Review on the human-specific α7-nicotinic acetylcholine receptor in leukocytes and human-specialized mechanisms to regulate inflammatory response to injury.
Keywords: neuroinflammation, vagus nerve, CHRNA7, α7nAChR, dupα7nAChR, SLURP1
Abstract
Conventional wisdom presumes that the α7nAChR product of CHRNA7 expression mediates the ability of the vagus nerve to regulate the inflammatory response to injury and infection. Yet, 15 years ago, a 2nd structurally distinct and human-specific α7nAChR gene was discovered that has largely escaped attention of the inflammation research community. The gene, originally called dupα7nAChR but now known as CHRFAM7A, has been studied exhaustively in psychiatric research because of its association with mental illness. However, dupα7nAChR/CHRFAM7A expression is relatively low in human brain but elevated in human leukocytes. Furthermore, α7nAChR research in human tissues has been confounded by cross-reacting antibodies and nonspecific oligonucleotide primers that crossreact in immunoblotting, immunohistochemistry, and RT-PCR. Yet, 3 independent reports show the human-specific CHRFAM7A changes cell responsiveness to the canonical α7nAChR/CHRNA7 ion-gated channel. Because of its potential for the injury research community, its possible significance to human leukocyte biology, and its relevance to human inflammation, we review the discovery and structure of the dupα7nAChR/CHRFAM7A gene, the distribution of its mRNA, and its biologic activities and then discuss its possible role(s) in specifying human inflammation and injury. In light of emerging concepts that point to a role for human-specific genes in complex human disease, the existence of a human-specific α7nAChR regulating inflammatory responses in injury underscores the need for caution in extrapolating findings in the α7nAChR literature to man. To this end, we discuss the translational implications of a uniquely human α7nAChR-like gene on new drug target discovery and therapeutics development for injury, infection, and inflammation.
Introduction
It is generally accepted that the vagus nerve regulates the inflammatory response after injury by acetylcholine binding to, and activation of, the homopentameric α7nAChR ion channel on leukocytes, which in turn, gauges the systemic and local anti-inflammatory responses to injury [1–3]. As Wang et al. [4] showed that vagus nerve stimulation is ineffective in mice lacking the α7nAChR gene, there is also a general consensus that the α7nAChR ligand-gated channel is an absolute requirement for the anti-inflammatory actions of vagus nerve stimulation.
Unlike all other species, however, the human genome has a 2nd structurally distinct α7nAChR gene, originally called “dupα7nAChR,” and now known as “CHRFAM7A” [5]. First and foremost, it is uniquely human. Second, it is preferentially expressed in peripheral tissues. These observations raise the possibility that there exist uniquely human mechanisms to gauge the α7nAChR anti-inflammatory response. For the purposes of this review, α7nAChR is called either “α7nAChR” or by its official name, “CHRNA7,” whereas the newly recognized, human-specific, partially duplicated gene is called dupα7nAChR or by its official name, CHRFAM7A.
VAGUS NERVE CONTROL OF INFLAMMATION
The vagal anti-inflammation model proposes that on one hand, afferent projections of the vagus nerve monitor the immune response by sensing peripheral cytokine levels. On the other, the Tracey laboratories [1, 3, 6–8] have clearly established that efferent signaling via α7nAChR activation regulates systemic cytokine responses by controlling spleen inflammatory cell function. Accordingly, the vagus nerve is presumed to play a critical role in maintaining homeostasis in humans by regulating cytokine responsiveness of the host and gauging the ability of the human immune response to modulate the systemic inflammatory effects of injury and ultimately, to attenuate host tissues damage (see Fig. 1).
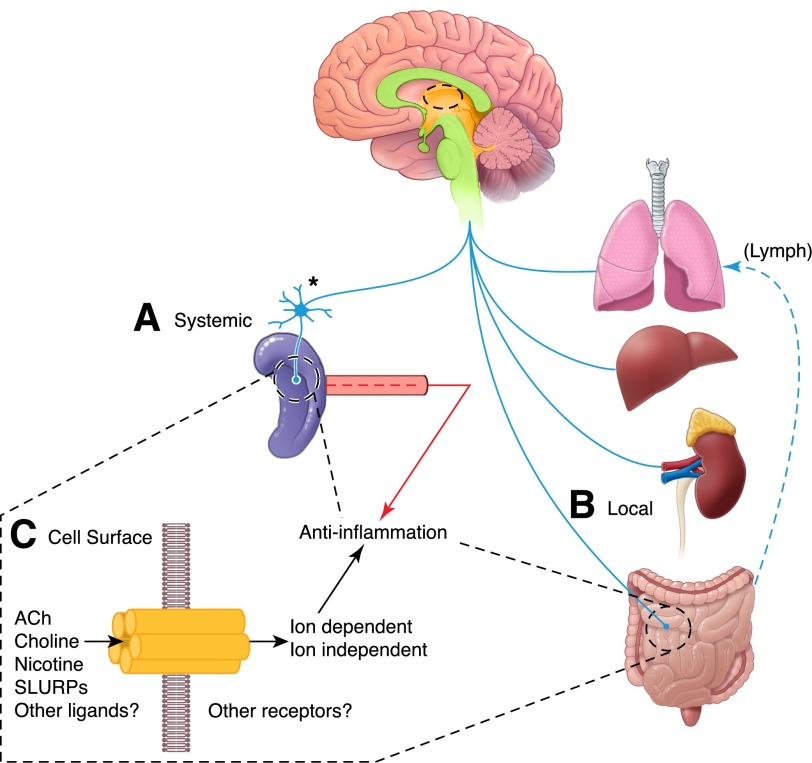
Figure 1. Vagus nerve control of inflammation.
There are 2 anti-inflammatory pathways activated after vagus nerve stimulation. The 1st (A) elicits systemic changes in circulating cytokines and in splenic cells that produce inflammatory and anti-inflammatory cytokines. In this spleen-dependent response, the vagus nerve acts by stimulating a sympathetic response in neurons of the celiac-mesenteric ganglia, which in turn, innervate the spleen where T cells release ACh to alter monocyte homeostasis. In the 2nd pathway (B), a local release of ACh by cholinergic neuronal projections of the vagus nerve targets inflammatory and epithelial cells locally in the lung, liver, kidney, and gut. This pathway, which is spleen independent, regulates local homeostasis and cytokine production in the target tissue, while also gauging cytokines and cells in the lymphatic vasculature (Lymph). In both instances, ACh is released (C) and presumed to act through the α7nAChR, but there is evidence for peptide hormone-like ligands (e.g., SLURP1/2 [26, 27] that can also affect function; see text for further details).
In this model, cholinergic agonists, including ACh and nicotine, activate the cell surface α7nAChR homopentameric channel (see below) and thus, modulate the cellular immune response to proinflammatory challenges by altering the responsiveness of NF-κB and Jak2/STAT3 inflammatory signaling pathways [9, 10]. The link between the vagus nerve and the spleen is indirect, however, as the vagus nerve does not directly innervate the spleen (see Fig. 1). To function, the vagus nerve regulation of cytokine signaling in splenocytes must invoke adrenergic neuron signaling in the celiac ganglion [11–13]. There, celiac ganglionic adrenergic neurons project into the spleen to stimulate T cells, which in turn, release ACh to regulate splenic macrophages [14–16]. Accordingly, with stimulation of the vagus nerve above or the splenic nerve below, the celiac ganglion inhibits TNF-α production by splenocytes [11, 17, 18]. In this way, indirect vagal innervation presumably stimulates ACh-producing memory T cells and thereby, mediates the vagus nerve effects on the inflammation response by binding α7nAChR on splenic macrophages to regulate splenic production and systemic distribution of cytokines [19].
It is also important to note that in addition to these indirect, spleen-dependent, anti-inflammatory effects of the vagus nerve [17], the Coimbra laboratories demonstrated that there exists a spleen-independent pathway that also regulates the local inflammatory response of vagus target tissues [20–24], and this has been recently confirmed by others [25]. In these instances, the vagus nerve locally modulates local cytokine production by direct innervation of tissues, such as gut (Fig. 1). As with spleen–mediated responsiveness, the α7nAChR appears essential in mediating vagal responsiveness, as vagus nerve signaling fails to decrease proinflammatory cytokine release after vagotomy or to prevent gut inflammation in knockout mice that lack the α7nAChR gene [4, 20–25]. Therefore, it is critical to understand the role of α7nAChR in the anti-inflammatory activity of vagus nerve stimulation.
THE HUMAN α7nAChR GENE (CHRNA7) AND THE HUMAN-SPECIFIC, PARTIALLY DUPLICATED CHRFAM7A GENE
The official name of the gene encoding the α7nAChR monomer is CHRNA7. Because of the importance of α7nAChR in calcium signaling of neuronal and synaptic cell–cell communication in the CNS, this α7nAChR/CHRNA7 gene, its mRNA(s), and the activities of the α7nAChR ligand-gated ion channel have been studied extensively by neuroscientists (see Papke [28]). The α7nAChR is distinguished from other nAChRs by the high permeability to calcium that it confers to cells when activated and its reversible desensitization [29–31]. Interestingly, there is also a long history of detecting α7nAChR proteins and gene expression in non-neuronal tissues, particularly in the immune system [4, 32, 33]. As these cells are not traditionally viewed as excitable, the nonionic, and metabotropic activities, functions for α7nAChRs have been suspected in non-neuronal tissues [9, 34, 35]. Unlike other heteromeric ligand-gated ion channels composed of α3, α4, α5, β2 and β4 nAChR monomers [28, 30, 31, 36, 37], α7nAChRs can also behave like canonical ligand receptors, and maximal activation occurs with a fraction of agonist binding [36–42].
That being said, the α7nAChR/CHRNA7 is a member of a superfamily of ligand-gated ion channels, and the α7nAChR cell-surface protein is composed of 5 homologous subunits that localize to the cell surface (Fig. 2A) to mediate fast signal transmission at neuronal synapses [29, 38]. For several years, α7nAChR was thought to be a CNS-specific, ligand-gated ion channel, but noncanonical, nicotinic α7nAChR-like channel activities have been detected with antibodies, ligands, and PCR primers over the last 20 years in several peripheral tissues, including numerous different subtypes of leukocytes (see below). The proposed structure of the α7nAChR includes an N-terminal extracellular domain, followed by 3 transmembrane domains, a cytoplasmic loop, a 4th transmembrane domain, and a short C-terminal extracellular region (Fig. 2A). Once translocated to the cell surface, the α7nAChR protein forms a homo-oligomeric pentameric channel with other α7nAChR proteins (Fig. 2B). Upon ligand binding, for example, by nicotine and/or acetylcholine, the α7nAChR proteins change conformation, and a calcium-conducting channel opens across the cell’s plasma membrane (Fig. 2C). With this opening, there is a measurable change in voltage that accompanies the flux of calcium across the plasma membrane, and this change in voltage reflects channel activity. Pharmacophores that affect α7nAChR protein conformation on the cell surface can potentiate, sustain, and significantly alter channel activity. Unlike other ligand-gated channels, several agonists, including choline, can affect channel activity [28].
