Abstract
Purpose
To report acute/subacute vision loss and paracentral scotomata in patients with idiopathic multifocal choroiditis/punctate inner choroidopathy (MFC/PIC) due to large zones of acute photoreceptor attenuation surrounding the chorioretinal lesions.
Methods
Multimodal-imaging case-series
Results
Six females and 2 males were included (mean age 31.5±5.8 years). Vision ranged from 20/20-1 to hand motion (mean 20/364). SD-OCT demonstrated extensive attenuation of the external limiting membrane (ELM), ellipsoid and interdigitation zones, adjacent to the visible MFC/PIC lesions. The corresponding areas were hyperautofluorescent on fundus-autofluorescence (FAF), and were associated with corresponding visual field defects. Full-field ERG (available in 3 cases) showed markedly decreased cone/rod response and multifocal ERG revealed reduced amplitudes and increased implicit times in 2 cases. Three patients received no treatment, the remaining were treated with oral corticosteroids (n=4), oral acyclovir/valacyclovir (n=2), intravitreal/posterior subtenon triamcinolone-acetate (n=3) and anti-VEGF (n=2). Visual recovery occurred in only 3 cases, of whom 2 were treated. Varying morphological recovery was found in 6 cases, associated with decrease in hyperautofluorescence on FAF.
Conclusions
MFC/PIC can present with transient or permanent central photoreceptor attenuation/loss. This presentation is likely a variant of MFC/PIC with chorioretinal atrophy. Associated changes are best evaluated using multimodal imaging.
Keywords: Multifocal choroiditis, punctate inner choroidopathy, AZOOR, optical coherence tomography, acute visual function loss, autofluorescence, photoreceptor loss
Introduction
Idiopathic multifocal choroiditis (MFC) and punctate inner choroidopathy (PIC) are inflammatory disorders typically affecting young, myopic females with no associated systemic disease.1,2 Most of the current authors believe PIC, MFC and MFC and panuveitis represent the same underlying disease entity. 3,4 Although this is still controversial, they share the same cytokine polymorphism.5 With classic MFC/PIC, patients may note a decrease in visual acuity (VA) (dependent on the location of the chorioretinal lesions), floaters, photopsias and an enlarged blind spot.6 The vision loss is classically attributed to inflammation or choroidal neovascularisation (CNV). On examination MFC/PIC presents with yellow hazy (later punched out) lesions in the posterior pole or periphery; vitreous cells may be present.7 Our recent study described a variant of idiopathic MFC with concomitant zonal, multizonal or diffuse outer retinal or chorioretinal atrophy.8 In that study patients with discrete MFC lesions were found to have extensive outer retinal or chorioretinal atrophy which often progressed over time.8 Central vision was often spared or involved only late in the course. Previously some patients with MFC/PIC with disruption of the ellipsoid and interdigitation zones seen on SD-OCT in clinically uninvolved areas within macula have been described.3,9,10 In these cases, the outer retinal structures recovered and the hyper-autofluorescence seen on fundus autofluorescence (FAF) faded with corticosteroid treatment.3,9,10
The aim of this current study is to describe the acute presentation of MFC/PIC with a dramatic decrease in visual acuity or central scotomata due to associated photoreceptor dysfunction out of proportion to the clinically visible lesions. In the described cases recovery from this insult was variable.
Methods
Patient selection and setting
This retrospective observational case series study included 8 patients (9 eyes) from 5 referring institutions: Feinberg School of Medicine at Northwestern University in Chicago (Illinois), Vitreous Retina Macula Consultants of New York, Casey Eye Institute/Oregon Health and Science (Portland), National Eye Institute (National Institute of Health, Bethesda) and Medical University Vienna (Austria). The study adhered to the tenets of the Declaration of Helsinki and was granted a waiver of informed consent by the Northwestern University institutional review board.
Patients diagnosed with idiopathic MFC or PIC with acute/subacute visual acuity loss or acute/subacute onset of central scotomata and photoreceptor-dysfunction adjacent to the lesions visible on SD-OCT were included. Patients with underlying associated systemic disease or possible infectious causes were excluded. Medical workup in individual cases varied but was uniformly negative for infectious or immune ocular or systemic diseases. Data collection in all patients included age, sex, medical history (medication and history of therapy), best corrected visual acuity (BCVA), refraction, slit-lamp examination and ophthalmoscopy. Multimodal image analysis included SD-OCT, Infrared (IR), Red-free, short-wave blue (488nm) fundus autofluorescence (FAF) (HRA+OCT Spectralis; Heidelberg Engineering, Heidelberg, Germany), green near-infrared (787nm)-FAF (Optos, Marlborough, MA, USA and/or Topcon America, Paramus, NJ, USA), fluorescein angiography (FA), indocyanine angiography (ICGA) (Heidelberg Engineering, Heidelberg, Germany or Topcon America, Paramus, NJ, USA), color fundus photographs (Topcon America, Paramus, NJ, USA) and/or wide field color fundus images (Optos, Marlborough, MA, USA). Additional functional analysis included a full-field electroretinogram (ffERG) in 3 patients (case 2+5+7) and a multifocal (mf) ERG in 2 patients (case 1+2). One patient (2 eyes) also had visual evoked potentials (VEP) and electrooculography (EOG) completed (case 7). Six out of 8 patients had a Humphrey visual field (HVF) performed, 1 had a Goldmann visual field (GVF) and 1 had microperimetry. Medical records were reviewed at presentation and during follow-up.
Results
Demographic characteristics and multimodal evaluation
Seven patients (8 eyes; 5 women and 2 men) were diagnosed with idiopathic MFC/PIC and visual acuity loss, and one patient (1 woman) was diagnosed with MFC/PIC and paracentral scotomata due to photoreceptor attenuation beyond the ophthalmoscopically visible lesions. No patient showed vitreous cells and/or vitreous haze. Demographic characteristics and treatment can be found in Table 1. Six patients were seen at initial onset and two patients were first seen years after their acute VA decrease. Mean age at disease onset was 31.5±5.8 years. Mean age at initial presentation to us was 33.3±7.8 years.
Table 1.
Patient’s demographics
| Pat.# | Age onset (years) | Sex | Our duration of F/u (months) | BCVA presentation | BCVA last visit | CNV | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | 24 | f | 19 | HM | 20/600 | yes | corticosteriods, valacyclovir, IVTA, PSTK, clindamycin, co- trimoxazole |
| 2 | 34 | f | 22 | HM | HM | yes | corticosteriods, PSTK, oral albendazole |
| 3 | 26 | f | 6 | 20/20-1 | 20/20 | no | none |
| 4 | 38 | f | 9 | 20/400 | 20/125 | yes | bevacizumab, IVTA |
| 5 | 33 | m | 3 | 20/125 | 20/125 | no | none |
| 6 | 27 | f | 7 | CF | 20/25 | yes | corticosteroids, ranibizumab |
| 7OD | 40 | f | 82 | 20/200 | HM | no | none |
| 7OS | 40 | f | 82 | 20/200 | HM | no | none |
| 8 | 30 | m | 7 | 20/400 | 20/20 | no | acyclovir, corticosteriods |
Pat.=patient, age onset= age at initial onset of symptoms, F/u= follow up, BCVA= best corrected visual acuity, CNV= choroidal neovascularisation, IVTA= intravitreal triamcinolone acetonide, PSTK= posterior subtenon triamcinolone acetonide, HM= hand motion, CF= counting fingers
Mean distance BCVA at baseline was 20/364 (1.26±0.75logMAR), ranging from the severity of hand motion (HM) to 20/20-1 (depending on the extent of foveal involvement of the photoreceptor loss/irregularity). Mean distance BCVA at the last follow up visit was 20/252 (1.1±0.92logMAR, range from HM+ to 20/20-1) (Table 1). The mean duration of follow-up was 25±33months (range 3–82 months). Three of the patients eventually developed CNV during the observational period and 1 patient presented with an old extrafoveal scar suspicious for a previous active CNV. Three cases received no treatment, the remaining cases were treated with oral corticosteroids (n=4), oral acyclovir/valacyclovir (n=2), intravitreal/posterior subtenon triamcinolone-acetate (n=3) and anti-VEGF (n=2) (Table 1). Complete morphological and visual function recovery was found in 3 patients (1 untreated, 2 treated with oral corticosteroids). Another three patients had some restoration of the ELM and the ellipsoid zone, without visual functional recovery (2 treated with oral corticosteroids and intravitreal/posterior subtenon triamcinolone-acetate, 1 treated with intravitreal triamcinolone-acetate and bevacizumab). The remaining 2 cases by history displayed no recovery of the hyperreflective bands or visual function but these 2 patients were first seen by us years after initial onset of symptoms (both never received any treatment). No patient was documented to have new areas of PR-loss in SD-OCT during follow up. However, 3 cases (case 1+4 +7) showed increasing VF-defects over time. Visual acuity and imaging findings at baseline and during follow up are summarized in Table 1 and 2.
