Abstract
Laboratory animal models play an important role in the study of human diseases. Using appropriate animals is critical not only for basic research but also for the development of therapeutics and diagnostic tools. Rabbits are widely used for the study of human atherosclerosis. Because rabbits have a unique feature of lipoprotein metabolism (like humans but unlike rodents) and are sensitive to a cholesterol diet, rabbit models have not only provided many insights into the pathogenesis and development of human atherosclerosis but also made a great contribution to translational research. In fact, rabbit was the first animal model used for studying human atherosclerosis, more than a century ago. Currently, three types of rabbit model are commonly used for the study of human atherosclerosis and lipid metabolism: (1) cholesterol-fed rabbits, (2) Watanabe heritable hyperlipidemic rabbits, analogous to human familial hypercholesterolemia due to genetic deficiency of LDL receptors, and (3) genetically modified (transgenic and knock-out) rabbits. Despite their importance, compared with the mouse, the most widely used laboratory animal model nowadays, the use of rabbit models is still limited. In this review, we focus on the features of rabbit lipoprotein metabolism and pathology of atherosclerotic lesions that make it the optimal model for human atherosclerotic disease, especially for the translational medicine. For the sake of clarity, the review is not an attempt to be completely inclusive, but instead attempts to summarize substantial information concisely and provide a guideline for experiments using rabbits.
Keywords: Atherosclerosis, experimental animal models, hypercholesterolemia, transgenic rabbits, translational medicine
Introduction
Atherosclerotic diseases such as myocardial infarction and stroke are still major causes of mortality globally. Atherosclerosis develops slowly throughout the stages of human life and many genetic and environmental factors are concertedly involved in the pathogenesis of this disease (Libby, Ridker, & Hansson, 2011). For study of the pathophysiology and also for the development of therapeutic modalities and diagnostic tools, utilization of appropriate experimental animals is essential. In general, the ideal animal models of human atherosclerosis should possess several important characteristics (Fan & Watanabe, 2000; Vesselinovitch, 1988). They should be easy to acquire and maintain at a reasonable cost, easy to handle, and the proper size to allow for all anticipated experimental manipulations. Ideally, the animal should reproduce in a laboratory setting and have a well-defined genetic background. Finally, the animal model should share with humans the most important aspects of lipid metabolism and cardiovascular pathophysiology. Atherosclerotic lesions should develop gradually while the animal consumes a special diet, and features of the lesions (from the fatty streaks to complicated plaques with calcification, ulceration, hemorrhage and thrombosis) should mimic those of human patients with clinical sequelae (such as myocardial infarction, stroke and gangrene). Unfortunately, there is no single animal model that fulfills all of these requirements; therefore, many different animals have been used to illustrate the diverse aspects of atherosclerosis (Moghadasian, 2002). Among those used to date, rabbits are the first and one of the best models for the study of lipoprotein metabolism and atherosclerosis.
History of rabbit atherosclerosis models
The first experiment using rabbits to investigate atherosclerosis should date back to more than a century ago. In 1908, a Russian physician Ignatowski, fed rabbits with a diet enriched in animal proteins (milk, meat and eggs) and observed intimal lesions with large clear cell (now called foam cell) accumulation in the aorta (Ignatowski, 1908). Later, a Russian experimental pathologist, Anitschkow, used a cholesterol diet dissolved in vegetable oil to produce aortic atherosclerosis in rabbits similar to those seen in humans and proposed a causal role of cholesterol in atherosclerosis (Steinberg, 2004). Now, a consensus has been reached in this field that, in both humans and experimental animals, it is dietary cholesterol that leads to the development of atherosclerosis (Steinberg, 2004). These pioneering studies provided the first experimental evidence and basis for the establishment of the “lipid hypothesis” of atherosclerosis (Steinberg, 2004). Since then, the rabbit model has been widely used to elucidate many facets of the pathophysiology of human atherosclerosis along with the development of therapeutics. In this regard, several significant milestones in the history of the study of atherosclerosis should be specially mentioned, which are based on tremendous breakthroughs and insights to understand the molecular and cellular mechanisms of atherosclerosis provided by rabbit models.
In the 1980s, Mahley et al. showed that the major lipoproteins elevated in cholesterol-fed rabbits are hepatically derived remnant lipoproteins, called β-VLDL, and it is these lipoproteins that are atherogenic because they are cholesterol-rich and can induce macrophages to transform into foam cells (Mahley, Innerarity, Brown, Ho, & Goldstein, 1980). Watanabe et al. demonstrated that, in the early-stage lesions of aortic atherosclerosis of cholesterol-fed rabbits, the initial cellular event is the recruitment of intimal foam cells and these foam cells are primarily derived from blood monocytes (T. Watanabe, Hirata, Yoshikawa, Nagafuchi, & Toyoshima, 1985). Later, Hansson et al. found that, in addition to monocytes, T lymphocytes are also present in the lesions of cholesterol-fed rabbits similar to those of humans (Hansson, Seifert, Olsson, & Bondjers, 1991), suggesting that the immune response is involved in the pathogenesis of atherosclerosis. Gimbrone’s, Libby’s and Fogelman’s groups further identified the presence of the vascular cell adhesion molecule (VCAM-1) in aortic endothelial cells of the lesions, which can be up-regulated by atherogenic lipoproteins and enhances the adhesion of monocytes to endothelial cells; they thus provided evidence that endothelial cell dysfunction induced by atherogenic lipoproteins plays an important role in monocyte adherence to endothelium and migration into the intima during atherogenesis (Cybulsky & Gimbrone, 1991; H. Li, Cybulsky, Gimbrone, & Libby, 1993; Territo, Berliner, Almada, Ramirez, & Fogelman, 1989). Steinberg’s group performed a series of studies and demonstrated that oxidization of β-VLDL and LDL actually occurs in the arterial wall of cholesterol-fed rabbits, which constitutes an essential trigger for the mediation of monocyte chemoattractant protein-1 (Yla-Herttuala, et al., 1991) and macrophage colony-stimulating factor expression during lesion progression (Rosenfeld, et al., 1992). It was in the rabbit that the impact of lipoproteins of different sizes on atherosclerosis was first studied. For example, it was noted more than fifty years ago that alloxan-induced type I diabetes surprisingly inhibited cholesterol-induced atherosclerosis in rabbits (Duff & Mc, 1949). This is due to the fact that large-sized triglyceride-rich lipoproteins (>75 nm diameter) that accumulated in the plasma of diabetic rabbits failed to penetrate into the arterial wall (Nordestgaard & Zilversmit, 1988). These pioneering studies using rabbits along with other animal models established the basis for the modern theory of atherosclerosis: plasma cholesterol is a critical atherogenic factor (lipid hypothesis) and inflammatory cells such as macrophages and T lymphocytes (inflammatory hypothesis) are key cellular components for the initiation and progression of atherosclerosis (Moore & Tabas, 2011). Probably, there are no more striking findings obtained from rabbit models than those of the Watanabe heritable hyperlipidemic (WHHL) rabbits, which were developed by Dr. Yoshio Watanabe at Kobe University, in Japan (Y. Watanabe, 1980). WHHL rabbits allowed epoch-making contributions to understand LDL receptor deficiency as a cause of human familial hypercholesterolemia discovered by Goldstein and Brown (Goldstein, Kita, & Brown, 1983).
