Abstract
A breakdown in self-tolerance underlies autoimmune destruction of β-cells and type 1 diabetes. A cure by restoring β-cell mass is limited by the availability of transplantable β-cells and the need for chronic immunosuppression. Evidence indicates that inhibiting costimulation through the PD-1/PD-L1 pathway is central to immune tolerance. We therefore tested whether induction of islet neogenesis in the liver, protected by PD-L1–driven tolerance, reverses diabetes in NOD mice. We demonstrated a robust induction of neo-islets in the liver of diabetic NOD mice by gene transfer of Neurogenin3, the islet-defining factor, along with betacellulin, an islet growth factor. These neo-islets expressed all the major pancreatic hormones and transcription factors. However, an enduring restoration of glucose-stimulated insulin secretion and euglycemia occurs only when tolerance is also induced by the targeted overexpression of PD-L1 in the neo-islets, which results in inhibition of proliferation and increased apoptosis of infiltrating CD4+ T cells. Further analysis revealed an inhibition of cytokine production from lymphocytes isolated from the liver but not from the spleen of treated mice, indicating that treatment did not result in generalized immunosuppression. This treatment strategy leads to persistence of functional neo-islets that resist autoimmune destruction and consequently an enduring reversal of diabetes in NOD mice.
Introduction
Restoration of functional β-cell mass to cure type 1 diabetes (T1D) has been limited by a lack of long-lasting transplantable β-cells (1). The long-term success of islet transplantation is limited by the requirement for chronic immunosuppression, limited donor availability, and eventual graft failure (2). Although immunosuppressive regimens have been optimized, they lead to generalized immunosuppression, with some of the drugs themselves being β-cell toxic (3). Targeted immunomodulation, without systemic immunosuppression, to prevent islet destruction by autoimmunity still remains an elusive goal.
T-effector cells mediate the autoimmune destruction of β-cells in T1D, although the mechanisms underlying this loss of self-tolerance remains poorly understood. Studies have highlighted the central role of the programmed death-1/programmed death ligand-1 (PD-1/PD-L1) pathway in the induction and maintenance of peripheral tolerance in autoimmune diabetes (4–9). The engagement of PD-L1, expressed normally by β-cells, with PD-1 on T-effector cells leads to truncation of the T-cell receptor (TCR) signal by inhibiting the required costimulation pathways and limits cytolysis by local self-reactive T cells (10,11) in both native and transplanted islets (12–14). In addition, NOD transgenic mice constitutively expressing PD-L1 under the human insulin promoter were significantly protected from diabetes (15), attesting to the tolerogenic role of the PD-1/PD-L1 pathway.
Although induction of islet neogenesis is an attractive approach to the restoration of β-cell mass, it still requires immunomodulation to prevent autoimmune destruction of the induced neo-islets. We have demonstrated previously that delivery of the islet lineage–determining gene Neurogenin3 (Ngn3) with the islet growth factor gene betacellulin (Btc) using helper-dependent adenoviral (HDAd) vectors induces ectopic islet neogenesis in the periportal regions of the liver that is sufficient to reverse insulin-deficient diabetes in streptozotocin-induced diabetic mice (16,17). However, in NOD mice, this regimen does not lead to a diabetes reversal due to autoimmune-mediated destruction of the induced neo-islets. In this study, we demonstrate that targeted induction of tolerance by overexpression of PD-L1 in the newly induced β-cells promotes β-cell long-term survival, leading to a reversal of diabetes in NOD mice with restoration of glucose tolerance. We show that this tolerance is due to a local reduction in the number and activation of CD4+ T cells only in the periportal regions surrounding the neo-islets. This study demonstrates that tolerance can be conferred to Ngn3-induced islet neogenesis by inhibition of costimulation with PD-L1 to effectively reverse T1D.
Research Design and Methods
Animals
NOD/ShiLtJ and NOD.CB17-Prkdcscid/J (NOD-Scid) mice (The Jackson Laboratory) were housed under standard conditions. All animal protocols were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine. Nonfasting body weight and blood glucose were monitored weekly at ∼9 a.m. The vectors encoding Ngn3 (HDAd-Ngn3), Btc (HDAd-Btc), and RIP-PD-L1 (HDAd-PD-L1) were generated on serotype 5 as described previously (16). Total vector dose was maintained at 7 × 1011 viral particles (vp) in all treatment groups as follows: [5 × 1011 vp Ngn3 + 1 × 1011 vp Btc + 1 × 1011 vp empty vector]; [5 × 1011 vp Ngn3 + 1 × 1011 vp Btc + 1 × 1011 vp PD-L1]; or [1 × 1011 vp PD-L1 + 6 × 1011 vp empty vector]. HDAd vectors were injected intravenously through the tail vein within 48 h after diagnosis of diabetes (two blood glucose measurements between 250 and 500 mg/dL). A glucose tolerance test (GTT) was performed 4 and 8 weeks after treatment on 6-h fasted mice administered 1.5 g/kg d-glucose i.p., and glucose and insulin were assayed at 0, 15, 30, 60, 120 min. BrdU (15 μL/g) was injected 2 h before kill.
Immunofluorescence Microscopy and Immunohistochemistry
Immunofluorescence and immunostaining were performed on 5-μm–thick formalin-fixed paraffin-embedded liver and pancreas sections as previously described (16,18). Primary antibodies were used and their dilutions are available on request. For assessing the T-cell numbers and their colocalization with BrdU, TUNEL, and Foxp3 in the periportal clusters, 1,000–2,000 cells were counted from 30–40 clusters from three sections 100 μm apart in four to five mice from each group.
Flow Cytometry
Mononuclear cells from the liver and spleen 8 weeks after treatment (Ngn3-Btc or Ngn3-Btc+PD-L1) were isolated as previously described (19) and used for flow cytometry analysis immediately or after culture ex vivo. For ex vivo culture, cells were seeded in a 96-well flat-bottomed plate coated with anti-CD3 (4 μg/mL) overnight and cultured for 48 h in medium containing anti-CD28 (2 μg/mL). Cells were analyzed after a 6-h incubation period with 1 μL/mL GolgiPlug. For in vitro stimulation and coincubation of CD4+ cells and β-cells together, CD4+ cells were purified from the spleens of nondiabetic NOD mice and stimulated with anti-CD3 and anti-CD28 as aforementioned. β-Cells (rat insulinoma β-cell line [a gift from Akio Koizumi, Graduate School of Medicine, Kyoto University]) were infected with helper-dependent empty (HDAd-empty) or PD-L1 virus (HDAd-PD-L1) for 2 days. Purified CD4+ were incubated 1:1 with infected β-cells for 24 h. Cells were analyzed after a 6-h incubation period with 1 μL/mL GolgiPlug. Cells were acquired on an LSR II (BD Biosciences) flow cytometer and analyzed with FlowJo (Tree Star, Inc.) software.
Bio-Plex Tissue Cytokine Assay
Lysate from the livers of mice treated for 8 weeks and pancreas from diabetic and nondiabetic NOD mice were used at a final protein concentration of 0.5 mg/mL and assayed for cytokines using 23 cytokine multiplexed bead-based immunoassay kits and the Bio-Plex tissue cytokine assay system (Bio-Rad), using high photomultiplier tube settings and at least 100 bead count per analyte per manufacturer’s protocol.
Adoptive Transfer
Two million splenocytes (from Ngn3-Btc+PD-L1– or Ngn3-Btc–treated groups) in 250 μL serum-free media were injected intraperitoneally into 6-week-old female NOD-Scid mice. Three to five donors were used per treatment group with one to two recipients per donor. Two million splenocytes from diabetic NOD mice were injected into 6-week-old female NOD-Scid mice or Ngn3-Btc+PD-L1–treated mice. Weekly blood glucose levels were measured in the recipients to determine the diabetes transfer rate.
Skin Transplantation
Skin transplantation was performed as described previously (20). Briefly, ear skin (1.0 cm2) from the donor mice (8–12 weeks old, C57BL/6) was grafted onto the flank of recipient NOD mice (Ngn3-Btc+PD-L1– or Ngn3-Btc–treated mice). Grafts were scored daily until rejection (defined as 80% of grafted tissue becoming necrotic and reduced in size).
