Abstract
The completion of the Multicenter Silent Infarct Transfusion Trial demonstrated that children with pre-existing silent cerebral infarct and sickle cell anemia (SCA) who received regular blood transfusion therapy had a 58% relative risk reduction of infarct recurrence when compared to observation. However, the total benefit of blood transfusion therapy, as assessed by the parents, was not measured against the burden of monthly blood transfusion therapy. In this planned ancillary study, we tested the hypothesis that a patient centered outcome, health-related quality of life (HRQL), would be greater in participants randomly assigned to the blood transfusion therapy group than the observation group. A total of 89% (175 of 196) of the randomly allocated participants had evaluable entry and exit HRQL evaluations. The increase in Change in Health, measured as the child’s health being better, was significantly greater for the transfusion group than the observation group (Difference Estimate = −0.54, p ≤ 0.001). This study provides the first evidence that children with SCA who received regular blood transfusion therapy felt better and had better overall HRQL than those who did not receive transfusion therapy.
Keywords: blood transfusion, health-related quality of life, children, sickle cell anemia, pediatrics
Introduction
As patient-centered care advances, measurement of health-related quality of life (HRQL) has become increasingly important when assessing the impact of a disease or therapy on a child. [1, 2] Assessment of HRQL captures essential aspects of patients’ experiences such as how they are functioning and feeling. To obtain patients’ perspectives on the effectiveness of therapy and to provide evidence on how treatment will impact what patients likely care about most, HRQL measurement must become an essential outcome measurement in clinical trials. Historically, clinical trials of children with sickle cell anemia (SCA) have not reported HRQL outcomes. HRQL outcomes are particularly relevant for children with SCA as they are at high risk for acute complications such as stroke, pain, acute chest syndrome, and priapism. Furthermore, there is abundant research that children with SCA often have poor HRQL [3–6] thus supporting the need for clinical trials of therapeutics aimed at improving HRQL for these children.
Blood transfusion therapy is a primary medical treatment for reducing many of the risks noted above in children with SCA. [7–9] Transfusion therapy is used to reduce the hemoglobin S concentration with a goal to treat or prevent many acute sickle cell-related complications. [10] Regular blood transfusion therapy reduces the incidence of pain, acute chest syndrome [10, 11] and primary stroke prevention in children with elevated transcranial Doppler. [12] The recently published Silent Cerebral Infarct Transfusion (SIT) trial found a 58% relative risk reduction in the rate of infarct recurrence and a decreased incidence of pain events and acute chest syndrome in children who received blood transfusion therapy versus those who did not. [7] The primary outcome measure of the trial was the presence of a new infarct or stroke; however, the additional benefits and burden of blood transfusion therapy was not assessed in the primary analysis. The current study seeks to fill this gap in research by utilizing data from the SIT trial, a clinical trial cohort of transfused and non-transfused children with silent cerebral infarcts. [7] As a planned secondary analysis of the SIT Trial, we tested the hypothesis that the patient centered outcome of HRQL would be greater after completion of the trial in participants randomly assigned to the blood transfusion therapy group versus the observation group.
Methods
Study setting and patients
This study was a planned secondary analysis of data collected for the Silent Cerebral Infarct Transfusion (SIT) trial.[7] The SIT trial was a multi-site intervention study of children with SCA and silent cerebral infarcts that was designed to assess the impact of blood transfusion therapy on recurrent cerebral infarcts. The detailed study and clinical trial design methods for this study have been previously published. [13] Parents/guardians of children ages 5 to 15 years with SCA (hemoglobin SS disease or hemoglobin Sβ0 thalassemia) were recruited to participate. Children identified by brain MRI and neurological examination to have a silent cerebral infarct (SCI) were randomized (n = 196) into two groups: treatment and observation. The treatment group received blood transfusion therapy, while the observation group continued with usual care. All parents of participants in the SIT trial completed an assessment of their child’s HRQL via the Child Health Questionnaire Parent Form 50 at baseline as part of the trial after qualifying with a SCI, but prior to the initiation of blood transfusion therapy for those subjects randomized to the transfusion arm. Parents completed the assessment of HRQL again at the time of the child’s last visit on the trial (36 months) or at the time of a neurological event such as an overt stroke.