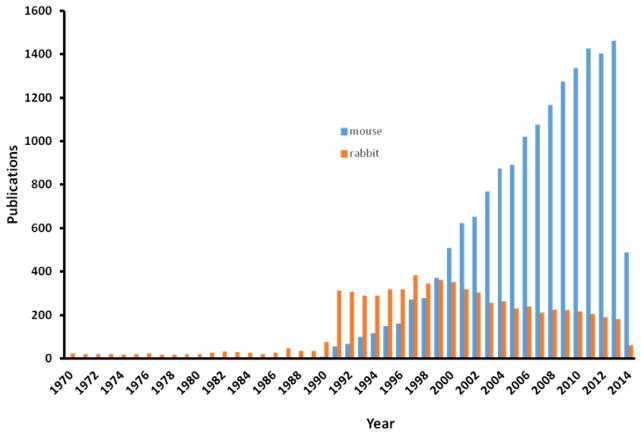
Although the rabbit model has undoubtedly brought about many breakthroughs in the history of atherosclerosis study, there has been a reduced trend of using this model since 2000, possibly due to the development of genetically modified apoE and LDL receptor gene knock-out (KO) mice (Fig. 1). Many researchers are even reluctant to use rabbits for studying atherosclerosis because they are sometimes wrongly considered as “giant rodents”. It is true that the mouse model has been a powerful tool for much research due in large part to the relative ease of genetic manipulation and the relatively short time for the development of atherosclerosis in the setting of apoE or LDL receptor deficiency (Getz & Reardon, 2012), but as an alternative model, rabbit is necessary in translational research of cardiovascular diseases. One of the best historic experiences is no less than the discovery of statin as a potent lipid-lowering drug (Shiomi, Koike, & Ito, 2013). When Dr. Akira Ando, a Japanese scientist who discovered statin, along with Beecham Pharmaceutical Research Laboratories in England discovered compactin (the first generation of statin), they failed to prove that compactin was therapeutically effective in lowering plasma cholesterol in rats and mice, even at high doses (>500 mg/kg), regardless of marked inhibition of hepatic cholesterol synthesis (Brown & Goldstein, 2004). Later, it was realized that rodents (mice, rats and hamsters) differ from humans in many aspects of lipoprotein metabolism, as discussed in detail below (Table 1). For example, approximately two-thirds of the cholesterol pool in humans is derived from in vivo synthesis, whereas a large part of the cholesterol is derived from dietary origin in rodents (Endo, 1980). Wild-type mouse and rat are HDL mammals, while humans as well as rabbit are LDL mammals. The ability of mice and rats to excrete bile acid, which is derived from cholesterol, is much higher than in human and rabbits (Names, 2004). Dr. Endo found that compactin was more effective in rabbits than in chickens, cats, dogs and monkeys, while it was ineffective in mice, rats and hamsters (Endo, 1980). It is not a matter of the size of the model but rather whether to use proper models precisely in order to translate or extrapolate research results into a clinical setting. Therefore, it is necessary to review the features of rabbit lipoprotein metabolism along with their atherosclerosis (either induced by cholesterol-diet or spontaneous atherosclerosis in WHHL rabbits) and find out how we can use them specifically and effectively for translational medicine.
Fig. 1.
Total number of research articles reporting the use of rabbits and mice in research on atherosclerosis from 1970 to 2014 (Source: Web of Science).
Table 1.
