Abstract
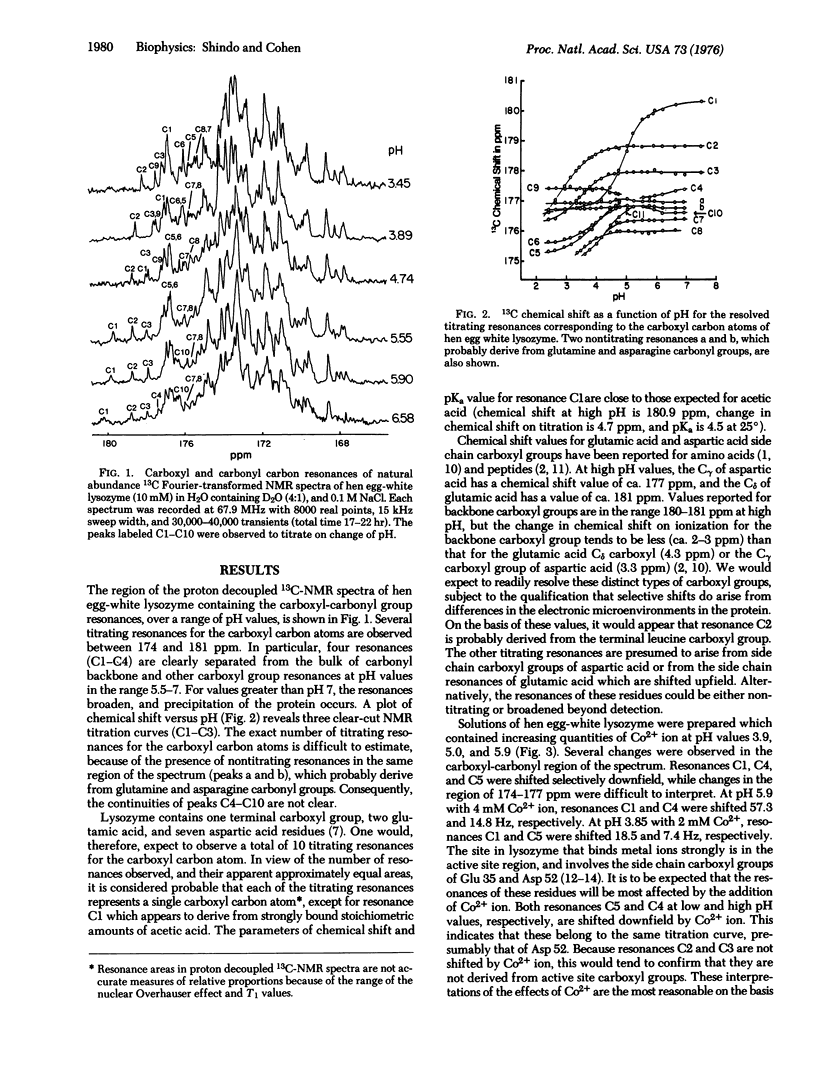
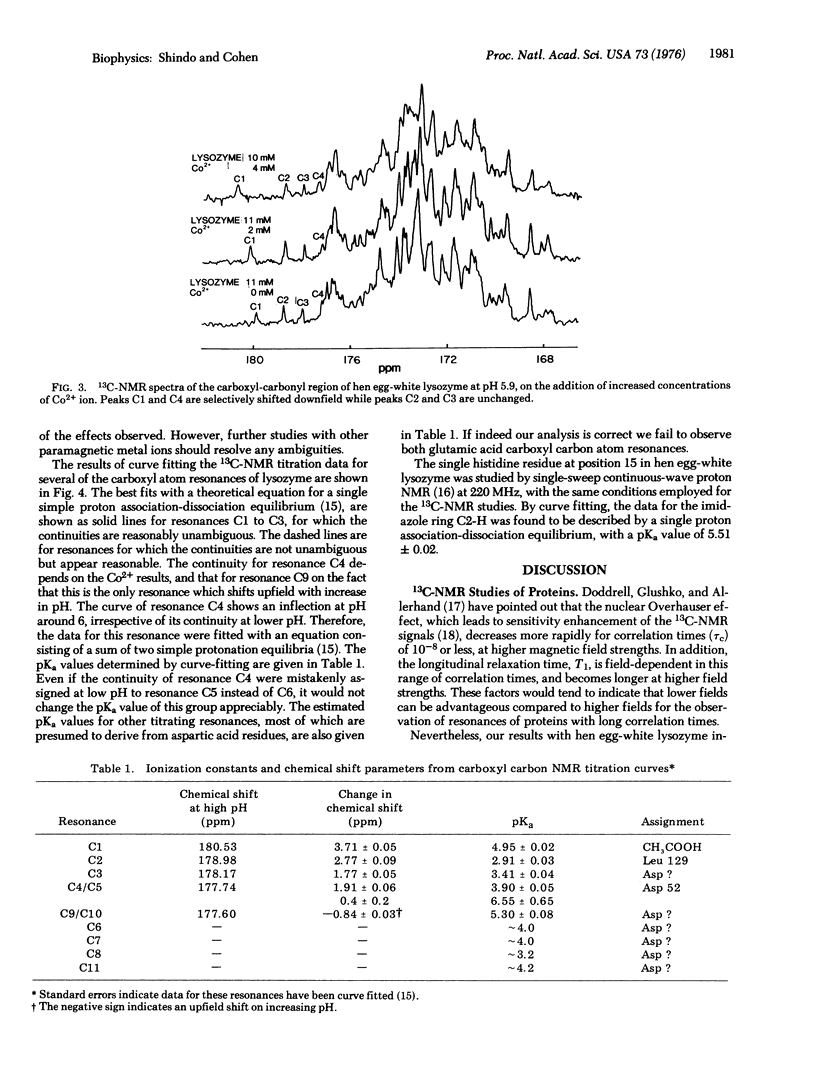
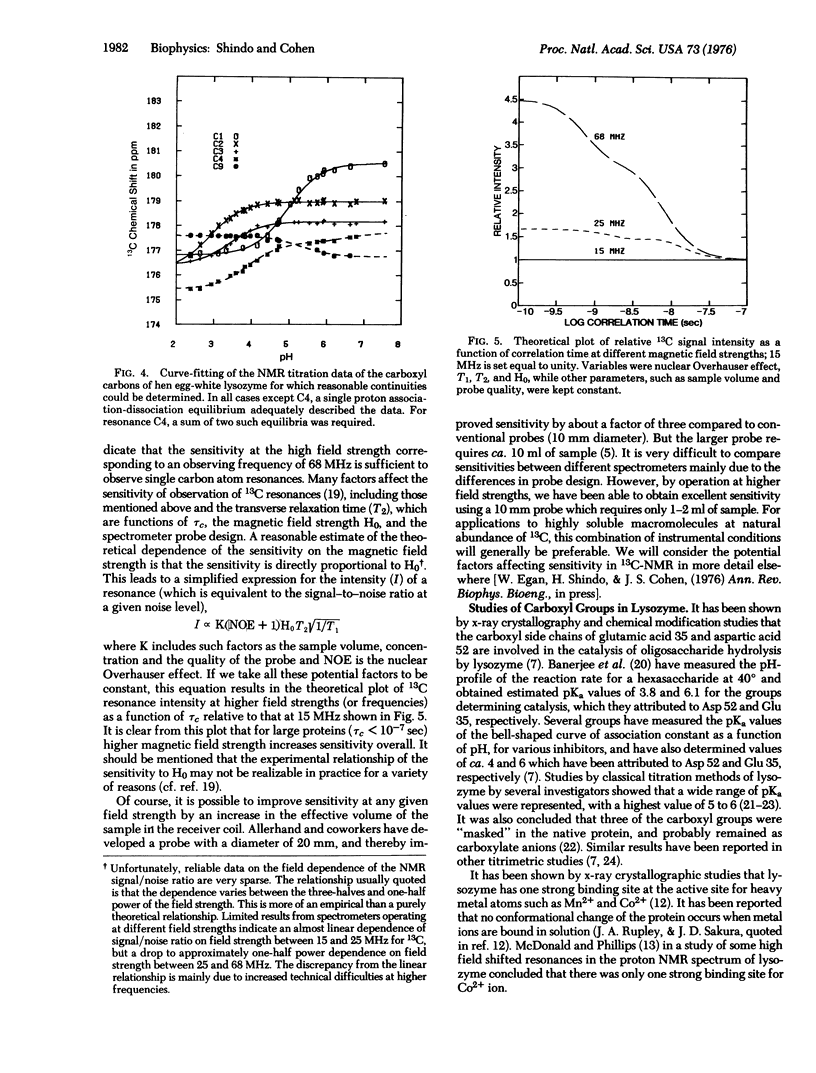
Several of the carboxyl carbon atom resonances of hen egg-white lysozyme (mucopeptide N-acetylmuramoyl hydrolase, EC 3.2.1.17) have been resolved by 13C-nuclear magnetic resonance (NMR) at 68 MHz. The change in chemical shift of the carboxyl carbon atom resonances, as a function of pH, has enabled the distinction of these resonances against the background of many nontitrating carbonyl group resonances. Several apparent microscopic ionization constants have been determined from the carboxyl group NMR titration curves, and possible assignments are discussed. Preliminary experiments were carried out in the presence of cobaltous ion, and selective shifts of several resonances were observed. Our results indicate the possibility of the direct observation of a wide range of single functional groups of proteins in solution by NMR techniques.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allerhand A., Childers R. F., Oldfield E. Natrual-abundance carbon-13 nuclear magnetic resonance studies in 20-mm sample tubes. Observation of numerous single-carbon resonances of hen egg-white lysozyme. Biochemistry. 1973 Mar 27;12(7):1335–1341. doi: 10.1021/bi00731a013. [DOI] [PubMed] [Google Scholar]
- Aune K. C., Tanford C. Thermodynamics of the denaturation of lysozyme by guanidine hydrochloride. I. Depdendence on pH at 25 degrees. Biochemistry. 1969 Nov;8(11):4579–4585. doi: 10.1021/bi00839a052. [DOI] [PubMed] [Google Scholar]
- Banerjee S. K., Kregar I., Turk V., Rupley J. A. Lysozyme-catalyzed reaction of the N-acetylglucosamine hexasaccharide. Dependence of rate on pH. J Biol Chem. 1973 Jul 10;248(13):4786–4792. [PubMed] [Google Scholar]