Demographic data were parent/guardian-reported for the child and self-reported for the parent/guardian at baseline. The Institutional Review Boards at each of the participating sites in the SIT trial approved the study. Scientists within the Division of Biostatistics at Washington University School of Medicine in St. Louis, Missouri analyzed the data and provided all authors with access to data when requested. The SIT trial was registered at www.clinicaltrials.gov as #NCT00072761.
Study groups: transfusion vs. observation
Participants were randomized to either the transfusion group (n = 99) or to the observation group (n = 97). Participants randomized to transfusion received monthly transfusions for 36 months, whereas participants randomized to observation received standard care for 36 months. Fifteen participants randomly assigned to transfusion crossed over to observation (e.g., declined blood transfusion), and six participants crossed over from observation to transfusion (Figure 1).
Figure 1.
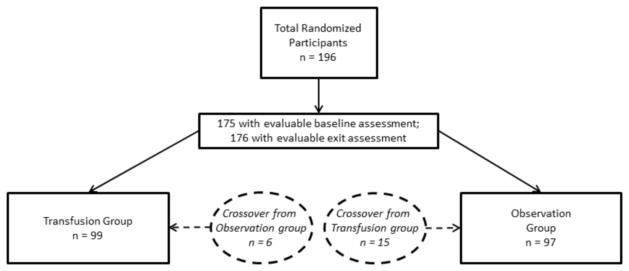
Distribution of participants. Displays the breakdown of participants including those who had enough baseline and exit assessments to be included in analyses, as well as those who crossed between groups.
Primary outcome
The primary outcome was HRQL as measured with the Child Health Questionnaire Parent Form 50 (CHQ_PF50) .[14] The CHQ_PF50 is a generic multi-dimensional measure of HRQL that is developmentally appropriate for the assessment of children ages 5 to 18 years. It requires a third grade reading level and utilizes a 1-month recall period. There are 15 concepts (11 multi-item scales and 4 single items) measured with the CHQ_PF50 that comprise 50 total items: Global Health (1-item), Physical Functioning, Role/Social Limitations-Emotional/Behavioral, Role/Social Limitations-Physical, Bodily Pain/Discomfort, Behavior, Global Behavior (1-item), Mental Health, Self-esteem, General Health Perceptions, Change in Health (1-item), Parental Impact-Emotional, Parental Impact-Time, Family-Limitations in Activities, and Family Cohesion (1-item). The majority of the items are anchored on a recall period of four weeks or less, except for the Change in Health item which asks, “Compared to one year ago, how would you rate your child’s health now?” The CHQ_PF50 was completed by parents/guardians of children ages 5 to 18 years at baseline and at the time of a neurological event (overt stroke) or at study exit at 36 months.
The CHQ_PF50 uses a 4 to 6 point response scale for items and scores are transformed to 0 to 100 for interpretation for all domains except Change in Health. The Change in Health domain consists of one item with a response scale of 1 to 5 and a final score that represents the raw score without transformation to a 0–100 scale. For all domains, a higher score represents a better quality of life. For example, a higher score on Bodily Pain would signify less pain indicating better quality of life. In addition, there are two summary scores: a Physical Health Summary score and Psychosocial Summary score. The scoring of the instrument allows a scaled score to be calculated if at least 50% of the items in each domain are answered [14].
The CHQ_PF50 was chosen as the instrument to measure HRQL in this study because it has been extensively tested, validated, and found to be reliable among children who have chronic illness (e.g. cancer and asthma) as well as childhood stroke [15] and has norms for a healthy comparison group. [14, 16–21] In addition, both the CHQ_PF50 and the CHQ_PF28 (the short form version) have been utilized in children with SCA and shown to be reliable in this population. [22–24]
Given the known impact of disease severity on HRQL, we examined whether the study groups were equally distributed in regards to severe disease at the baseline assessment. [3] Similar to our previous research, patients were classified as having severe SCA if they experienced additional, more severe complications of the disease – specifically, acute chest syndrome or three or more hospitalizations for painful events in the three years prior to study enrollment. [3, 25] All others were classified as having mild SCA for this analysis.