Comparison of lipid and lipoprotein metabolism features
| Human | Rabbit | Mouse | |
|---|---|---|---|
| Major plasma lipoproteins | LDL | LDL | HDL |
| CETP | Abundant | Abundant | None |
| Hepatic apoB mRNA editing | No | No | Yes |
| apoB-48 | Chylomicrons | Chylomicrons | VLDLs/LDLs and Chylomicrons |
| apoB-100 | Can be bound to apo(a) | Can be bound to apo(a) | Cannot be bound to apo(a) |
| HDL | Heterogeneous | Heterogeneous | Homogeneous |
| apoAII | Dimer | Absent | Monomer |
| Hepatic LDL receptor activity | Down-regulated | Down-regulated | Usually high |
| VLDL receptor in macrophages | Yes | Yes | No |
| Hepatic lipase | High, liver-bound | Low, liver-bound | High, 70% in circulation |
| Cholesterol pool | Mainly from hepatic synthesis | Mainly from hepatic synthesis | Mainly from dietary origin |
| Excretion of bile acid | Low | Low | High |
| Response to a cholesterol diet | Sensitive | Sensitive | Resistant |
Lipid metabolism features of normal rabbits
All laboratory rabbits (including NZW and JW) are originated from European rabbits (Oryctolagus cuniculus) and belong to the family Leporidae of the order Lagomorpha. The rabbit is an herbivore, and its typical laboratory chow diet contains ~15% protein, 40~50% carbohydrate, 2% vegetable fat and 15~25% fiber. Normally, the cholesterol (phytosterol) content in a regular chow diet is less than 0.01%. On this type of diet, plasma cholesterol levels for both New Zealand White (NZW) and Japanese White (JW) rabbits (the most commonly used laboratory rabbits) are in the range of 30~90 mg/dl at the age of 3–16 months (M. J. Taylor & J. Fan, 1997). The plasma cholesterol concentrations are higher in females than in males, decrease with age in males and remain unchanged in females. In addition, cholesterol levels show greater seasonal variation in females than in males and are lower in pregnant and lactating females than in non-pregnant, non-lactating ones (Roberts, West, Redgrave, & Smith, 1974). Owing to these features (possibly affected by estrogen) of plasma cholesterol levels, males are used more often than females for atherosclerosis study. On a standard chow diet, NZW and JW rabbits (up to five years old in our laboratory) do not develop spontaneous atherosclerosis because of their low cholesterol levels.
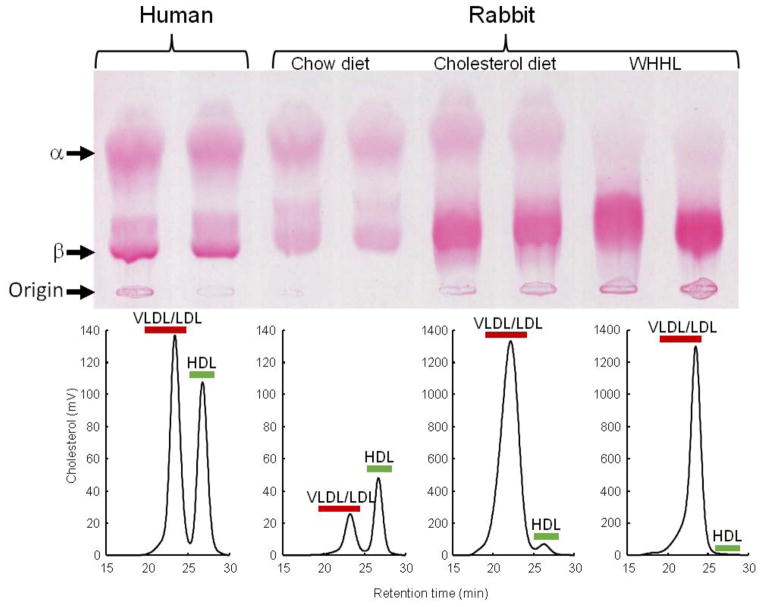
As described below, many features of lipid metabolism of rabbits make them particularly suitable for the study of human lipoprotein metabolism and atherosclerosis (Table 1). Being aware of these features is important for researchers who decide to choose rabbits rather than other models for any specific experiments. Unlike mice and rats in which HDL is the predominant plasma lipoprotein, ~40% of plasma cholesterol in chow-fed rabbits (depending on their age) and >90% in cholesterol-fed and WHHL rabbits is contained in apoB-containing particles, VLDL and LDL (Fig. 2). Rabbits have abundant plasma cholesteryl ester transfer protein (CETP) activity, an important regulator of cholesterol metabolism, whereas mice as well as rats do not have CETP in the plasma (Tall, 1986). As in humans, rabbit do not have hepatic apoB mRNA editing activity so that rabbit apoB-48 is only present in intestinally derived chylomicrons but not in hepatically derived VLDLs and LDLs. In the mouse, apoB-48 is also produced in the liver, so mouse VLDLs and LDLs contain apoB-48 in addition to apoB-100. Although the physiological significance of differences among species in terms of apoB-48-containing VLDLs and LDLs in rodents is not known, it has been reported that apoB-48-containing particles are catabolized faster than apoB-100-containing particles (X. Li, Catalina, Grundy, & Patel, 1996), which may help explain in part why most wild-type mice are resistant to an atherogenic diet. In human plasma, there is a specific LDL-like lipoprotein called lipoprotein (a) [Lp(a)], which is formed through a disulfate bond between apoB-100 and apo(a). Although Lp(a) is normally not present in either rabbit or mouse plasma, transgenic studies showed that rabbit apoB-100 but not mouse apoB-100 (Chiesa, et al., 1992) can be bound to human apo(a) to form Lp(a) particles, which enhance the development of atherosclerosis (Fan, Araki, et al., 1999; Fan, Shimoyamada, et al., 2001). In addition, hepatic LDL receptors in both humans and rabbits are highly down-regulated according to the level of cholesterol uptake in the liver. Furthermore, VLDL receptors, which are involved in the foam cell formation, are highly expressed in macrophages of rabbits and humans but not in mice (Takahashi, et al., 2011). Owing to these similarities between rabbits and humans, rabbits have been the first model for testing many hypolipidemic drugs, such as statins (Aikawa, et al., 1999; Shiomi, et al., 2013), fibrates (Corti, et al., 2007), and probucol (Daugherty, Zweifel, & Schonfeld, 1989; Kita, et al., 1987). Anti-atherogenic effects of infusion HDLs were also first proved in cholesterol-fed rabbits (Badimon, Badimon, & Fuster, 1990; Badimon, Badimon, Galvez, Dische, & Fuster, 1989).
Fig. 2.
Lipoprotein profiles of wild-type rabbits on a normal chow diet and cholesterol-fed, as well as WHHL rabbits, compared with human. Agarose gel electrophoresis of plasma lipoproteins (upper panel) and FPLC analysis of lipoproteins (lower panel).