Virus Clearance
Helper virus serotype 2 (1 × 1011 vp/mouse) was injected intravenously (21) into Ngn3-Btc+PD-L1 or Ngn3-Btc mice. Mice were killed at day 3 or 3 weeks after injection, and liver tissue was harvested. DNA was extracted using a DNeasy Blood & Tissue Kit (QIAGEN), and viral copy number was analyzed by quantitative PCR using serotype-specific primers
Statistical Methods
Student t test or ANOVA was used for significance testing. For the adoptive transfer experiment, Kaplan-Meier survival analysis using log-rank test was used in SigmaPlot. P < 0.05 was considered significant.
Results
Ngn3-Btc+PD-L1 Gene Transfer Reverses Diabetes in NOD Mice
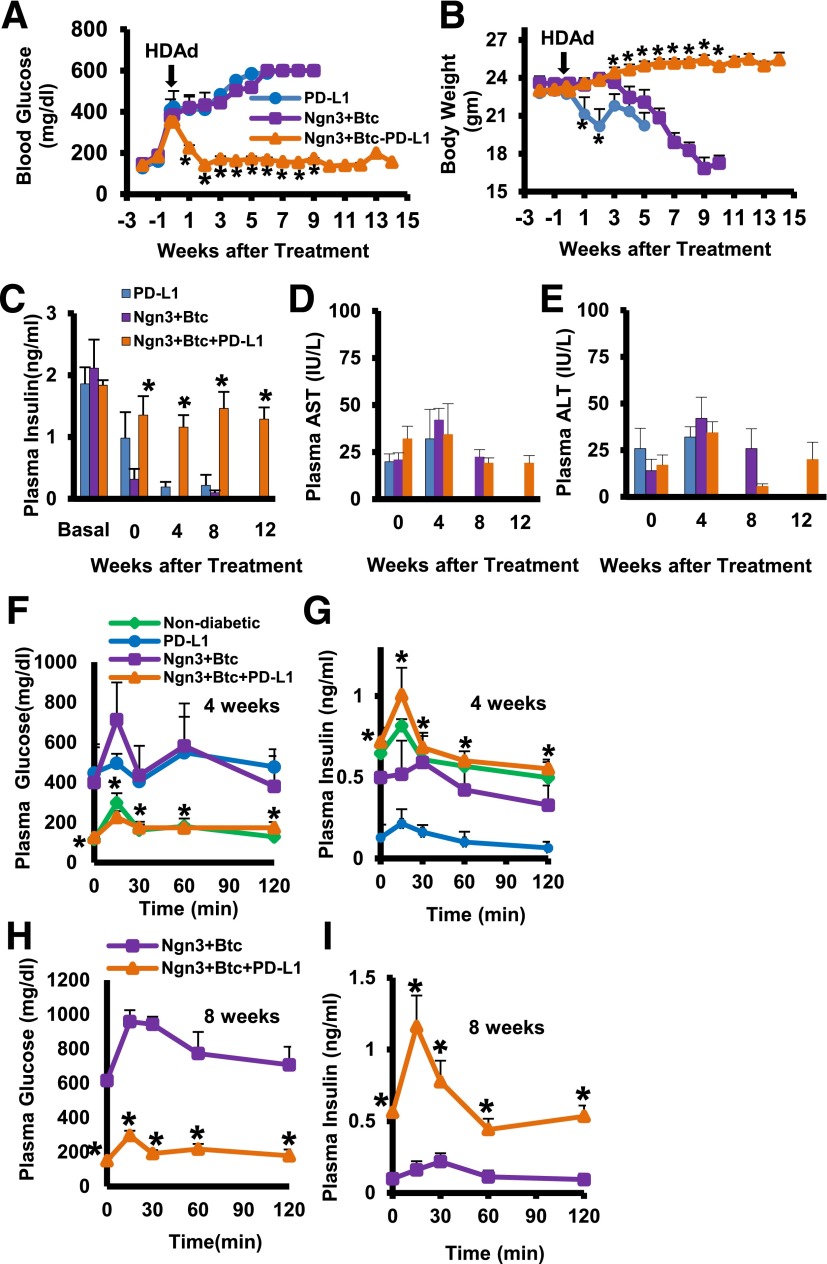
We have shown before that Ngn3-Btc gene transfer using HDAd vectors leads to the induction of insulin-expressing neo-islets in the liver and reverses diabetes in streptozotocin-induced diabetic mice (16,17). However, this did not ameliorate the hyperglycemia in overtly diabetic NOD mice, which continued to lose weight with time (Fig. 1A and B). We therefore combined Ngn3-Btc with RIP-driven PD-L1 so that the expression of PD-L1 is limited only to insulin-expressing cells induced in the liver of treated mice. In contrast to Ngn3-Btc or PDL-1 gene transfer alone, the combination therapy led to a rapid reversal of hyperglycemia and promoted weight gain in >75% of treated mice. This was sustained for at least 14 weeks, the duration of the study (Fig. 1A and B). The treatment restored normal circulating plasma insulin (Fig. 1C) with no deleterious effects on liver function (Fig. 1D and E). A GTT demonstrated restoration of normal glucose tolerance and in vivo glucose-stimulated insulin secretion to the levels seen in nondiabetic mice by 4 weeks (Fig. 1F and G), and this was sustained at 8 weeks after treatment (Fig. 1H and I) only when treatment with Ngn3-Btc was combined with PD-L1.
Figure 1.
Ngn3-Btc+PD-L1 reverses hyperglycemia and restores glucose tolerance. A and B: Blood glucose and body weight in diabetic NOD mice (Ngn3-Btc+PD-L1 group, n = 16; other groups, n = 4–7). C: Nonfasting plasma insulin at indicated time points (n = 4–8). D and E: Plasma aspartate aminotransferase (AST) (D) and alanine aminotransferase (ALT) (E) at the indicated time points (n = 4–6). F–I: Plasma glucose and insulin during an intraperitoneal GTT at 4 weeks (F and G) and 8 weeks (H and I). Data are mean ± SEM. *P ≤ 0.05.
Neo-islets Induced in the Liver of Ngn3-Btc–Treated NOD Mice Persist Only When PD-L1 Is Also Expressed
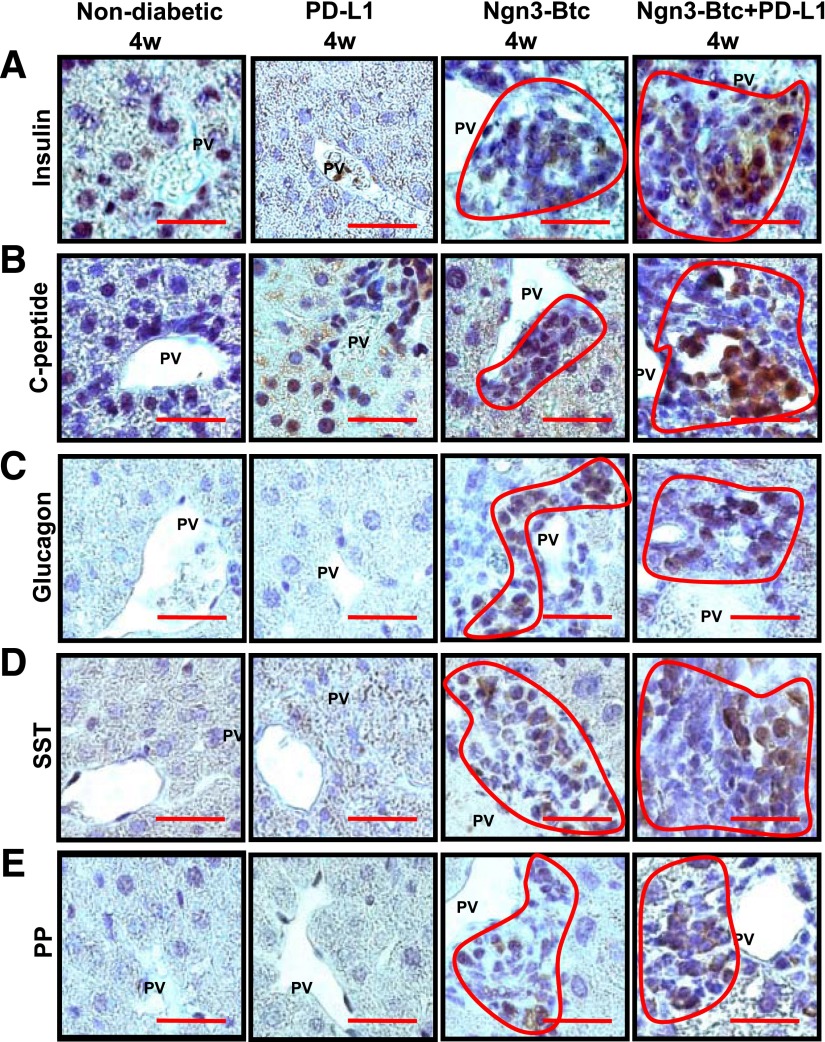
Ngn3-Btc transiently induced insulin-expressing cells in the periportal regions of the liver in overtly diabetic NOD mice (Fig. 2A), which were quickly destroyed by an immune infiltrate. In contrast, the periportal neo-islets induced by Ngn3-Btc+PD-L1 combination therapy in the liver are similar to pancreatic islets in that they express the four major islet hormones that were clearly identified by 4 weeks and sustained when re-examined at 8 and 12 weeks after treatment (Fig. 2A–E and Supplementary Fig. 1A). These neo-islets stained robustly for both insulin and C-peptide (Fig. 2A and B), indicating that they produced proinsulin that was processed to mature insulin. The induction and persistence of neo-islets was limited to the liver because the pancreas did not show any recovery of islets (Supplementary Fig. 1B). Strong staining for PD-L1 was limited only to the insulin-expressing neo-islets (Supplementary Fig. 2A and B).
