Abstract
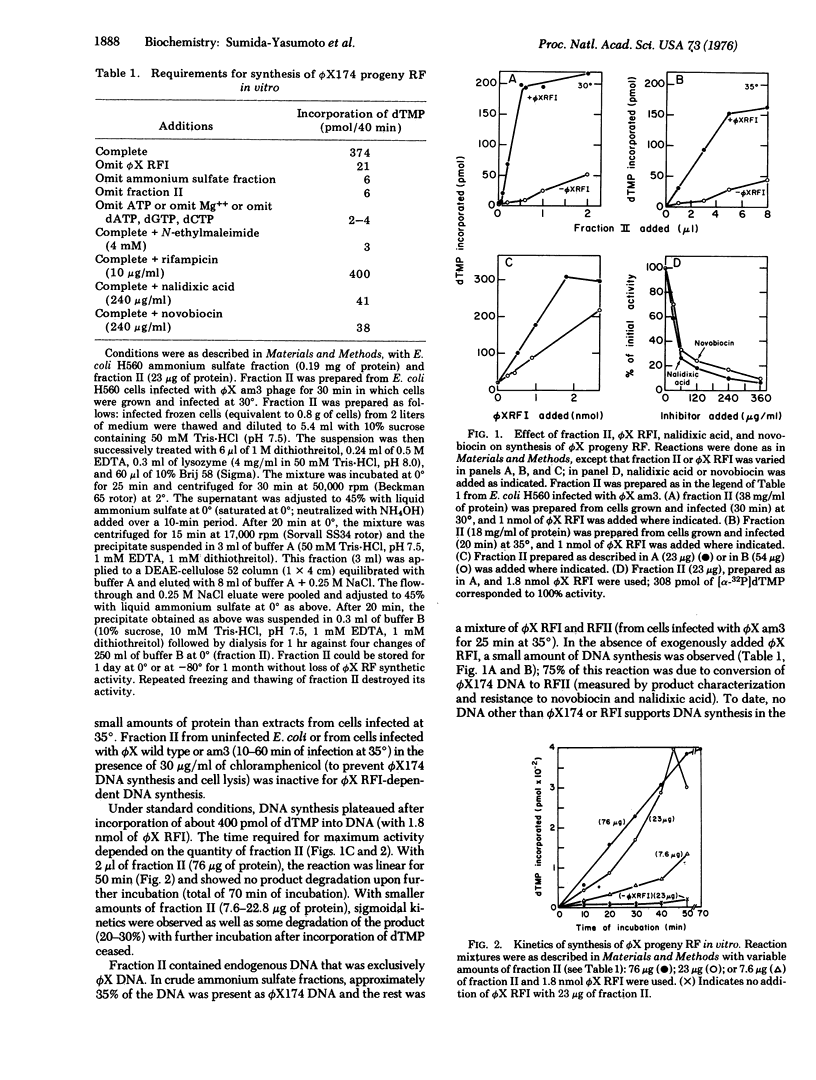
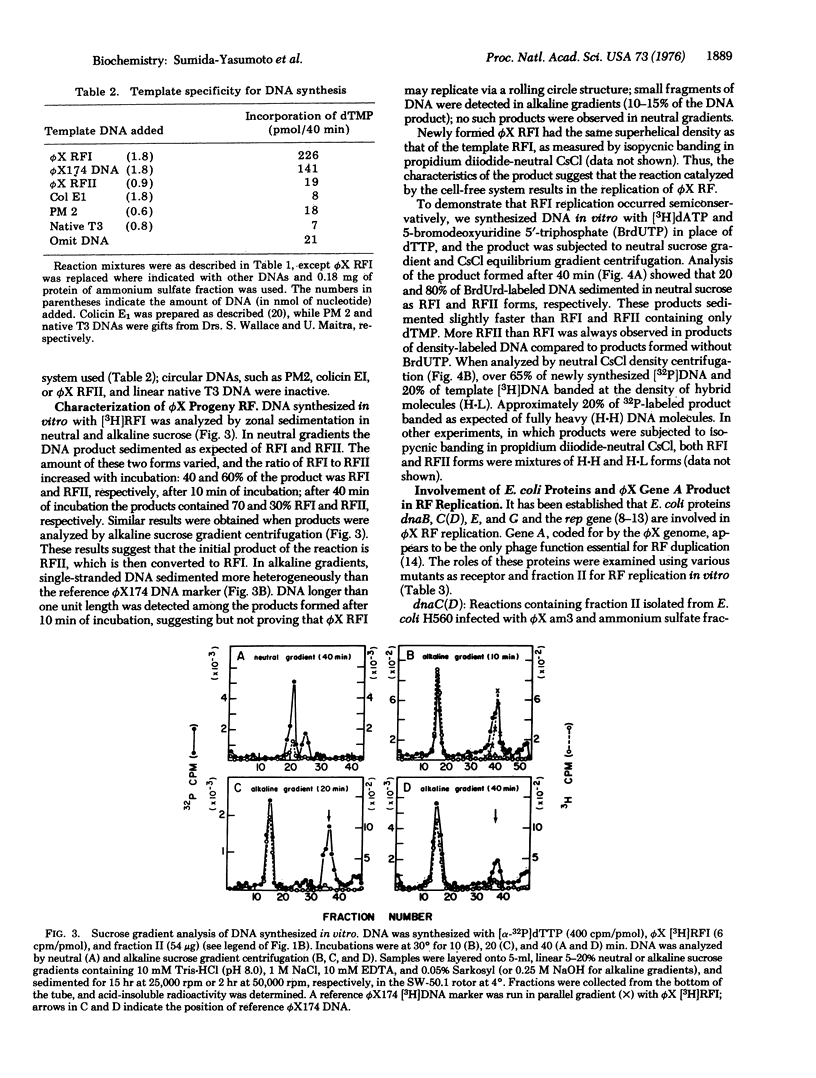
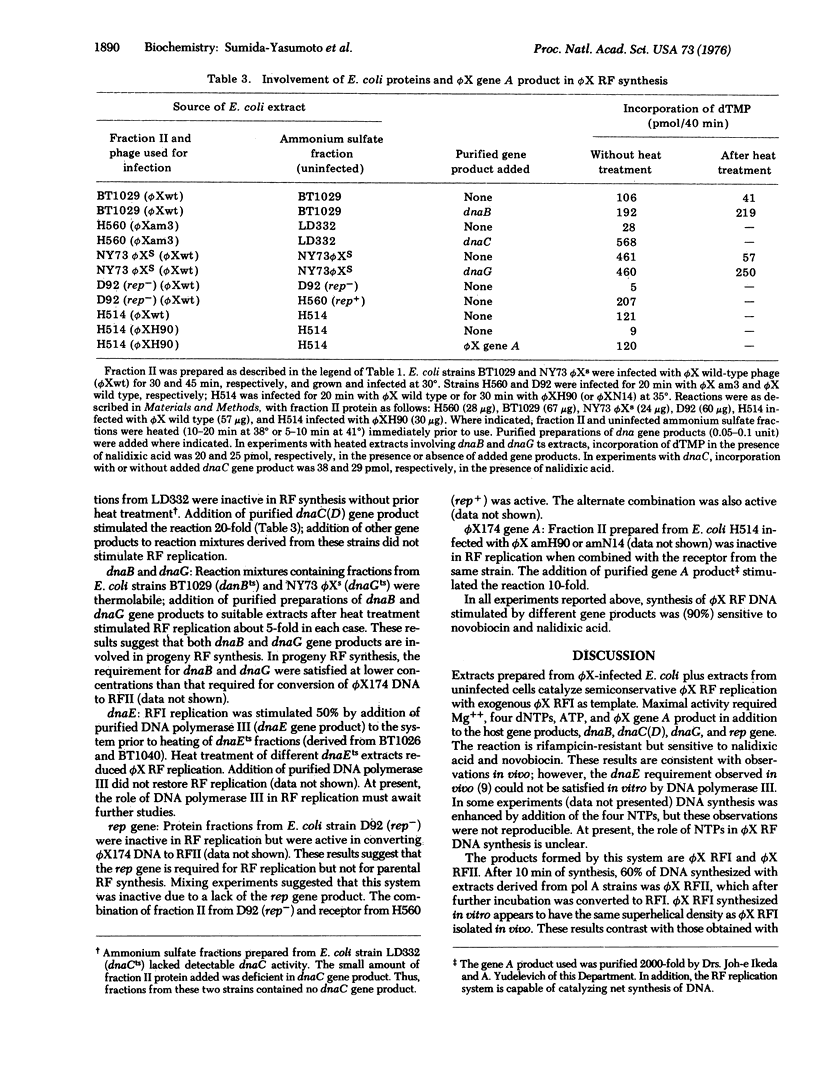
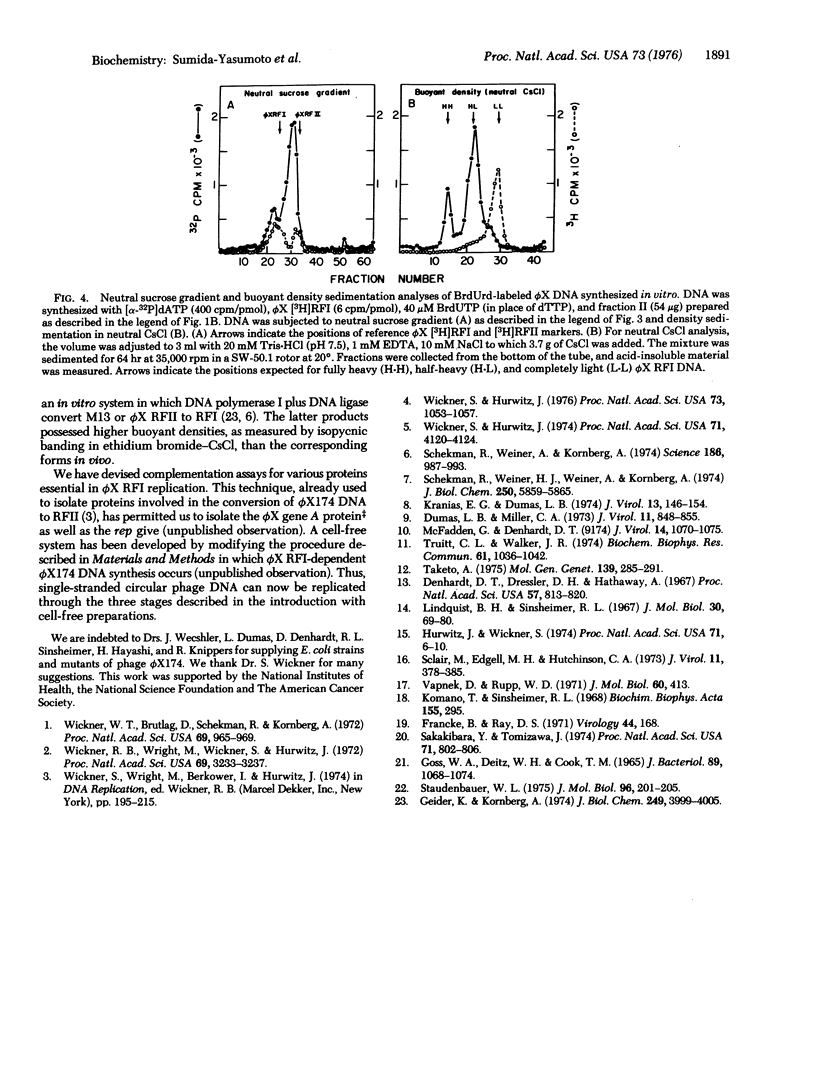
Extracts of Escherichia coli strains infected with bacteriophage phiX174 catalyze DNA synthesis dependent on double-stranded, circular phiX174 replicative form I (phiX RFI) by a semiconservative process. The reaction required Mg++, ATP, all four dNTP, and exogenous phiX RFI DNA as template and yielded phiX RFI and phiX RFII. The reaction was inhibited by nalidixic acid and novobiocin but not by rifampicin. DNA synthesis required the phiX174 gene A product and E. coli gene products dnaB, dnaC(D), dnaG, and rep.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Denhardt D. T., Dressler D. H., Hathaway A. THE ABORTIVE REPLICATION OF PhiX174 DNA IN A RECOMBINATION-DEFICIENT MUTANT OF Escherichia coli. Proc Natl Acad Sci U S A. 1967 Mar;57(3):813–820. doi: 10.1073/pnas.57.3.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas L. B., Miller C. A. Replication of bacteriophage phiX174 DNA in a temperature-sensitive dnaE mutant of Escherichia coli C. J Virol. 1973 Jun;11(6):848–855. doi: 10.1128/jvi.11.6.848-855.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke B., Ray D. S. Fate of parental phi X174-DNA upon infection of starved thymine-requiring host cells. Virology. 