < 0.005. B, Ras induces arginase II promoter activity. RAW 264.7 cells were transfected with pGL3e-Arg2P, pRL-SV40, and combination of a constitutively active Ras-expressing vector (pSRa-Ha-p21RasV12 mutant, designated as Ras) and either pSRa-3xFlag-SrfI (pSRa), pSRa-3xFlag-MKP3 (MKP3), or pSRa-HA-ERK2K52R (ERK2KR). *, different from control, p < 0.001; #, different from the combination of RasV12 and pSRa-3xFlag-SrfI.
On the other hand, co-transfection of pGL3e-Arg2P and pRL-SV40 with a constitutive Ras mutant construct (RasV12), which persistently activates the Raf/ERK cascade (15, 18), resulted in an increase in the arginase II-luciferase reporter activity (Fig. 7B). Importantly, the stimulatory effect of the dominant positive Ras mutant on arginase II promoter activity was substantially attenuated when the dominant negative ERK2K52R or MKP3 was co-expressed in these cells (Fig. 7B). These results strongly indicate that ERK activation is crucial for the induction of arginase II in LPS-stimulated RAW264.7 cells.
To determine if U0126 or SB203580 treatment would have an effect on arginase II mRNA half-life following LPS stimulation, RAW 264.7 cells treated with LPS for 24 h, then either vehicle, U0126, or SB203580 were added for 1 h to inhibit either the ERK or the p38 pathway. Subsequently, actinomycin D was added to the cells to block mRNA synthesis, and total RNA was harvested at 0, 1, 2, 4, 8, and 12 h for real-time PCR determination of Arginase II mRNA levels. The arginase II mRNA from RAW 264.7 cells treated with LPS had a relatively long half-life of ∼9.5 h (Fig. 8). However, in RAW 264.7 cells pretreated with SB203580 the arginase II mRNA half-life was only ∼3.2 h, while in RAW cells pretreated with U0126 the arginase II mRNA half-life was only ∼1.2 h (Fig. 8). These results suggest that arginase II mRNA stability is also regulated by the ERK and p38 pathways.
FIGURE 8.
Inhibiting the ERK or p38 pathway decreased arginase II mRNA half-life in LPS treated RAW cells. RAW cells were incubated for 24 h with LPS, and then either vehicle (closed circles), SB203580 (gray triangles), or U0126 (gray circles) added 60 min prior to adding actinomycin D to the medium, and RNA was harvested at 0, 1, 2, 4, 8, and 12 h for real-time PCR determination of arginase II mRNA normalized to 18 S RNA. The half-life from the exponential fit of the data were 9.5 h for LPS-treated (solid line), 3.2 h for LPS + SB203580-treated (dashed line), and 1.2 h for LPS + U0126-treated cells (gray line).
Preventing LPS-induced Arginase II Expression Increases LPS-induced NO Production
To investigate whether down-regulation of arginase II affects NO production in LPS-stimulated macrophages, RAW 264.7 cells were transfected with an arginase II siRNA or a scramble siRNA, and then treated with LPS for 24 h. Western blot results showed that the arginase II siRNA prevented LPS-induced arginase II expression with no significant effect on LPS-induced iNOS expression (Fig. 9A). As expected, nitrite production from LPS-treated cells was significantly greater than in control cells, which was associated with the induction of iNOS (Fig. 9, A and B). LPS-induced nitrite production was substantially greater in cells transfected with the arginase II siRNA compared with either scramble transfected or non-transfected cells (Fig. 9B).
FIGURE 9.
Preventing LPS-induced arginase II expression using an siRNA results in augmented LPS-induced NO production. RAW 264.7 cells were transfected with an arginase II siRNA or a scramble control siRNA and then treated with vehicle or 0.1 μg/ml LPS for 24 h. Medium was harvested for nitrite measurement and protein was harvested for Western blotting. A, arginase II and iNOS protein expression were examined by Western blot, and the arginase II siRNA prevented LPS-induced arginase II expression. The bar graphs show the densitometric data. *, different from scramble no LPS, p < 0.05; #, different from scramble +, LPS, p < 0.01; $, ArgIIsiRNA + LPS different from ArgIIsiRNA, p < 0.05. B, nitrite production in RAW 264.7 cells following treatment with either a scramble or arginase II siRNA. *, different from control, p < 0.001; #, ArgIIsiRNA different from either scramble or no transfection, p < 0.005.