Figure 2.
Ngn3-Btc+PD-L1 induces neo-islets in the periportal regions of the liver. A–E: Representative sections of Ngn3-Btc+PD-L1–treated diabetic NOD mouse liver stained by immunohistochemistry for insulin (A); C-peptide (B); glucagon (C); somatostatin (SST) (D); and pancreatic polypeptide (PP) (E) at 4 weeks (4w) after diabetes onset or treatment. Scale bar = 20 μm. Periportal clusters of cells were only occasionally seen in the PD-L1 group and are shown in A and B to contrast their staining with that of the Ngn3-Btc and Ngn3-Btc+PD-L1 groups. Most of the periportal areas in the PD-L1 group were similar to those shown in C–E. PV, portal vein.
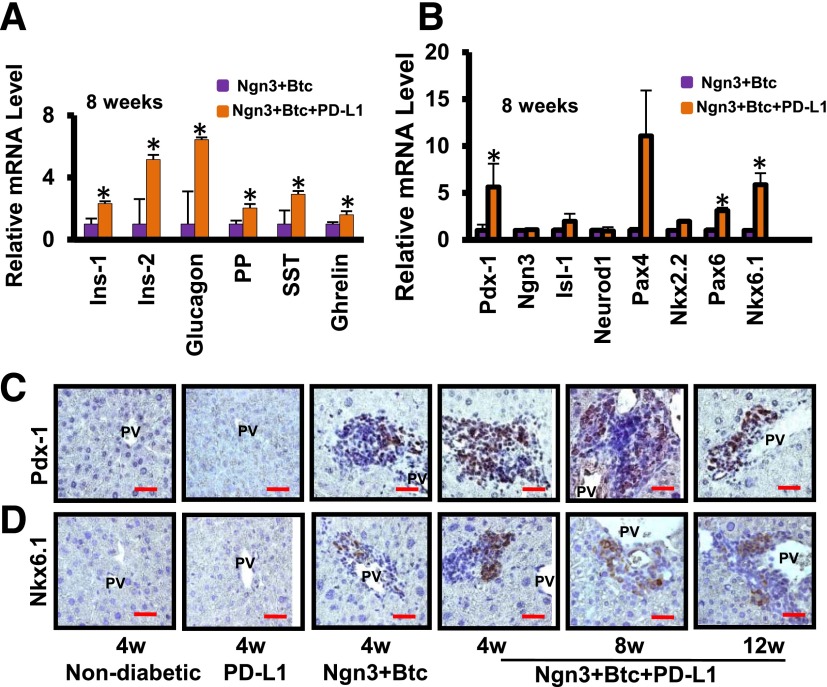
The periportal neo-islets also expressed transcripts of the various islet hormones (Fig. 3A). At 4 weeks, consistent with the data shown in Fig. 2, Ngn3-Btc, either alone or combined with PD-L1, induced many of these hormones (Supplementary Fig. 3A). However, by 8 weeks after treatment, the level of expression of these hormones was significantly higher in the mice treated with Ngn3-Btc+PD-L1, in parallel with the persistence and expansion of the neo-islets when PD-L1 was added to Ngn3-Btc (Fig. 3A and Supplementary Fig. 3A). A similar pattern of gene expression was observed with the islet transcription factors (Fig. 3B and Supplementary Fig. 3B) and in the progressive increase of Pdx-1 and Nkx6.1 protein levels, transcription factors critical to the normal development and function of β-cells (Fig. 3C and D). Of note, expression of Ngn3 itself and many of its immediate downstream target genes, such as Neurod1, persisted in mice treated only with Ngn3-Btc, despite the autoimmune-mediated destruction of insulin-expressing cells. This observation supports the hypothesis that Ngn3-Btc is effective in inducing the transcription factors required for neo-islet development, but PD-L1 is required only to allow their persistence in the face of an autoimmune attack. These neo-islets also expressed hepatic oval cell markers OC2-1D11 (22) and A6 (Supplementary Fig. 4A and B), consistent with our previous study that these neo-islets arose from the reprogramming of hepatic oval cells (16).
Figure 3.
Neo-islets express islet hormones and transcription factors. Quantitative RT-PCR showing the expression of islet hormones (A) and transcription factors (B) involved in islet development at 8 weeks (n = 4–5). Data are mean ± SEM. *P ≤ 0.05. C and D: Representative sections of liver stained for Pdx-1 and Nkx 6.1 in various groups. Scale bar = 20 μm. 4w, 4 weeks; 8w, 8 weeks; 12w, 12 weeks; Ins-1, rat insulinoma cell line INS-1; Ins-2, rat insulinoma cell line INS-2; PP, pancreatic polypeptide; PV, portal vein; SST, somatostatin.
Targeted PD-L1 Expression in Neo-islets Leads to a Decrease in CD4+ T Cells Locally in and Around Neo-islets
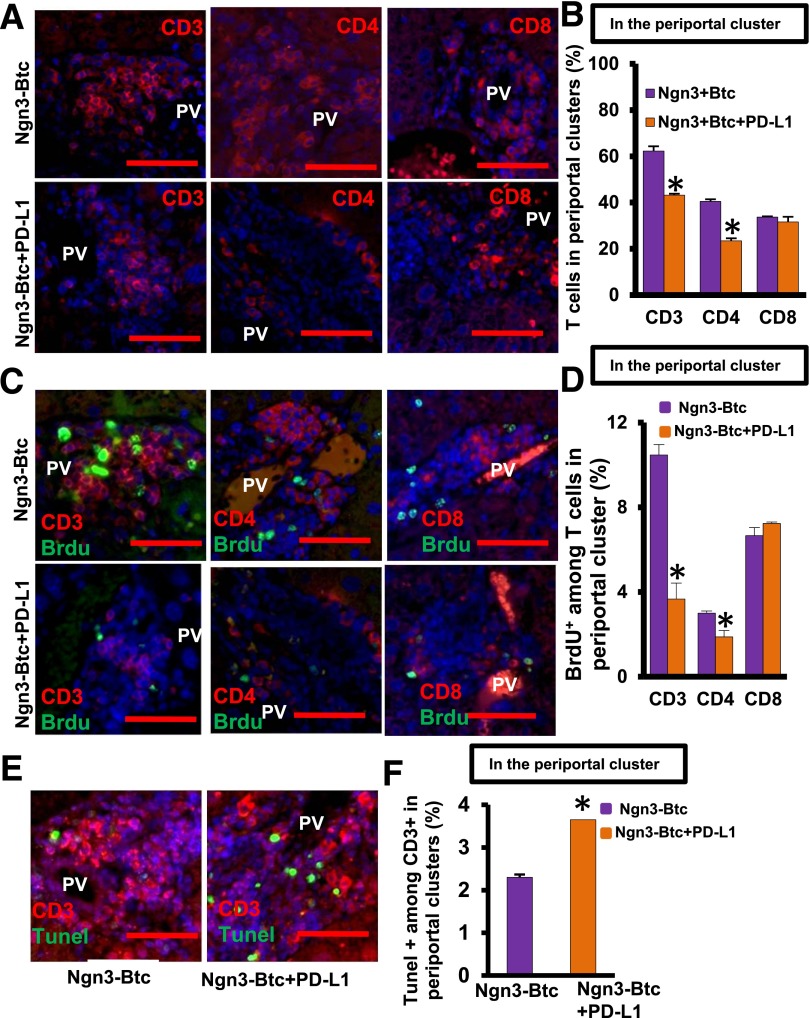
To elucidate mechanisms underlying PD-L1–induced tolerance of these neo-islets in the liver, we quantified the number of T cells and their subsets in liver sections from mice treated with Ngn3-Btc with and without PD-L1. Immunostaining revealed that CD3+ T cells were significantly reduced in and around the induced neo-islets in the periportal regions of the livers of Ngn3-Btc+PD-L1–treated mice (Fig. 4A and B). Of note, this was due to a decrease in the CD4+ T cells but not CD8+ T cells. We then tested whether neo-islet–restricted expression of PD-L1 had an effect on T cells beyond the periportal areas. When single-cell suspensions of the whole liver were assessed by flow cytometry, the total number of CD4+ and CD8+ T cells was not different in the two groups treated with Ngn3-Btc with or without PD-L1 (Supplementary Fig. 5A–D), in contrast to that observed only in the periportal areas surrounding the neo-islets (Fig. 4A and B). This suggests that expression of insulin promoter–driven PD-L1 resulted only in local immunomodulation in the periportal regions.