However, there are some distinct differences between rabbits and humans in terms of lipoprotein metabolism. Rabbit plasma does not contain apoAII (Koike, Kitajima, Yu, Li, et al., 2009), an important protein component of HDL in humans; therefore, their HDL contains only apoAI. However, we recently found that there is an apoAII analogous gene in the rabbit genome, although it is still unclear whether it is truly functional or a pseudogene (Zhang, J., unpublished data). Furthermore, rabbit hepatic lipase activity is naturally low, with about 1/10 as much activity as that of the rat due to the low expression of hepatic lipase mRNA (Warren, Ebert, Mitchell, & Barter, 1991). It is not yet known whether these differences are responsible for the high susceptibility of rabbits to a cholesterol diet and the rapid development of atherosclerosis in this model. Nevertheless, these differences present in rabbits also provide a unique chance to investigate these protein functions using transgenic methods since transgenic rabbits expressing either human apoAII gene (Koike, Kitajima, Yu, Li, et al., 2009) or hepatic lipase (Fan, et al., 1994b) have been developed, as described below.
Cholesterol-fed rabbits
As mentioned above, rabbits are sensitive to dietary cholesterol and rapidly develop severe hypercholesterolemia leading to prominent aortic atherosclerosis. Therefore, cholesterol-fed rabbits are widely used for atherosclerosis studies. When rabbits are fed a chow diet containing up to 2% cholesterol, they show rapid elevation of plasma cholesterol, which can exceed 2,000 mg/dl. This response can be further enhanced by adding extra fat in the diet, with saturated fats increasing both plasma cholesterol and the extent of aortic lesions (Kritchevsky, 1970). Cholesterol diet feeding leads to increased plasma levels of cholesteryl ester-rich, β-migrating very low-density lipoproteins (β-VLDL) derived from the liver and intestine (Fig. 2) due to the relatively efficient absorption of dietary cholesterol, limited hepatic conversion of cholesterol to bile acids and down-regulated hepatic lipoprotein receptors (J. M. Taylor & J. Fan, 1997).
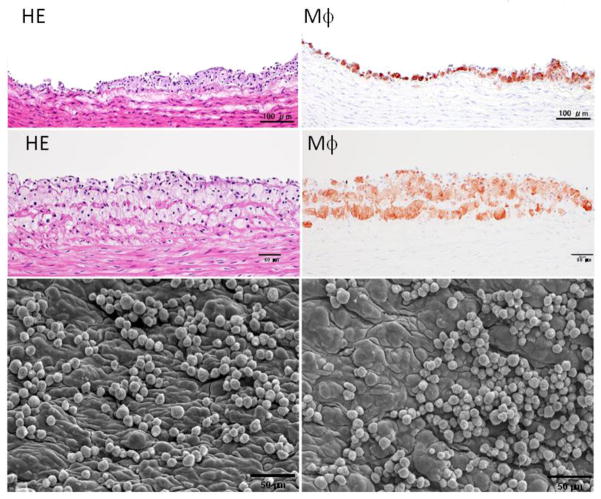
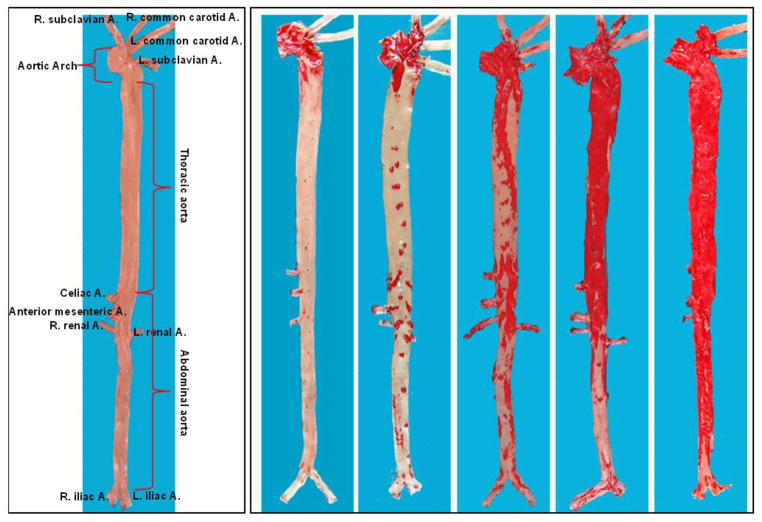
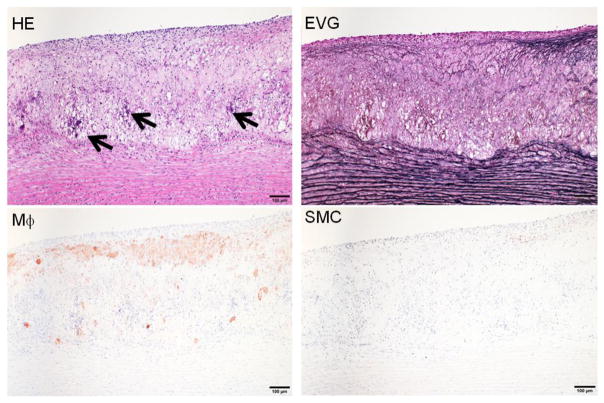
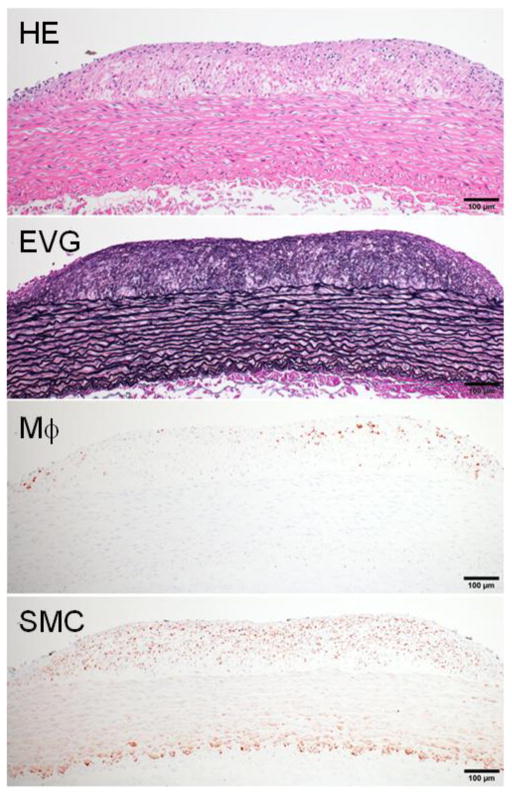
Microscopic features of atherosclerosis exhibited in cholesterol-fed rabbits include monocyte adhesion to intimal endothelial cells and migration of monocytes into the subintima of the aorta, which can be observed under microscopy as early as several weeks after cholesterol diet feeding (Fig. 3). Aortic lesions can be grossly visualized by Sudan IV staining after cholesterol diet feeding for about 12 weeks. The lesions (sudanophilic area) start from the aortic arch and extend to