Table 2.
Multimodal imaging findings corresponding to the areas of attenuation of the external limiting membrane (ELM), ellipsoid (EZ) and interdigitation zones (IZ) in spectral domain optical coherence tomography (SD-OCT)
| Pat.# | FAF (488nm/787nm) | Corr. VF defect | Indocyanine green (ICGA) | Infrared | Recovery of ELM/EZ/IZ during F/u in SD-OCT |
|---|---|---|---|---|---|
| 1 | Hyper-autofluorescence | yes | No corr. changes | Hyporeflectivity | partial recovery |
| 2 | Hyper-autofluorescence | No VF done | No corr. changes | no corr. changes | partial recovery |
| 3 | Hyper-autofluorescence | yes | No ICGA performed | Hyporeflectivity | Minimal residue inferior to fovea |
| 4 | Hyper-autofluorescence | yes | Hypo-cyanescence | Hyporeflectivity | partial recovery |
| 5 | Hyper-autofluorescence | yes | No ICGA performed | no corr. changes | no recovery |
| 6 | Hyper-autofluorescence | yes | No ICGA performed | no corr. changes | full recovery |
| 7OD | Hyper-autofluorescence | No VF done | No ICGA performed | no corr. changes | no recovery |
| 7OS | Hyper-autofluorescence | No VF done | No ICGA performed | no corr. changes | no recovery |
| 8 | Hyper-autofluorescence | yes | No corr. changes | no corr. changes | full recovery |
Pat.=patient, FAF=fundus autofluorescence, corr.=corresponding, VF= visual field, F/u=follow up,
Case reports
Case 1
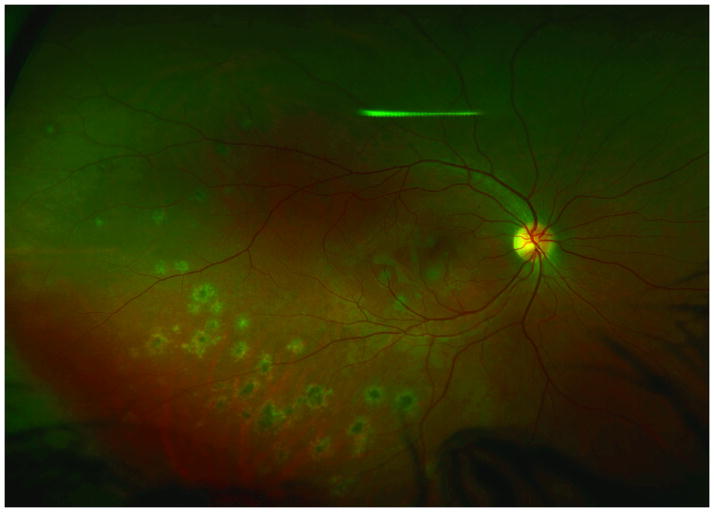
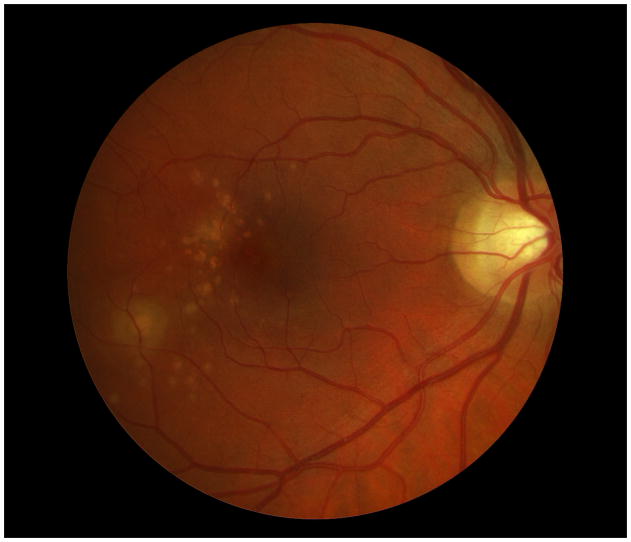
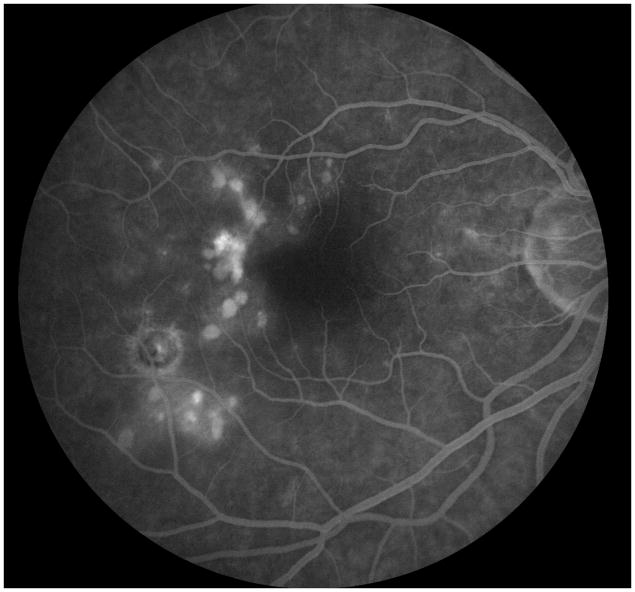
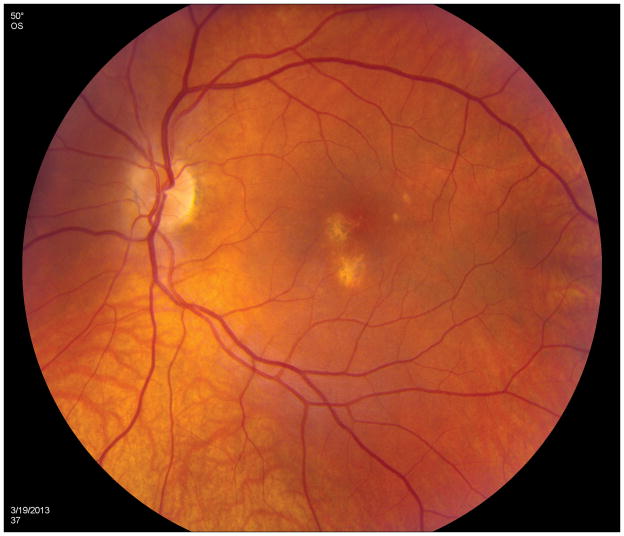
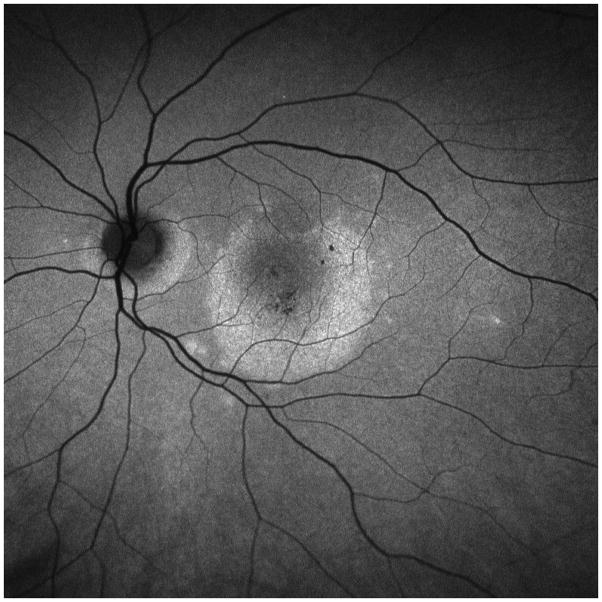
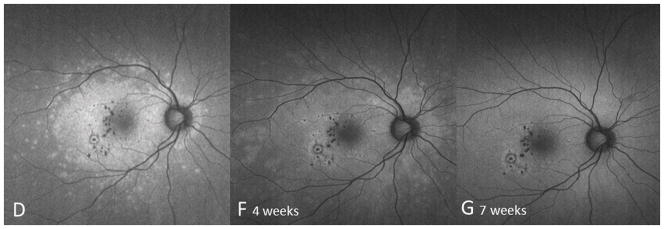
A 24 year old Caucasian woman noted a black cloud OD for 5 days. An ophthalmologist detected chorioretinal lesions in the posterior pole with a BCVA of 20/125 and started her on oral prednisone 75mg. Within 5 days her vision dropped to counting fingers (CF) and she was referred for evaluation. At presentation she was on 25mg prednisone and her BCVA was CF-/HM+ OD at 1m and 20/20 OS. The anterior chamber was quiet and there were no vitreous cells. Ophthalmoscopy revealed multiple chorioretinal lesions OD, at various stages of evolution; some old and pigmented others new with a creamy poorly circumscribed appearance (Figure 1A). Oral prednisone was increased to 50mg, valacyclovir (2000 mg) was started and she briefly received oral clindamycin and co-trimoxazole. An extensive work-up was negative. SD-OCT revealed subRPE lesions and extensive ELM-, ellipsoid and interdigitation zones -loss (Figure 1B). The disruption of these respective layers corresponded to hyporeflectivity on the IR image (Figure 1B). FAF showed diffuse hyperautofluorescence. Amsler grid, GVF and HVF revealed extensive central and peripheral visual field loss with a temporal island of preserved visual field. Pattern ERG demonstrated a significantly reduced P50 amplitude OD and mfERG revealed markedly reduced amplitudes sub- and parafoveally indicating a loss of macular visual function. FA showed late staining and late ICGA showed hypofluorescence corresponding to the visible lesions. Over the next two months, prednisone was slowly tapered. Valacyclovir was stopped after 4 weeks. Two months later, VA was 20/800 and SD-OCT showed incomplete recovery of the hyperreflective bands. The hyporeflectivity on IR remained (Figure 1C). At 4 months she experienced further VA decrease down to CF. New MFC/PIC lesions and old bridging scar tissue were seen (Figure 1D). Late FA images revealed some new leakage around the chorioretinal lesions (Figure 1E) and SD-OCT revealed further photoreceptor-loss. FAF demonstrated an increase in hyperautofluorescence. GVF revealed further constriction of the temporal visual field island. The patient was restarted on 50mg prednisone and received a posterior subtenon injection of triamcinolone acetate OD. Her BCVA increased slightly to 20/800. Oral Prednisone therapy was tapered over the next 5 months and then stopped. Despite some central visual function improvement and some morphological recovery of the photoreceptors on SD-OCT (Figure 1F), the hyperautofluorescence on FAF (Figure 1G) remained and the GVF showed no recovery. Four months later, she presented again with a sudden onset of VA decrease down to CF- OD, however no new lesions were found. One month thereafter the patient presented with central CNV accompanied by subretinal fluid and intraretinal cysts. She was treated with a single intravitreal triamcinolone acetate injection (IVTA). Her BCVA returned to 20/600 and at the last follow-up visit the intraretinal cysts as well as subretinal fluid had almost completely resolved.