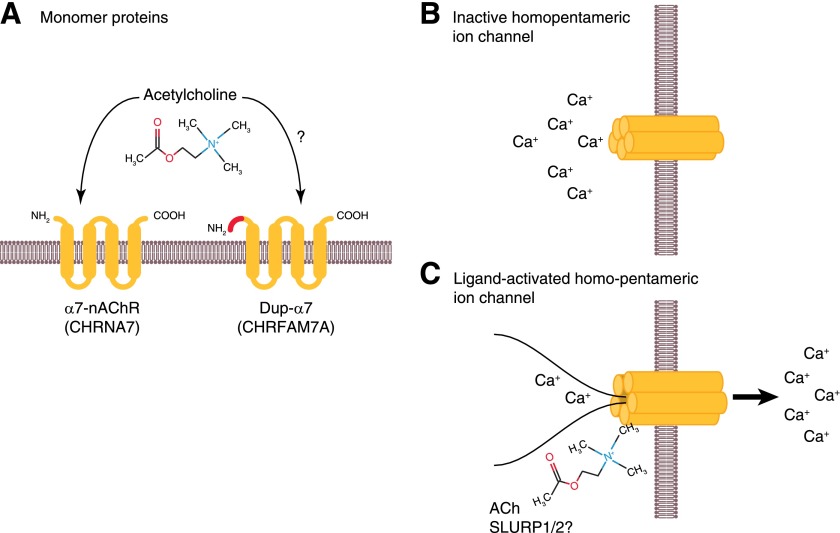
Figure 2. Mechanism of action of the α7nAChR.
The monomeric α7nAChR protein (A) that is ultimately expressed by the CHRNA7 gene encodes a protein with 4 transmembrane domains that form a complex pentameric ligand-binding domain when 5 α7nAChR proteins are translocated to the cell surface (B). As the homopentameric complex binds ACh (C), a conformational change enables translocation of calcium across the plasma membrane, inducing a change in voltage that is measured via patch-clamping of cells. The structural similarities with the dupα7nAChR protein discussed later in the text are presented on the right side of A. Colors represent the domains that are the shared original α7nAChR sequence, but the unique amino terminus dupα7nAChR sequence is shown in red.
Historically, the ligand-gating α7nAChR function was thought to reflect its function as a synaptic ion-gated channel. More recently, however, the detection of α7nAChR in endothelial and epithelial cells [28, 33, 43–46] and then the discovery of hormone-like peptide ligands that can bind α7nAChR complexes [26, 27] raised the possibility that there might exist alternative, metabotropic mechanisms for α7nAChR that are independent of its traditional, ligand-gated ion channeling [47]. α7nAChR/CHRNA7 is clearly detected by PCR, immunostaining, and immunoblotting of peripheral tissues, but the protein identified has been rarely characterized. Could the 1998 discovery of a unique and human-specific α7nAChR gene [5] that is highly expressed in peripheral tissues, including leukocytes [48], be a candidate α7nAChR/CHRNA7 receptor? CHRFAM7A is a structurally related α7nAChR that cross-reacts with antibodies to human α7nAChR, and its mRNA is equally detected by α7nAChR PCR when amplified with commonly used primers (see below).
DISCOVERY OF CHRFAM7A
In 1998, Gault et al. [5] sequenced the human α7nAChR gene on chromosome 15q13,14 and found it to be structurally similar to that of all other species. It has 10 exons that localize to highly conserved splice-junction positions (Fig. 3A). With high GC content (77%) in its putative promoter, the α7nAChR gene is epigenetically regulated by DNA methylation and contains consensus transcription factor-binding sites for specificity protein 1 (transcription factor), AP2, early growth response factor 1 (transcription factor), and CREB [5]. Unlike all other species, however, Gault et al. [5] noted that the human α7nAChR/CHRNA7 locus appeared unusually large, and in the course of extending their structural analyses, they discovered the presence of duplicated exons 5–10 of the human α7nAChR gene that are >1.6 Mb 5′ upstream from the intact α7nAChR/CHRNA7 gene (Fig. 3B). Also present in this newly identified and partially duplicated α7nAChR/CHRNA7 sequence, Gault et al. [5] identified 4 completely novel exons that, surprisingly, derived, in part, from an unrelated sequence, itself originating, in part, from human chromosome 3 [49]. These “new” exons were rearranged in place of the missing exons 1–4 of human α7nAChR (Fig. 3C), and the 4 new exons of this FAM7 sequence created a new ORF to form a new hybrid gene. The fused, partially duplicated α7nAChR sequence and new FAM sequence created a new human α7nAChR gene, now called CHRFAM7A (Fig. 3D).
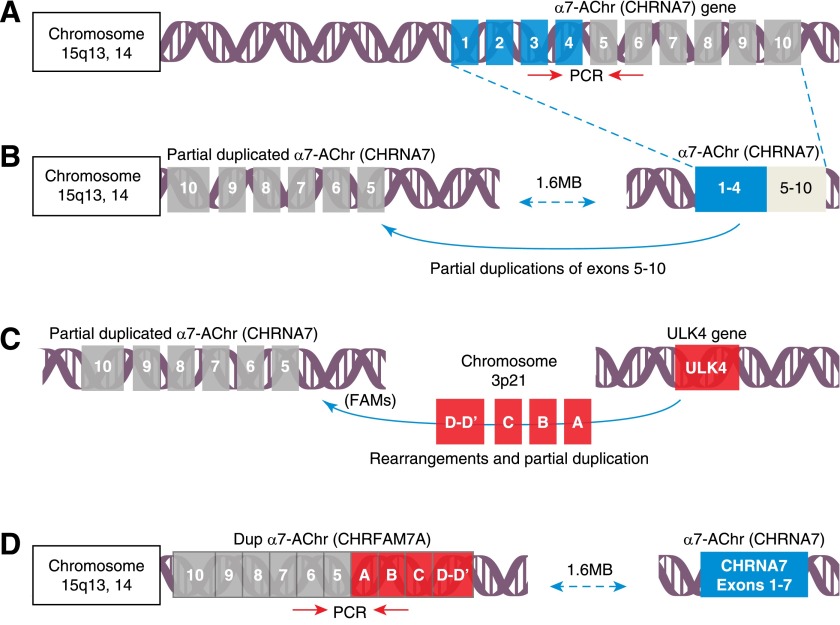
Figure 3. Discovery of the partially duplicated human α7nAChR.
As illustrated (A) α7nAChR/CHRNA7, the human gene encoding the α7nAChR, is localized to chromosome 15q13,14, where it spans 10 exons. In 1 evolution mechanism, it is assumed that shortly after the divergence of humans from other primates, a rearrangement and perhaps simultaneously, a partial duplication of the CHRNA7 gene (B) occurred, ∼1.6 MB 5′ from the original gene. Shortly thereafter, several partially duplicated DNA sequences, including parts the ULK4 kinase of chromosome 3, rearranged in several copies onto the q13,14 locus of chromosome 15 (C). One copy derived from FAM7 duplicated and rearranged 3′ to the partially duplicated gene encoding exons 5–10 of the α7nAChR gene on chromosome 15 to create a new hybrid gene, called dupα7nAChR (D), now CHRFAM7A. To differentiate mRNA expression, it is critical to use primers that hybridize unique sequences at junctions (as illustrated by red arrows).
This newly emerged and partially duplicated human-specific CHRFAM7A gene was present in all 4 human samples available to Gault et al. [5], but future research would find that the gene is sometimes absent or only present as a single copy in 5–15% of patients. The significance of these findings and the identification of a 2 bp deletion polymorphism that puts the C-terminus α7nAChR/CHRNA7 moiety out of frame are unknown in regard to the inflammatory response but have gained significant interest in neuropsychiatric research. The findings could point to the possibility of significant variability in leukocyte responses to Ach in human populations but has not been investigated. Interestingly, it cannot be studied in animal models, as the gene is human specific. The emergence of this dupα7nAChR/CHRFAM7A gene from an α7nAChR duplication and rearrangement is also recent and occurred after the divergence of humans from other primates [50, 51]. This makes the dupα7nAChR/CHRFAM7A gene human specific [52]. Other species (e.g., mice) have variants of α7nAChR/CHRNA7 (see below) generated by alternative splicing that can confer new pharmacological properties to α7nAChR, but none have a distinct dupα7nAChR/CHRFAM7A gene that encodes a uniquely human part-kinase sequence (FAM/ULK4) and part-functional α7nAChR.
As the α7nAChR/CHRNA7 gene has traditionally been viewed as regulating synaptic Ca2+ in the CNS, the discovery of a human-specific dupα7nAChR/CHRFAM7A gene gained the immediate attention of the psychiatric research community. Its chromosomal location (chromosome 15 q13,14) is within 1 MB of a significant genotypic disequilibrium in schizophrenia [53–58] and associated with bipolar disorders [59–61], episodic memory performance [62], dementia [63–66], hyperactivity [67], autism [68], Tourette syndrome [69], juvenile myoclonic epilepsy [70], and autosomal-dominant nocturnal frontal-lobe epilepsy and idiopathic-generalized epilepsy [71, 72]. However, it was in the course of more detailed, comparative genetic mapping when investigators realized that dupα7nAChR/CHRFAM7A was overexpressed significantly in human leukocytes, particularly when its levels were compared with the amount expressed in the CNS or with the relative expression of the “wild-type” human α7nAChR/CHRNA7 gene in human leukocytes [48].
STRUCTURE OF THE CHRFAM7 GENE AND mRNA EXPRESSION
The dupα7nAChR/CHRFAM7A gene consists of 5 FAM7-derived exons (D′, D, C, B, and A) and exons 5–10 of CHRNA7 (Fig. 3D). Analyses of dupα7nAChR/CHRFAM7A gene expression using AceView [73] show that dupα7nAChR/CHRFAM7A gene expression is comparable with many other genes in the human genome and that it occurs at ∼1.1× the mean expression level of all genes. dupα7nAChR/CHRFAM7A mRNA has also been characterized from cDNA clones acquired from testis, brain, colon, tumors, eye, and over 30 other tissues, confirming a wide distribution of expression that extends well beyond its original detection in the CNS and identification in leukocytes. These data suggest that like CHRNA7, the biologic significance of dupα7nAChR/CHRFAM7A is not restricted to the CNS but extends to potential regulatory functions in peripheral tissues. Its activities are currently unknown.