the thoracic (around the intercostal ostia) and finally the abdominal aorta (less frequent) (Fig. 4). In contrast to the early-stage lesions in which only a small number of macrophages are present in the intima (Fig. 3), there are diverse types of lesions in this stage. One of the major lesions is the fatty streaks as seen in human atherosclerosis, which are composed of macrophage-derived foam cells intermingled with smooth muscle cells and extracellular matrix (Figs. 5 and 6). It is not unusual to observe those so-called advanced lesions, depending on the diet and the length of cholesterol feeding. Such advanced lesions contain deposition of calcium or calcification (see below on WHHL rabbits). There are some fibrotic lesions that are almost completely composed of smooth muscle cells and extracellular matrix with few macrophages, but this kind of lesion is rare in cholesterol-fed rabbits (Fig. 7). It should be pointed out, however, that all these lesions can be observed in the same rabbit, while some lesions can be predominant depending on the cholesterol levels in plasma, duration of atherogenic diet feeding and location in the aorta.
Fig. 3.
Early pathological changes of aortic surface of cholesterol-fed rabbits. Two representative lesions are shown (the top and middle panels) and were subjected to either hematoxylin and eosin (HE)staining (left) or immunohistochemical staining with RAM11 antibody against rabbit macrophage (Mϕ) (right). Aortic lesions are also visible under a scanning electron microscope (lower panels). Many monocytes either singly or in clusters adhere to the endothelial cells of the aorta.
Fig. 4.
Gross lesions of aortic atherosclerosis in cholesterol-fed rabbits. The normal aorta is cut open and shown as a reference (left) to illustrate rabbit aortic tree anatomy. Five aortas stained by Sudan IV show different degrees of aortic lesions (red areas stained with Sudan IV).
Fig. 5.
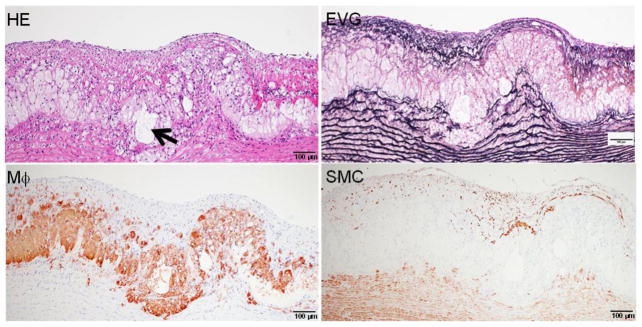
Representative fatty streaks of aortic atherosclerosis in cholesterol-fed rabbits. The lesions are composed of accumulated macrophage-derived foam cells in the center and smooth muscle cells on the top. Arrowhead indicates the necrotic core. The specimen is stained with HE and elastic van Gieson (EVG) or immunohistochemically stained with RAM11 antibody against rabbit macrophage (Mϕ) and HHF35 antibody for smooth muscle cells (SMC).
Fig. 6.
Advanced lesions of aortic atherosclerosis in cholesterol-fed rabbits. The lesions contain a small number of macrophages and calcium deposition can be seen, as indicated by arrowheads.
Fig. 7.
“Fibrotic lesions” of aortic atherosclerosis in cholesterol-fed rabbits. This lesion is less frequent and characterized by fibrosis and smooth muscle cell accumulation with fewer macrophages.