Figure 1. Case 1.
Optomap (A) of the right eye reveals pigmented multifocal choroiditis (MFC) lesions in the inferotemporal periphery. Centrally there are several new creamy chorioretinal lesions inferior to the fovea. Spectral-domain optical coherence tomography (SD-OCT) (B) has extensive photoreceptor loss/irregularity: the central cross-section scan demonstrates extensive loss of the external limiting membrane (ELM), ellipsoid- and interdigitation zones, more pronounced temporally. The SD-OCT through active chorioretinal lesions highlights adjacent loss of the respective layers. On infrared (IR) a hyporeflective area is noted which corresponds to the photoreceptor disruption on SD-OCT (yellow arrow). Follow-up 2 months later shows some recovery of the photoreceptors: SD-OCT (C) outlines some regeneration of the ELM, ellipsoid and interdigitation zones. The hyporeflective IR area remained (yellow arrow). 6 months from baseline: The late indocyanine angiography (ICGA) (D) reveals new hypofluorescent lesions. Fluorescein angiography (FA) image (E) shows new, hyperfluorescent multifocal lesions that leak in the late phase. 12 months after initial presentation: SD-OCT (F) shows that the chorioretinal hyperreflective nodules representing MFC lesions have grown in size. Although the interdigitation zone is still not well defined the ellipsoid zone shows further recovery and is now distinguishable. Hyperautofluorescence (near infrared 788nm fundus autofluorescence) (G) is still visible, but could be also due to a photobleaching effect.
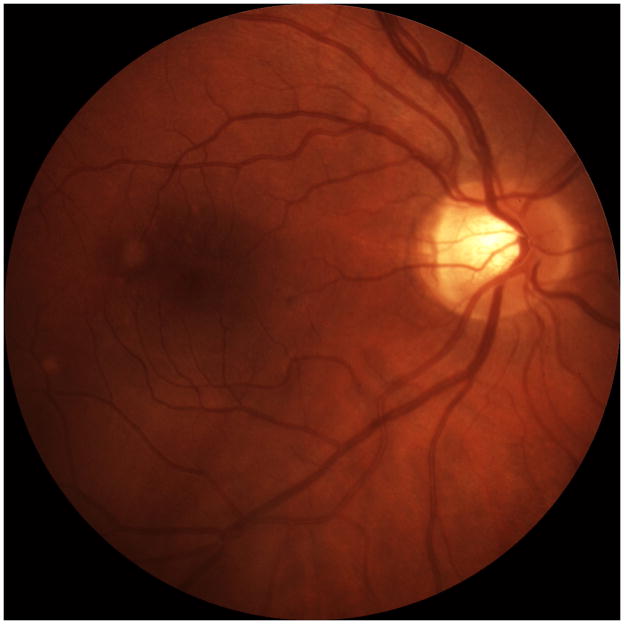
Case 2
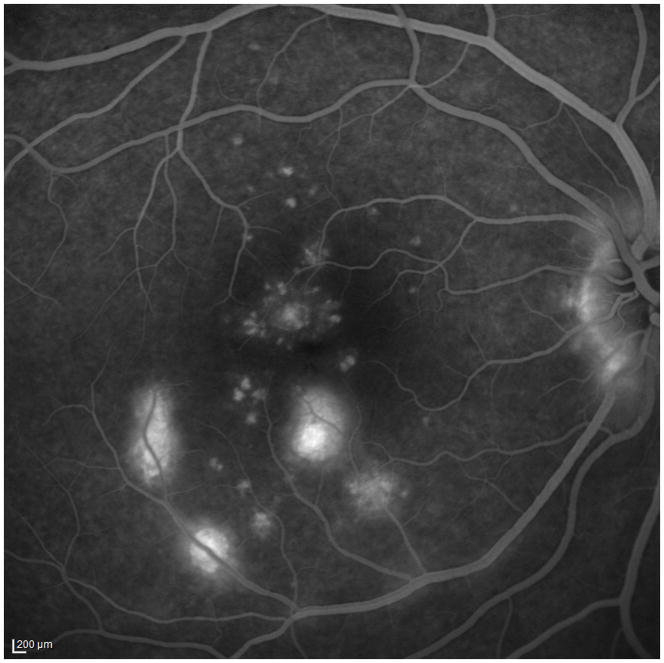
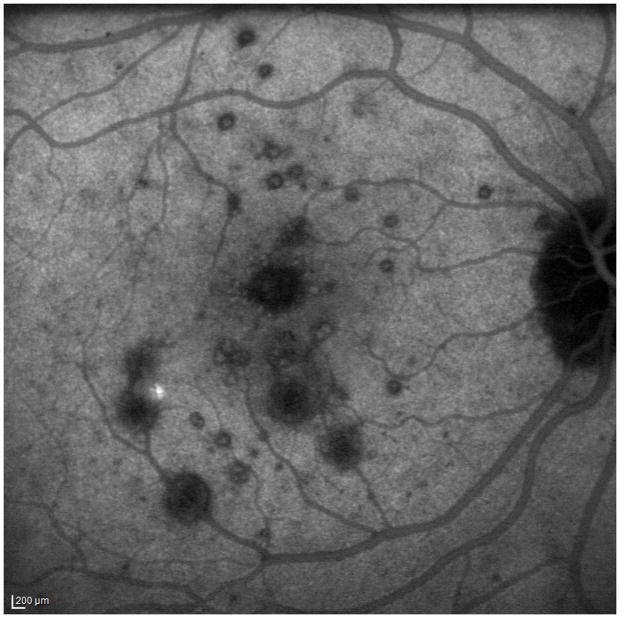
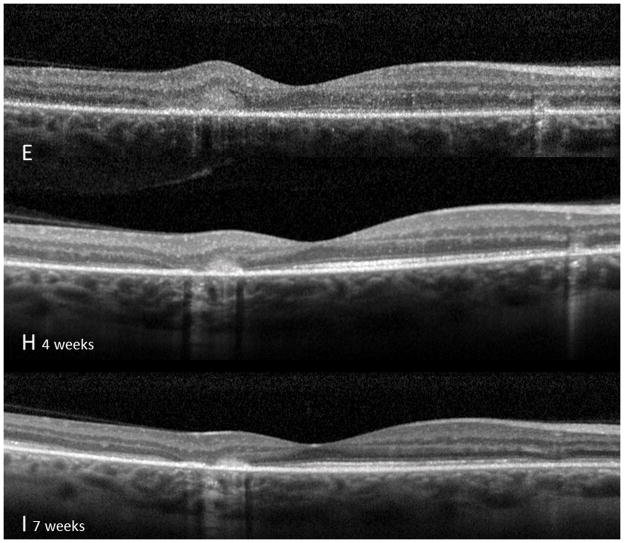
A 34 year old Caucasian woman with moderate myopia (spherical equivalent (SE): −2.75) experienced monocular visual loss OD. Her symptoms first began with a small paracentral scotoma OD that progressed in density over the following year. Her VA during this time period was 20/20 OU (Figure 2A). Examination revealed creamy yellow round spots in her right macula that had increased in number over this year. Her left eye was unaffected except for one small yellow macular lesion. Her subjective scotoma faded over the next 6 months, but she then suddenly awoke with acute central vision loss and a visual acuity of HM in the right eye. Retinal examination revealed further increase in size and number of creamy yellow spots OD (Figure 2B). FA revealed staining of the chorioretinal lesions with some mild surrounding late leakage inferotemporally. There was a hypofluorescent lesion at the inferior arcade which did not leak but was suspicious for inactive CNV (Figure 2C). The day following her acute vision loss, she received a posterior subtenon triamcinolone acetate injection without subsequent visual improvement. Nine days later she presented for evaluation. Imaging was performed including blue short-wave (488nm) and green near infrared (787nm) FAF which was remarkable for a diffuse area of hyper-autofluorescence with mottled borders in the right macula and peripapillary area (Figure 2D). Her chorioretinal lesions corresponded to hypoautofluorescent spots on FAF. In addition to multiple outer chorioretinal lesions, SD-OCT showed extensive and diffuse ELM- and ellipsoid-/interdigitation zones loss and thinning of the RPE layer OD (Figure 2E). The ffERG showed decreased amplitudes OU consistent with mild rod and cone dysfunction, worse OD. The mfERG revealed subnormal amplitudes and prolonged implicit times OD and was normal OS. The extensive work-up was negative. Although there was no evidence or clinical finding of a helminthic infection the patient was empirically treated for thirty days with oral albendazole. The patient was also started on oral prednisone 80mg daily for two weeks which was then slowly tapered over two months. Subsequent examinations and imaging over the following two months revealed no increase in vision, but a decrease in the number and size of chorioretinal spots and a decreased intensity of the fundus hyper-autofluorescence (Figure 2F+G). OCT-imaging demonstrated minor recovery of the ELM, ellipsoid - and interdigitation zones (Figure 2H and I). At the last visit, 21 months after initial presentation, the patient’s vision was still HM OD.