Overexpressing Arginase II Decreases LPS-induced NO Production
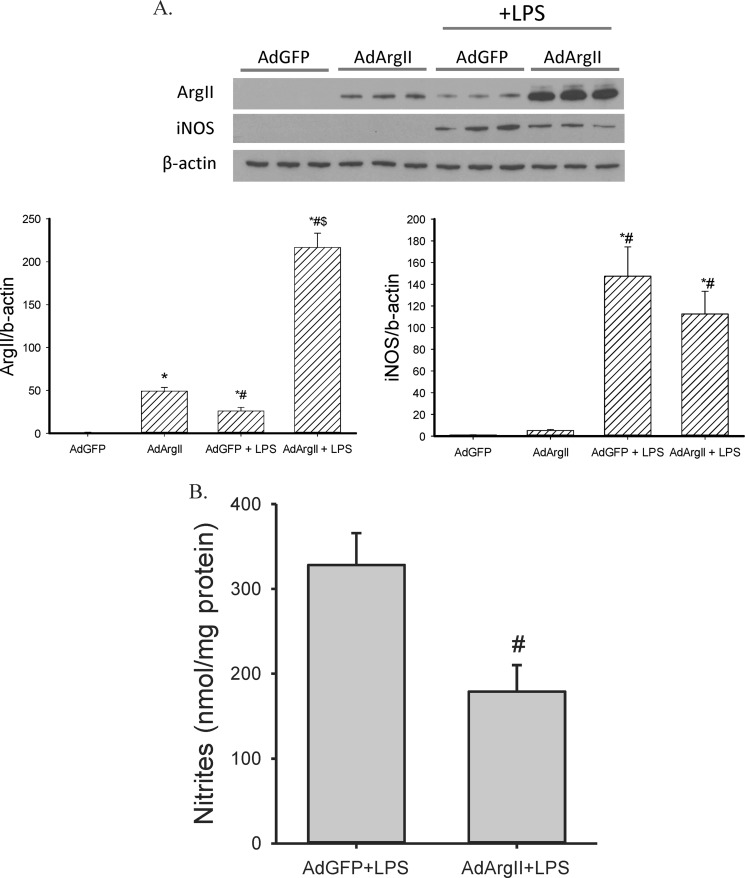
As the expression of αV integrins is low in resting macrophages, we utilized the transfection methods of Foxwell et al. (19) to overexpress arginase II. We first cultured RAW264.7 cells in medium containing M-CSF for 48 h, and then infected these cells with an adenovirus expressing arginase II (AdArgII), using an MOI of 100. In the absence of LPS treatment, arginase II was only detected in cells transfected with AdArgII but not in cells transfected with AdGFP. Following LPS stimulation, arginase II levels were substantially elevated in cells transfected with either AdGFP or AdArgII. Note that while AdGFP-transfected cells expressed detectable levels of arginase II, arginase II levels were substantially higher in the AdArgII-transfected cells following LPS treatment (Fig. 10A). Transfection of AdArgII had no significant effect on LPS-induced iNOS protein levels (Fig. 10A). Nitrite production following LPS stimulation was significantly lower in AdArgII transfected cells then in AdGFP-transfected cells (Fig. 10B).
FIGURE 10.
Overexpressing arginase II suppressed LPS-induced NO production in RAW 264.7 cells. Cells were incubated in conditioned media for 48 h, then transfected with an adenoviral construct containing either arginase II (AdArgII) or green fluorescent protein (AdGFP) for 2 h, washed, and allowed to recover overnight, then the cells were treated with vehicle or 0.1 μg/ml LPS for 24 h. Media were harvested for nitrite measurement and protein was harvested for Western blot. A, arginase II and iNOS protein expression was examined by Western blot, and AdArgII resulted in greater Arginase II protein expression without affecting iNOS protein levels. The bar graphs show the densitometric data. *, different from AdGFP no LPS, p < 0.05; #, AdArgII+LPS different from AdGFP+LPS, p < 0.01; $, AdArgII +LPS different from AdArgII, p < 0.01. B, nitrite production from LPS-treated RAW 264.7 cells transfected with AdGFP or AdArgII. #, AdArgII different from AdGFP, p < 0.05.