Expressed sequence-tag cloning, sequencing, and further analyses of dupα7nAChR/CHRFAM7A gene expression in cells and tissues show that the dupα7nAChR/CHRFAM7A gene can lead to transcription of at least 11 different mRNAs, of which 8 are alternatively spliced variants, and 3 are unspliced. The ORFs encoded within these transcripts predict the existence of proteins that range from 104 to 461 aa, but there is really only 1 transcript that encodes a dupα7nAChR/CHRFAM7A that has sufficient Kozak consensus sequences to predict that it is translated into a protein. Bioinformatic analyses (Fig. 4) point to the existence of a 42–45 kDa dupα7nAChR/CHRFAM7A protein (depending on glycosylation) that only differs by the substitution of 146 amino terminus in α7nAChR/CHRNA7 by a peptide of 27 aa in dupα7nAChR/CHRFAM7A (see GenBank: NP_647536.1). A 2nd predicted dupα7nAChR/CHRFAM7A protein encoded by a “mRNA-b transcript” has been described in some curated genomic databases, such as AceView [73], but its existence has not been confirmed. This hypothetical dupα7nAChR/CHRFAM7A-like protein differs by the substitution of the 146 amino terminus of CHRNA7 with 32 aa that also derive from FAM7/ULK4 (Fig. 4). The absence of a canonical leader sequence predicts them to be inefficient in reaching the cell surface, but hydrophobic transmembrane sequences can serve to direct trafficking to the endoplasmic reticulum and extracellular secretion. The predicted dupα7nAChR/CHRFAM7A (GenBank: NP_647536) is 412 aa and the 1st 140 aa contain a significant portion of the original α7nAChR/CHRNA7 neurotransmitter-gated, ion-channel, ligand-binding domain, as analyzed by PFAM (pfam02931). This predicts that dupα7nAChR/CHRFAM7A can participate in the pentameric arrangement of the ion channel with retention of ligand and bungarotoxin binding. This Variant 1 of dupα7nAChR/CHRFAM7A also contains the neurotransmitter-gated, ion-channel transmembrane region and transmembrane domain at aa 147–397 (pfam02932).
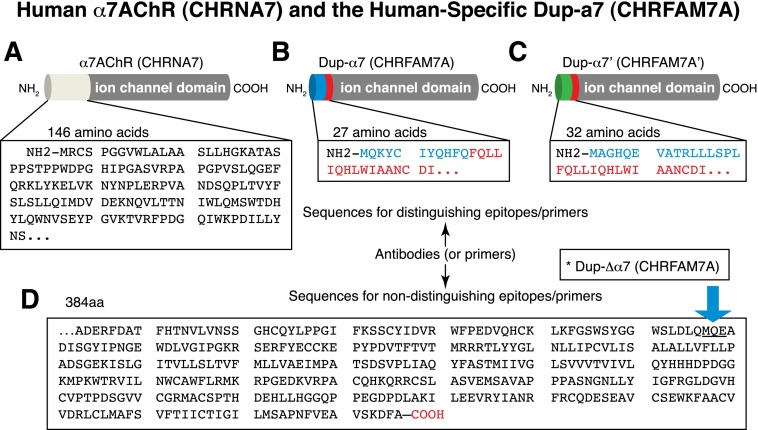
Figure 4. Sequence differences between α7nAChR and its partially duplicated homolog (CHRFAM7A).
(A) As illustrated, 146 aa of the human α7nAChR that are normally on the outer cell surface and in the extracellular space were lost during partial duplication of exons 5–10 and the loss of exons 1–4. In their place, a 27 aa sequence derived from the FAM7 gene localizes to the amino terminus (B). A hypothetical alternative (C) protein with sequence common to CHRFAM7A (red amino acids) is reported in reconstructed human genome databases, but its existence is still unproven (see text). In all instances, (D) the proteins share the same 384 aa containing 4 transmembrane domains and the ligand-gated, ion-channel transmembrane region. Polyclonal antibodies and mAb that recognize the last 386 aa of α7nAChR cannot distinguish human α7nAChR from CHRFAM7A, and predicted molecular weights are confounded by glycosylation sites. The sequence encoded by a Variant 2 mRNA encodes a duplicated C-terminus fragment of α7nAChR that is 100% identical to α7nAChR (see text for details).
A 2nd Variant 2 dupα7nAChR/CHRFAM7A mRNA (GenBank: NP_683709.1) encodes a protein that is truncated by 91 aa at its amino terminus and accordingly, does not contain any FAM sequence (Fig. 4D, arrow). The resulting 321 aa protein initiates from translation at a downstream ATG from Variant 1 and encodes a protein with no FAM sequence and only 20 aa of the original α7nAChR ligand-binding domain. Recently called dupΔα7 [74], the mRNA encodes an amino terminus truncated AChR with a sequence indistinguishable from truncated α7nAChR. The distance of the Kozak consensus sequencing from initiation methionine makes native translation from the dupα7nAChR/CHRFAM7A mRNA unlikely, although not totally impossible. It is only included here for the purposes of discussing its hypothetical existence, but its expression can be forced as a GFP-fusion protein regulated by a CMV promoter [74]. Together, these findings underscore the need to identify native forms of the dupα7nAChR/CHRFAM7A protein present in cells and in tissue lysates rather than force over expression. This would be an important 1st step forward to help determine the biologic and physiologic significance of dupα7nAChR/CHRFAM7A gene expression. Based on molecular-weight estimates, cell lysates contain proteins of 42–45 kDa MW, which correspond to the predicted CHRNFAM7A Variant 1 (Fig. 4B).
THE MAIN CHRNA7 TRANSCRIPT DETECTED BY PCR OF HUMAN LEUKOCYTES IS CHRFAM7A
Historically, the existence of an α7nAChR/CHRNA7 in peripheral tissues has been contentious, as the α7nAChR was thought to be a neuron-specific ion channel [29, 33, 38, 41, 42]. Shortly after Gault’s discovery of CHRFAM7A in 1998 [5], however, Villiger et al. [48] confirmed the presence of dupα7nAChR/CHRFAM7A expression in the CNS but also reported that human leukocytes were an unexpectedly rich source of dupα7nAChR/CHRFAM7A gene expression. With the use of 2 pairs of primers that were specific for the CHRNA7 or the newly discovered dupα7nAChR/CHRFAM7A (then called dupα7), these investigators confirmed a differential α7nAChR/CHRNA7 and dupα7nAChR/CHRFAM7A expression in brain and found dupα7nAChR/CHRFAM7A to be a disproportionately significant transcript in human leukocytes. Unlike bone marrow cells, which yielded a positive and significant hybridizing signal for α7nAChR/CHRNA7 and dupα7nAChR/CHRFAM7A, HL60 cells (a leukemic cell line) and even PBMCs, purified from human blood, expressed almost exclusively the dupα7nAChR/CHRFAM7A gene. Both HL60 cells and PBMCs also immunostain dupα7nAChR/CHRFAM7A with anti-human α7nAChR/CHRNA7 mAb (mAb306) and immunoblot a 45 kDa protein with anti-human α7nAChR/CHRNA7 polyclonal antibodies (pAb C-20). Accordingly, the authors concluded that the dupα7nAChR/CHRFAM7A mRNA that is detected by RT-PCR is translated to protein. The molecular weights (42–45 kDa) observed also predict that the dupα7nAChR/CHRFAM7A mRNA in PBMCs translates the mRNA encoding the Variant 1 protein (see Fig. 4B). The identity of the transcript was confirmed by sequencing of the amplified fragment and the amplicon proven identical to the dupα7nAChR/CHRFAM7A sequence identified by Gault et al. [5]. This made PCR identification of the new human-specific gene in leukocytes unequivocal. Ironically, the significance of this finding was overlooked by the α7nAChR/CHRNA7 research community, as the dupα7nAChR/CHRFAM7A shares peptide epitopes with α7nAChR and has a similar molecular weight in immunoblotting, staining in immunohistochemistry, and even the PCR primers cross-hybridize within RT-PCR without careful selection (Fig. 5). Therefore, the literature is confounded by the misidentification of α7nAChR/CHRNA7 and dupα7nAChR/CHRFAM7A expression, distribution, and identity in human tissues and cells (see the following section below).
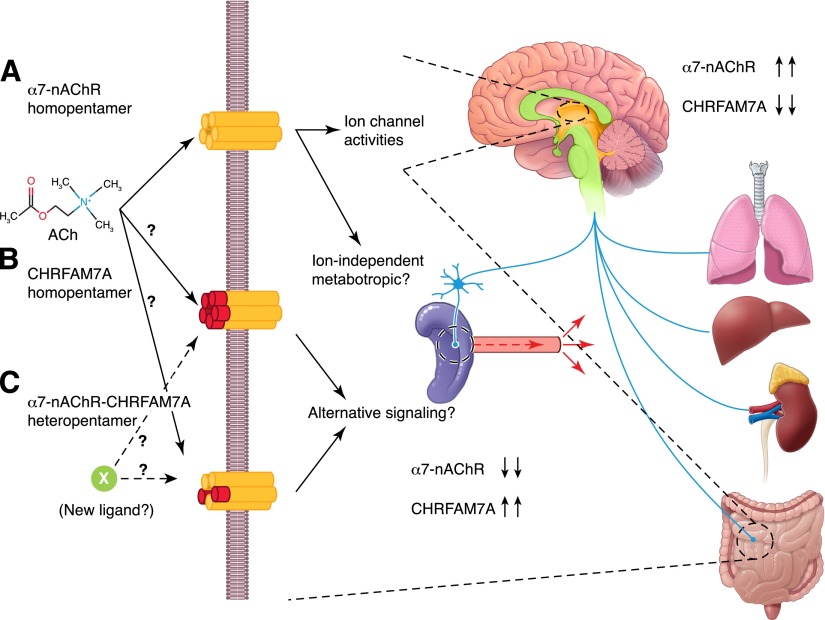
Figure 5. Vagus nerve control of inflammation may be unique in humans.
Traditional models of ACh action presume its binding to the α7nAChR, thereby inducing a conformational change in the homopentamer channel that allows calcium entry into the cells and changes in plasma membrane voltage (A). The overexpression of CHRFAM7 in cells suggests that a novel homopentamer might form on the cell surface that replaces the 186 aa N terminus of α7nAChR with 27 aa of FAM7A (B). Its function is unknown, and it might have decreased affinity for Ach or alternative affinity for a new, currently unidentified ligand. In cells that express similar levels of α7nAChR/CHRNA7 and dupα7nAChR/CHRFAM7A (C), it is reasonable to propose that a heteropentameric channel might form on the surface with altered sensitivity to Ach or perhaps affinity for a heretofore unidentified ligand, such as SLURP1 and -2. Examining the relative distribution of α7nAChR/CHRNA7 and dupα7nAChR/CHRFAM7A gene expression, the differences are most striking in the comparison of CNS with peripheral tissues. Current models of α7nAChR activity do not distinguish the preponderance of α7nAChR in the brain (see arrows) and the overexpression of CHRFAM7A in leukocytes, epithelial cells, and peripheral tissues.