Data analysis
Descriptive statistics were calculated for CHQ_PF50 summary scores and CHQ_PF50 scale scores both at baseline and at exit time periods. Chi-square tests for independence were conducted to determine whether study groups were equally distributed in regards to demographic characteristics at baseline. Additionally, differences between groups at baseline were tested for rates of hospitalization for pain and acute chest syndrome using independent t-tests. Differences in mean HRQL scores were calculated between groups at baseline and exit as well as over time using independent-samples and paired-samples t-tests. To control for the possibility of false positives due to multiple testing, the false discovery rate (FDR) approach was applied to the subscale analysis, with control set to 5%. [26] An estimated least squares means (LSM) test, based on two-way repeated-measures analysis of variance, was used to compare the change in HRQL over time, from baseline to exit, between the transfusion group and the observation group.
Results
Study sample
Participants included 196 children, of whom 21 did not have any baseline assessment and 20 did not have any exit assessment, leaving 175 evaluable participants at baseline and 176 at exit (Figure 1). In addition, some of the remaining participants did not have the minimum 50% of items necessary to calculate each scale score (i.e., did not earn a score for that particular scale). Specifically, 29 participants had up to 6 scale scores missing at baseline. At exit, 47 participants had up to 2 scale scores missing. Thus, of evaluable data, 1.5% was missing at baseline and 1.9% was missing at exit. The final analysis included a maximum of 92 and 84 participants in the treatment and observation groups, respectively, and varied based on questions answered by each participant.
Children (43% female) ranged in age from 6 to 16 (M = 9.55) years at study enrollment, and 92% were Black. Overall, 48% of children were classified as having severe disease. Additional baseline demographics are displayed by study group in Table I. There were no differences between groups at baseline in regards to rate of hospitalization for pain [t = −0.471, p = 0.638] or rate of hospitalization for acute chest syndrome, t = −0.675, p = 0.500. Means and standard deviations for HRQL reported at baseline and exit for both study groups are displayed in Table II.
Table I.
Baseline characteristics of participants by study group
| Transfusion (N = 99) | Observation (N = 97) | |
|---|---|---|
| Age in years | ||
| Mean | 9.97 | 9.97 |
| 25th Percentile | 7.80 | 7.60 |
| 50th Percentile | 10.00 | 9.90 |
| 75th Percentile | 11.50 | 12.20 |
| Ethnicity N(%) | ||
| African Origin | 90 (92.8) | 91 (91.9) |
| White | 0 (0.0) | 2 (2.0) |
| Other | 7 (7.2) | 6 (6.1) |
| Gender N(%) | ||
| Male | 59 (59.6) | 52 (53.6) |
| Female | 40 (40.4) | 45 (46.4) |
| Disease severity N(%) | ||
| Mild | 57 (57.6) | 45 (46.4) |
| Severe | 42 (42.4) | 52 (53.6) |
No differences were detected between study groups at baseline.
Table II.