- Banerjee S. K., Pogolotti A., Jr, Rupley J. A. Self-association of lysozyme. Thermochemical measurements: effect of chemical modification of Trp-62, Trp-108, and Glu-35. J Biol Chem. 1975 Oct 25;250(20):8260–8266. [PubMed] [Google Scholar]
- Christl M., Roberts J. D. Nuclear magnetic resonance spectroscopy. Carbon-13 chemical shifts of small peptides as a function of pH. J Am Chem Soc. 1972 Jun 28;94(13):4565–4573. doi: 10.1021/ja00768a026. [DOI] [PubMed] [Google Scholar]
- Cohen J. S., Shrager R. I., McNeel M., Schechter A. N. Proton magnetic resonance studies at 220 MHz of the histidine residues of staphylococcal nuclease. Nature. 1970 Nov 14;228(5272):642–644. doi: 10.1038/228642a0. [DOI] [PubMed] [Google Scholar]
- Dobson C. M. Comments on the proposed rigidity of staphylococcal protease. Biochemistry. 1975 Nov 4;14(22):4905–4906. doi: 10.1021/bi00693a019. [DOI] [PubMed] [Google Scholar]
- Freedman M. H., Lyerla J. R., Jr, Chaiken I. M., Cohen J. S. Carbon-13 nuclear-magnetic-resonance studies on selected amino acids, peptides, and proteins. Eur J Biochem. 1973 Jan 15;32(2):215–226. doi: 10.1111/j.1432-1033.1973.tb02599.x. [DOI] [PubMed] [Google Scholar]
- Jones R., Dwek R. A. The mechanism of water-proton relaxation in enzyme paramagnetic-ion complexes. 1. The Gd(3)-lysozyme complex. Eur J Biochem. 1974 Sep 1;47(2):271–283. doi: 10.1111/j.1432-1033.1974.tb03691.x. [DOI] [PubMed] [Google Scholar]
- Keim P., Vigna R. A., Morrow J. S., Marshall R. C., Gurd F. R. Carbon 13 nuclear magnetic resonance of pentapeptides of glycine containing central residues of serine, threonine, aspartic and glutamic acids, asparagine, and glutamine. J Biol Chem. 1973 Nov 25;248(22):7811–7818. [PubMed] [Google Scholar]
- Kurachi K., Sieker L. C., Jensen L. H. Metal ion binding in triclinic lysozyme. J Biol Chem. 1975 Oct 10;250(19):7663–7667. [PubMed] [Google Scholar]