Figure 2. Case 2.
Color fundus image (A) of the right eye reveals yellowish circumscribed chorioretinal lesions temporal to the fovea. Best corrected visual acuity (BCVA) was 20/20 One year later the patient presents with sudden visual acuity loss down to hand motion (HM) (B): There is a visible increase in the size and number of yellowish creamy chorioretinal lesions within the macula. Fluorescein angiography (FA) (C) shows staining and some mild late leakage. The inferotemporal hypofluorescent lesion is surrounded by a subtle hyperfluorescent ring suspicious for a possible inactive choroidal neovascular membrane. Near infrared (787nm) (D) fundus autofluorescence (FAF) is remarkable for a distinctive area of hyperautofluorescence throughout the posterior pole. The spectral domain optical coherence tomography (SD-OCT) scan (E) has extensive loss of the external limiting membrane (ELM), ellipsoid and interdigitation zones. The retinal pigment epithelium is thinner and less reflective. Near infrared (787nm) FAF (F+G) highlights the decrease in hyper-autofluorescence over the next two months. The chorioretinal lesions corresponding to hypoautofluorescent spots on FAF have decreased in number as well as in size. Correspondingly, SD-OCT (H+I) shows partial restoration of the ELM and ellipsoid zones. Hereby the respective bands remain attenuated and less hyperreflective and the photoreceptor-complex and the outer nuclear layer appears significantly thinner when compared to the non-affected fellow eye. The interdigitation zone is not distinguishable. The BCVA remains HM.
Case 3
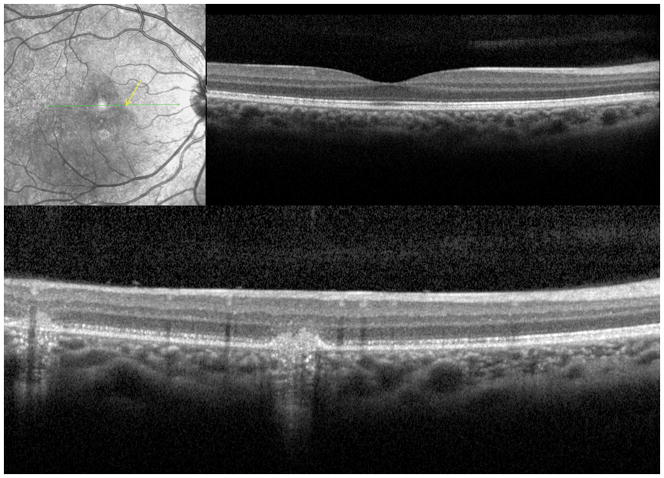
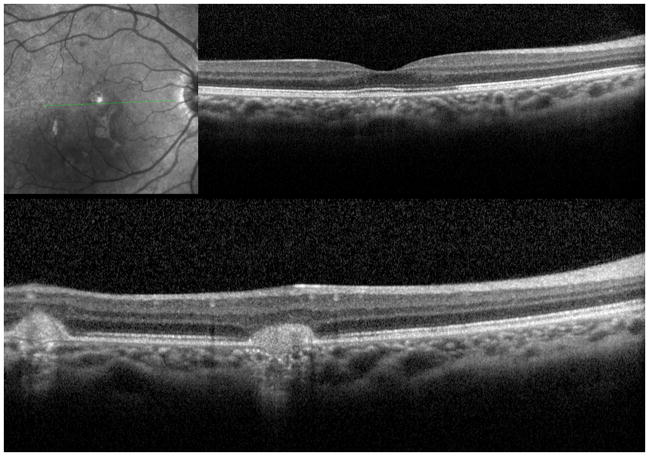
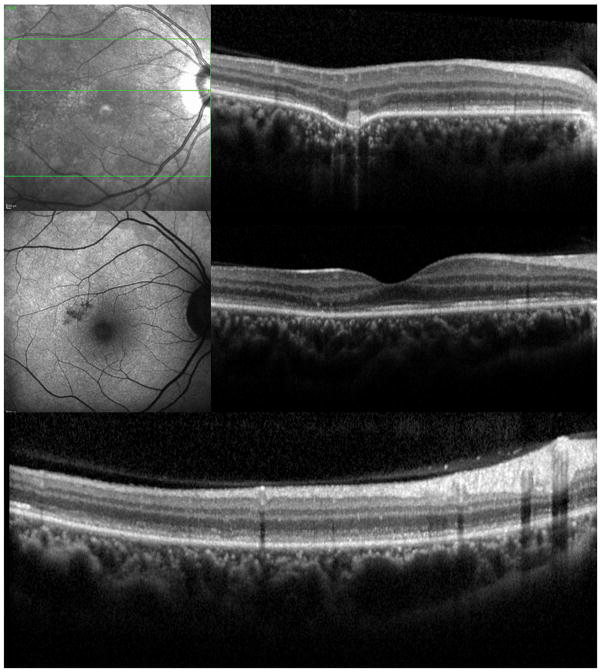
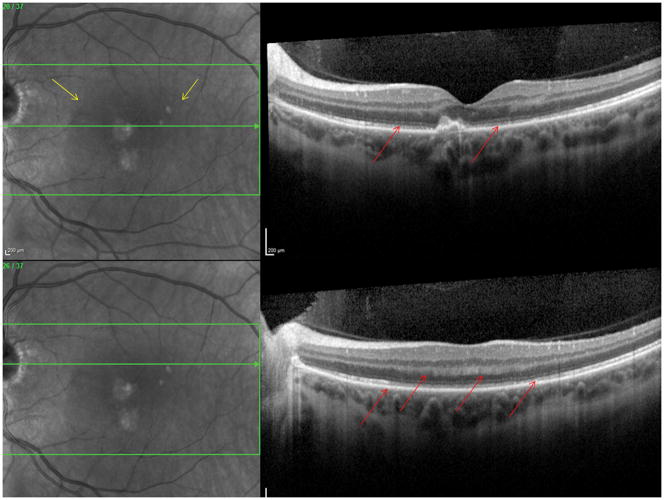
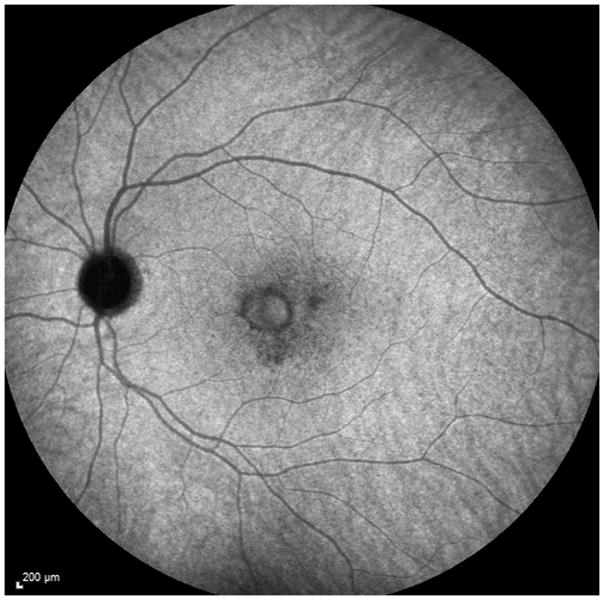
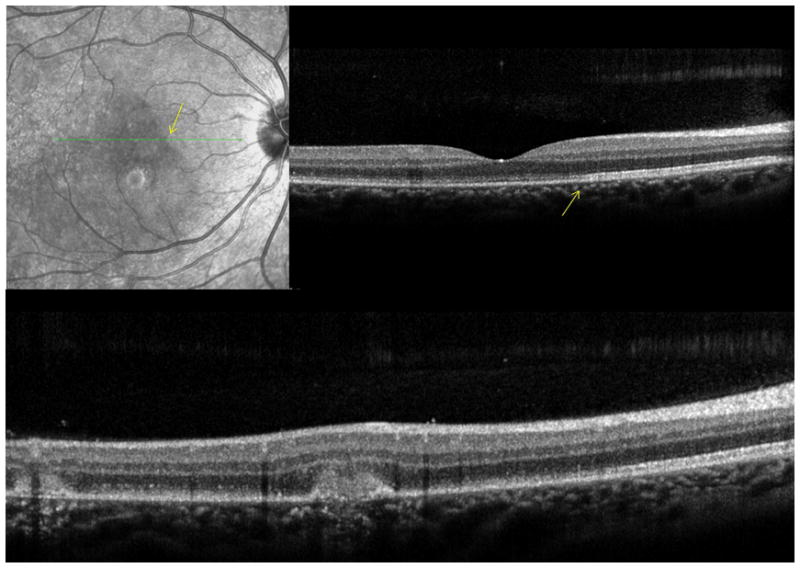
A 26 year old Caucasian woman with a scotoma and macular lesions OD for 1 month was referred for evaluation. The patient described blurred vision and a paracentral scotoma after waking up accompanied by flashes and floaters in the right eye. Her medical history was negative. Her BCVA was 20/20 OU. Indirect ophthalmoscopy revealed peripapillary atrophy and MFC/PIC lesions superior to the fovea OD. SD-OCT showed a juxtafoveal choroidal lesion with choroidal excavation and demonstrated loss and irregularity of the ELM, ellipsoid- and interdigitation zones adjacent to the MFC/PIC lesion (Figure 3A). This photoreceptor-irregularity/loss corresponded to hyperautofluorescence on short wave blue (488nm) FAF (Figure 3A) and with hyporeflectivity on the IR. Follow-up visit 4 months later revealed improvement of the ELM, the ellipsoid and the interdigitation zones and the hyperautofluorescence and hyporeflectivity had decreased in intensity (Figure 3B). The BCVA was 20/20 OU and the patient reported a subjective improvement in her vision and fading of the paracentral scotoma. However, a small superotemporal scotoma remained which was delineated in the 24-2 HVF and which corresponded to a still attenuated but overall improved appearance of the ELM, ellipsoid and interdigitation zones. Two months thereafter this scotoma also had nearly vanished.
Figure 3. Case 3.
3A: Top: Spectral domain optical coherence tomography (SD-OCT) outlines the chorioretinal lesion presenting as a hyperreflective deposit which has broken through the RPE disrupting the overlying retina. The adjacent external limiting membrane (ELM), ellipsoid and interdigitation zones are not distinguishable. Middle: Fundus autofluorescence reveals hyperautofluorescence and the central macular scan shows a relative sparing of the fovea. Bottom: SD-OCT B-scan superior to the lesion verifies the adjacent loss of the ELM, the ellipsoid- and interdigitation zone.