Figure 4.
Ngn3-Btc+PD-L1 treatment reduces the local number of CD4+ T cells infiltrating the periportal neo-islet clusters. A: Representative sections in control and Ngn3-Btc+PD-L1–treated NOD mouse liver stained for CD3, CD4, and CD8. B: CD3+, CD4+, and CD8+ cells represented as a percentage of all cells only in periportal clusters. C: Representative sections in the liver of control and Ngn3-Btc+PD-L1–treated NOD mice costained for BrdU with CD3, CD4, or CD8. D: Percentage of BrdU+ T cells in the periportal clusters. E: Representative sections in the liver of control and Ngn3-Btc+PD-L1–treated NOD mice costained for TUNEL and CD3. F: Percentage of TUNEL+ T cells in periportal clusters. Scale bar = 50 μm. Data are mean ± SEM (n = 4–5 per group). *P ≤ 0.05. PV, portal vein.
Targeted PD-L1 Expression in Neo-islets Inhibits Proliferation and Promotes Apoptosis of Local CD4+ T Cells by a Foxp3-Independent Mechanism
To determine the mechanism of the decrease in local CD4+ T cells in Ngn3-Btc+PD-L1–treated mice, we tested the rate of proliferation and apoptosis of the infiltrating T cells in periportal regions of the liver. BrdU incorporation, indicative of active proliferation, was significantly lower in CD3+ T cells (Fig. 4C and D) but not in CD8+ T cells (Fig. 4C and D), consistent with the reduction only in numbers of CD4+ T cells. Furthermore, an increase in apoptosis was detected by TUNEL staining in CD3+ T cells infiltrating the neo-islets in Ngn3-Btc+PD-L1–treated mice compared with the controls that received only Ngn3-Btc (Fig. 4E and F).
Because Foxp3+ regulatory T cells (Tregs) have been invoked as mediating tolerance induction by PD-L1 (23,24), we measured the number and expression of Foxp3+ cells in these mice. The number of Foxp3+ cells in the whole liver, as assessed by flow cytometry, was surprisingly not different between the mice that received PD-L1 and those that did not (Supplementary Fig. 6A and B), a finding that was corroborated by Western blotting of whole-liver lysate for Foxp3 protein (Supplementary Fig. 6C and D). Furthermore, the number of Foxp3+ cells among the infiltrating periportal T cells also was not different between the two treatment groups (Supplementary Fig. 6E). These data excluded Foxp3+ Tregs as playing a major role in the induction and maintenance of peripheral tolerance to the neo-islets with Ngn3-Btc+PD-L1 treatment.
Inactivation of Infiltrating T Cells in the Liver Results From Targeted PD-L1 Expression in Neo-islets
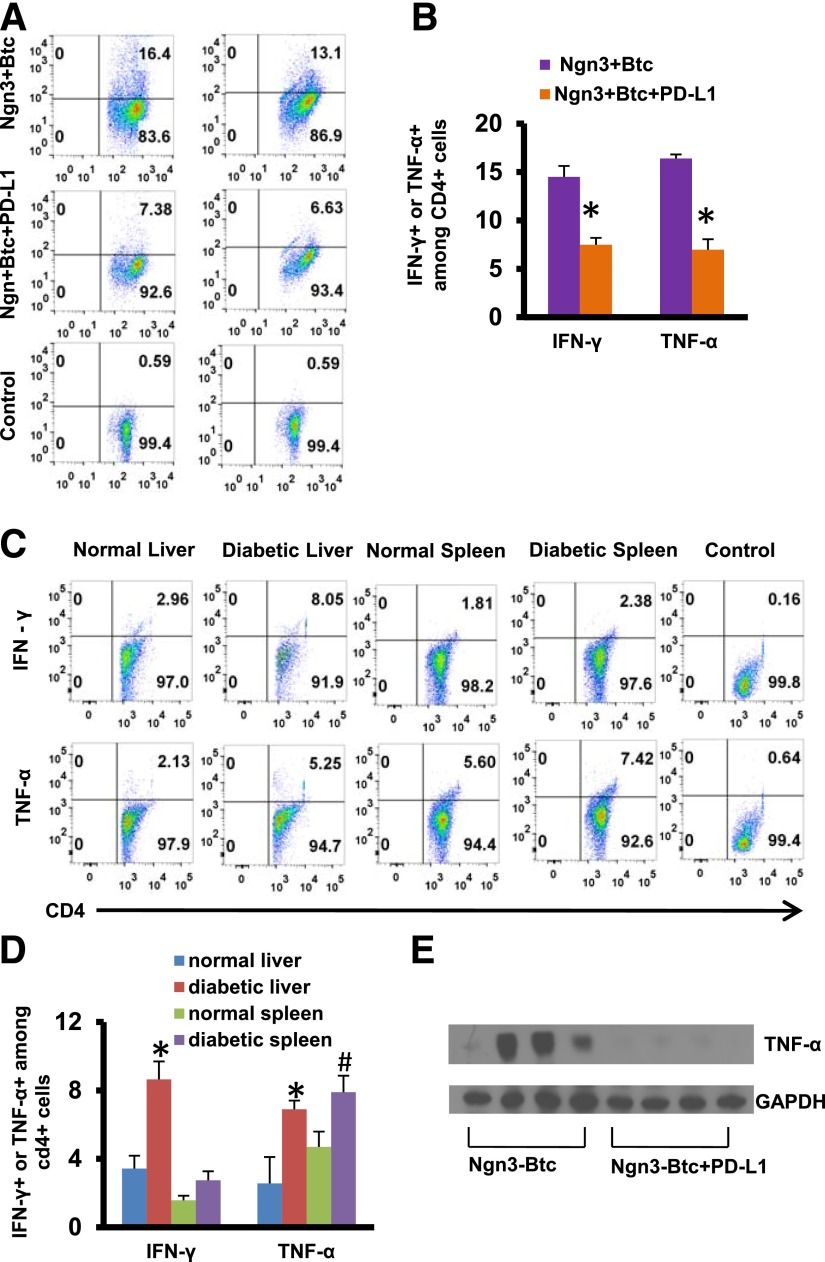
We then investigated whether PD-L1 expressed on the neo-islets was also leading to local T-cell inactivation. We assessed by flow cytometry the expression of cytokines in T cells isolated from mice 8 weeks after treatment and stimulated ex vivo using anti-CD3 and anti-CD28. CD4+ T cells expressing interferon-γ (IFN-γ) (7.38%) or tumor necrosis factor-α (TNF-α) (6.63%) were significantly decreased in the livers of mice that received Ngn3-Btc+PD-L1 (Fig. 5A and B) compared with mice that received Ngn3-Btc but no PD-L1 (16.4% and 13.1%, respectively). We thus concluded that the addition of PD-L1 to the neo-islet–inducing Ngn3-Btc regimen is sufficient to decrease the activation of the liver T cells in the periportal “fighting” zone. In parallel experiments, we also show that this degree of CD4+ activation is similar to that seen in the liver of untreated diabetic NOD mice (Fig. 5C and D). This was also consistent with a significantly lower TNF-α protein on Western blotting of Ngn3-Btc+PD-L1–treated mouse liver lysates compared with controls that received only Ngn3+Btc (Fig. 5E).