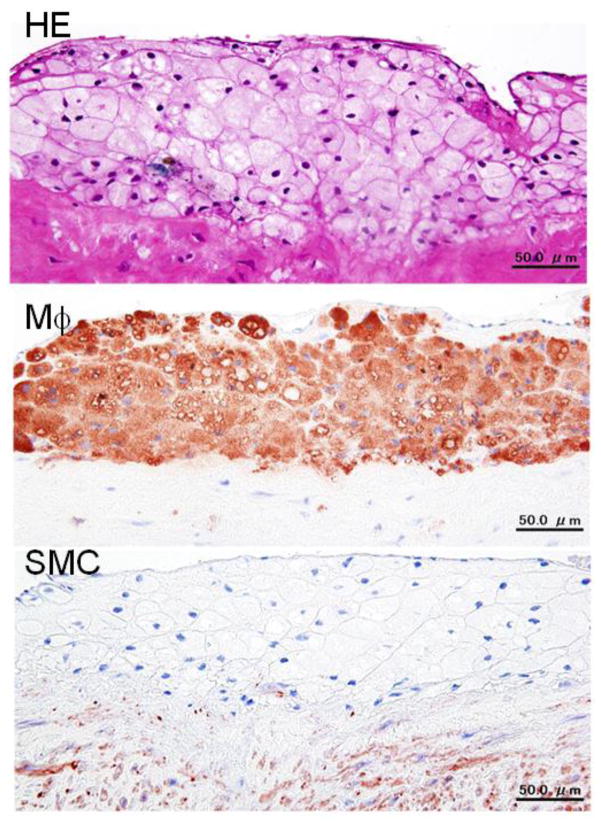
Common atherogenic diets consist of 0.3 to 2% cholesterol and 4 to 8% fat by weight. However, on a diet containing more than 1% cholesterol for a long period (more than a month), rabbits suffer from extraordinarily “high” hypercholesterolemia and show massive lipid accumulation in many organs, in addition to the aorta. In such a case, cholesterol-fed rabbits are often criticized as “not physiological” or “a poor model for human atherosclerosis” because “cholesterol concentration is too high in the plasma” and “too unusual foamy lesions in the aorta”. Such high plasma cholesterol (exceeding 2000 mg/dl or 51.8 mmol/L) is never seen in human hypercholesterolemic patients. Moreover, the atherosclerotic lesions in rabbits fed cholesterol diets consist almost completely of foam cells (Fig. 8), which are seldom seen in humans. Therefore, it is generally recommended that feeding rabbits a 0.3~0.5% cholesterol diet results in reasonable elevation (compatible to human familial hypercholesterolemia) of plasma cholesterol (averaging 1,000 mg/dl or 25.9 mmol/L) without affecting the animal’s general health. There is an important relationship between dietary cholesterol and fat in the production of atherosclerosis in rabbits (Bocan, et al., 1993). Feeding on a cholesterol diet without additional fat usually results in the development of more severe atherosclerosis than in rabbits fed on the diet that contains both cholesterol and fat; presumably, failure to add supplementary dietary fat leads to mobilization of endogenous fat stores, which are more saturated than common dietary fats. Furthermore, saturated fat in the diet is more atherogenic than unsaturated fat. Currently, we recommend using a diet supplemented with 0.3~0.5% cholesterol and 3% soybean or corn oil by weight for 16 weeks fed either ad libitum or restricted for most rabbit experiments. The average cholesterol levels of these rabbits rise to ~800 mg/dl after 4 weeks (Fan, Shimoyamada, et al., 2001). Although feeding on a cholesterol diet can lead to the formation of fatty streaks and advanced lesions in the aorta of rabbits, plaque ruptures or aortic aneurysms have not been observed, suggesting that either the cholesterol feeding period is insufficient or other additional factors may be required for inducing these complications in rabbits.
Fig. 8.
A typical foam cell-rich lesion of aortic atherosclerosis in cholesterol-fed rabbits with extremely high hypercholesterolemia. The lesions are almost completely composed of foam cells, which can be stained with RAM11 antibody.
There are clear differences in arterial susceptibility to atherosclerosis among humans, rabbits and mice, as shown in Table 2. In humans, abdominal aorta is the first and major location of atherosclerosis, whereas in cholesterol-fed rabbits as well as WHHL rabbits, aortic lesions appear first in the aortic arch and then in the thoracic aorta (usually starting from the intercostal artery orifice) (Fig. 4). Abdominal aortic lesions are last seen in rabbits when the whole aortic lesions are severe. In addition to aortic lesions, coronary atherosclerosis is also observed in cholesterol-fed rabbits, but is usually restricted to the left coronary arterial trunks (Fig. 9) (Hirata & Watanabe, 1988). It is almost impossible to induce cerebral atherosclerosis in rabbits by feeding on a cholesterol diet alone. However, if hypertension is present simultaneously, cerebral atherosclerosis can be produced (Kato, Tokunaga, Watanabe, & Sunaga, 1991).
Table 2.
Features of atherosclerotic lesion sites in human, rabbit and mouse
| Human | Rabbit | Mouse | |
|---|---|---|---|
| Aortic lesions | Abdominal aorta | Aortic arch and thoracic aorta | Aortic root and aortic arch |
| Coronary lesions | Yes | Yes | Rare |
| Cerebral lesions | Yes | Yes* | ND |
With hypertension. ND: not done.
Fig. 9.
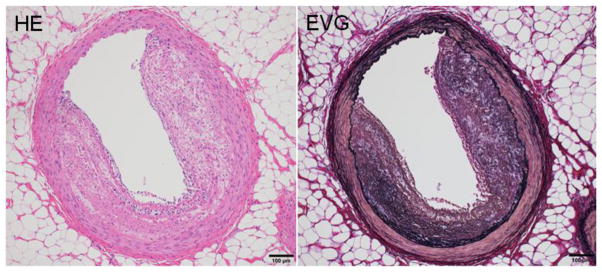
Typical coronary atherosclerosis in cholesterol-fed rabbits. The lesions are mainly composed of smooth muscle cells and occupy about 50% of the lumen.