1971 Apr;44(1):168–187. doi: 10.1016/0042-6822(71)90163-2. [DOI] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI.II. INHIBITION OF DEOXYRIBONUCLEIC ACID SYNTHESIS. J Bacteriol. 1965 Apr;89:1068–1074. doi: 10.1128/jb.89.4.1068-1074.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geider K., Kornberg A. Conversion of the M13 viral single strand to the double-stranded replicative forms by purified proteins. J Biol Chem. 1974 Jul 10;249(13):3999–4005. [PubMed] [Google Scholar]
- Hurwitz J., Wickner S. Involvement of two protein factors and ATP in in vitro DNA synthesis catalyzed by DNA polymerase 3 of Escherichia coli. Proc Natl Acad Sci U S A. 1974 Jan;71(1):6–10. doi: 10.1073/pnas.71.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komano T., Sinsheimer R. L. Preparation and purification of phi X-RF component I. Biochim Biophys Acta. 1968 Jan 29;155(1):295–298. doi: 10.1016/0005-2787(68)90360-2. [DOI] [PubMed] [Google Scholar]
- Kranias E. G., Dumas L. B. Replication of bacteriophage phiX174 DNA in a temperature-sensitive dnaC mutant of escherichia coli C. J Virol. 1974 Jan;13(1):146–154. doi: 10.1128/jvi.13.1.146-154.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist B. H., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. XV. Bacteriophage DNA synthesis in abortive infections with a set of conditional lethal mutants. J Mol Biol. 1967 Nov 28;30(1):69–80. doi: 10.1016/0022-2836(67)90244-6. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y., Tomizawa J. I. Replication of colicin E1 plasmid DNA in cell extracts. Proc Natl Acad Sci U S A. 1974 Mar;71(3):802–806. doi: 10.1073/pnas.71.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schekman R., Weiner A., Kornberg A. Multienzyme systems of DNA replication. Science. 1974 Dec 13;186(4168):987–993. doi: 10.1126/science.186.4168.987. [DOI] [PubMed] [Google Scholar]
- Schekman R., Weiner J. H., Weiner A., Kornberg A. Ten proteins required for conversion of phiX174 single-stranded DNA to duplex form in vitro. Resolution and reconstitution. J Biol Chem. 1975 Aug 10;250(15):5859–5865. [PubMed] [Google Scholar]
- Sclair M., Edgell M. H., Hutchinson C. A., 3rd Mapping of new Escherichia coli K and 15 restriction sites on specific fragments of bacteriophage phi X174. J Virol. 1973 Mar;11(3):378–385. doi: 10.1128/jvi.11.3.378-385.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W. L. Letters to the editor: Novobiocin-a specific inhibitor of semiconservative DNA replication in permeabilized Escherichia coli cells. J Mol Biol. 1975 Jul 25;96(1):201–205. doi: 10.1016/0022-2836(75)90191-6. [DOI] [PubMed] [Google Scholar]
- Taketo A. Replication of phiA and phiX174 in Escherichia coli mutants thermosensitive in DNA synthesis. Mol Gen Genet. 1975 Sep 8;139(4):285–291. doi: 10.1007/BF00267968. [DOI] [PubMed] [Google Scholar]
- Truitt C. L., Walker J. R. Growth of phages lambda, phiX174, and Ml3 requires the dnaZ (previously dnaH) gene product of Escherichia coli. Biochem Biophys Res Commun. 1974 Dec 11;61(3):1036–1042. doi: 10.1016/0006-291x(74)90259-9. [DOI] [PubMed] [Google Scholar]
- Vapnek D., Rupp W. D. Identification of individual sex-factor DNA strands and their replication during conjugation in thermosensitive DNA mutants of Escherichia coli. J Mol Biol. 1971 Sep 28;60(3):413–424. doi: 10.1016/0022-2836(71)90178-1. [DOI] [PubMed] [Google Scholar]
- Wickner R. B., Wright M., Wickner S., Hurwitz J. Conversion of phiX174 and fd single-stranded DNA to replicative forms in extracts of Escherichia coli. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3233–3237. doi: 10.1073/pnas.69.11.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Conversion of phiX174 viral DNA to double-stranded form by purified Escherichia coli proteins. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4120–4124. doi: 10.1073/pnas.71.10.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner S., Hurwitz J. Involvement of escherichia coli dnaZ gene product in DNA elongation in vitro. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1053–1057. doi: 10.1073/pnas.73.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]