Figure 5.
PD-L1 protects neo-islets by inactivating T cells. A and B: IFN-γ– and TNF-α–positive CD4+ T cells isolated from livers of treated mice after ex vivo stimulation with anti-CD3 and anti-CD28 on FACS analysis. Representative dot plot from one set of mice is shown in A, and quantification from three to five separate mice is shown in B. C and D: IFN-γ– and TNF-α–positive CD4+ T cells isolated from liver or spleen of nondiabetic or diabetic mice after ex vivo stimulation with anti-CD3 and anti-CD28 on FACS analysis. Representative dot plot from one set of mice is shown in C, and quantification from three to four separate mice is shown in D. Control shown is unstimulated CD4+ T cells from the spleen of Ngn3-Btc–treated (A) or untreated diabetic (C) mice. E: Western blotting of whole-liver lysate for TNF-α at 8 weeks after treatment. Data are mean ± SEM. *P ≤ 0.05; #P ≤ 0.06.
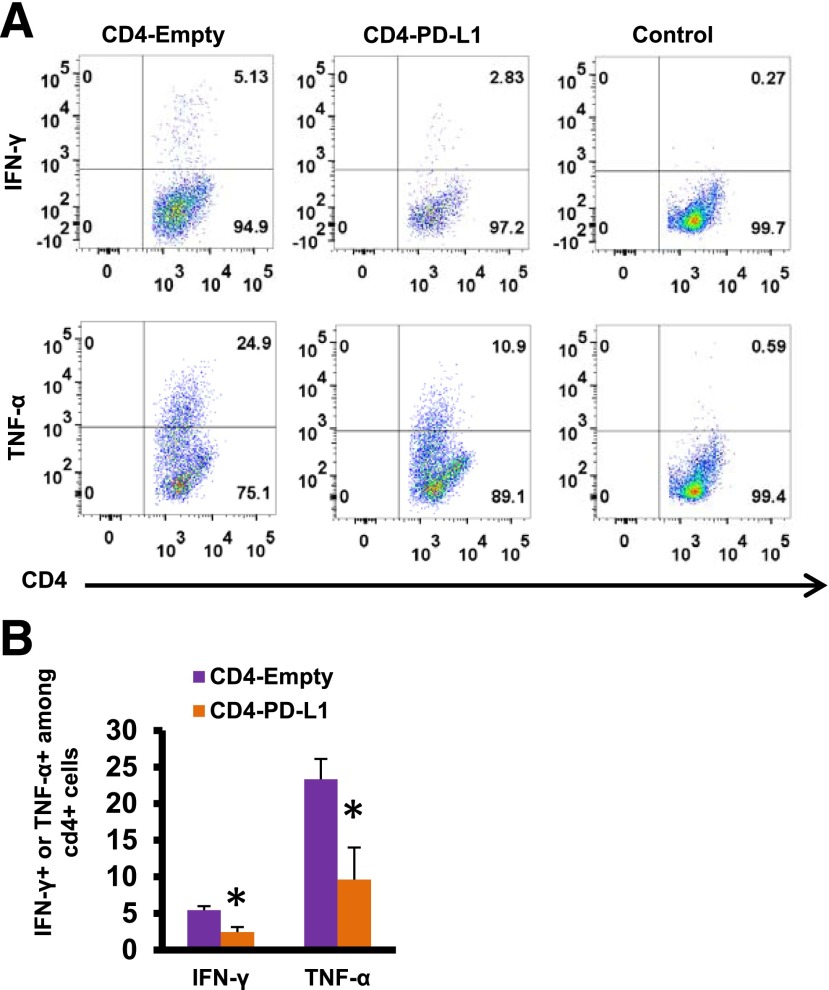
We then explored the molecular mechanisms underlying this local T-cell inactivation by PD-L1 expression on neo-islets. PD-L1–mediated costimulation inhibition occurs by inhibiting the TCR/MHC class II (MHC-II) costimulation pathways. However, the neo-islets do not express MHC-II antigens (Supplementary Fig. 7A). This raised the possibility that a simultaneous spatiotemporal TCR engagement may not be required for PD-L1–mediated inhibition of costimulation. To test this, we stimulated isolated CD4+ T cells with anti-CD3 and anti-CD28 antibodies to activate the TCR/MHC-II costimulation pathways. When these stimulated CD4+ T cells were subsequently coincubated with PD-L1 overexpressing mouse insulinoma cells, there was a significant decrease in IFN-γ– and TNF-α–positive cells compared with controls (Fig. 6A and B). This demonstrated that PD-L1–mediated T-cell inactivation can be spatiotemporally separated from TCR/MHC-II engagement. We also identified MHC-II–expressing B cells infiltrating the neo-islets that may be providing the stimulus to activate the TCR signaling cascade in these infiltrating CD4+ T cells (Supplementary Fig. 7).
Figure 6.
IFN-γ and TNF-α protein was assessed in isolated CD4+ T cells purified from the spleen of nondiabetic NOD mice that were first stimulated with anti-CD3 and anti-CD28 for 2 days and then coincubated with HDAd-empty or HDAd-PD-L1 virus-infected β-cells (rat insulinoma cell line INS-2 cells) for 24 h. Representative dot plot of one set of experiments is shown in A and B. Data are mean ± SEM. *P ≤ 0.05. CD4-empty, stimulated CD4+ cells coincubated with β-cells infected with HDAd-empty; CD4-PD-L1, stimulated CD4+ cells coincubated with β-cells infected with HDAd-empty PD-L1; control, unstimulated CD4+ cells.
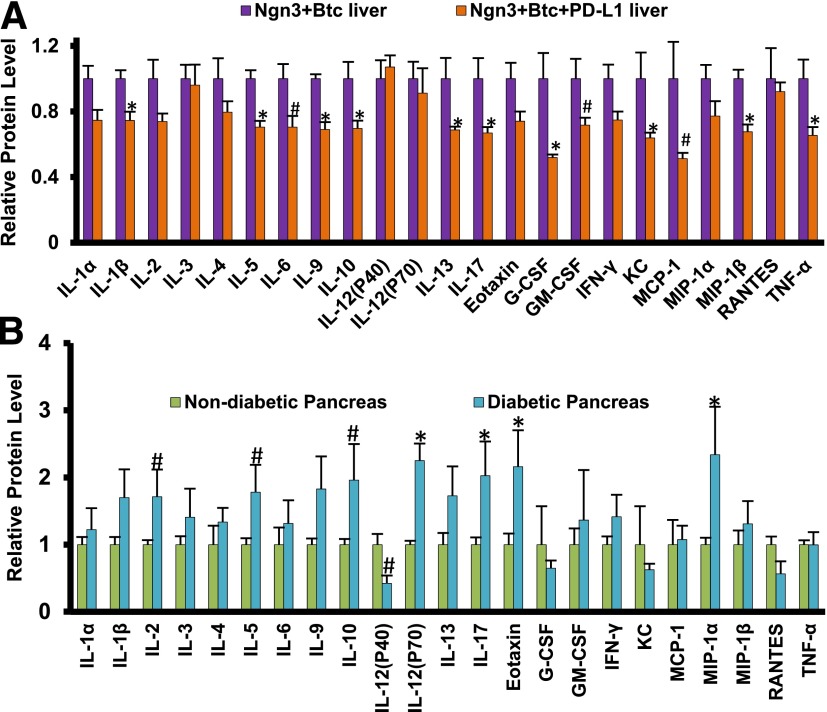
To comprehensively test the effect of the targeted expression of PD-L1 in the neo-islet on the liver cytokine profile, we assessed 23 cytokines in whole-liver lysate using the Bio-Plex tissue cytokine assay system. Many cytokines involved in the pathogenesis of diabetes in NOD mice, including interleukin (IL)-1β, TNF-α, IL-10, and IL-17, were downregulated in the liver when PD-L1 was added to Ngn3-Btc (Fig. 7A). Of note, when compared with the results from that of diabetic NOD mouse pancreas (Fig. 7B), there was a remarkable inverse correlation in that most of the cytokines that were increased in NOD diabetic pancreas were decreased in the livers of mice treated with Ngn3-Btc+PD-L1, indicating that mechanisms that destroy β-cells in the pancreas have been specifically attenuated by PD-L1 and supporting this as a mechanism for the tolerance induction by PD-L1 in this setting.