Means for health-related quality of life at baseline and exit
| Group | Variable | Baseline
|
Exit
|
||
|---|---|---|---|---|---|
| N | Mean | N | Mean | ||
| Transfusion (n=99) | General Health | 82 | 65.43 | 82 | 68.35 |
| Global Behavior | 82 | 79.09 | 87 | 75.57 | |
| Change In Health | 83 | 3.45 | 88 | 4.30 | |
| Family Cohesion | 85 | 78.82 | 90 | 75.61 | |
| Physical Function | 85 | 82.97 | 92 | 88.82 | |
| Role-Emotion | 84 | 82.74 | 92 | 84.12 | |
| Role-Physical | 84 | 79.96 | 92 | 88.04 | |
| Bodily Pain | 85 | 73.18 | 92 | 78.70 | |
| Behavior | 84 | 72.86 | 91 | 74.54 | |
| Mental Health | 84 | 75.95 | 92 | 80.01 | |
| Self Esteem | 84 | 83.86 | 92 | 82.22 | |
| General Health | 84 | 46.42 | 92 | 51.69 | |
| Parental Impact-Emotion | 84 | 57.84 | 90 | 65.79 | |
| Parent Time | 85 | 75.69 | 92 | 79.05 | |
| Family-Limitations in Activities | 85 | 73.17 | 92 | 79.33 | |
| Physical Summary | 82 | 41.03 | 89 | 46.16 | |
| Psychosocial Summary | 82 | 48.94 | 89 | 50.05 | |
| Observation (n=97) | General Health | 84 | 63.33 | 72 | 66.53 |
| Global Behavior | 83 | 75.48 | 78 | 78.33 | |
| Change In Health | 83 | 3.51 | 80 | 3.83 | |
| Family Cohesion | 89 | 78.76 | 81 | 78.89 | |
| Physical Function | 90 | 76.56 | 84 | 77.31 | |
| Role-Emotion | 90 | 74.44 | 83 | 82.13 | |
| Role-Physical | 90 | 77.22 | 84 | 81.55 | |
| Bodily Pain | 89 | 66.07 | 84 | 67.62 | |
| Behavior | 89 | 71.71 | 84 | 75.79 | |
| Mental Health | 89 | 76.53 | 84 | 78.88 | |
| Self Esteem | 90 | 84.44 | 84 | 79.84 | |
| General Health | 90 | 47.22 | 84 | 48.73 | |
| Parental Impact-Emotion | 90 | 63.33 | 84 | 66.67 | |
| Parent Time | 90 | 74.57 | 84 | 76.72 | |
| Family-Limitations in Activities | 90 | 72.93 | 84 | 76.78 | |
| Physical Summary | 88 | 38.62 | 83 | 40.16 | |
| Psychosocial Summary | 88 | 49.36 | 83 | 50.76 | |
No differences in HRQL between groups at baseline; some differences between groups detected at exit
As expected, no significant differences were found in HRQL between the study groups at baseline; however, differences in some areas of HRQL were detected between study groups at exit. Specifically, children’s scores at exit for Physical Function were 11.50 points higher for those in the transfusion group compared to those in the observation group, t = 3.25, p ≤ 0.001. Also, compared to children who did not receive transfusion therapy, children who received transfusions scored an average of 11.08 points higher on Bodily Pain, [t = 2.93, p = 0.004] and 0.47 points higher (on a 1–5 point scale) on Change in Health, t = 3.29, p ≤ 0.001. Additionally, children in the transfusion group scored 5.99 points higher on Physical Summary Scores at exit than children in the observation group, t = 3.06, p = 0.003. Thus, parents of children who received transfusions reported better overall physical functioning, less bodily pain, and more improved overall health than children who were in the observation group.
Transfusion group reported changes over time in distinct areas of HRQL
Observation Group
There were no significant changes over time for the observation group.
Transfusion Group
Children who were transfused had increases (MD = 0.97) in their Change in Health scores [t = 7.71, p ≤ 0.001] as well as increases (MD = 5.03) in their General Health scores [t = 2.97, p = 0.004] between baseline and exit, reflecting better health than when compared to the previous year and greater belief that the child’s health is good. The Physical Summary Scores were significantly improved (MD = 4.86) from beginning to end of the study, t = 2.89, p = 0.005 reflecting overall better physical functioning.
Transfusion group reported greater change in health than observation group
Significant differences in the change in HRQL were detected between study groups from baseline to exit in one area. An estimated LSM test (Table III) revealed the increase in Change in Health over time for the transfusion group was significantly greater than the increase in Change in Health over time for the observation group. There were no other statistically significant differences in the change in HRQL between study groups over time.
Table III.