3B: Follow-up at 4 months: Recovery of the hyperreflective bands on SD-OCT can be observed. Compared to the left eye, however, the ELM, ellipsoid and interdigitation zones still appear partially disrupted. Middle: Autofluorescence reveals a decrease in hyperautofluorescence
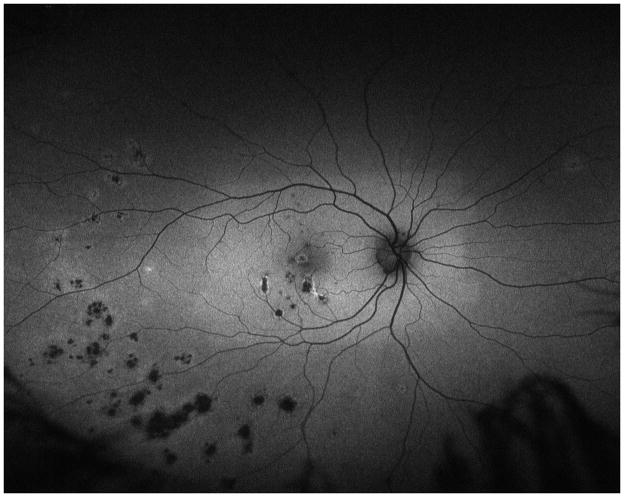
Case 4
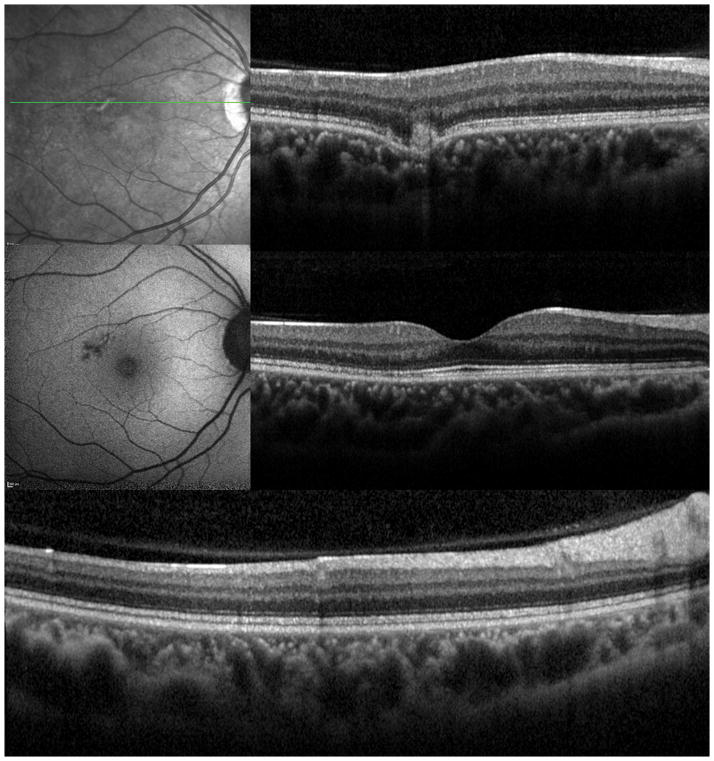
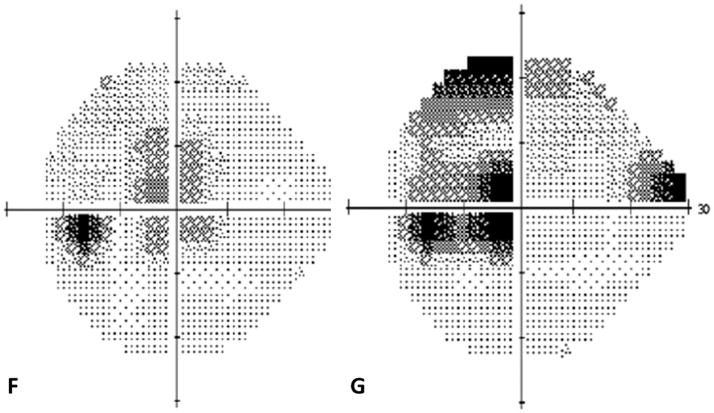
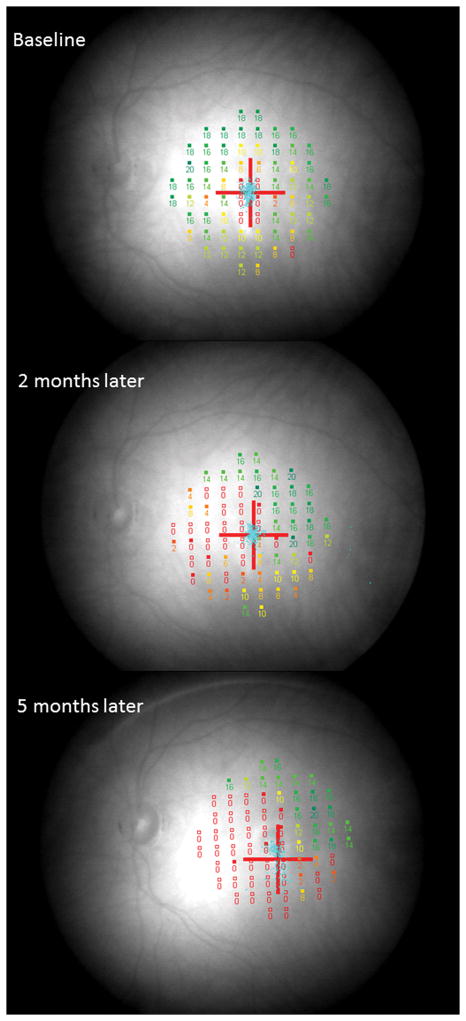
A 38 year old Caucasian woman with myopia (SE: −3.25D OU) and a two year history of decreased visual function and metamorphopsia OS was referred for evaluation. She had been previously treated with 7 injections of intravitreal bevacizumab OS. At initial presentation her BCVA was 20/16 OD and 20/400 OS. No active CNV was identified. She had peripapillary atrophy with 2 large parafoveal chorioretinal lesions and 3 smaller lesions superior to the fovea (Figure 4A). FA demonstrated window defects adjacent to the disc and late staining of the hypofluorescent lesions (Figure 4B). On ICGA more chorioretinal lesions were identified compared to examination and FA (Figure 4C). Beside the chorioretinal lesions, a plaque of hypofluorescence on ICGA was noted in the early and in the late frames, which was in turn surrounded by a discrete hyperfluorescent ring. Blue short wave (488nm) - as well as near infrared (787nm) FAF revealed a paracentral and peripapillary hyperautofluorescence which corresponded to the hypofluorescent area on ICGA (Figure 4D) and to a hyporeflective area on IR. SD-OCT changes (Figure 4E) displayed blurred and indistinct ellipsoid and interdigitation zones and a hyperreflective Henle’s layer. These areas corresponded to the FAF hyperautofluorescence, the ICGA hypofluorescence, the IR hyporeflectivity (Figure 3E) and to the VF defect (Figure 4F). The patient was treated with IVTA. One month later BCVA was 20/80. Although the hyperautofluorescence had decreased in intensity and the photoreceptor status had improved, the VF defect and the absolute scotomata on microperimetry had increased in size (Figure 4G+H). Four months after IVTA and initial presentation to us BCVA worsened again to 20/160 and CNV was suspected. The patient was treated again with IVTA and intravitreal bevacizumab. Central vision improved, but the microperimetry again revealed an increase in absolute scotomata (Figure 4H). As intraocular pressure (IOP) pressure was not controllable with topical medications a tube shunt surgery was performed 5 month after initial presentation. At the last visit the BCVA was 20/125 OS and IOP was 14. Despite improvement of the ellipsoid and the interdigitation zones, resolution of the hypofluorescent plaque on ICGA and further decrease of the FAF hyperautofluorescence, the scotomata seen with microperimetry and visual field defects continued to enlarge (Figure 4H+4I). It remains unclear whether the increased IOP contributed to the increase in VF defects.
Figure 4. Case 4.
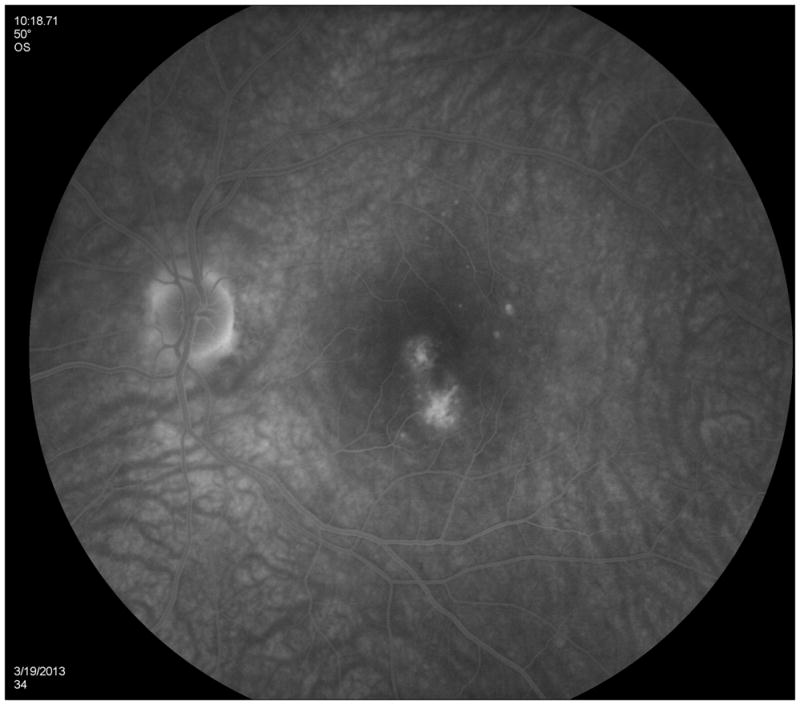
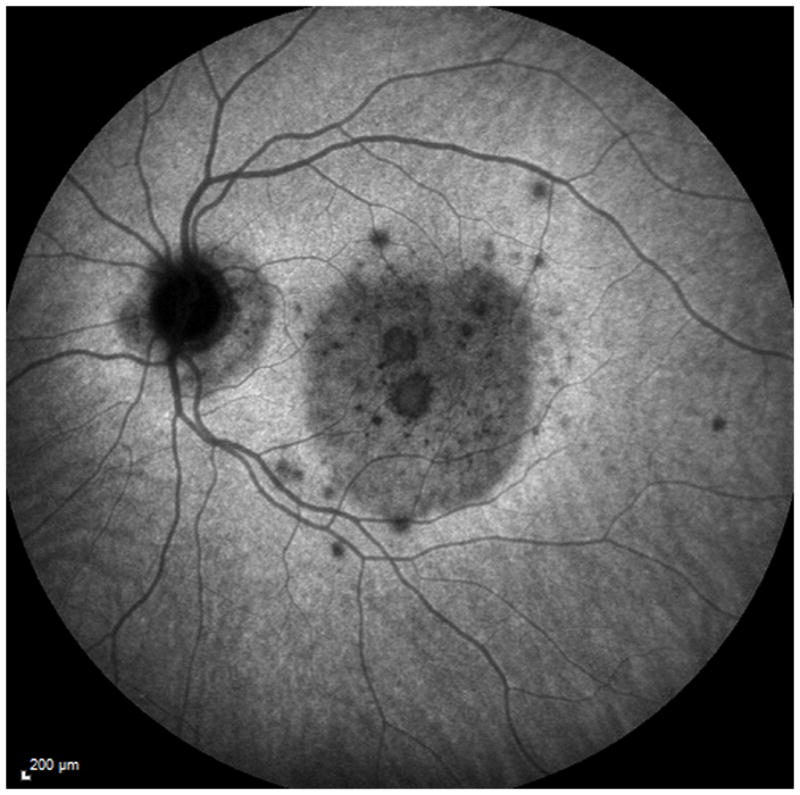
Color photographs of the left eye (A) reveals peripapillary atrophy, 2 large yellow parafoveal chorioretinal lesions and 3 smaller lesions superior to the fovea. Fluorescein angiography (FA) (B) identifies window defects and late staining of the chorioretinal lesions. Indocyanine green angiography (ICGA) highlights (C) the hypofluorescent lesions but also has a plaque-like hypofluorescent area which corresponds to the hyper-autofluorescent area seen with near infrared- (787nm) autofluorescence (FAF) (D): Near infrared-FAF outlines the clear ring-shaped hyperautofluorescence visible around the optic disc and around the fovea. Corresponding spectral domain optical coherence tomography (SD-OCT) changes (E) include an indistinct external limiting membrane (ELM), ellipsoid and interdigitation zones adjacent to the chorioretinal lesions and a hyperreflective Henle’s layer, which seems to correspond to the hypofluorescence on ICGA (red arrows). Corresponding to the