Watanabe heritable hyperlipidemic (WHHL) rabbits
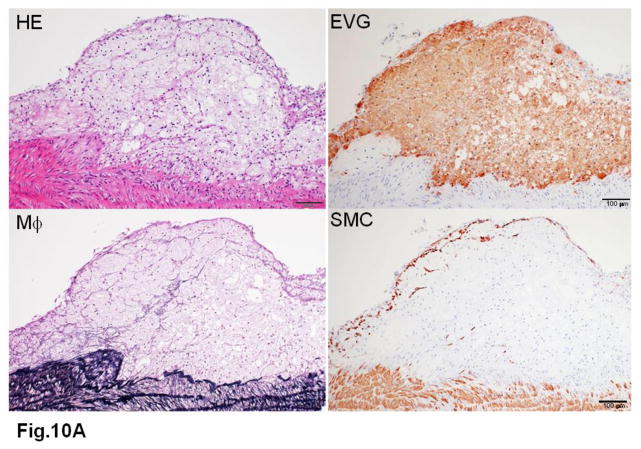
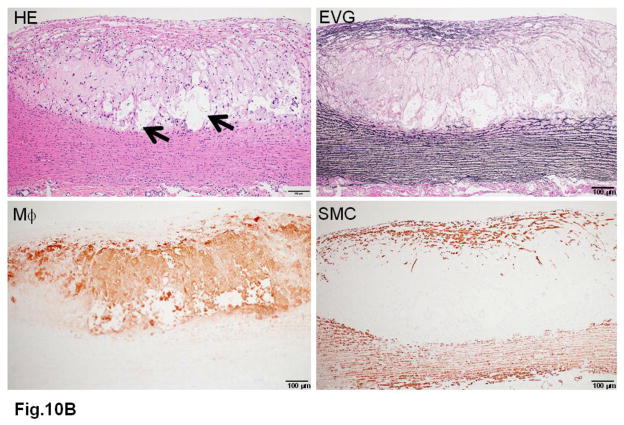
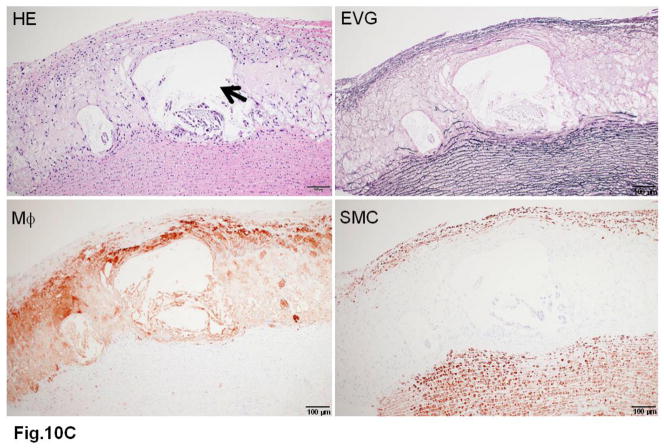
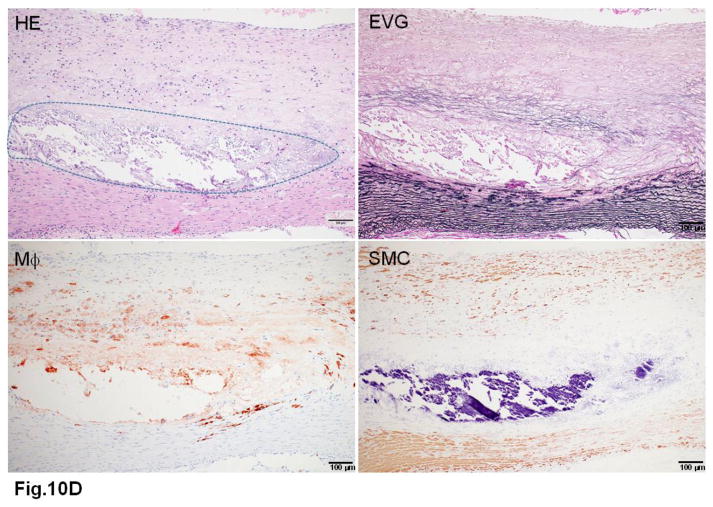
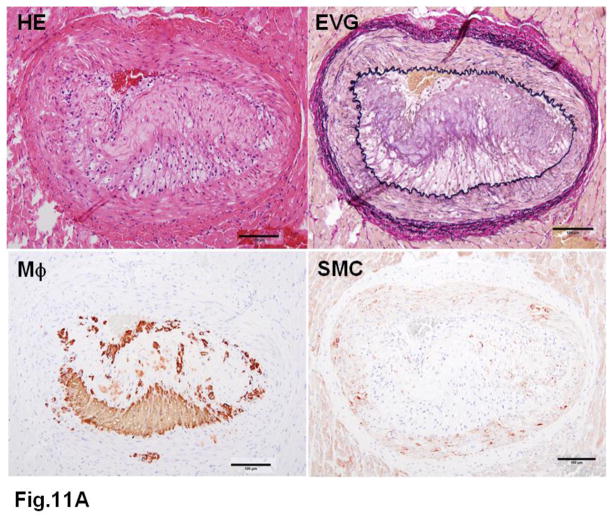
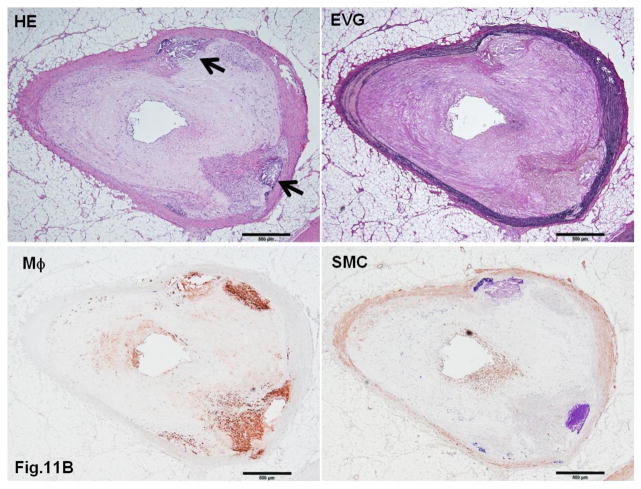
WHHL rabbits as an excellent animal model of human familial hypercholesterolemia were established by Dr. Yoshio Watanabe at Kobe University, Japan, in 1980 (Kobayashi, Ito, & Shiomi, 2011; Shiomi & Ito, 2009; Y. Watanabe, 1980). WHHL rabbits are genetically deficient in LDL receptor functions due to a spontaneously arising deletion in exon 4 of the LDL receptor gene that encodes a 4-amino-acid deletion in the cysteine-rich ligand-binding domain (Yamamoto, Bishop, Brown, Goldstein, & Russell, 1986). Homozygous WHHL rabbits on a chow diet exhibit marked elevations of plasma cholesterol and triglycerides from birth and suffer from atherosclerosis and tendon xanthoma, both of which exhibit remarkable pathological resemblance to those observed in human familial hypercholesterolemia. Heterozygous WHHL rabbits on a chow diet, however, do not show apparent hyperlipidemia. As shown in Fig. 10, mature WHHL rabbits develop diverse aortic atherosclerosis ranging from early fatty streaks to advanced lesions. Importantly, WHHL rabbits also develop coronary atherosclerosis and spontaneous myocardial infarction (Fig. 11), which is rarely observed in other animal models (Shiomi & Fan, 2008). Ten years ago, Dr. Masashi Shiomi demonstrated that some WHHL rabbits (designated as WHHL-MI) show higher incidences of coronary atherosclerosis and myocardial infarction at old age (Shiomi & Fan, 2008; Shiomi, Ito, Yamada, Kawashima, & Fan, 2003). Advantages of using WHHL rabbits over cholesterol-fed rabbits are as follows: (1) the lipoprotein profiles of WHHL rabbits are characterized by high LDLs and extremely low HDLs, whereas the major lipoproteins in cholesterol-fed rabbits are those of β-VLDLs but triglycerides and HDL levels are usually unchanged (Fig. 2); (2) hypercholesterolemia is consistently present in all homozygous WHHL rabbits on a chow diet, so there is less variation in the plasma cholesterol than in cholesterol-fed rabbits; (3) WHHL rabbits > 9 months old develop more advanced aortic atherosclerotic lesions, such as calcification and lipid core formation, which are more similar to those observed in humans. The advanced lesions consist of a typical lipid or necrotic core covered by a fibrotic cap (smooth muscle cells and extracellular matrix), a typical atherosclerotic plaque similar to human atherosclerosis (Fig. 10C–D); (4) coronary atherosclerosis and myocardial infarction can be observed, which may be more relevant to human situations (Fig. 11). WHHL rabbits are especially useful for the development of lipid-lowering drugs and image diagnostic tools, as reviewed previously (Kobayashi, et al., 2011). WHHL rabbits are commercially available from Kitayama Labes Co., Japan. On the basis of collaborative research, Dr. Shiomi at Kobe University provides WHHL rabbits for academic researchers.
Fig. 10. Different features of aortic atherosclerotic lesions in WHHL rabbits.