DISCUSSION
In the present study, we demonstrated that different members of the MAPK family play different roles in modulating iNOS and arginase expression in LPS-stimulated RAW 264.7 macrophages. Inhibition of p38 not only completely prevented iNOS protein expression but also markedly attenuated arginase II protein expression in LPS-stimulated RAW 264.7 cells (Fig. 4, A and B). On the other hand, inhibition of the ERK pathway abolished arginase II expression while it actually modestly enhanced iNOS expression in these cells (Fig. 4, A and C). Consistent with the differential effects of MAPKs on the protein expression of these two arginine utilizing enzymes, we also observed that arginase II promoter activity following LPS-stimulation was primarily dependent on the ERK pathway, with little contribution from the p38 or JNK pathway (Fig. 6A). The critical role of the ERK pathway in mediating arginase II induction was further supported by the finding that arginase II promoter activity in LPS-activated RAW 264.7 macrophages is significantly inhibited by expressing either a dominant negative ERK2 mutant (ERK2K52R) or MKP-3, an ERK-specific phosphatase (Fig. 7). Additionally, we found that expression of a constitutively active RasV12 mutant enhances arginase II promoter activity in the absence of LPS (Fig. 7, lower graph). RasV12 has been shown to activate several pathways including ERK, JNK, and p38 as well as the PI3 kinase pathways (15, 20, 21, 22, 23). Because the stimulatory effect of Ras activation is largely abolished by the dominant negative ERK2 mutant and MKP-3, both of which selectively target the ERK pathway (Fig. 7B), we conclude that ERK is likely the mediator for arginase promoter activation by the oncogenic Ras mutant. Since arginase II competes with iNOS to modulate NO production, differential inhibition of the MAPK pathways may represent a potential therapeutic strategy to regulate macrophage phenotype. Indeed, we found that siRNA-mediated knockdown of arginase II enhanced the production of nitrites (Fig. 9). In contrast, adenovirus-mediated arginase II overexpression significantly attenuated the production of nitrites (Fig. 10). Taken together, our studies raise the interesting possibility that the balance and interplay between the ERK and p38 pathways may influence arginine metabolism by LPS-activated macrophages.
Signaling Pathways Regulating Arginase II Expression
It is clear that the ERK pathway is crucial for arginase II expression. U0126 almost completely abolished arginase II induction by LPS, significantly inhibited the promoter activity of arginase II, and significantly decreased arginase II mRNA half-life in LPS-stimulated cells (Figs. 4, 6A, and 8). However, JNK does not appear to play a significantly role in the regulation of arginase II. On the other hand, p38 also plays a role in arginase II induction. The pre-treatment of cells with SB203580 markedly decreased LPS-induced arginase protein and mRNA levels (Fig. 4, A and B), while having little effect on arginase II promoter activity (Fig. 6A) SB203580 did decrease arginase II mRNA half-life (Fig. 8). We speculate that it is possible that the arginase II promoter may lack a p38-responsive element regulating the induction of the endogenous arginase II gene. Although the promoter we used in our assays is 2113 bp long, it is always possible that a p38-responsive element may be located further upstream or downstream of the sequence we utilized. Second, our data are consistent with p38 regulating arginase II expression via a post-transcriptional mechanism as has been reported for p38 regulation of COX-2 (24). Consistent with our findings, p38 has been shown to enhance the stability of mRNA species containing AU-rich elements (ARE) at their 3′-un-translational regions (25). Moreover, p38 can also promote the translation of mRNA species containing AREs (25). Since murine arginase II mRNA in RAW264.7 cells has an ARE (AUUUA) in its 3′-un-translated region, it is plausible that p38 may regulate the expression of arginase II through a similar mechanism. This is further supported by the observation that the arginase II level in AdArgII-transfected RAW264.7 cells following LPS stimulation is far higher than the LPS-induced endogenous arginase II protein levels, and the levels of exogenous arginase II protein in unstimulated AdArgII-transfected cells (Fig. 10A). Expression of the human arginase II in the AdArgII construct is driven by a CMV promoter, which should not be regulated by LPS. The dramatic increase in arginase II protein levels is likely to be due to the stimulatory effects of LPS on the exogenously expressed human arginase mRNA or protein. The exogenously expressed human arginase II contains four AREs: three AUUUA and one WWAUUUAWW elements (where W = A or U), predicted by the ARE searching program. The AREs interact with ARE-binding proteins, including TTP, AUF1, and HuR, and regulate mRNA stability and translation in a p38-dependent manner (25). By modulating the stability and translation of arginase II mRNA, p38 may enhance arginase II expression of both the endogenously and exogenously expressed arginase II mRNA. This mechanism may also explain the observation that SB203580 partially blocks the induction of arginase II in LPS-stimulated cells (Fig. 4A) but has no effect on arginase II promoter activity (Fig. 6A).