hyperreflectivity in the Henle’s layer, the hyperreflectivity of the ELM, ellipsoid and interdigitation zones is further attenuated (red arrows). There is also hyporeflectivity on the IR imaging visible (yellow arrows) corresponding to the hypofluorescence on ICGA and the hyperautofluorescence with near infrared FAF. The visual field (F) defect corresponds to the area of hyperautofluorescence. Two months later: The hyperautofluorescence has decreased in intensity but the visual field defect (G) as well as the central scotoma (H) has increased in size. Five months later: The hypofluorescence on ICGA has faded (I) but the absolute scotoma has continuously grown in size (H)
Discussion
These 8 patients diagnosed with MFC/PIC presented with an acute/subacute onset of decreased visual acuity and scotomata with vision loss as severe as CF- and HM+. This decrease in visual acuity and visual field loss was secondary to central photoreceptor attenuation. These changes involved retina adjacent to the clinically visible lesions as well as other locations. As far as evaluable (limited to the extension of the SD-OCT scans) the photoreceptor irregularity corresponded to hyperautofluorescence on FAF and scotomata on VF testing. In addition the areas of photoreceptor attenuation corresponded to hyporeflectivity on IR imaging in 3 cases and ICGA hypofluorescence in 1 case. In contrast to previous reports, which described a transient decrease in VA with concomitant temporary photoreceptor attenuation in MFC/PIC followed by complete recovery, 3,9–11 we demonstrate that severe, persistent visual function loss can occur. The spectrum of the clinical course in our patients ranged from total visual recovery and concomitant restoration of the outer retinal layers and decrease in hyperautofluorescence without any treatment to persistent severe visual function loss despite maximal and immediate therapy.
Idiopathic MFC/PIC may be an immune mediated disease occurring in genetically susceptible individuals.2,5 Previous reports have noted MFC/PIC with photoreceptor attenuation beyond the chorioretinal lesions or have described MFC/PIC accompanied by enlarged blind spots.9–11,13–18 Some authors have described this MFC/PIC variant as part of the AZOOR complex.13,14,19 The original Gass case series, which first described AZOOR, included patients with MFC lesions.20 Spaide et al. reported 11 patients with MFC and blind spot enlargement and found that the scotomata correlated with the loss of the photoreceptor outer segments on SD-OCT.16 Chen et al. reported in their series of PIC patients that the scotomata seen in these patients also corresponded to the photoreceptor-loss visible on SD-OCT.10 Kramer et al. found that these areas of photoreceptor-loss in patients with MFC corresponded to hyperautofluorescence, which was also verified by reports from Jung et al. and Freund et al.9,11,12 All these findings are consistent with our observations. The underlying cause of the hyperautofluorescence in areas of photoreceptor attenuation associated with MFC/PIC remains unclear. Hyperautofluorescence may be due to active inflammation in this disease entity. 9,11,12 It may be secondary to increased accumulation of fluorophores within the RPE due to inflammation 21 or caused by a reduction of the optical pigment density due to disruption of the photoreceptors.12,22,23 The cause of the plaque-like hypofluorescence on ICGA, previously reported in MFC remains also uncertain. 9, 17 Choroidal hypo-perfusion, choroidal ischemia or a blockage-effect were assumed to cause this finding.17, 24–26 Hyperreflectivity in the Henle’s layer corresponding to the hypofluorescent area in this case and the fact that increased choroidal thickness is found in areas corresponding to the photoreceptor irregularity in MFC/PIC with secondary photoreceptor loss in the acute inflammatory stage,10 made us believe that a blockage effect rather than choroidal hypo-perfusion is responsible for the hypofluorescence on ICGA found in our patient.