Four years after Villiger et al. [48] identified dupα7nAChR/CHRFAM7A in human PBMCs, Perl et al. [75] would inadvertently confirm the differential expression of dupα7nAChR/CHRFAM7A and α7nAChR/CHRNA7 in human leukocytes by use of sequence-specific primers that distinguish α7nAChR/CHRNA7 from dupα7nAChR/CHRFAM7A on human blood samples. Soon thereafter, Severance et al. [76] would be the 1st to combine sequence-specific primers and quantitative PCR to quantify α7nAChR/CHRNA7, α7nAChR/CHRNA7 splice variants (e.g., exon 4 deletion, exon 5 deletion, exon 4 and 5 deletion, and exon 4a insertion), and dupα7nAChR/CHRFAM7A expression in human PBMCs. None of the splice variants was detected, thereby confirming that dupα7nAChR/CHRFAM7A Variant 1 is the major transcript in human leukocytes. Furthermore, when analyzing blood from 20 patients, they found that whereas the presence of dupα7nAChR/CHRFAM7A was readily detectable in human leukocytes, α7nAChR/CHRNA7 was not [76]. Interestingly, they also evaluated dupα7nAChR/CHRFAM7A expression levels in smokers and reported significantly (P < 0.001) decreased dupα7nAChR/CHRFAM7A expression.
BIOLOGIC SIGNIFICANCE OF CHRFAM7 IN HUMAN LEUKOCYTES
When Gault et al. [77] originally compared polymorphisms in α7nAChR/CHRNA7 and dupα7nAChR/CHRFAM7A genes, they hypothesized that a partially duplicated fusion gene, such as dupα7nAChR/CHRFAM7A, might interfere with CHRNA7 expression, assembly, or function of the pentameric α7nAChR/CHRNA7 ligand-gated channel on the cell surface (Fig. 5). For example, as α7nAChR/CHRNA7 gene expression is decreased in schizophrenia, they postulated that the presence of dupα7nAChR/CHRFAM7A mRNA could also lead to dysfunctional α7nAChR/CHRNA7 expression by altered transcriptional priming and promoting sequence misalignment. It is also interesting to note that differential expression of α7nAChR/CHRNA7 and dupα7nAChR/CHRFAM7A in leukocytes and brain could equally point to differential activities. For example, the new FAM7 peptide sequence (encoded by exons A–D of FAM7), fused to the extracellular domain of α7nAChR/CHRNA7, could alter normal channel activity (Fig. 5A) by altering the channel’s ligand tropism and conferring new activity to a pentameric dupα7nAChR/CHRFAM7A-containing channel in leukocytes (Fig. 5B). To this end, it is interesting to note that an analogous transcriptional modification of the mouse α7nAChR/CHRNA7 gene has been identified and generates functional diversity to mouse α7nAChR/CHRNA7 function. This isoform of mouse α7nAChR/CHRNA7, called α7nAChR/CHRNA7-2 [78], incorporates a novel, 87 bp exon onto mouse α7nAChR/CHRNA7 that preserves the mouse AChR/CHRNA7 reading frame. This cell-surface pentameric protein binds bungarotoxin, a specific marker of α7nAChR/CHRNA7, but exhibits slower rates of desensitization. Could this gene be argued a functional mouse equivalent to dupα7nAChR/CHRFAM7A?
In attempts to establish the possible significance of selective dupα7nAChR/CHRFAM7A expression in human leukocytes, Villager et al. [48] recorded ion currents during exposure of cells to ACh and nicotine. They noted that neither could elicit a response from their freshly isolated leukocytes. They also reported that these leukocytes do not bind large amounts of bungarotoxin. Without detectable α7nAChR/CHRNA7 expression in PBMCs, Villager et al. [48] speculated that the dupα7nAChR/CHRFAM7A could interact with an alternative and as yet, currently undefined endogenous ligand that is distinct from Ach and presumably bind the FAM7 moiety of dupα7nAChR/CHRFAM7A.
In 1989, Kaufman and Oger [79] had found little high-affinity bungarotoxin binding to human leukocytes, but 10 years earlier, Richman and Arnason [80], along with their colleagues [81] had nevertheless detected a functional nAChR. It was this finding that led Atweh et al. [82] to characterize an unusual nAChR on human leukocytes, and they reported that whereas these receptors bound muscarinic ligands, they were blocked by the nicotinic cholinergic ligand, o-tubocurarine. To this end, several functional studies showed the existence of a α7nAChR/CHRNA7-like protein in human leukocytes [80, 81, 83, 84]. Direct binding studies, however, have been equivocal, even though nicotine binding to human PBMCs is saturable and specific [85]. Carbachol and other classic nicotinic antagonists, for example, do not inhibit nicotine binding, and leukocytes do not bind classic antagonists with high affinity [86]. The identity of this aberrant AChR and its relationship with dupα7nAChR/CHRHFAM7A are not known, but the dupα7nAChR/CHRHFAM7A not α7nAChR/CHRNA7 is unquestionably the main α7nAChR-like transcript detected by the PCR of human leukocytes. On this note, it is particularly interesting to speculate that the “functionally distinct” α7nAChR that was 1st described on human lymphocytes by Richman and colleagues [80–82], and others [84–87], more than 30 years ago, might be attributable to the newly recognized dupα7nAChR/CHRFAM7A.
Ultimately, the 2002 discovery [48] that peripheral blood lymphocytes express large amounts of dupα7nAChR/CHRFAM7A mRNA and little to no α7nAChR/CHRNA7 was confirmed in 2009 [76] by use of normal human leukocytes and followed up later, in 2011 [88], by use of 3 monocytic cell lines. As Villager et al. [48] and Benfante et al. [88] were also able to detect 42–43 kDa and 54–55 kDa dupα7nAChR/CHRFAM7A proteins that correspond to the predicted molecular weights of a dupα7nAChR/CHRFAM7A channel, they concluded that the dupα7nAChR/CHRFAM7A mRNA is translated. Interestingly, Benfante et al. [88] was also able to detect very low levels of α7nAChR/CHRNA7 in freshly purified human monocytes and in human macrophages grown over 6 days in culture. The levels of this α7nAChR/CHRNA7 expression, however, were 200–1000 times lower then dupα7nAChR/CHRFAM7A. The relative levels of dupα7nAChR/CHRFAM7A gene expression were also highly dependent on the donor. Whether this is because the duplicated gene is absent or present in only 1 copy in 5–15% of the population is not known (see above). Regardless, they confirmed that LPS (100 ng/ml) inhibited dupα7nAChR/CHRFAM7A gene expression [88, 89] and extended their findings to include nicotine (1 μM) and IL-1β (25 ng/ml). They also proposed that proinflammatory signals might change the expression ratios of α7nAChR/CHRFAM7A:α7nAChR/CHRNA7 and thus, differentially regulate inflammatory responsiveness.
Generally, there is a paucity of functional and/or gene expression experiments examining CHRFAM7A, presumably because its existence has not been discussed extensively outside of the neuropsychiatry literature. In one exception, van der Zanden et al. [89] reported that nicotine treatment of monocytes attenuated LPS sensitivity of THP1 cells. In these experiments, they also suggested that nicotine priming increases α7nAChR/CHRNA7 expression but decreases dupα7nAChR/CHRFAM7A. In a cautionary note of interpretation, however, neither primers distinguish α7nAChR/CHRNA7 from dupα7nAChR/CHRFAM7A gene expression (Fig. 5). The results are in contrast to Severence et al. [76], who by use of appropriate specific primers was able to distinguish α7nAChR/CHRNA7 and dupα7nAChR/CHRFAM7A gene expression and found that dupα7nAChR/CHRFAM7A gene expression was decreased (P < 0.001) in leukocytes from smoking patients. In contrast, van der Zanden et al. [89] reported that α7nAChR/CHRNA7 gene expression increased. The effects of smoking and nicotine on dupα7nAChR/CHRFAM7A expression point to the importance of understanding the differential regulation of these 2 proximal and structurally related genes, particularly in CNS disease [90]. Although the dupα7nAChR/CHRFAM7A promoter has not been characterized, it appears to be inhibited in leukocytes via NF-κB activation of LPS in vitro [48, 88].
The functional consequence(s) of the elevated expression of dupα7nAChR/CHRFAM7A in human leukocytes have emerged as an important question in inflammation but have only been addressed in 3 studies [74, 91, 92]. Two studies [91, 92] reported that overexpression of dupα7nAChR/CHRFAM7A in cells acts as a dominant-negative inhibitor of α7nAChR/CHRNA7 function, while the other study [74] found altered activity. None evaluated the effects of expression in normal human leukocytes, a natural source of dupα7nAChR/CHRFAM7A expression. In the 1st study, de Lucas-Cerrillo et al. [92] expressed dupα7nAChR/CHRFAM7A in rat pituitary GH4C1 cells, Xenopus oocytes, or human promyeloid leukemic HL60 cells. They used polysome translation assays and immunohistochemical staining (but not immunoblotting) with anti-α7nAChR/CHRNA7 antibodies to demonstrate that the dupα7nAChR/CHRFAM7A transcripts are translated into protein in HL60 cells. Presumably, as these cells are very difficult to transduce, they used rat GH4C1 cells to show that the protein translated can traffic to the cell surface, although it appears to segregate at the inner, not outer, plasma membrane. In the most convincing experiments, these authors transfected Xenopus oocytes with dupα7nAChR/CHRFAM7A, α7nAChR/CHRNA7, or both dupα7nAChR/CHRFAM7A and α7nAChR/CHRNA7 mRNAs and measured the ability of nicotine to alter current across the plasma membrane. Whereas dupα7nAChR/CHRFAM7A overexpression failed to confer nicotine responsiveness with increased current, expression with α7nAChR/CHRNA7 was highly effective. In contrast, coexpression of mRNAs encoding CHRFAM7A with α7nAChR/CHRNA7 inhibited the effects of α7nAChR/CHRNA. As no bungarotoxin binding was detected on the cell surface of transfected oocytes, they proposed that CHRFAM7A is a dominant-negative inhibitor of α7nAChR/CHRNA7 that inhibits α7nAChR/CHRNA7 trafficking to the cell surface.
A 2nd study, published 6 months later [91], substantially confirmed that dupα7nAChR/CHRFAM7A encodes a functional ORF and produces a protein. Neuroblastoma-derived SH-EP cells were transfected with plasmids encoding an epitope-tagged dupα7nAChR/CHRFAM7A to differentiate it from α7nAChR/CHRNA7. Protein was then detected with specific antibodies. The dominant-negative effects of dupα7nAChR/CHRFAM7 coexpression in Xenopus oocytes [91, 92] were also confirmed, although they suggested that the dupα7nAChR/CHRFAM7A protein localized to the cell surface was a functionally inactive modulator of α7nAChR/CHRNA7, as coexpression of dupα7nAChR/CHRFAM7A with α7nAChR/CHRNA7 significantly inhibited the ACh-evoked current measured in Xenopus oocytes by >50%. An allosteric modulator of α7nAChR/CHRNA7, called PNU-120596 [93], was used to point to the mechanism of current inhibition. Hill coefficients and EC50, derived from linear regression analyses of binding and current, showed a decrease in ACh-induced current amplitude but no change in receptor sensitivity to its natural ligand.