While the ERK pathway is crucial for the induction of arginase II in response to LPS, this pathway does not play a significant role in arginase induction in response to 8-bromo-cAMP. The MEK1/2 inhibitor U0126 had little effect on cAMP-induced arginase expression (Figs. 2 and 4D), suggesting that LPS and cAMP utilize distinct pathways to activate arginase II expression. A plausible explanation for the lack of contribution from the ERK pathway in 8-bromo-cAMP-stimulated cells is that 8-bromo-cAMP leads to a weak and transient ERK activation while LPS leads to a robust and sustained ERK activation. Because the ERK pathway is only mildly activated for a relatively short time window, it is not surprising that U0126 did not significantly affect arginase II expression in response to 8-bromo-cAMP. Additionally, in the arginase II promoter there are putative cis-elements for a variety of transcription factors, including cAMP-response elements, AP-1, C/EBP, and SP-1 sites.
Interplay between the p38 and ERK Pathways and the Expression of Arginase versus iNOS
An interesting observation from this study is that the MEK1/2 inhibitor U0126 actually augmented LPS-induced iNOS expression (Fig. 4, A and C), although it almost completely prevented arginase II induction. A possible explanation is that U0126 abolishes ERK-mediated MKP-1 induction, thus prolonging the activity of p38. Previously, we have shown that MKP-1 induction in response to LPS is substantially attenuated in the presence of U0126, which coincides with increased p38 activity (13). Additionally, knock-out of MKP-1 in macrophages markedly enhances iNOS expression induced by LPS (26). Thus, it is likely that by regulating the expression of MKP-1, the ERK pathway inhibits the p38 pathway, effectively attenuating iNOS expression. Additionally, by enhancing the expression of arginase II, the ERK pathway may further limit the bioavailability of arginine to iNOS and thereby restrain the production of NO. In the scenario of bacterial infection, this could be very important. Upon the detection of invading bacteria, the early function of macrophages is to kill the invading bacteria primarily through the production of reactive oxygen and reactive nitrogen species (27). Following the elimination of the invading bacteria the macrophages participate in the repair of damaged tissues resulting from the bacterial invasion by up-regulating arginase activity (5, 8). The regulation of iNOS versus arginase expression through the interplay of the ERK and p38 pathways offers a perfect window to study intricate immune regulatory mechanisms.
Arginase versus iNOS in Macrophage Phenotype
In terms of the biological function of arginase induction in activated macrophages, most studies have examined the role of arginase in the regulation of iNOS-derived NO production via substrate limitation to iNOS (6, 28, 29) or via translational regulation (9). IL-13 has been found to decrease NO production due to both arginase induction and iNOS down-regulation (4). One effect of LPS-induced arginase expression in macrophages could be to decrease iNOS-derived NO-mediated apoptosis (30) by decreasing NO production via substrate competition with iNOS (1, 7). Consistent with a role of LPS-induced arginase II in regulating NO production we found that suppressing arginase II expression increased NO production and overexpressing arginase II decreased NO production in LPS-treated RAW 264.7 macrophages. It could also be that NO-induced apoptosis in LPS-treated macrophages causes the release of some factor or factors from apoptotic cells. Candidate factors include apoptotic cell-derived factor sphingosine-1-phosphate (S1P), which activates CREB through ERK signaling leading to arginase II induction and thereby inhibiting further iNOS activity (4, 8, 31). In any case, switching arginine metabolism from iNOS to arginase would effectively change the macrophage phenotype from M1 to M2 (10, 11).