Estimated Least Squares Means (LSM) comparing the change in HRQL over time, from baseline to exit, between transfusion and observation groups
| Transfusion
|
Observation
|
Est. | 95% CI [Lower, Upper] | P | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline LSM | Exit LSM | Baseline LSM | Exit LSM | |||||
| Primary Outcome | Change in health | 3.44 | 4.30 | 3.50 | 3.82 | −0.54 | [−0.92, −0.17] | 0.00 |
| Secondary Outcomes | Global health | 65.70 | 68.29 | 63.30 | 66.00 | 0.11 | [−7.52, 7.73] | 0.98 |
| Global behavior | 79.37 | 68.29 | 75.59 | 77.67 | 5.84 | [−1.73, 13.42] | 0.13 | |
| Family cohesion | 79.05 | 75.53 | 78.61 | 78.81 | 3.72 | [−3.39, 10.83] | 0.30 | |
| Physical function | 82.98 | 88.67 | 76.75 | 76.90 | −5.54 | [−13.37, 2.29] | 0.16 | |
| Role-emotion | 82.87 | 84.06 | 74.53 | 81.76 | 6.05 | [−3.71, 15.80] | 0.22 | |
| Role-physical | 80.06 | 87.88 | 77.30 | 81.31 | −3.82 | [−13.84, 6.21] | 0.45 | |
| Bodily pain | 73.18 | 78.51 | 66.21 | 67.56 | −3.98 | [−12.85, 4.89] | 0.38 | |
| Behavior | 72.80 | 74.49 | 71.91 | 75.02 | 1.42 | [−4.37, 7.20] | 0.63 | |
| Mental health | 75.93 | 79.86 | 76.43 | 78.25 | −2.10 | [−7.31, 3.11] | 0.43 | |
| Self esteem | 84.01 | 82.24 | 84.34 | 79.34 | −3.22 | [−8.73, 2.29] | 0.25 | |
| General health | 46.45 | 51.60 | 47.12 | 48.68 | −3.59 | [−8.71, 1.53] | 0.17 | |
| Parental Impact-Emotional | 57.86 | 65.94 | 63.22 | 66.04 | −5.26 | [−15.52, 5.01] | 0.31 | |
| Parent time | 75.41 | 78.83 | 74.51 | 76.67 | −1.27 | [−11.53, 9.00] | 0.81 | |
| Family-Limitations in Activities | 72.92 | 79.16 | 73.08 | 76.40 | −2.93 | [−10.17, 4.31] | 0.43 | |
| Physical summary | 41.04 | 46.06 | 38.68 | 39.90 | −3.79 | [−7.99, 0.42] | 0.08 | |
| Psychosocial summary | 48.96 | 50.08 | 49.33 | 50.24 | −0.22 | [−3.44, 3.01] | 0.89 | |
CI, confidence interval; Est., Difference estimate.
Discussion
The SIT trial was designed primarily to assess the impact of blood transfusion therapy on recurrent cerebral infarct; thus, the study was not designed to determine the overall impact of transfusion therapy on HRQL. However, even with this design, this study provides the first evidence from a parent’s perspective that their child’s HRQL improves with blood transfusion therapy. Because children with SCA typically have poor HRQL in comparison to children without SCA, [5] interventions that improve their HRQL are important. Participants in the current study who received transfusion therapy to prevent infarct recurrence had improvements in many important areas of HRQL. Specifically, parents reported that their children had greater physical health functioning and better health than one year prior. Parents also reported that their children had overall good health.
Our primary finding revealed that the transfusion group reported greater improvement in health than the observation group. Specifically, parents reported their children’s health was better on the transfusion treatment arm of this randomized trial. This distinction is important, as it shows the impact of the transfusion therapy from the parents’ perspective. Although the domain that measures this, the Change in Health item, is a single-item global assessment of the patients’ perception of “feeling better,” recent research has demonstrated the relevance of this type of global health item to measure a patient’s well-being. [27] For example, individuals who had worse scores on a single-item of general self-rated health had higher healthcare expenditures. [28] Further, a meta-analysis revealed that single-item health measures have shown a strong association with mortality, such that those individuals reporting worse health had a higher risk of mortality. [29] These studies demonstrate the ability of a single-item to capture important information about an individual’s health status. Therefore, the significant between-groups effect for Change in Health is considered a meaningful finding that fits with clinical expectations of blood transfusion therapy.