These findings were significantly different from Wang et al. [74], who recently published that the duplicated α7nAChR/CHRFAM7A assembles and then forms functional nicotinic receptors with full-length α7nAChR/CHRNA7. They also reported altered responses to choline in cells when they were re-engineered to overexpress the dupα7nAChR/CHRFAM7A with α7nAChR/CHRNA7. They concluded that the human-specific dupα7nAChR/CHRFAM7A gene encodes a functionally active channel. Whether this represents physiologic activity of the cell-surface complex in cells that normally overexpress dupα7nAChR/CHRFAM7A (e.g., leukocytes), however, is unknown. First, the studies could not control for gene expression, cell-surface translocation, channel assembly, or the contribution of cotransduced chaperones [74]. Second, they also found identical effects by use of overexpression of dupΔα7nAChR/CHRFAM7A, which is identical to amino-truncated α7nAChR/CHRNA7 (see Fig. 4) and has no novel peptide sequences that differ from α7nAChR/CHRNA7. This suggests that functional differences could be a result of subunit overexpression rather than the FAM moiety fused onto the truncated α7nAChR sequence. Ongoing studies will be needed to clarify dupα7nAChR/CHRFAM7A activity and determine whether there are differential effects of the truncated gene in cells that naturally produce and respond to dupα7nAChR/CHRFAM7A.
FUTURE DIRECTIONS AND THE SIGNIFICANCE OF A HUMAN-SPECIFIC CHRFAM7A FOR INJURY/INFLAMMATION RESEARCH
Over the next several years, it will be critical to define the biologic significance, if any, of a human-specific α7nAChR and specifically, the possibility that it can alter human α7nAChR activities in a species-specific fashion. On one hand, CHRFAM7A could have novel activities, presumably as a homopentamer or alternatively, as a heteropentamer with α7nAChR. On the other hand, CHRFAM7A could be a mechanism to gauge cellular responsiveness to α7nAChR. For example, whereas there is significant evidence that α7nAChR agonists activate intracellular signal transduction cascades [9, 28, 34, 35], emerging research is now pointing to ion channel-independent signal transduction that resembles muscarinic AChRs but anti-inflammatory responses that are presumed to involve α7nAChR [27, 34, 42]. To this end, it is interesting to note that CHRFAM7A is also highly expressed in human epithelial cells and most notably, cells from gut, lung, kidney, pancreas, prostate, ovary, and skin. Accordingly, the impact of CHRFAM7A gene expression in human peripheral tissues may not be restricted to human leukocytes, and its possible physiologic significance to human inflammation clearly needs further investigation.
The emergence of human-specific genes is rare, but it is neither new or unexpected [94–99]. In fact, gene duplication, rearrangement, and the horizontal transfer of DNA are cornerstones of evolution and speciation among all taxa. The possibility that specific human genes, such as CHRFAM7A, might contribute to human-specific physiologic responses, however, is a relatively new concept, particularly as applied to injury and inflammation. Its recognition can be attributed to 2 significant advances in human biology. First, progress in human genome analyses has identified numerous genes that evolved in humans but not in other species or alternatively, lost to the human genome but retained as pseudogenes [100–103]. Together, these genes are thought to help define differential genetic responses to complex human diseases [100, 102, 104]. Second, in early 2001, the National Institute of General Medical Sciences set a course to define the genetic basis to the inflammatory response to injury [105] and sponsored the mapping of gene expression in leukocytes of trauma, burn, and sepsis patients. This Inflammation and the Host Response to Injury large-scale collaborative research program has provided important insight (and unexpected controversy) into the strengths and limitations of preclinical animal models of human disease [106–109]. The very existence of human-specific genes, such as CHRFAM7A, has significant implications for injury and inflammation research that underscores the call for “translational research that specifically focuses on human studies” (and responses) by Seok et al. [107]. Just as genes, such as cmah, which are absent in humans [110], can confer “nonhuman” responsiveness in preclinical models, human genes, such as CHRFAM7A, may well do the opposite and gauge human-specific responses. Accordingly, whereas human-specific genes raise exciting opportunities for drug discovery and new therapeutic development in injury and inflammation research, they will require new preclinical modeling to study their physiologic significance, for example, by use of emerging, humanized mouse technologies, human clinical research, and human clinical trials.
ACKNOWLEDGMENTS
This work was prepared with support from the U.S. National Institutes of Health (NIH) Grant CA170140 and a U.S. Department of Defense grant administered by the American Burn Association (W81XWH-10-1-0527), with research originating with seed funding from NIH P20 Center for Exploratory Wound Healing Research (NIGM78421).
Glossary
- α7nAChR
α7-nicotinic acetylcholine receptor (protein)
- CHRNA7
α7-nicotinic acetylcholine receptor gene
- ACh
acetylcholine
- CHRFAM7A
duplicated α7-nicotinic acetylcholine receptor (protein)
- dupα7nAChR/CHRFAM7A
duplicated α7-nicotinic acetylcholine receptor gene
- FAM7
family with sequence similarity
- nAChR
nicotinic acetylcholine receptor
- ORF
open reading frame
- SLURP
secreted mammalian Ly6/urokinase plasminogen activator receptor-related protein
- ULK4
unc-51-like kinase 4
AUTHORSHIP
T.C., X.D., and A.B. assembled all papers published to date that mention CHRFAM7A since its discovery in 1998. R.C. and B.E. evaluated and then prepared a critical evaluation of this body of work and put it into context regarding the biology of inflammation. A.B wrote the 1st draft of the review, and T.C. and B.E. reviewed and made extensive revisions. X.D. and R.C. verified statements for accuracy and assisted in editing the final document.
DISCLOSURES
The authors declare no competing financial interests.
REFERENCES
- 1.Andersson U., Tracey K. J. (2012) Neural reflexes in inflammation and immunity. J. Exp. Med. 209, 1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Metz C. N., Tracey K. J. (2005) It takes nerve to dampen inflammation. Nat. Immunol. 6, 756–757. [DOI] [PubMed] [Google Scholar]
- 3.Tracey K. J. (2009) Reflex control of immunity. Nat. Rev. Immunol. 9, 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang H., Yu M., Ochani M., Amella C. A., Tanovic M., Susarla S., Li J. H., Wang H., Yang H., Ulloa L., Al-Abed Y., Czura C. J., Tracey K. J. (2003) Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature 421, 384–388. [DOI] [PubMed] [Google Scholar]
- 5.Gault J., Robinson M., Berger R., Drebing C., Logel J., Hopkins J., Moore T., Jacobs S., Meriwether J., Choi M. J., Kim E. J., Walton K., Buiting K., Davis A., Breese C., Freedman R., Leonard S. (1998) Genomic organization and partial duplication of the human alpha7 neuronal nicotinic acetylcholine receptor gene (CHRNA7). Genomics 52, 173–185. [DOI] [PubMed] [Google Scholar]
- 6.Tracey K. J. (2002) The inflammatory reflex. Nature 420, 853–859. [DOI] [PubMed] [Google Scholar]
- 7.Tracey K. J. (2007) Physiology and immunology of the cholinergic antiinflammatory pathway. J. Clin. Invest. 117, 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olofsson P. S., Rosas-Ballina M., Levine Y. A., Tracey K. J. (2012) Rethinking inflammation: neural circuits in the regulation of immunity. Immunol. Rev. 248, 188–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Jonge W. J., van der Zanden E. P., The F. O., Bijlsma M. F., van Westerloo D. J., Bennink R. J., Berthoud H. R., Uematsu S., Akira S., van den Wijngaard R. M., Boeckxstaens G. E. (2005) Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 6, 844–851. [DOI] [PubMed] [Google Scholar]
- 10.Yoshikawa H., Kurokawa M., Ozaki N., Nara K., Atou K., Takada E., Kamochi H., Suzuki N. (2006) Nicotine inhibits the production of proinflammatory mediators in human monocytes by suppression of I-kappaB phosphorylation and nuclear factor-kappaB transcriptional activity through nicotinic acetylcholine receptor alpha7. Clin. Exp. Immunol. 146, 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kees M. G., Pongratz G., Kees F., Schölmerich J., Straub R. H. (2003) Via beta-adrenoceptors, stimulation of extrasplenic sympathetic nerve fibers inhibits lipopolysaccharide-induced TNF secretion in perfused rat spleen. J. Neuroimmunol. 145, 77–85. [DOI] [PubMed] [Google Scholar]
- 12.Brandon K. W., Rand M. J. (1961) Acetylcholine and the sympathetic innervation of the spleen. J. Physiol. 157, 18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leaders F. E., Dayrit C. (1965) The cholinergic component in the sympathetic innervation to the spleen. J. Pharmacol. Exp. Ther. 147, 145–152. [PubMed] [Google Scholar]
- 14.Bellinger D. L., Felten S. Y., Lorton D., Felten D. L. (1989) Origin of noradrenergic innervation of the spleen in rats. Brain Behav. Immun. 3, 291–311. [DOI] [PubMed] [Google Scholar]
- 15.Bellinger D. L., Lorton D., Hamill R. W., Felten S. Y., Felten D. L. (1993) Acetylcholinesterase staining and choline acetyltransferase activity in the young adult rat spleen: lack of evidence for cholinergic innervation. Brain Behav. Immun. 7, 191–204. [DOI] [PubMed] [Google Scholar]
- 16.Berthoud H. R., Powley T. L. (1993) Characterization of vagal innervation to the rat celiac, suprarenal and mesenteric ganglia. J. Auton. Nerv. Syst. 42, 153–169. [DOI] [PubMed] [Google Scholar]