TLRs play a crucial role in the detection of invading microbial pathogens and regulation of innate and adaptive immunity. As the immune defense system needs to maintain an adequate balance between activation to eliminate pathogens and inhibition to avoid detrimental inflammatory responses, the TLR signaling cascade must be tightly regulated. At least five levels of negative regulation on the TLR signaling cascade have so far been uncovered. These range from extracellular decoy receptors, to membrane-bound suppressors, degradation of TLRs, dissociation of adaptor complexes, intracellular inhibitors, and TLR-induced apoptosis (32, 33). By constraining the signaling process via constitutively acting negative regulators or eliciting negative-feedback mechanisms, the immune system synchronizes the positive activation and negative regulation of signal transduction to avert potentially harmful immunological consequences (34). An interesting common feature of the regulation is that when a positive pathway driving the inflammatory response is evoked a negative pathway limiting the response is often simultaneously enlisted. For example, TLR4 activation elicits the production of cytokines via activating NF-κB and MAPKs, it also enhances the expression of the negative regulators IκB and MKP-1 which terminate NF-κB and MAPKs. TLR4 activation also stimulates the PI3K/Akt pathway, which serve to terminate the inflammatory responses (35). Our finding that TLR activation induces both iNOS and arginase further highlights the intricacy of the regulatory mechanisms at the iNOS level to ensure the balance between activating the antimicrobial immune defense and limiting collateral tissue damage.
Summary
We found that in RAW 246.7 macrophage cells that LPS treatment results in iNOS induction largely via p38 and arginase II induction via ERK. The LPS-induced up-regulation of arginase II protein resulted in lower levels of NO production by iNOS. These findings suggest that LPS-induced arginase II induction is vital for regulating NO production in LPS stimulated macrophages, and that ERK activation may be necessary for regulating macrophage phenotype. Our results suggest that the differential effects of p38 and ERK on iNOS and arginase II induction, respectively, may offer potential therapeutic targets to influence macrophage phenotype in inflammatory diseases. Taken together, our results demonstrate for the first time that ERK activation is necessary and sufficient for arginase II induction in LPS-stimulated RAW 264.7 macrophages, while iNOS induction depends on p38 activation.
Acknowledgments
We thank Leonard I. Zon for providing the SEK1 expression vector, Michael Wigler for providing the constitutively active Ras mutant, and Michael Weber for providing the dominant negative ERK2 mutant construct.
This study was supported by Grant Number R01HL075261 from the NHLBI and Grant Number R01AI068956 from the NIAID, National Institutes of Health.
- iNOS
- inducible nitric-oxide synthase
- LPS
- lipopolysaccharide
- ROS
- reactive oxygen species
- NO
- nitric oxide.
REFERENCES
- 1. Mori M. (2007) Regulation of nitric oxide synthesis and apoptosis by arginase and arginine recycling. J. Nutr. 137, 1616S-1620S [DOI] [PubMed] [Google Scholar]
- 2. Pelgrin P., Surprenant A. (2009) Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1P release through pyrophosphates. EMBO J. 28, 2114–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ricardo S. D., van Goor H., Eddy A. A. (2008) Macrophage diversity in renal injury and repair. J. Clin. Invest. 118, 3522–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chang C., Zoghi B., Liao J. C., Kuo L. (2000) The involvement of tyrosine kinases, cyclic AMP/protein kinase A, and p38 mitogen-activated protein kinase in IL-13-mediated arginase I induction in macrophages: its implications in IL-13-inhibited nitric oxide production. J. Immunol. 165, 2134–2141 [DOI] [PubMed] [Google Scholar]