During follow-up the FAF hyper-autofluorescence, the ICGA hypofluorescence as well as the IR hyporeflectivity faded corresponding to the restoration of the ELM, the ellipsoid- and interdigitation zones. This correlates with previous published observations.9,11,17 However, in previous reports the photoreceptor-attenuation and hyperautofluorescence resolved completely under immune modulating therapy and BCVA returned back to normal.3,9,10 In our series, BCVA fully recovered only in 3 out of 8 cases. The remaining patients displayed persistent decreased BCVA and photoreceptor attenuation. A recent report by our group described a variant of MFC with significant outer retinal and or chorioretinal atrophy. This atrophy often progressed over time despite therapy.8 In contrast to the current observations, in these patients foveal sparing was common until late in the disease course and the majority of the patients maintained good VA.8 In our cohort, 3 cases (case 3, 6 and 8) recovered similar to the reports of Kramer et al., Spaide et al. and Chen et al., 3,10,11 two while undergoing systemic corticosteroid and anti-VEGF treatment and one patient recovered without treatment and retained a very small discrete relative paracentral scotoma. Two of our cases (case 5+7) presented with already existing central vision- and corresponding photoreceptor loss and with extinguished or diminished cone and rod response on ERG testing. The remaining 3 cases (case 1, 2+4) displayed persistent decrease in VA despite immediate treatment. We believe that the patients presented here represent a MFC/PIC variant with adjacent photoreceptor-attenuation. MFC/PIC with adjacent photoreceptor attenuation may vary in severity and may lead to chorioretinal atrophic lesions,11 acute persistent central photoreceptor loss and outer retinal atrophy, diffuse peripheral or zonal outer retinal and chorioretinal atrophy, 8 or to transient photoreceptor attenuation with recovery of morphology and function.3,10 Depending on the severity of the process, changes may be self-limiting and/or apparently respond to immune modulating therapy. In some cases, the acute photoreceptor attenuation may be permanent. The exact mechanisms involved in the outer retinal dysfunction and death remain unclear and no obvious factors indicating severity or permanence of photoreceptor loss was identifiable. The only parameter noteworthy may be that patients, who developed new lesions over time (case 1, 2 and 7) showed the worst VA outcome.
Interestingly, 3 cases (case 1, 2 and 4) with persistent decrease in VA showed some anatomical recovery of the outer retinal layers. In these cases the ELM and ellipsoid zone showed some restoration, however the interdigitation zone remained non-distinguishable. This picture may resemble re-integration of the inner segments with persistently damaged cone outer segments, which may explain the permanent visual function loss despite the partial recovery of the 1st and 2nd hyperreflective band. In order to solve the apparent discrepancy between morphology and function, imaging approaches such as adaptive optics scanning laser ophthalmoscopy (AO-SLO), AO-OCT or AO using split detection are warranted, to precisely illustrate the status of the photoreceptors.
The efficacy of treatment for this presentation of MFC/PIC is uncertain: Spaide et al. as well as Chen et al. and Kramer et al. noted an improvement after treatment initiation and therefore stated that immune modulating therapy induced respective recovery.3,9,10 In contrast, Jung et al. reported progression of outer retinal atrophy in the variant of MFC with outer retinal and chorioretinal atrophy in some patients despite systemic immune-modulating therapy.8 Five out of the 8 patients in our cohort were treated, but only two regained their visual function after treatment initiation. Although some studies have reported that immunomodulatory therapy of MFC/PIC can effectively control inflammation, 27 other studies have shown that the inflammation can be difficult to control.28 Although some cases of idiopathic MFC with secondary acute photoreceptor loss can be self-limiting it is worth considering immune modulating treatment, especially in progressive cases that develop new chorioretinal lesions over time. However, this therapy may not result in recovery.