- 17.Huston J. M., Ochani M., Rosas-Ballina M., Liao H., Ochani K., Pavlov V. A., Gallowitsch-Puerta M., Ashok M., Czura C. J., Foxwell B., Tracey K. J., Ulloa L. (2006) Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J. Exp. Med. 203, 1623–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosas-Ballina M., Ochani M., Parrish W. R., Ochani K., Harris Y. T., Huston J. M., Chavan S., Tracey K. J. (2008) Splenic nerve is required for cholinergic antiinflammatory pathway control of TNF in endotoxemia. Proc. Natl. Acad. Sci. USA 105, 11008–11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosas-Ballina M., Olofsson P. S., Ochani M., Valdés-Ferrer S. I., Levine Y. A., Reardon C., Tusche M. W., Pavlov V. A., Andersson U., Chavan S., Mak T. W., Tracey K. J. (2011) Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science 334, 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Costantini T. W., Bansal V., Krzyzaniak M., Putnam J. G., Peterson C. Y., Loomis W. H., Wolf P., Baird A., Eliceiri B. P., Coimbra R. (2010) Vagal nerve stimulation protects against burn-induced intestinal injury through activation of enteric glia cells. Am. J. Physiol. Gastrointest. Liver Physiol. 299, G1308–G1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costantini, T. W., Bansal, V., Peterson, C. Y., Loomis, W. H., Putnam, J. G., Rankin, F., Wolf, P., Eliceiri, B. P., Baird, A., Coimbra, R. (2010) Efferent vagal nerve stimulation attenuates gut barrier injury after burn: modulation of intestinal occludin expression. J. Trauma 68, 1349–1354; discussion 1354–1346. [DOI] [PMC free article] [PubMed]
- 22.Costantini T. W., Krzyzaniak M., Cheadle G. A., Putnam J. G., Hageny A. M., Lopez N., Eliceiri B. P., Bansal V., Coimbra R. (2012) Targeting α-7 nicotinic acetylcholine receptor in the enteric nervous system: a cholinergic agonist prevents gut barrier failure after severe burn injury. Am. J. Pathol. 181, 478–486. [DOI] [PubMed] [Google Scholar]
- 23.Costantini T. W., Loomis W. H., Putnam J. G., Drusinsky D., Deree J., Choi S., Wolf P., Baird A., Eliceiri B., Bansal V., Coimbra R. (2009) Burn-induced gut barrier injury is attenuated by phosphodiesterase inhibition: effects on tight junction structural proteins. Shock 31, 416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Costantini T. W., Putnam J. G., Sawada R., Baird A., Loomis W. H., Eliceiri B. P., Bansal V., Coimbra R. (2009) Targeting the gut barrier: identification of a homing peptide sequence for delivery into the injured intestinal epithelial cell. Surgery 146, 206–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matteoli G., Gomez-Pinilla P. J., Nemethova A., Di Giovangiulio M., Cailotto C., van Bree S. H., Michel K., Tracey K. J., Schemann M., Boesmans W., Vanden Berghe P., Boeckxstaens G. E. (2014) A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut 63, 938–948. [DOI] [PubMed] [Google Scholar]
- 26.Kawashima K., Fujii T., Moriwaki Y., Misawa H., Horiguchi K. (2012) Reconciling neuronally and nonneuronally derived acetylcholine in the regulation of immune function. Ann. N. Y. Acad. Sci. 1261, 7–17. [DOI] [PubMed] [Google Scholar]
- 27.Grando S. A. (2008) Basic and clinical aspects of non-neuronal acetylcholine: biological and clinical significance of non-canonical ligands of epithelial nicotinic acetylcholine receptors. J. Pharmacol. Sci. 106, 174–179. [DOI] [PubMed] [Google Scholar]
- 28.Papke R. L. (2014) Merging old and new perspectives on nicotinic acetylcholine receptors. Biochem. Pharmacol. 89, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Séguéla P., Wadiche J., Dineley-Miller K., Dani J. A., Patrick J. W. (1993) Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J. Neurosci. 13, 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams D. K., Peng C., Kimbrell M. R., Papke R. L. (2012) Intrinsically low open probability of α7 nicotinic acetylcholine receptors can be overcome by positive allosteric modulation and serum factors leading to the generation of excitotoxic currents at physiological temperatures. Mol. Pharmacol. 82, 746–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Williams D. K., Wang J., Papke R. L. (2011) Investigation of the molecular mechanism of the α7 nicotinic acetylcholine receptor positive allosteric modulator PNU-120596 provides evidence for two distinct desensitized states. Mol. Pharmacol. 80, 1013–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma G., Vijayaraghavan S. (2002) Nicotinic receptor signaling in nonexcitable cells. J. Neurobiol. 53, 524–534. [DOI] [PubMed] [Google Scholar]
- 33.Wessler I., Kirkpatrick C. J. (2008) Acetylcholine beyond neurons: the non-neuronal cholinergic system in humans. Br. J. Pharmacol. 154, 1558–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arredondo J., Chernyavsky A. I., Jolkovsky D. L., Pinkerton K. E., Grando S. A. (2006) Receptor-mediated tobacco toxicity: cooperation of the Ras/Raf-1/MEK1/ERK and JAK-2/STAT-3 pathways downstream of alpha7 nicotinic receptor in oral keratinocytes. FASEB J. 20, 2093–2101. [DOI] [PubMed] [Google Scholar]
- 35.De Jonge W. J., Ulloa L. (2007) The alpha7 nicotinic acetylcholine receptor as a pharmacological target for inflammation. Br. J. Pharmacol. 151, 915–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papke R. L., Bencherif M., Lippiello P. (1996) An evaluation of neuronal nicotinic acetylcholine receptor activation by quaternary nitrogen compounds indicates that choline is selective for the alpha 7 subtype. Neurosci. Lett. 213, 201–204. [DOI] [PubMed] [Google Scholar]
- 37.Papke R. L., Porter Papke J. K. (2002) Comparative pharmacology of rat and human alpha7 nAChR conducted with net charge analysis. Br. J. Pharmacol. 137, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orr-Urtreger A., Göldner F. M., Saeki M., Lorenzo I., Goldberg L., De Biasi M., Dani J. A., Patrick J. W., Beaudet A. L. (1997) Mice deficient in the alpha7 neuronal nicotinic acetylcholine receptor lack alpha-bungarotoxin binding sites and hippocampal fast nicotinic currents. J. Neurosci. 17, 9165–9171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parrish W. R., Rosas-Ballina M., Gallowitsch-Puerta M., Ochani M., Ochani K., Yang L. H., Hudson L., Lin X., Patel N., Johnson S. M., Chavan S., Goldstein R. S., Czura C. J., Miller E. J., Al-Abed Y., Tracey K. J., Pavlov V. A. (2008) Modulation of TNF release by choline requires alpha7 subunit nicotinic acetylcholine receptor-mediated signaling. Mol. Med. 14, 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pavlov V. A., Ochani M., Yang L. H., Gallowitsch-Puerta M., Ochani K., Lin X., Levi J., Parrish W. R., Rosas-Ballina M., Czura C. J., Larosa G. J., Miller E. J., Tracey K. J., Al-Abed Y. (2007) Selective alpha7-nicotinic acetylcholine receptor agonist GTS-21 improves survival in murine endotoxemia and severe sepsis. Crit. Care Med. 35, 1139–1144. [DOI] [PubMed] [Google Scholar]
- 41.Pavlov V. A., Parrish W. R., Rosas-Ballina M., Ochani M., Puerta M., Ochani K., Chavan S., Al-Abed Y., Tracey K. J. (2009) Brain acetylcholinesterase activity controls systemic cytokine levels through the cholinergic anti-inflammatory pathway. Brain Behav. Immun. 23, 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pavlov V. A., Tracey K. J. (2012) The vagus nerve and the inflammatory reflex—linking immunity and metabolism. Nat. Rev. Endocrinol. 8, 743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Macklin K. D., Maus A. D., Pereira E. F., Albuquerque E. X., Conti-Fine B. M. (1998) Human vascular endothelial cells express functional nicotinic acetylcholine receptors. J. Pharmacol. Exp. Ther. 287, 435–439. [PubMed] [Google Scholar]
- 44.Nastrucci C., Russo P. (2012) α7 nAChR in airway respiratory epithelial cells. Curr. Drug Targets 13, 666–670. [DOI] [PubMed] [Google Scholar]
- 45.Pillai S., Chellappan S. (2012) α7 Nicotinic acetylcholine receptor subunit in angiogenesis and epithelial to mesenchymal transition. Curr. Drug Targets 13, 671–679. [DOI] [PubMed] [Google Scholar]
- 46.Cardinale A., Nastrucci C., Cesario A., Russo P. (2012) Nicotine: specific role in angiogenesis, proliferation and apoptosis. Crit. Rev. Toxicol. 42, 68–89. [DOI] [PubMed] [Google Scholar]
- 47.Kabbani N., Nordman J. C., Corgiat B. A., Veltri D. P., Shehu A., Seymour V. A., Adams D. J. (2013) Are nicotinic acetylcholine receptors coupled to G proteins? BioEssays 35, 1025–1034. [DOI] [PubMed] [Google Scholar]
- 48.Villiger Y., Szanto I., Jaconi S., Blanchet C., Buisson B., Krause K. H., Bertrand D., Romand J. A. (2002) Expression of an alpha7 duplicate nicotinic acetylcholine receptor-related protein in human leukocytes. J. Neuroimmunol. 126, 86–98. [DOI] [PubMed] [Google Scholar]
- 49.Zody M. C., Garber M., Sharpe T., Young S. K., Rowen L., O’Neill K., Whittaker C. A., Kamal M., Chang J. L., Cuomo C. A., Dewar K., FitzGerald M. G., Kodira C. D., Madan A., Qin S., Yang X., Abbasi N., Abouelleil A., Arachchi H. M., Baradarani L., Birditt B., Bloom S., Bloom T., Borowsky M. L., Burke J., Butler J., Cook A., DeArellano K., DeCaprio D., Dorris L. III, Dors M., Eichler E. E., Engels R., Fahey J., Fleetwood P., Friedman C., Gearin G., Hall J. L., Hensley G., Johnson E., Jones C., Kamat A., Kaur A., Locke D. P., Madan A., Munson G., Jaffe D. B., Lui A., Macdonald P., Mauceli E., Naylor J. W., Nesbitt R., Nicol R., O'Leary S. B., Ratcliffe A., Rounsley S., She X., Sneddon K. M., Stewart S., Sougnez C., Stone S. M., Topham K., Vincent D., Wang S., Zimmer A. R., Birren B. W., Hood L., Lander E. S., Nusbaum C. (2006) Analysis of the DNA sequence and duplication history of human chromosome 15. Nature 440, 671–675. [DOI] [PubMed] [Google Scholar]