- 5. Gordon S. (2003) Alternative activation of macrophages. Nat Rev Immunol 3, 23–35 [DOI] [PubMed] [Google Scholar]
- 6. Chang C. I., Liao J. C., Kuo L. (1998) Arginase modulates nitric oxide production in activated macrophages. Am. J. Physiol. Heart Circ. Physiol. 274, H342-H348 [DOI] [PubMed] [Google Scholar]
- 7. Gotoh T., Mori M. (1999) Arginase II downregulates nitric oxide (NO) production and prevents NO-mediated apoptosis in murine macrophage-derived RAW 264.7 cells. J. Cell Biol. 144, 427–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Johann A. M., Barra V., Kuhn A., Weigert A., von Knethen A., Brüne B. (2007) Apoptotic cells induce arginase II in macrophages, thereby attenuating NO production. FASEB J. 21, 2704–2712 [DOI] [PubMed] [Google Scholar]
- 9. Lee J., Ryu H., Ferrante R. J., Morris S. M., Jr., Ratan R. R. (2003) Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc. Natl. Acad. Sci. U.S.A. 100, 4843–4848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Douda D. N., Palaniyar N. (2010) Pulmonary collectins, arginases and inducible NOS regulate nitric oxide-mediated antibacterial defense and macrophage polarization. The Open Nitric Oxide J. 2, 69–76 [Google Scholar]
- 11. Nelin L. D., Wang X., Zhao Q., Chicoine L. G., Young T. L., Hatch D. M., English B. K., Liu Y. (2007) MKP-1 switches arginine metabolism from nitric oxide synthase to arginase following endotoxin challenge. Am. J. Physiol. Cell Physiol. 293, 632–640 [DOI] [PubMed] [Google Scholar]
- 12. Chen C., Wang J. (1999) p38 but not p44/42 mitogen-activated protein kinase is required for nitric oxide synthase induction mediated by lipopolysaccharide in RAW 264.7 macrophages. Mol. Pharmacol. 55, 481–488 [PubMed] [Google Scholar]
- 13. Chen P., Hutter D., Yang X., Gorospe M., Davis R. J., Liu Y. (2001) Discordance between the binding affinity of mitogen-activated protein kinase subfamily members for MAP kinase phosphatase-2 and their ability to activate the phosphatase catalytically. J. Biol. Chem. 276, 29440–29449 [DOI] [PubMed] [Google Scholar]
- 14. Chen P., Hutter D., Liu P., Liu Y. (2002) A mammalian expression system for rapid production and purification of active MAP kinase phosphatases. Protein Expr. Purif. 24, 481–488 [DOI] [PubMed] [Google Scholar]
- 15. White M. A., Nicolette C., Minden A., Polverino A, Van Aelst L., Karin M., Wigler M. H. (1995) Multiple Ras functions can contribute to mammalian cell transformation. Cell 80, 533–541 [DOI] [PubMed] [Google Scholar]
- 16. Rossomando A., Wu J., Weber M. J., Sturgill T. W. (1992) The phorbol ester-dependent activator of the mitogen-activated protein kinase p42mapk is a kinase with specificity for the threonine and tyrosine regulatory sites. Proc. Natl. Acad. Sci. U.S.A. 89, 5221–5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sánchez I., Hughes R. T., Mayer B. J., Yee K., Woodgett J. R., Avruch J., Kyriakis J. M., Zon L. I. (1994) Role of SAPK/ERK kinase-1 in the stress-activated pathway regulating transcription factor c-Jun. Nature 372, 794–798 [DOI] [PubMed] [Google Scholar]
- 18. Joneson T., White M. A., Wigler M. H., Bar-Sagi D. (1996) Stimulation of membrane ruffling and MAP kinase activation by distinct effectors of RAS. Science 271, 810–812 [DOI] [PubMed] [Google Scholar]
- 19. Foxwell B., Browne K., Bondeson J., Clarke C., de Martin R., Brennan F., Feldmann M. (1998) Efficient adenoviral infection with IκBα reveals that macrophage tumor necrosis factor-α production in rheumatoid arthritis is NF-KB dependent. Proc. Natl. Acad. Sci. U.S.A. 95, 8211–8215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dérijard B., Hibi M., Wu I. H., Barrett T., Su B., Deng T., Karin M., Davis R. J. (1994) JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell 76, 1025–1037 [DOI] [PubMed] [Google Scholar]