- Kuramitsu S., Ikeda K., Hamaguchi K., Fujio H., Amano T. Ionization constants of Glu 35 and Asp 52 in hen, turkey, and human lysozymes. J Biochem. 1974 Oct;76(4):671–683. [PubMed] [Google Scholar]
- McDonald C. C., Phillips W. D. Perturbation of the PMR spectrum of lysozyme by Co+2. Biochem Biophys Res Commun. 1969 Apr 10;35(1):43–51. doi: 10.1016/0006-291x(69)90480-x. [DOI] [PubMed] [Google Scholar]
- Meadows D. H., Markley J. L., Cohen J. S., Jardetzky O. Nuclear magnetic resonance studies of the structure and binding sites of enzymes. I. Histidine residues. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1307–1313. doi: 10.1073/pnas.58.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield E., Norton R. S., Allerhand A. Studies of individual carbon sites of proteins in solution by natural abundance carbon 13 nuclear magnetic resonance spectroscopy. Strategies for assignments. J Biol Chem. 1975 Aug 25;250(16):6381–6402. [PubMed] [Google Scholar]
- Quirt A. R., Lyerla J. R., Jr, Peat I. R., Cohen J. S., Reynolds W. F., Freedman M. H. Carbon-13 nuclear magnetic resonance titration shifts in amino acids. J Am Chem Soc. 1974 Jan 23;96(2):570–574. doi: 10.1021/ja00809a038. [DOI] [PubMed] [Google Scholar]
- Shrager R. I., Cohen J. S., Heller S. R., Sachs D. H., Schechter A. N. Mathematical models for interacting groups in nuclear magnetic resonance titration curves. Biochemistry. 1972 Feb 15;11(4):541–547. doi: 10.1021/bi00754a010. [DOI] [PubMed] [Google Scholar]
- Sophianopoulos A. J. Association sites of lysozyme in solution. I. The active site. J Biol Chem. 1969 Jun 25;244(12):3188–3193. [PubMed] [Google Scholar]
- Tanford C., Roxby R. Interpretation of protein titration curves. Application to lysozyme. Biochemistry. 1972 May 23;11(11):2192–2198. doi: 10.1021/bi00761a029. [DOI] [PubMed] [Google Scholar]