- 50.Le Novère N., Corringer P. J., Changeux J. P. (2002) The diversity of subunit composition in nAChRs: evolutionary origins, physiologic and pharmacologic consequences. J. Neurobiol. 53, 447–456. [DOI] [PubMed] [Google Scholar]
- 51.Locke D. P., Archidiacono N., Misceo D., Cardone M. F., Deschamps S., Roe B., Rocchi M., Eichler E. E. (2003) Refinement of a chimpanzee pericentric inversion breakpoint to a segmental duplication cluster. Genome Biol. 4, R50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Riley B., Williamson M., Collier D., Wilkie H., Makoff A. (2002) A 3-Mb map of a large segmental duplication overlapping the alpha7-nicotinic acetylcholine receptor gene (CHRNA7) at human 15q13-q14. Genomics 79, 197–209. [DOI] [PubMed] [Google Scholar]
- 53.Flomen R. H., Davies A. F., Di Forti M., La Cascia C., Mackie-Ogilvie C., Murray R., Makoff A. J. (2008) The copy number variant involving part of the alpha7 nicotinic receptor gene contains a polymorphic inversion. Eur. J. Hum. Genet. 16, 1364–1371. [DOI] [PubMed] [Google Scholar]
- 54.Freedman R., Leonard S., Gault J. M., Hopkins J., Cloninger C. R., Kaufmann C. A., Tsuang M. T., Farone S. V., Malaspina D., Svrakic D. M., Sanders, A., Gejman, P. (2001) Linkage disequilibrium for schizophrenia at the chromosome 15q13-14 locus of the alpha7-nicotinic acetylcholine receptor subunit gene (CHRNA7). Am. J. Med. Genet. 105, 20–22. [PubMed] [Google Scholar]
- 55.Freedman R., Leonard S., Waldo M., Gault J., Olincy A., Adler L. E. (2006) Characterization of allelic variants at chromosome 15q14 in schizophrenia. Genes Brain Behav. 5 (Suppl 1), 14–22. [DOI] [PubMed] [Google Scholar]
- 56.Iwata Y., Nakajima M., Yamada K., Nakamura K., Sekine Y., Tsuchiya K. J., Sugihara G., Matsuzaki H., Suda S., Suzuki K., Takei N., Mori N., Iwayama Y., Takao H., Yoshikawa T., Riley B., Makoff A., Sham P., Chen R., Collier D. (2007) Linkage disequilibrium analysis of the CHRNA7 gene and its partially duplicated region in schizophrenia. Neurosci. Res. 57, 194–202. [DOI] [PubMed] [Google Scholar]
- 57.Petrovsky N., Schmechtig A., Flomen R. H., Kumari V., Collier D., Makoff A., Wagner M., Ettinger U. (2009) CHRFAM7A copy number and 2-bp deletion polymorphisms and antisaccade performance. Int. J. Neuropsychopharmacol. 12, 267–273. [DOI] [PubMed] [Google Scholar]
- 58.Sinkus M. L., Lee M. J., Gault J., Logel J., Short M., Freedman R., Christian S. L., Lyon J., Leonard S. (2009) A 2-base pair deletion polymorphism in the partial duplication of the alpha7 nicotinic acetylcholine gene (CHRFAM7A) on chromosome 15q14 is associated with schizophrenia. Brain Res. 1291, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Luca V., Likhodi O., Van Tol H. H., Kennedy J. L., Wong A. H. (2006) Regulation of alpha7-nicotinic receptor subunit and alpha7-like gene expression in the prefrontal cortex of patients with bipolar disorder and schizophrenia. Acta Psychiatr. Scand. 114, 211–215. [DOI] [PubMed] [Google Scholar]
- 60.Flomen R. H., Collier D. A., Osborne S., Munro J., Breen G., St Clair D., Makoff A. J. (2006) Association study of CHRFAM7A copy number and 2 bp deletion polymorphisms with schizophrenia and bipolar affective disorder. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 141B, 571–575. [DOI] [PubMed] [Google Scholar]
- 61.Hong C. J., Lai I. C., Liou L. L., Tsai S. J. (2004) Association study of the human partially duplicated alpha7 nicotinic acetylcholine receptor genetic variant with bipolar disorder. Neurosci. Lett. 355, 69–72. [DOI] [PubMed] [Google Scholar]
- 62.Dempster E. L., Toulopoulou T., McDonald C., Bramon E., Walshe M., Wickham H., Sham P. C., Murray R. M., Collier D. A. (2006) Episodic memory performance predicted by the 2bp deletion in exon 6 of the “alpha 7-like” nicotinic receptor subunit gene. Am. J. Psychiatry 163, 1832–1834. [DOI] [PubMed] [Google Scholar]
- 63.Fehér A., Juhász A., Rimanóczy A., Csibri E., Kálmán J., Janka Z. (2009) Association between a genetic variant of the alpha-7 nicotinic acetylcholine receptor subunit and four types of dementia. Dement. Geriatr. Cogn. Disord. 28, 56–62. [DOI] [PubMed] [Google Scholar]
- 64.Swaminathan S., Huentelman M. J., Corneveaux J. J., Myers A. J., Faber K. M., Foroud T., Mayeux R., Shen L., Kim S., Turk M., Hardy J., Reiman E. M., Saykin A. J.; Alzheimer’s Disease Neuroimaging Initiative and NIA-LOAD/NCRAD Family Study Group (2012) Analysis of copy number variation in Alzheimer’s disease in a cohort of clinically characterized and neuropathologically verified individuals. PLoS One 7, e50640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Swaminathan S., Kim S., Shen L., Risacher S. L., Foroud T., Pankratz N., Potkin S. G., Huentelman M. J., Craig D. W., Weiner M. W., Saykin A. J.; The Alzheimer’s Disease Neuroimaging Initiative Adni (2011) Genomic Copy Number Analysis in Alzheimer’s Disease and Mild Cognitive Impairment: An ADNI study. Int. J. Alzheimers Dis. 2011, 729478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Swaminathan S., Shen L., Kim S., Inlow M., West J. D., Faber K. M., Foroud T., Mayeux R., Saykin A. J.; Alzheimer’s Disease Neuroimaging Initiative; NIA-LOAD/NCRAD Family Study Group (2012) Analysis of copy number variation in Alzheimer’s disease: the NIALOAD/NCRAD family study. Curr. Alzheimer Res. 9, 801–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Manchia M., Viggiano E., Tiwari A. K., Renou J., Jain U., De Luca V., Kennedy J. L. (2010) Smoking in adult attention-deficit/hyperactivity disorder: interaction between 15q13 nicotinic genes and Temperament Character Inventory scores. World J. Biol. Psychiatry 11, 506–510. [DOI] [PubMed] [Google Scholar]
- 68.Casey J. P., Magalhaes T., Conroy J. M., Regan R., Shah N., Anney R., Shields D. C., Abrahams B. S., Almeida J., Bacchelli E., Bailey A. J., Baird G., Battaglia A., Berney T., Bolshakova N., Bolton P. F., Bourgeron T., Brennan S., Cali P., Correia C., Corsello C., Coutanche M., Dawson G., de Jonge M., Delorme R., Duketis E., Duque F., Estes A., Farrar P., Fernandez B. A., Folstein S. E., Foley S., Fombonne E., Freitag C. M., Gilbert J., Gillberg C., Glessner J. T., Green J., Guter S. J., Hakonarson H., Holt R., Hughes G., Hus V., Igliozzi R., Kim C., Klauck S. M., Kolevzon A., Lamb J. A., Leboyer M., Le Couteur A., Leventhal B. L., Lord C., Lund S. C., Maestrini E., Mantoulan C., Marshall C. R., McConachie H., McDougle C. J., McGrath J., McMahon W. M., Merikangas A., Miller J., Minopoli F., Mirza G. K., Munson J., Nelson S. F., Nygren G., Oliveira G., Pagnamenta A. T., Papanikolaou K., Parr J. R., Parrini B., Pickles A., Pinto D., Piven J., Posey D. J., Poustka A., Poustka F., Ragoussis J., Roge B., Rutter M. L., Sequeira A. F., Soorya L., Sousa I., Sykes N., Stoppioni V., Tancredi R., Tauber M., Thompson A. P., Thomson S., Tsiantis J., Van Engeland H., Vincent J. B., Volkmar F., Vorstman J. A., Wallace S., Wang K., Wassink T. H., White K., Wing K., Wittemeyer K., Yaspan B. L., Zwaigenbaum L., Betancur C., Buxbaum J. D., Cantor R. M., Cook E. H., Coon H., Cuccaro M. L., Geschwind D. H., Haines J. L., Hallmayer J., Monaco A. P., Nurnberger J. I. Jr., Pericak-Vance M. A., Schellenberg G. D., Scherer S. W., Sutcliffe J. S., Szatmari P., Vieland V. J., Wijsman E. M., Green A., Gill M., Gallagher L., Vicente A., Ennis S. (2012) A novel approach of homozygous haplotype sharing identifies candidate genes in autism spectrum disorder. Hum. Genet. 131, 565–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Melchior L., Bertelsen B., Debes N. M., Groth C., Skov L., Mikkelsen J. D., Brøndum-Nielsen K., Tümer Z. (2013) Microduplication of 15q13.3 and Xq21.31 in a family with Tourette syndrome and comorbidities. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 162B, 825–831. [DOI] [PubMed] [Google Scholar]
- 70.Taske N. L., Williamson M. P., Makoff A., Bate L., Curtis D., Kerr M., Kjeldsen M. J., Pang K. A., Sundqvist A., Friis M. L., Chadwick D., Richens A., Covanis A., Santos M., Arzimanoglou A., Panayiotopoulos C. P., Whitehouse W. P., Rees M., Gardiner R. M. (2002) Evaluation of the positional candidate gene CHRNA7 at the juvenile myoclonic epilepsy locus (EJM2) on chromosome 15q13-14. Epilepsy Res. 49, 157–172. [DOI] [PubMed] [Google Scholar]
- 71.Rozycka A., Dorszewska J., Steinborn B., Kempisty B., Lianeri M., Wisniewska K., Jagodzinski P. P. (2013) A transcript coding for a partially duplicated form of α7 nicotinic acetylcholine receptor is absent from the CD4+ T-lymphocytes of patients with autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE). Folia Neuropathol. 51, 65–75. [DOI] [PubMed] [Google Scholar]
- 72.Rozycka A., Dorszewska J., Steinborn B., Lianeri M., Winczewska-Wiktor A., Sniezawska A., Wisniewska K., Jagodzinski P. P. (2013) Association study of the 2-bp deletion polymorphism in exon 6 of the CHRFAM7A gene with idiopathic generalized epilepsy. DNA Cell Biol. 32, 640–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Thierry-Mieg D., Thierry-Mieg J. (2006) AceView: a comprehensive cDNA-supported gene and transcripts annotation. Genome Biol. 7 (Suppl 1), S12.1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y., Xiao C., Indersmitten T., Freedman R., Leonard S., Lester H. A. (2014) The duplicated alpha7 subunits assemble and form functional nicotinic receptors with the full-length alpha7. J. Biol. Chem. 289, 26451–26463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Perl O., Strous R. D., Dranikov A., Chen R., Fuchs S. (2006) Low levels of alpha7-nicotinic acetylcholine receptor mRNA on peripheral blood lymphocytes in schizophrenia and its association with illness severity. Neuropsychobiology 53, 88–93. [DOI] [PubMed] [Google Scholar]