Figure 7.
The levels of 23 cytokines in the livers (A) of Ngn3-Btc– and Ngn3-Btc+PD-L1–treated NOD mice or in the pancreas (B) of diabetic NOD mice (5–6 weeks after diabetes onset) and nondiabetic NOD mice (27 weeks old) analyzed by Bio-Plex tissue cytokine assay. Data are mean ± SEM (n = 3–5 per group). *P ≤ 0.05; #P = 0.05–0.06. G-CSF, granulocyte colony-stimulating factor; GM-CSF, granulocyte macrophage colony-stimulating factor; KC, keratinocyte-derived chemokine; MIP-1α, macrophage inflammatory protein-1α; MIP-1β, macrophage inflammatory protein-1β; RANTES, regulated on activation, normal T cell expressed and secreted.
Ngn3-Btc+PD-L1 Treatment Is Not Associated With Systemic Immunosuppression
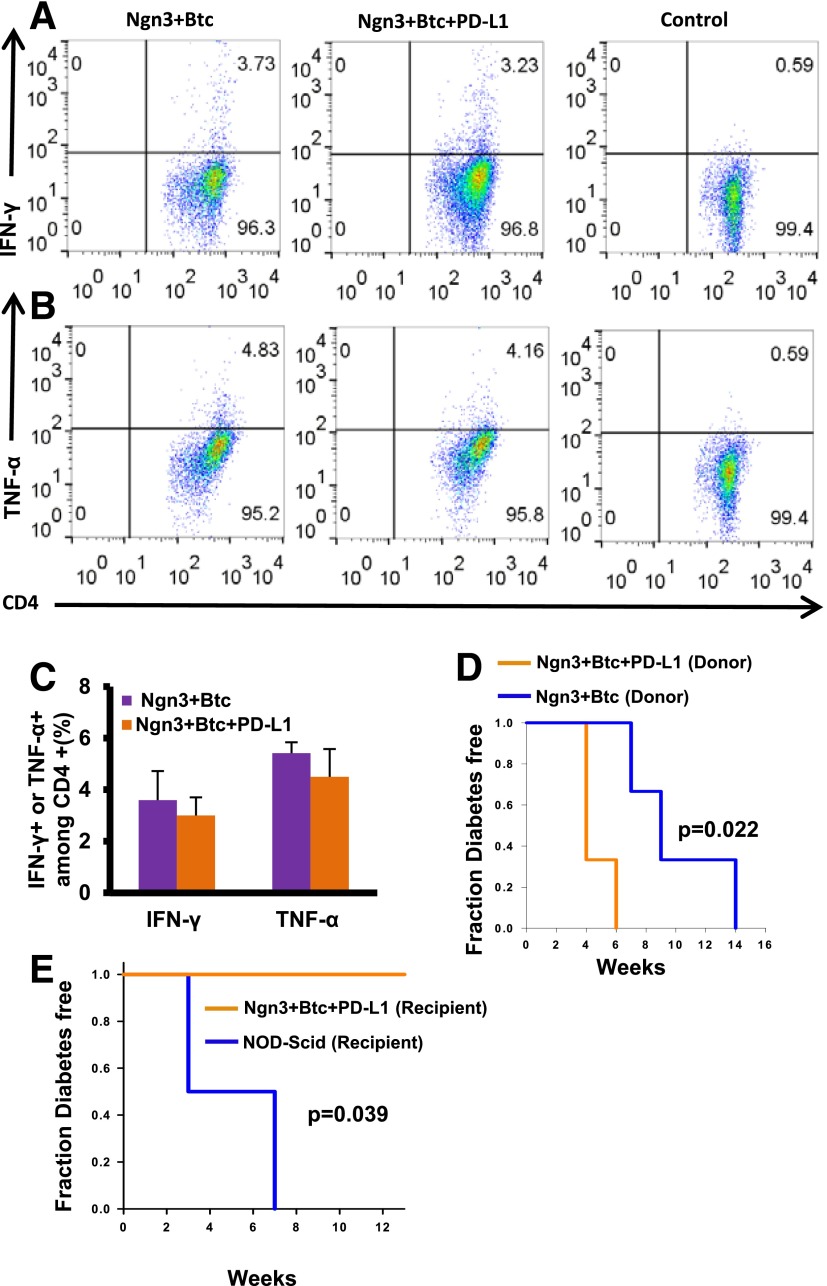
To assess whether systemic immunosuppression could have contributed to the observed tolerance in Ngn3-Btc+PD-L1–treated mice, we isolated lymphocytes from the spleen of mice treated with Ngn3-Btc with and without PD-L1 and compared the expression of TNF-α and IFN-γ upon TCR-dependent stimulation with anti-CD3 and anti-CD28. Splenocytes from mice treated with Ngn3-Btc with or without PD-L1 exhibited a similar response upon stimulation in terms of IFN-γ and TNF-α expression (Fig. 8A–C). Other controls for this experiment performed are shown in Supplementary Fig. 8A–D. We also performed skin allografts from C57BL/6 mice into diabetic NOD mice that had received Ngn3-Btc with or without PD-L1. Ngn3-Btc+PD-L1 mice rejected the skin graft at the same rate and time course as the controls that did not receive PD-L1 (data not shown), confirming that generalized immunosuppression was not induced by Ngn3-Btc+PD-L1 therapy. Having thus excluded systemic immunosuppression as a mechanism for the tolerance to neo-islets in Ngn3-Btc+PD-L1, we tested whether the immune system in the liver could clear other antigens. We injected mice treated with Ngn3-Btc with or without PD-L1 with an adenovirus (of a serotype that was different from the original vectors) and assessed viral clearance rate after 3 weeks. This experiment indicated that both groups had similar viral clearance rates (Supplementary Fig. 9), indicating that PD-L1 expression limited to the neo-islets in the liver did not impair the ability of the liver to clear other antigens.
Figure 8.
No peripheral immunosuppression in the mice treated with Ngn3-Btc+PD-L1. A–C: IFN-γ– and TNF-α–positive CD4+ T cells isolated from spleens of treated mice after ex vivo stimulation with anti-CD3 and anti-CD28 on FACS analysis. Representative dot plot from one set of mice is shown in A and B, and quantification from three to five separate mice is shown in C. D: Diabetes induction in NOD-Scid mice after adoptive transfer of splenocytes from Ngn3-Btc+PD-L1 or controls. E: Diabetes induction in Ngn3-Btc+PD-L1 or control mice after adoptive transfer of splenocytes from newly diabetic NOD mice. Data are mean ± SEM (n = 3–5 per group).
