Abstract
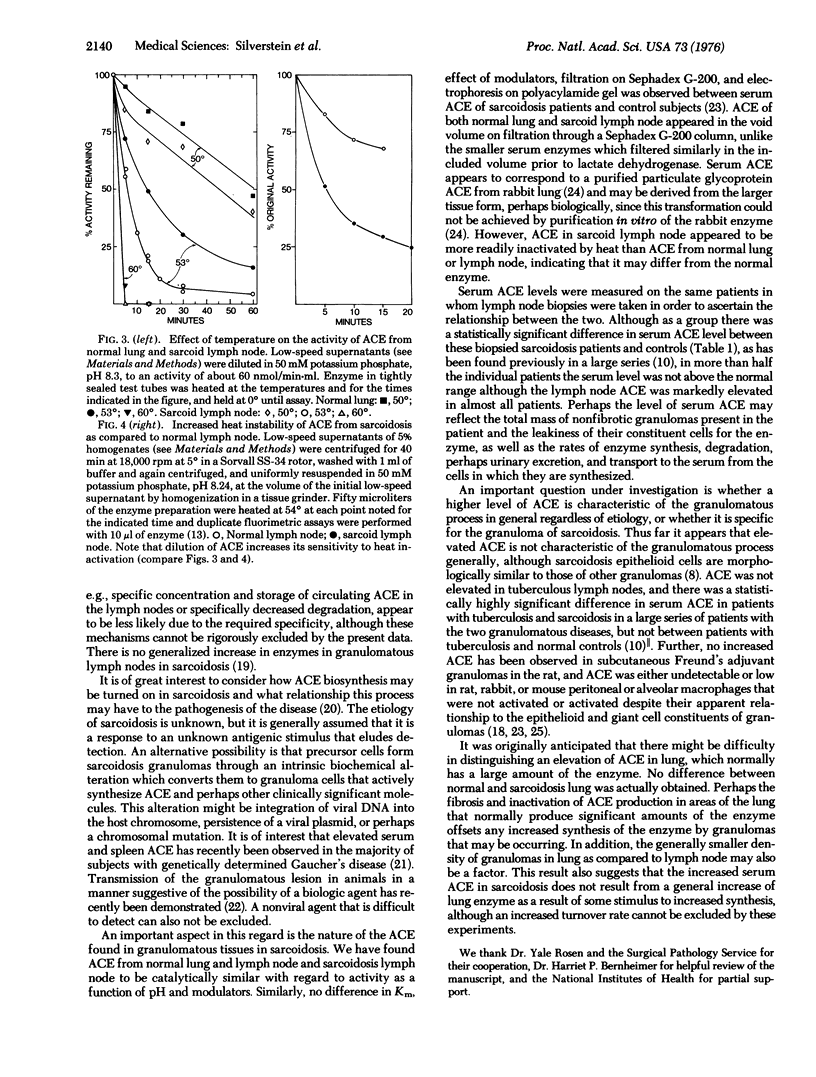
Sarcoidosis is a disease of unknown etiology that is characterized by the generalized formation of granulomas and is accompanied by elevation in the serum in less than half the patients of angiotensin converting enzyme, a dipeptidyl carboxypeptidase that catalyzes the conversion of the decapeptide, angiotensin I, to the pressor octapeptide, angiotensin II, and L-histidyl-L-leucine. Mean activity of angiotensin converting enzyme was elevated generally more than 10-fold in granuloma-containing lymph nodes, but not in lung in which normally it is abundant, in 19 of 20 patients with sarcoidosis. Angiotensin converting enzyme in lymph nodes from subjects with sarcoidosis was similar to the enzyme from normal lung and lymph node with respect to activity as a function of pH, inhibition of activity by EDTA and o-phenanthroline, gel filtration on Sephadex G-200, and requirement for chloride for activity, but appeared to be more heat labile. The data suggest that the granulomas in sarcoidosis may be the source of the elevated serum enzyme and that cells of the granulomas, particularly the epitheloid cells which appear by electron microscopy to have active protein biosynthesis, may be actively synthesizing the enzyme.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biberfeld P., Hedfors E. Atypical blood lymphocytes in sarcoidosis: morphology, cytochemistry, and membrane properties. Scand J Immunol. 1974;3(5):615–625. doi: 10.1111/j.1365-3083.1974.tb01295.x. [DOI] [PubMed] [Google Scholar]
- Cushman D. W., Cheung H. S. Concentrations of angiotensin-converting enzyme in tissues of the rat. Biochim Biophys Acta. 1971 Oct;250(1):261–265. doi: 10.1016/0005-2744(71)90142-2. [DOI] [PubMed] [Google Scholar]
- Das M., Soffer R. L. Pulmonary angiotensin-converting enzyme. Structural and catalytic properties. J Biol Chem. 1975 Sep 10;250(17):6762–6768. [PubMed] [Google Scholar]
- Hirshaut Y., Glade P., Vieira B. D., Ainbender E., Dvorak B., Siltzbach L. E. Sarcoidosis, another disease associated with serologic evidence for herpes-like virus infection. N Engl J Med. 1970 Sep 3;283(10):502–506. doi: 10.1056/NEJM197009032831002. [DOI] [PubMed] [Google Scholar]