It is unknown what minimal clinically important differences are for the CHQ_PF50 and this study was not designed to determine this difference. However, the fact that parents reported their child’s Change in Health score increased by an average of nearly one point demonstrates that parents noticed an overall improvement in their child’s HRQL. Thus, even though our study was not designed to determine longitudinal validity, it is likely the CHQ_PF50 is capable of such a calculation in future studies.
The current study was limited by a pre/post design. Incorporating more assessments of HRQL during the study period may have allowed us to see more detailed changes over time. With a pre/post design, we were unable to determine when the transfusion therapy had the most impact over the three years of the study. Detecting this information would require multiple assessments over the course of the study. Despite this limitation, these results are still the largest cohort to demonstrate a measurable difference in HRQL for children receiving chronic transfusions versus those under observation alone.
It is also important to note that patients enrolled in the SIT trial were recommended to attend clinic every three months if they were in the observation group and every three to four weeks for those in the transfusion group. [13] By participating in the trial, children and their parents may have received more medical attention than non-clinical trial patients. Additionally, this clinical trial was comprised of children who otherwise did not have clinical indications to receive transfusion therapy when they entered the trial (e.g., prior stroke, elevated cerebral blood flow velocities, or recurrent vaso-occlusive painful events). Compared to previous research using the CHQ to assess HRQL in children with SCD, children in the current study had comparable, but slightly higher, HRQL scores at baseline. [3, 23, 24]
Despite the prior use of multiple forms of the CHQ in the SCA population [22–24], the CHQ is not a disease-specific measure. However, at the time the study was opened in 2003, no disease-specific assessment of HRQL existed for SCA. Although the CHQ_PF50 detected overall improvement in HRQL, it is likely that the CHQ_PF50 could not detect disease-related HRQL effects that are relevant in this population. While parents of transfused children reported improvement in pain and physical functioning with the CHQ_PF50, a tool specific to sickle cell disease may provide more details about changes in functioning related to transfusion therapy. Future studies could benefit by using a disease-specific measure, such as the PedsQL™ Sickle Cell Disease Module. [30]
In the era of the Affordable Care Act and with the creation of the Patient Centered Outcomes Research Institute (PCORI), it has become increasingly evident that clinical trials must incorporate the patient’s perspective of their own functioning to determine the overall effectiveness of therapy. This study provides evidence that parents of children with SCA believe chronic blood transfusion therapy improves the health of their children despite the known risks of transfusion therapy. These findings support the use of HRQL as a primary clinical trial outcome, in conjunction with traditional medical outcomes, to understand how a treatment impacts patients’ overall health and functioning from their own perspective. By including HRQL as a primary outcome, research and clinical practice can fully embrace patient-centered care.
Children with SCA who received chronic blood transfusion therapy had better overall parent-reported HRQL than children in the observation group. This novel finding is consistent with the reduction in hospitalizations for pain and acute chest syndrome experienced by children with SCA receiving blood transfusion therapy in prior clinical trials [31, 32] and in the SIT trial which includes this cohort of patients. [7] Blood transfusion therapy made children feel better. Including measurement of HRQL as a primary outcome in clinical trials in the future will help to further elucidate the impact of therapy on patient functioning by providing the essential perspective of the patient on the effectiveness of therapy.
Acknowledgments
L.M.B., J.A.P., and M.R.D. designed the research; all authors interpreted results; L.M.B. and J.A.P. made the figure and tables and wrote the paper; all authors contributed to revisions of the paper. This work was supported by the SIT trial investigators [7]. Funding was provided by SIT trial, 5U01-NS042804, M.R.D., American Recovery Reinvestment ACT supplementary grant funded by NINDS (3U01NS042804-06S1), and K23 HL80092, J.A.P.