Summary statement.
Idiopathic multifocal choroiditis/punctate inner choroidopathy can present with acute central outer photoreceptor disruption associated with decreased vision and visual field defects. Multimodal imaging highlights the acute damage seen in this disease process.
Acknowledgments
This work was supported in part by an unrestricted grant from Research to Prevent Blindness Inc., New York and by Kevin Hitzeman and Mary Demsey
We want to thank Dr. Maberly and Dr. Balaratnasingam who provided us with the images of one of the patients.
Footnotes
Financial disclosures or proprietary interest: none
References
- 1.Amer R, Lois N. Punctate inner choroidopathy. Surv Ophthalmol. 2011;56:36–53. doi: 10.1016/j.survophthal.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 2.Jampol LM, Becker KG. White spot syndromes of the retina: a hypothesis based on the common genetic hypothesis of autoimmune/inflammatory disease. Am Journal Ophthalmol. 2003;135:376–379. doi: 10.1016/s0002-9394(02)02088-3. [DOI] [PubMed] [Google Scholar]
- 3.Spaide RF, Goldberg N, Freund KB. Redefining multifocal choroiditis and panuveitis and punctate inner choroidopathy through multimodal imaging. Retina. 2013;33:1315–1324. doi: 10.1097/IAE.0b013e318286cc77. [DOI] [PubMed] [Google Scholar]
- 4.Essex RW, Wong J, Jampol LM, et al. Idiopathic multifocal choroiditis: a comment on present and past nomenclature. Retina. 2013;33:1–4. doi: 10.1097/IAE.0b013e3182641860. [DOI] [PubMed] [Google Scholar]
- 5.Atan D, Fraser-Bell S, Plskova J, et al. Punctate inner choroidopathy and multifocal choroiditis with panuveitis share haplotypic associations with IL10 and TNF loci. Invest Ophthalmol Vis Sci. 2011;52:3573–3581. doi: 10.1167/iovs.10-6743. [DOI] [PubMed] [Google Scholar]
- 6.Thorne JE, Wittenberg S, Jabs DA, et al. Multifocal choroiditis with panuveitis incidence of ocular complications and of loss of visual acuity. Ophthalmology. 2006;113:2310–2316. doi: 10.1016/j.ophtha.2006.05.067. [DOI] [PubMed] [Google Scholar]
- 7.Dreyer RF, Gass DJ. Multifocal choroiditis and panuveitis. A syndrome that mimics ocular histoplasmosis. Arch Ophthalmol. 1984;102:1776–1784. doi: 10.1001/archopht.1984.01040031440019. [DOI] [PubMed] [Google Scholar]
- 8.Jung JJ, Khan S, Mrejen S, et al. Idiopathic Multifocal Choroiditis with Outer Retinal or Chorioretinal Atrophy. Retina. 2013 doi: 10.1097/IAE.0000000000000079. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 9.Kramer M, Priel E. Fundus Autofluorescence Imaging in Multifocal Choroiditis: Beyond the Spots. Ocul Immunol Inflamm. 2013 doi: 10.3109/09273948.2013.855797. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Chen SN, Hwang JF. Ocular Coherence Tomographic and Clinical Characteristics in Patients of Punctuate Inner Choroidopathy Associated with Zonal Outer Retinopathy. Ocul Immunol Inflamm. 2013 doi: 10.3109/09273948.2013.844264. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Jung JJ, Mrejen S, Freund KB, Yannuzzi L. IDIOPATHIC MULTIFOCAL CHOROIDITIS WITH PERIPAPILLARY ZONAL INFLAMMATION: A Multimodal Imaging Analysis. Retinal cases and Brief reports. 2014;8:141–144. doi: 10.1097/ICB.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 12.Freund KB, Mrejen S, Jung J, et al. Increased fundus autofluorescence related to outer retinal disruption. JAMA Ophthalmol. 2013;131:1645–1649. doi: 10.1001/jamaophthalmol.2013.5030. [DOI] [PubMed] [Google Scholar]
- 13.Taira K, Nakazawa M, Takano Y, Ota T. Acute zonal occult outer retinopathy in the fellow eye 5 years after presentation of punctate inner choroidopathy. Graefes Arch Clin Exp Ophthalmol. 2006;244:880–2. doi: 10.1007/s00417-005-0172-7. [DOI] [PubMed] [Google Scholar]
- 14.Holz FG, Kim RY, Schwartz SD, et al. Acute zonal occult outer retinopathy (AZOOR) associated with multifocal choroidopathy. Eye. 1994;8:77–83. doi: 10.1038/eye.1994.15. [DOI] [PubMed] [Google Scholar]
- 15.Saito A, Saito W, Furudate N, Ohno S. Indocyanine green angiography in a case of punctate inner choroidopathy associated with acute zonal occult outer retinopathy. Jpn J Ophthalmol. 2007;51:295–300. doi: 10.1007/s10384-007-0451-4. [DOI] [PubMed] [Google Scholar]
- 16.Spaide RF, Koizumi H, Freund KB. Photoreceptor outer segment abnormalities as a cause of blind spot enlargement in acute zonal occult outer retinopathy-complex diseases. Am J Ophthalmol. 2008;146:111–120. doi: 10.1016/j.ajo.2008.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Slakter JS, Giovannini A, Yannuzzi LA, et al. Indocyanine green angiography of multifocal choroiditis. Ophthalmology. 1997;104:1813–1819. doi: 10.1016/s0161-6420(97)30022-0. [DOI] [PubMed] [Google Scholar]
- 18.Volpe NJ, Rizzo JF, 3rd, Lessell S. Acute idiopathic blind spot enlargement syndrome: a review of 27 new cases. Arch Ophthalmol. 2001;119:59–63. [PubMed] [Google Scholar]
- 19.Gass JD, Agarwal A, Scott IU. Acute zonal occult outer retinopathy: a long-term follow-up study. Am J Ophthalmol. 2002;134:329–339. doi: 10.1016/s0002-9394(02)01640-9. [DOI] [PubMed] [Google Scholar]
- 20.Gass JD. Acute zonal occult outer retinopathy. J Clin Neuroophthalmol; Donders Lecture; June 19, 1992; Maastricht, Holland: The Netherlands Ophthalmological Society; 1993. Jun, pp. 79–97. [PubMed] [Google Scholar]
- 21.Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina. 2008;28:385–409. doi: 10.1097/IAE.0b013e318164a907. [DOI] [PubMed] [Google Scholar]
- 22.Chuang EL, Sharp DM, Fitzke FW, et al. Retinal dysfunction in central serous retinopathy. Eye. 1987;1:120–125. doi: 10.1038/eye.1987.18. [DOI] [PubMed] [Google Scholar]
- 23.Burns SA, Eisner AE, Lobes LA., Jr Foveal cone photopigment bleaching in central serous retinopathy. Appl Opt. 1988;27:1045–1049. doi: 10.1364/AO.27.001045. [DOI] [PubMed] [Google Scholar]
- 24.Howe LJ, Woon H, Graham EM, et al. Choroidal hypoperfusion in acute posterior multifocal placoid pigment epitheliopathy. An indocyanine green angiography study. Ophthalmology. 1995;102:790–798. doi: 10.1016/s0161-6420(95)30955-4. [DOI] [PubMed] [Google Scholar]
- 25.Golchet PR, Jampol LM, Wilson D, et al. Persistent placoid maculopathy: a new clinical entity. Ophthalmology. 2007;114:1530–1540. doi: 10.1016/j.ophtha.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 26.Khairallah M, Ben Yahia S. Persistent placoid maculopathy. Ophthalmology. 2008;115:220–221. doi: 10.1016/j.ophtha.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Michel SS, Ekong A, Baltatzis S, Foster CS. Multifocal choroiditis and panuveitis: immunomodulatory therapy. Ophthalmology. 2002;109:378–383. doi: 10.1016/s0161-6420(01)00901-0. [DOI] [PubMed] [Google Scholar]
- 28.Benitez-del-Castillo JM, Martinez-de-la-Casa JM, Pato-Cour E, et al. Long-term treatment of refractory posterior uveitis with anti-TNFalpha (infliximab) Eye. 2005;19:841–845. doi: 10.1038/sj.eye.6701689. [DOI] [PubMed] [Google Scholar]