- James D. G. Editorial: Modern concepts of sarcoidosis. Chest. 1973 Dec;64(6):675–677. doi: 10.1378/chest.64.6.675. [DOI] [PubMed] [Google Scholar]
- James E. M., Williams W. J. Fine structure and histochemistry of epithelioid cells in sarcoidosis. Thorax. 1974 Jan;29(1):115–120. doi: 10.1136/thx.29.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lanzillo J. J., Fanburg B. L. Membrane-bound angiotensin-converting enzyme from rat lung. J Biol Chem. 1974 Apr 10;249(7):2312–2318. [PubMed] [Google Scholar]
- Lieberman J. Elevation of serum angiotensin-converting-enzyme (ACE) level in sarcoidosis. Am J Med. 1975 Sep;59(3):365–372. doi: 10.1016/0002-9343(75)90395-2. [DOI] [PubMed] [Google Scholar]
- Mitchell D. N., Rees R. J. A transmissible agent from sarcoid tissue. Lancet. 1969 Jul 12;2(7611):81–84. doi: 10.1016/s0140-6736(69)92392-7. [DOI] [PubMed] [Google Scholar]
- Mitchell D. N., Scadding J. G. Sarcoidosis. Am Rev Respir Dis. 1974 Dec;110(6):774–802. doi: 10.1164/arrd.1974.110.6P1.774. [DOI] [PubMed] [Google Scholar]
- Naito M., Akiyama Y., Kato S., Tachibana T., Sakai T. Studies on antibody surveillance to EB virus-induced antigen in patients with sarcoidosis and nasopharyngeal carcinoma by indirect immunofluorescence. Biken J. 1973 Dec;16(4):141–148. [PubMed] [Google Scholar]
- Palva T., Dammert K., Palva A. On enzyme histochemistry of sarcoidosis. Acta Pathol Microbiol Scand A. 1973 Mar;81(2):189–194. doi: 10.1111/j.1699-0463.1973.tb00011.x. [DOI] [PubMed] [Google Scholar]
- Topilsky M., Siltzbach L. E., Williams M., Glade P. R. Lymphocyte response in sarcoidosis. Lancet. 1972 Jan 15;1(7742):117–120. doi: 10.1016/s0140-6736(72)90680-0. [DOI] [PubMed] [Google Scholar]
- WEISS L. P., FAWCETT D. W. Cytochemical observations on chicken monocytes macrophages and giant cells in tissue culture. J Histochem Cytochem. 1953 Jan;1(1):47–65. doi: 10.1177/1.1.47. [DOI] [PubMed] [Google Scholar]
- Wahren B., Carlens E., Espmark A., Lundbeck H., Löfgren S., Madar E., Henle G., Henle W. Antibodies to various herpesviruses in sera from patients with sarcoidosis. J Natl Cancer Inst. 1971 Oct;47(4):747–755. [PubMed] [Google Scholar]