Footnotes
Conflict-of-interest disclosure: Dr. Casella has received honoraria and travel expenses in the past and receives salary support through Johns Hopkins for providing consultative advice to Mast Therapeutics, previously Adventrx Pharmaceuticals. He is an inventor and named party on a patent and licensing agreement to ImmunArray. Neither company had any input into the design, analysis, interpretation, or content of this study and did not influence the decision to submit this manuscript. The contents of this article represent the personal opinion of the authors and should not be construed as the opinion or position of the National Institutes of Health or its affiliates. The other authors have no significant financial disclosures to report.
References
- 1.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118:622–629. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- 2.Varni JW, Burwinkle TM, Lane MM. Health-related quality of life measurement in pediatric clinical practice: An appraisal and precept for future research and application. Health Qual Life Outcomes. 2005;3:34–42. doi: 10.1186/1477-7525-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panepinto JA, O'Mahar KM, DeBaun MR, et al. Health-related quality of life in children with sickle cell disease: Child and parent perception. Br J Haematol. 2005;130:437–444. doi: 10.1111/j.1365-2141.2005.05622.x. [DOI] [PubMed] [Google Scholar]
- 4.Panepinto JA. Health-related quality of life in sickle cell disease. Pediatr Blood Cancer. 2008;51:5–9. doi: 10.1002/pbc.21557. [DOI] [PubMed] [Google Scholar]
- 5.Panepinto JA, Pajewski NM, Foerster LM, et al. The performance of the PedsQL generic core scales in children with sickle cell disease. J Pediatr Hematol Oncol. 2008;30:666–673. doi: 10.1097/MPH.0b013e31817e4a44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panepinto JA, Bonner M. Health-related quality of life in sickle cell disease: Past, present, and future. Pediatr Blood Cancer. 2012;59:377–385. doi: 10.1002/pbc.24176. [DOI] [PubMed] [Google Scholar]
- 7.DeBaun MR, Gordon M, McKinstry RC, et al. Controlled trial of transfusions for silent cerebral infarcts in sickle cell anemia. N Engl J Med. 2014;371:699–710. doi: 10.1056/NEJMoa1401731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hulbert ML, McKinstry RC, Lacey JL, et al. Silent cerebral infarcts occur despite regular blood transfusion therapy after first strokes in children with sickle cell disease. Blood. 2011;117:772–779. doi: 10.1182/blood-2010-01-261123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Styles LA, Vichinsky E. Effects of a long-term transfusion regimen on sickle cell-related illnesses. J Pediatr. 1994;125:909–911. doi: 10.1016/s0022-3476(05)82006-2. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg MH. Management of sickle cell disease. N Engl J Med. 1999;340:1021–1030. doi: 10.1056/NEJM199904013401307. [DOI] [PubMed] [Google Scholar]
- 11.Emre U, Miller ST, Gutierez M, et al. Effect of transfusion in acute chest syndrome of sickle cell disease. J Pediatr. 1995;127:901–904. doi: 10.1016/s0022-3476(95)70025-0. [DOI] [PubMed] [Google Scholar]
- 12.Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 13.Casella JF, King AA, Barton B, et al. Design of the silent cerebral infarct transfusion (SIT) trial. J Pediatr Hematol Oncol. 2010;27:69–89. doi: 10.3109/08880010903360367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Landgraf JM, Abetz LN, Ware JE. The CHQ user's manual. Boston, MA: HealthAct; 1999. [Google Scholar]
- 15.Gordon AL, Ganesan V, Towell A, et al. Functional outcome following stroke in children. J Child Neurol. 2002;17:429–434. doi: 10.1177/088307380201700606. [DOI] [PubMed] [Google Scholar]
- 16.Landgraf JM, Abetz LN. Functional status and well-being of children representing three cultural groups: Initial self-reports using the CHQ-CF87. Psychology and Health. 1997;12:839–854. [Google Scholar]