- 76.Severance E. G., Dickerson F. B., Stallings C. R., Origoni A. E., Sullens A., Monson E. T., Yolken R. H. (2009) Differentiating nicotine- versus schizophrenia-associated decreases of the alpha7 nicotinic acetylcholine receptor transcript, CHRFAM7A, in peripheral blood lymphocytes. J. Neural Transm. 116, 213–220. [DOI] [PubMed] [Google Scholar]
- 77.Gault J., Hopkins J., Berger R., Drebing C., Logel J., Walton C., Short M., Vianzon R., Olincy A., Ross R. G., Adler L. E., Freedman R., Leonard S. (2003) Comparison of polymorphisms in the alpha7 nicotinic receptor gene and its partial duplication in schizophrenic and control subjects. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 123B, 39–49. [DOI] [PubMed] [Google Scholar]
- 78.Severance E. G., Zhang H., Cruz Y., Pakhlevaniants S., Hadley S. H., Amin J., Wecker L., Reed C., Cuevas J. (2004) The alpha7 nicotinic acetylcholine receptor subunit exists in two isoforms that contribute to functional ligand-gated ion channels. Mol. Pharmacol. 66, 420–429. [DOI] [PubMed] [Google Scholar]
- 79.Kaufman R. L., Oger J. (1989) Search for nicotinic acetylcholine receptors on human leukocytes: absence of alpha-bungarotoxin binding in studies of healthy individuals and myasthenia gravis patients. J. Neuroimmunol. 23, 83–87. [DOI] [PubMed] [Google Scholar]
- 80.Richman D. P., Arnason B. G. (1979) Nicotinic acetylcholine receptor: evidence for a functionally distinct receptor on human lymphocytes. Proc. Natl. Acad. Sci. USA 76, 4632–4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Richman D. P., Antel J. P., Burns J. B., Arnason B. G. (1981) Nicotinic acetylcholine receptor on human lymphocytes. Ann. N. Y. Acad. Sci. 377, 427–435. [DOI] [PubMed] [Google Scholar]
- 82.Atweh S. F., Grayhack J. J., Richman D. P. (1984) A cholinergic receptor site on murine lymphocytes with novel binding characteristics. Life Sci. 35, 2459–2469. [DOI] [PubMed] [Google Scholar]
- 83.Engel E. K., Trotter J. L., McFarlin D. E., McIntosh C. L. (1977) Thymic epithelial cell contains acetylcholine receptor. Lancet 1, 1310–1311. [DOI] [PubMed] [Google Scholar]
- 84.Mizuno Y., Dosch H. M., Gelfand E. W. (1982) Carbamycholine modulation of E-rosette formation: identification of nicotinic acetylcholine receptors on a subpopulation of human T lymphocytes. J. Clin. Immunol. 2, 303–308. [DOI] [PubMed] [Google Scholar]
- 85.Davies B. D., Hoss W., Lin J. P., Lionetti F. (1982) Evidence for a noncholinergic nicotine receptor on human phagocytic leukocytes. Mol. Cell. Biochem. 44, 23–31. [DOI] [PubMed] [Google Scholar]
- 86.Marks M. J., Collins A. C. (1982) Characterization of nicotine binding in mouse brain and comparison with the binding of alpha-bungarotoxin and quinuclidinyl benzilate. Mol. Pharmacol. 22, 554–564. [PubMed] [Google Scholar]
- 87.Whaley K., Lappin D., Barkas T. (1981) C2 synthesis by human monocytes is modulated by a nicotinic cholinergic receptor. Nature 293, 580–583. [DOI] [PubMed] [Google Scholar]
- 88.Benfante R., Antonini R. A., De Pizzol M., Gotti C., Clementi F., Locati M., Fornasari D. (2011) Expression of the α7 nAChR subunit duplicate form (CHRFAM7A) is down-regulated in the monocytic cell line THP-1 on treatment with LPS. J. Neuroimmunol. 230, 74–84. [DOI] [PubMed] [Google Scholar]
- 89.Van der Zanden E. P., Hilbers F. W., Verseijden C., van den Wijngaard R. M., Skynner M., Lee K., Ulloa L., Boeckxstaens G. E., de Jonge W. J. (2012) Nicotinic acetylcholine receptor expression and susceptibility to cholinergic immunomodulation in human monocytes of smoking individuals. Neuroimmunomodulation 19, 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yasui D. H., Scoles H. A., Horike S., Meguro-Horike M., Dunaway K. W., Schroeder D. I., Lasalle J. M. (2011) 15q11.2-13.3 Chromatin analysis reveals epigenetic regulation of CHRNA7 with deficiencies in Rett and autism brain. Hum. Mol. Genet. 20, 4311–4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Araud T., Graw S., Berger R., Lee M., Neveu E., Bertrand D., Leonard S. (2011) The chimeric gene CHRFAM7A, a partial duplication of the CHRNA7 gene, is a dominant negative regulator of α7*nAChR function. Biochem. Pharmacol. 82, 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.De Lucas-Cerrillo A. M., Maldifassi M. C., Arnalich F., Renart J., Atienza G., Serantes R., Cruces J., Sánchez-Pacheco A., Andrés-Mateos E., Montiel C. (2011) Function of partially duplicated human α77 nicotinic receptor subunit CHRFAM7A gene: potential implications for the cholinergic anti-inflammatory response. J. Biol. Chem. 286, 594–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hurst R. S., Hajós M., Raggenbass M., Wall T. M., Higdon N. R., Lawson J. A., Rutherford-Root K. L., Berkenpas M. B., Hoffmann W. E., Piotrowski D. W., Groppi V. E., Allaman G., Ogier R., Bertrand S., Bertrand D., Arneric S. P. (2005) A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization. J. Neurosci. 25, 4396–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kaessmann H. (2010) Origins, evolution, and phenotypic impact of new genes. Genome Res. 20, 1313–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li C. Y., Zhang Y., Wang Z., Zhang Y., Cao C., Zhang P. W., Lu S. J., Li X. M., Yu Q., Zheng X., Du Q., Uhl G. R., Liu Q. R., Wei L. (2010) A human-specific de novo protein-coding gene associated with human brain functions. PLOS Comput. Biol. 6, e1000734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Long M., Betrán E., Thornton K., Wang W. (2003) The origin of new genes: glimpses from the young and old. Nat. Rev. Genet. 4, 865–875. [DOI] [PubMed] [Google Scholar]
- 97.Ohno S. (1970) Evolution by Gene Duplication. Springer-Verlag, Heidelberg, Germany. [Google Scholar]
- 98.Toll-Riera M., Bosch N., Bellora N., Castelo R., Armengol L., Estivill X., Albà M. M. (2009) Origin of primate orphan genes: a comparative genomics approach. Mol. Biol. Evol. 26, 603–612. [DOI] [PubMed] [Google Scholar]
- 99.Wu D. D., Irwin D. M., Zhang Y. P. (2011) De novo origin of human protein-coding genes. PLoS Genet. 7, e1002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Stahl P. D., Wainszelbaum M. J. (2009) Human-specific genes may offer a unique window into human cell signaling. Sci. Signal. 2, pe59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nahon J. L. (2003) Birth of ‘human-specific’ genes during primate evolution. Genetica 118, 193–208. [DOI] [PubMed] [Google Scholar]
- 102.Huang H., Winter E. E., Wang H., Weinstock K. G., Xing H., Goodstadt L., Stenson P. D., Cooper D. N., Smith D., Albà M. M., Pointing C. P., Fechtel K. (2004) Evolutionary conservation and selection of human disease gene orthologs in the rat and mouse genomes. Genome Biol. 5, R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tu Z., Wang L., Xu M., Zhou X., Chen T., Sun F. (2006) Further understanding human disease genes by comparing with housekeeping genes and other genes. BMC Genomics 7, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cooper D. N., Kehrer-Sawatzki H. (2011) Exploring the potential relevance of human-specific genes to complex disease. Hum. Genomics 5, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.National Institute of General Medical Sciences, National Institutes of Health (2001) Glue Grant Inflammation and the Host Response to Injury. Massachusetts General Hospital, Boston, from https://www.gluegrant.org/. [Google Scholar]
- 106.Osuchowski M. F., Remick D. G., Lederer J. A., Lang C. H., Aasen A. O., Aibiki M., Azevedo L. C., Bahrami S., Boros M., Cooney R., Cuzzocrea S., Jiang Y., Junger W. G., Hirasawa H., Hotchkiss R. S., Li X. A., Radermacher P., Redl H., Salomao R., Soebandrio A., Thiemermann C., Vincent J. L., Ward, P., Yao Y. M., Yu H. P., Zingarelli B., Chaudry I. H. (2014) Abandon the mouse research ship? Not just yet! Shock 41, 463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Seok J., Warren H. S., Cuenca A. G., Mindrinos M. N., Baker H. V., Xu W., Richards D. R., McDonald-Smith G. P., Gao H., Hennessy L., Finnerty C. C., López C. M., Honari S., Moore E. E., Minei J. P., Cuschieri J., Bankey P. E., Johnson J. L., Sperry J., Nathens A. B., Billiar T. R., West M. A., Jeschke M. G., Klein M. B., Gamelli R. L., Gibran N. S., Brownstein B. H., Miller-Graziano C., Calvano S. E., Mason P. H., Cobb J. P., Rahme L. G., Lowry S. F., Maier R. V., Moldawer L. L., Herndon D. N., Davis R. W., Xiao W., Tompkins R. G.; Inflammation and Host Response to Injury, Large Scale Collaborative Research Program (2013) Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 110, 3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Takao K., Miyakawa T. (2014) Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA doi:10.1073/pnas.1401965111 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xiao W., Mindrinos M. N., Seok J., Cuschieri J., Cuenca A. G., Gao H., Hayden D. L., Hennessy L., Moore E. E., Minei J. P., Bankey P. E., Johnson J. L., Sperry J., Nathens A. B., Billiar T. R., West M. A., Brownstein B. H., Mason P. H., Baker H. V., Finnerty C. C., Jeschke M. G., López M. C., Klein M. B., Gamelli R. L., Gibran N. S., Arnoldo B., Xu W., Zhang Y., Calvano S. E., McDonald-Smith G. P., Schoenfeld D. A., Storey J. D., Cobb J. P., Warren H. S., Moldawer L. L., Herndon D. N., Lowry S. F., Maier R. V., Davis R. W., Tompkins R. G.; Inflammation and Host Response to Injury Large-Scale Collaborative Research Program (2011) A genomic storm in critically injured humans. J. Exp. Med. 208, 2581–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hedlund M., Tangvoranuntakul P., Takematsu H., Long J. M., Housley G. D., Kozutsumi Y., Suzuki A., Wynshaw-Boris A., Ryan A. F., Gallo R. L., Varki N., Varki A. (2007) N-Glycolylneuraminic acid deficiency in mice: implications for human biology and evolution. Mol. Cell. Biol. 27, 4340–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]