Ngn3-Btc+PD-L1–Induced Neo-islets Resist Destruction by Adoptively Transferred Diabetogenic Splenocytes
To exclude the possibility that a loss of diabetogenicity of the T cells in mice that received PD-L1 underlies the tolerance, we performed an adoptive transfer of splenocytes from the Ngn3-Btc+PD-L1– and Ngn3-Btc–treated mice into NOD-Scid recipients. Splenocytes from Ngn3-Btc+PD-L1–treated donor mice robustly transferred diabetes (Fig. 8D), indicating that there was no reduction in the diabetogenicity of the splenocytes and no peripheral tolerance to islet antigens in the donor Ngn3-Btc+PD-L1 mice. Indeed, they actually led to a significantly earlier onset of diabetes in the recipients than in the control NOD-Scid mice receiving splenocytes from Ngn3-Btc–treated donors, which may be a reflection of the persistent islet antigenic presence in the Ngn3-Btc+PD-L1 condition. We then did the converse experiment wherein we performed adoptive transfer using diabetogenic splenocytes from newly diabetic NOD mice and transferred them to NOD-Scid mice (controls) and to euglycemic Ngn3-Btc+PD-L1–treated diabetic NOD mice. The Ngn3-Btc+PD-L1 mice remained completely resistant to diabetes up to 15 weeks, whereas all the NOD-Scid recipients became diabetic by 7 weeks (Fig. 8E). These experiments demonstrate that Ngn3-Btc+PD-L1 treatment induced a local tolerance in the periportal regions despite the presence of diabetogenic T cells, leading to the persistence of the neo-islets and a long-term reversal of diabetes in NOD mice.
Discussion
Although innumerable studies have reported decreasing the incidence of diabetes in NOD mice (25), only a few successful interventions have reversed diabetes after disease onset. The most notable success has been achieved by targeting the TCR complex by anti-CD3 antibodies (26–29) and antithymocyte globulin (30–33), although generalized immunosuppression limits the translational applicability of these approaches. Other targeted therapies, including antigen-specific approaches by using dendritic cell–expanded islet-specific Tregs (34) and IL-2 therapy–expanded Tregs (35), have had some success in restoring euglycemia, although an enduring response was rarely seen and never with high efficacy. Mixed hematopoietic chimerism achieved by an anti-CD3/anti-CD8 conditioning regimen with bone marrow transplantation to inhibit autoimmunity reversed diabetes in 60% of NOD mice when combined with 2 months of daily injections of epidermal growth factor and gastrin to induce β-cell formation (36).
In the current study, we tested whether engineered neo-islets that specifically resist T-cell–targeted destruction offer a cure for diabetic NOD mice. With targeted expression of PD-L1 in the Ngn3-Btc–induced insulin-expressing cells, we demonstrate that this approach is sufficient to reverse diabetes by persistent functional neo-islets in the liver. In the data not shown, these treated mice remained euglycemic for prolonged periods up to 1 year without any untoward effects, such as liver dysfunction, tumors, or hypoglycemia. We show that this occurs as a result of PD-L1–mediated reduction in activated T cells locally around the neo-islets, without systemic immunosuppression. These data are consistent with previous studies demonstrating that the expression of PD-L1 induces tolerance by a reduction in the number of infiltrating T cells by inducing apoptosis while inhibiting proliferation of T cells (37).
Although Foxp3+ Tregs have also been shown to mediate the tolerance induction by PD-L1 in some settings (24), the current data indicate that the locally induced tolerance to the neo-islets is Foxp3 independent. This is also consistent with other studies revealing that systemic development of Tregs may not occur when manipulation of immune responses is done in an organ-specific or -limited microenvironment (38–40). Although the liver microenvironment has been suggested to be more tolerogenic than other tissues (41), it may play a contributory but not a determinant role in this study because the neo-islets induced in the control mice that received Ngn3-Btc were rapidly destroyed and the expression of PD-L1 on the neo-islets was required for their continued survival. The persistent diabetogenicity of splenocytes in the mice that expressed PD-L1 on the neo-islets and the resistance of these neo-islets to destruction by extraneous diabetogenic splenocytes demonstrate the critical tolerogenic role played by modulation of the microenvironment surrounding the neo-islets without perturbing the systemic immune system. These experiments also demonstrate that PD-L1 expression is not required to be on the same cell that carries the MHC-II to engage the TCR on the CD4+ cells.
Some paradoxical observations in transgenic models have been made wherein PD-L1 was overexpressed in β-cells in various genetic backgrounds: Overexpression in a B6 background led to an increase in allograft rejection and induction of autoimmune diabetes (42), whereas in the NOD background, there was a clear protection from diabetes (15). However, the preponderance of evidence in NOD mice points to a significant inhibition of T-cell response and protection with the activation of the PD/PD-L1 pathway in both transgenic and transplant models (12,15,43,44). These studies are consistent with the present observation that expression of PD-L1 in target cells leads to a decrease in activation of T cells and an inhibition of T-cell proliferation.
One other study using a first-generation adenoviral vector–mediated gene transfer of Pdx-1 to the liver reported induction of insulin expression in hepatocytes with a decrease in hyperglycemia in fewer than one-half of the mice treated (45). A decreased diabetogenicity of the splenocytes from treated mice was reported as the reason for the tolerance, although the mechanism of this decreased diabetogenicity was not defined. In contrast, splenocytes from the treated mice in the current study showed increased diabetogenicity in destroying recipient pancreatic islets in adoptive transfer experiments. This is likely to be secondary to the persistent antigen exposure from the neo-islets that would perpetuate diabetogenic lymphocytes in the body.
Cell-intrinsic requirement of PD-1 expression on islet-reactive CD4+ T cells in diabetes induction has been demonstrated (44,46). This is consistent with a model wherein the PD-L1 expressed on the neo-islets engages the PD-1 on the infiltrating T cells to inactivate them for tolerance induction. However, other studies identified the important role of PD-L1 binding to B7-1–mediating T-cell inhibition (47,48), specifically in diabetogenesis in NOD mice (49). The current study was not designed to address this question, and future experiments will need to address whether the binding of PD-L1 to PD-1 or B7-1 mediates the tolerance induction seen in this study.
With the regimen of Ngn3-Btc+PD-L1, 24% of the mice did not respond to tolerance induction and remained diabetic while showing significant insulitis and loss of insulin-positive neo-islets similar to mice that did not receive PD-L1. It is likely that other pathways that are not regulated by the inhibition of costimulation by PD-L1 exist in these mice, and unraveling the mechanisms that underlie the resistance to tolerance induction will require further study.
In summary, we demonstrate that Ngn3-Btc gene transfer is adequate to induce ectopic islet neogenesis in the liver of diabetic NOD mice and reverse diabetes when combined with targeted PD-L1 expression to inhibit the costimulatory pathway selectively in T cells infiltrating the newly induced β-cells. To our knowledge, this report is the first of a successful enduring reversal of diabetes achieved in a majority of overtly diabetic NOD mice by inducing islet neogenesis with concurrent β-cell–specific tolerance without systemic immunosuppression. This treatment strategy may be an attractive and viable approach to β-cell replacement therapy of autoimmune T1D.
Supplementary Material
Article Information
Acknowledgments. The authors thank Shixia Huang and Myra Custorio (both from Baylor College of Medicine, Houston, TX) for assistance with the Bio-Plex tissue cytokine assay. The authors also thank Marcus Grompe (Oregon Health & Science University, Portland, OR) for providing the OC2-1D11 antibody for oval cells.
Funding. This work was supported by National Institutes of Health grants K08-HL-091176 (to M.M.), R03-DK-089061-01 (to V.Y.), K08-DK-068391 (to V.Y.), and R56-DK-089061 (to V.Y.); the Betty Rutherford Chair from St. Luke’s Episcopal Hospital and the Charles and Barbara Close Foundation (to L.C.); Diabetes Research Center pilot and feasibility grant P30-DK-079638 (to V.Y.); and JDRF awards 46-2010-752 (to L.C.) and 5-2006-134 (to V.Y.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. R.L. contributed to the study design, performance of experiments, and data analysis. M.M., Q.Y., W.C., K.O., and L.C. contributed to the study design and data analysis. J.L., M.-s.K., V.L., H.L., A.X., L.Y., Y.L., and T.H.T. contributed to the performance of experiments. V.Y. supervised the project and contributed to the study design and data analysis. V.Y. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-1737/-/DC1.