- 17.Levi RB, Drotar D. Health-related quality of life in childhood cancer: Discrepancy in parent-child reports. Int J Cancer Suppl. 1999;12:58–64. doi: 10.1002/(sici)1097-0215(1999)83:12+<58::aid-ijc11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 18.Asmussen L, Olson LM, Grant EN, et al. Use of the child health questionnaire in a sample of moderate and low-income inner-city children with asthma. Am J Respir Crit Care Med. 2000;162:1215–1221. doi: 10.1164/ajrccm.162.4.2001067. [DOI] [PubMed] [Google Scholar]
- 19.Wake M, Hesketh K, Cameron F. The child health questionnaire in children with diabetes: Cross-sectional survey of parent and adolescent-reported functional health status. Diabet Med. 2000;17:700–707. doi: 10.1046/j.1464-5491.2000.00360.x. [DOI] [PubMed] [Google Scholar]
- 20.Schneider JW, Gurucharri LM, Gutierrez AL, et al. Health-related quality of life and functional outcome measures for children with cerebral palsy. Dev Med Child Neurol. 2001;43:601–608. doi: 10.1017/s0012162201001098. [DOI] [PubMed] [Google Scholar]
- 21.Waters EB, Wake MA, Hesketh KD, et al. Health-related quality of life of children with acute lymphoblastic leukaemia: Comparisons and correlations between parent and clinician reports. Int J Cancer. 2003;103:514–518. doi: 10.1002/ijc.10815. [DOI] [PubMed] [Google Scholar]
- 22.Panepinto JA, O'Mahar KM, DeBaun MR, et al. Validity of the child health questionnaire for use in children with sickle cell disease. J Pediatr Hematol Oncol. 2004;26:574–578. doi: 10.1097/01.mph.0000136453.93704.2e. [DOI] [PubMed] [Google Scholar]
- 23.Palermo TM, Riley CA, Mitchell BA. Daily functioning and quality of life in children with sickle cell disease pain: Relationship with family and neighborhood socioeconomic distress. J Pain. 2008;9:833–840. doi: 10.1016/j.jpain.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palermo TM, Schwartz L, Drotar D, et al. Parental report of health-related quality of life in children with sickle cell disease. J Behav Med. 2002;25:269–283. doi: 10.1023/a:1015332828213. [DOI] [PubMed] [Google Scholar]
- 25.Beverung LM, Varni JW, Panepinto JA. Clinically meaningful interpretation of pediatric health-related quality of life in sickle cell disease. J Pediatr Hematol Oncol. 2014 doi: 10.1097/MPH.0000000000000177. ePub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 27.Forrest CB, Bevans KB, Pratiwadi R, et al. Development of the PROMIS® pediatric global health (PGH-7) measure. Qual Life Res. 2014;23:1221–1231. doi: 10.1007/s11136-013-0581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeSalvo KB, Jones TM, Peabody J, et al. Health care expenditure prediction with a single item, self-rated health measure. Med Care. 2009;47:440–447. doi: 10.1097/MLR.0b013e318190b716. [DOI] [PubMed] [Google Scholar]
- 29.DeSalvo KB, Bloser N, Reynolds K, et al. Mortality prediction with a single general self-rated health question: A meta-analysis. J Gen Intern Med. 2006;21:267–275. doi: 10.1111/j.1525-1497.2005.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Panepinto JA, Torres S, Bendo CB, et al. PedsQLtm sickle cell disease module: Feasibility, reliability, and validity. Pediatr Blood Cancer. 2013;60:1338–1344. doi: 10.1002/pbc.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvarez O, Yovetich NA, Scott JP, et al. Pain and other non-neurological adverse events in children with sickle cell anemia and previous stroke who received hydroxyurea and phlebotomy or chronic transfusions and chelation: Results from the SWiTCH clinical trial. Am J Hematol. 2013;88:932–938. doi: 10.1002/ajh.23547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller ST, Wright E, Abboud M, et al. Impact of chronic transfusion on incidence of pain and acute chest syndrome during the stroke prevention trial (STOP) in sickle-cell anemia. J Pediatr. 2001;139:785–789. doi: 10.1067/mpd.2001.119593. [DOI] [PubMed] [Google Scholar]