- 21. Deng Q., Liao R., Wu B. L., Sun P. (2004) High intensity ras signaling induces premature senescence by activating p38 pathway in primary human fibroblasts. J. Biol. Chem. 279, 1050–1059 [DOI] [PubMed] [Google Scholar]
- 22. Yan J., Roy S., Apolloni A., Lane A., Hancock J. F. (1998) Ras isoforms vary in their ability to activate Raf-1 and phosphoinositide 3-kinase. J. Biol. Chem. 273, 24052–24056. [DOI] [PubMed] [Google Scholar]
- 23. Downward J. (1998) Mechanisms and consequences of activation of protein kinase B/Akt. Curr. Opin. Cell Biol. 10, 262–267 [DOI] [PubMed] [Google Scholar]
- 24. Monick M. M., Robeff P. K., Butler N. S., Flaherty D. M., Carter A. B., Peterson M. W., Hunninghake G. W. (2002) Phosphatidylinositol 3-kinase activity negatively regulates stability of cyclooxygenase 2 mRNA. J. Biol. Chem. 277, 32992–33000 [DOI] [PubMed] [Google Scholar]
- 25. Dean J. L., Sully G., Clark A. R., Saklatvala J. (2004) The involvement of AU-rich element-binding proteins in p38 mitogen-activated protein kinase pathway-mediated mRNA stabilisation. Cell Signal 16, 1113–1121 [DOI] [PubMed] [Google Scholar]
- 26. Wang X., Zhao Q., Matta R., Meng X., Liu X., Liu C. G., Nelin L. D., Liu Y. (2009) Inducible nitric oxide synthase expression is regulated by mitogen-activated protein kinase phposphatase-1. J. Biol. Chem. 284, 27123–27134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee T. Y., Lee K. C., Chen S. Y., Chang H. H. (2009) 6-gingerol inhibits ROS and iNOS through the suppression of PKC-α and NF-κB pathways in lipopolysaccaride-stimulated mouse macrophages. Biochem. Biophys. Res. Commun. 382, 134–139 [DOI] [PubMed] [Google Scholar]
- 28. Das P., Lahiri A., Lahiri A., Chakravortty D. (2010) Modulation of the arginase pathway in the context of microbial pathogenesis: a metabolic enzyme moonlighting as an immune modulator. PLoS Pathogens 6, 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kasmi K. C. E., Qualls J. E., Pesce J. T., Smith A. M., Thompson R. W., Henao-Tamayo M., Basaraba R. J., König T., Schleicher U., Koo M., Kaplan G., Fitzgerald K. A., Tuomanen E. I., Orme I. M., Kanneganti T., Bogdan C., Wynn T. A., Murray P. J. (2008) Toll-like receptor-induced arginase I in macrophages thwarts effective immunity against intracellular pathogens. Nat. Immunol. 9, 1399–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gotoh T., Oyadomari S., Mori K., Mori M. (2002) Nitric oxide-induced apoptosis in RAW 264.7 macrophages is mediated by endoplasmic reticulum stress pathway involving ATF6 and CHOP. J. Biol. Chem. 277, 12343–12350 [DOI] [PubMed] [Google Scholar]
- 31. Barra V., Kuhn A., von Knethen A., Weigert A., Brüne B. (2011) Apoptotic cell-derived factors induce arginase II expression in murine macrophages by activating ERK5/CREB. Cell Mol Life Sci 68, 1815–1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liew F. Y., Xu D., Brint E. K., O'Neill L. A. (2005) Negative regulation of toll-like receptor-mediated immune responses. Nat Rev Immunol 5, 446–458 [DOI] [PubMed] [Google Scholar]
- 33. Kondo T., Kawai T., Akira S. (2012) Dissecting negative regulation of Toll-like receptor signaling. Trends Immunol 33, 449–458 [DOI] [PubMed] [Google Scholar]
- 34. Lang T., Mansell A. (2007) The negative regulation of Toll-like receptor and associated pathways. Immunol Cell Biol. 85, 425–434 [DOI] [PubMed] [Google Scholar]
- 35. Morris M. C., Gilliam E. A., Button J., Li L. (2014) Dynamic modulation of innate immune response by varying dosages of lipopolysaccharide (LPS) in human monocytic cells. J. Biol. Chem. 289, 21584–21590 [DOI] [PMC free article] [PubMed] [Google Scholar]