References
- 1.Shapiro AM, Ricordi C, Hering BJ, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med 2006;355:1318–1330 [DOI] [PubMed] [Google Scholar]
- 2.Robertson RP. Islet transplantation a decade later and strategies for filling a half-full glass. Diabetes 2010;59:1285–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest 2007;117:2553–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ansari MJ, Salama AD, Chitnis T, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med 2003;198:63–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1-/- mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci U S A 2005;102:11823–11828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin-Orozco N, Wang YH, Yagita H, Dong C. Cutting edge: programmed death (PD) ligand-1/PD-1 interaction is required for CD8+ T cell tolerance to tissue antigens. J Immunol 2006;177:8291–8295 [DOI] [PubMed] [Google Scholar]
- 7.Guleria I, Gubbels Bupp M, Dada S, et al. Mechanisms of PDL1-mediated regulation of autoimmune diabetes. Clin Immunol 2007;125:16–25 [DOI] [PubMed] [Google Scholar]
- 8.Rajasalu T, Brosi H, Schuster C, et al. Deficiency in B7-H1 (PD-L1)/PD-1 coinhibition triggers pancreatic beta-cell destruction by insulin-specific, murine CD8 T-cells. Diabetes 2010;59:1966–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fiorina P. β-Cells step up in controlling the autoimmune response. Diabetes 2010;59:1861–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev 2010;236:219–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med 2006;203:883–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keir ME, Freeman GJ, Sharpe AH. PD-1 regulates self-reactive CD8+ T cell responses to antigen in lymph nodes and tissues. J Immunol 2007;179:5064–5070 [DOI] [PubMed] [Google Scholar]
- 14.Lee I, Wang L, Wells AD, et al. Blocking the monocyte chemoattractant protein-1/CCR2 chemokine pathway induces permanent survival of islet allografts through a programmed death-1 ligand-1-dependent mechanism. J Immunol 2003;171:6929–6935 [DOI] [PubMed] [Google Scholar]
- 15.Wang CJ, Chou FC, Chu CH, et al. Protective role of programmed death 1 ligand 1 (PD-L1) in nonobese diabetic mice: the paradox in transgenic models. Diabetes 2008;57:1861–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yechoor V, Liu V, Espiritu C, et al. Neurogenin3 is sufficient for transdetermination of hepatic progenitor cells into neo-islets in vivo but not transdifferentiation of hepatocytes. Dev Cell 2009;16:358–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yechoor V, Liu V, Paul A, et al. Gene therapy with neurogenin 3 and betacellulin reverses major metabolic problems in insulin-deficient diabetic mice. Endocrinology 2009;150:4863–4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J, Kim MS, Li R, et al. Loss of Bmal1 leads to uncoupling and impaired glucose-stimulated insulin secretion in β-cells. Islets 2011;3:381–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Dong Z, Zhou R, Luo D, Wei H, Tian Z. Isolation of lymphocytes and their innate immune characterizations from liver, intestine, lung and uterus. Cell Mol Immunol 2005;2:271–280 [PubMed] [Google Scholar]
- 20.Miyahara Y, Khattar M, Schroder PM, et al. Anti-TCRβ mAb induces long-term allograft survival by reducing antigen-reactive T cells and sparing regulatory T cells. Am J Transplant 2012;12:1409–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parks R, Evelegh C, Graham F. Use of helper-dependent adenoviral vectors of alternative serotypes permits repeat vector administration. Gene Ther 1999;6:1565–1573 [DOI] [PubMed] [Google Scholar]
- 22.Dorrell C, Erker L, Lanxon-Cookson KM, et al. Surface markers for the murine oval cell response. Hepatology 2008;48:1282–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang W, Lau R, Yu D, Zhu W, Korman A, Weber J. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol 2009;21:1065–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med 2009;206:3015–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shoda LK, Young DL, Ramanujan S, et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity 2005;23:115–126 [DOI] [PubMed] [Google Scholar]
- 26.Herold KC, Hagopian W, Auger JA, et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N Engl J Med 2002;346:1692–1698 [DOI] [PubMed] [Google Scholar]
- 27.Chatenoud L, Thervet E, Primo J, Bach JF. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc Natl Acad Sci U S A 1994;91:123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belghith M, Bluestone JA, Barriot S, Mégret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med 2003;9:1202–1208 [DOI] [PubMed] [Google Scholar]
- 29.Ablamunits V, Henegariu O, Hansen JB, et al. Synergistic reversal of type 1 diabetes in NOD mice with anti-CD3 and interleukin-1 blockade: evidence of improved immune regulation. Diabetes 2012;61:145–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogawa N, List JF, Habener JF, Maki T. Cure of overt diabetes in NOD mice by transient treatment with anti-lymphocyte serum and exendin-4. Diabetes 2004;53:1700–1705 [DOI] [PubMed] [Google Scholar]
- 31.Simon G, Parker M, Ramiya V, et al. Murine antithymocyte globulin therapy alters disease progression in NOD mice by a time-dependent induction of immunoregulation. Diabetes 2008;57:405–414 [DOI] [PubMed] [Google Scholar]
- 32.Parker MJ, Xue S, Alexander JJ, et al. Immune depletion with cellular mobilization imparts immunoregulation and reverses autoimmune diabetes in nonobese diabetic mice. Diabetes 2009;58:2277–2284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vergani A, D’Addio F, Jurewicz M, et al. A novel clinically relevant strategy to abrogate autoimmunity and regulate alloimmunity in NOD mice. Diabetes 2010;59:2253–2264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarbell KV, Petit L, Zuo X, et al. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J Exp Med 2007;204:191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grinberg-Bleyer Y, Baeyens A, You S, et al. IL-2 reverses established type 1 diabetes in NOD mice by a local effect on pancreatic regulatory T cells. J Exp Med 2010;207:1871–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang M, Racine JJ, Song X, et al. Mixed chimerism and growth factors augment β cell regeneration and reverse late-stage type 1 diabetes. Sci Transl Med 2012;4:133ra59 [DOI] [PubMed] [Google Scholar]
- 37.Wen X, Zhu H, Li L, et al. Transplantation of NIT-1 cells expressing pD-L1 for treatment of streptozotocin-induced diabetes. Transplantation 2008;86:1596–1602 [DOI] [PubMed] [Google Scholar]
- 38.Sung HH, Juang JH, Lin YC, et al. Transgenic expression of decoy receptor 3 protects islets from spontaneous and chemical-induced autoimmune destruction in nonobese diabetic mice. J Exp Med 2004;199:1143–1151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shieh SJ, Chou FC, Yu PN, et al. Transgenic expression of single-chain anti-CTLA-4 Fv on beta cells protects nonobese diabetic mice from autoimmune diabetes. J Immunol 2009;183:2277–2285 [DOI] [PubMed] [Google Scholar]
- 40.Huang SH, Chu CH, Yu JC, et al. Transgenic expression of haem oxygenase-1 in pancreatic beta cells protects non-obese mice used as a model of diabetes from autoimmune destruction and prolongs graft survival following islet transplantation. Diabetologia 2010;53:2389–2400 [DOI] [PubMed] [Google Scholar]
- 41.Tiegs G, Lohse AW. Immune tolerance: what is unique about the liver. J Autoimmun 2010;34:1–6 [DOI] [PubMed] [Google Scholar]
- 42.Subudhi SK, Zhou P, Yerian LM, et al. Local expression of B7-H1 promotes organ-specific autoimmunity and transplant rejection. J Clin Invest 2004;113:694–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao W, Demirci G, Strom TB, Li XC. Stimulating PD-1-negative signals concurrent with blocking CD154 co-stimulation induces long-term islet allograft survival. Transplantation 2003;76:994–999 [DOI] [PubMed] [Google Scholar]
- 44.Fife BT, Pauken KE, Eagar TN, et al. Interactions between PD-1 and PD-L1 promote tolerance by blocking the TCR-induced stop signal. Nat Immunol 2009;10:1185–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shternhall-Ron K, Quintana FJ, Perl S, et al. Ectopic PDX-1 expression in liver ameliorates type 1 diabetes. J Autoimmun 2007;28:134–142 [DOI] [PubMed] [Google Scholar]
- 46.Pauken KE, Jenkins MK, Azuma M, Fife BT. PD-1, but not PD-L1, expressed by islet-reactive CD4+ T cells suppresses infiltration of the pancreas during type 1 diabetes. Diabetes 2013;62:2859–2869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Butte MJ, Peña-Cruz V, Kim MJ, Freeman GJ, Sharpe AH. Interaction of human PD-L1 and B7-1. Mol Immunol 2008;45:3567–3572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity 2007;27:111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paterson AM, Brown KE, Keir ME, et al. The programmed death-1 ligand 1:B7-1 pathway restrains diabetogenic effector T cells in vivo. J Immunol 2011;187:1097–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.