A. The foam cell-rich lesion is composed of macrophages and covered by a few smooth muscle cells on the top.
B. A fibrous plaque contains a lipid core (arrowheads) in the center.
C. Another fibrous plaque contains a large necrotic core (arrowheads) in the center, which is covered by a thin fibrous cap.
D. Advanced lesions show marked calcification defined by dashed lines.
Fig. 11. Typical coronary atherosclerosis in WHHL rabbits.
A. A typical fatty streak with many macrophages leads to stenosis.
B. An advanced lesion with calcification (arrowheads) shows remarkable stenosis.
Transgenic rabbits
In addition to cholesterol-fed rabbits and WHHL rabbits, transgenic (Tg) rabbits have also been used for the study of human cardiovascular disease during the last two decades. The technology for producing Tg rabbits was simultaneously developed by German (Brem, et al., 1985) and US (Hammer, et al., 1985) groups in 1985, but the actual use of Tg rabbits as an experimental tool in the field of cardiovascular diseases was not realized until 1994 when the first Tg rabbit expressing human hepatic lipase was generated by John Taylor’s laboratory at the Gladstone Institute of Cardiovascular Disease in San Francisco (Fan, et al., 1994b). Later, they produced Tg rabbits expressing human apoB-100 (Fan, et al., 1995a), apoE (Fan, et al., 1998; Huang, Schwendner, Rall, Sanan, & Mahley, 1997) and apoB mRNA editing protein (Yamanaka, et al., 1995b). To date, 16 genes have been introduced into Tg rabbits and have provided considerable insight into the molecular mechanisms associated with their functions in lipoprotein metabolism and atherosclerosis (Peng, 2012). The transgenes expressed in Tg rabbits can generally be classified into three categories: (1) those proteins that are directly associated with lipoproteins such as apo(a) (Fan, Araki, et al., 1999; Rouy, et al., 1998), apoAI (Duverger, Kruth, et al., 1996), apoAII (Koike, Kitajima, Yu, Li, et al., 2009; Y. Wang, M. Niimi, et al., 2013), apoB-100 (Fan, et al., 1995a), apoCIII (Ding, et al., 2011) and apoE (Fan, et al., 1998; Huang, et al., 1997); (2) those enzymes that are involved in the lipid metabolism [hepatic lipase (Fan, et al., 1994b), lipoprotein lipase (Fan, Unoki, et al., 2001), phospholipid transfer protein (PLTP) (Masson, et al., 2011), apoB-100 mRNA editing enzyme catalytic polypeptide protein (Yamanaka, et al., 1995b), lecithin:cholesterol acyltransferase (LCAT)] (J. M. Hoeg, et al., 1996); and (3) those proteins that may participate in the pathogenesis of atherosclerosis (matrix metalloproteinase-12 (Liang, et al., 2006), 15-lipoxygenase (Shen, et al., 1996), C-reactive protein (Koike, Kitajima, Yu, Nishijima, et al., 2009) and vascular endothelial growth factor (Kitajima, et al., 2005) (Table 3). Tg rabbits have also been used for the study of human heart diseases, such as LQT syndrome (Brunner, et al., 2008), hypertrophic cardiomyopathy (Marian, et al., 1999) and tachycardia-induced cardiomyopathy (Suzuki, et al., 2009) because, compared with rodents, the rabbit heart is structurally and functionally more similar to that of humans (Table 4) (King, et al., 2010; Pogwizd & Bers, 2008; Tanaka, et al., 2008).
Table 3.
Transgenic rabbits for the study of human cardiovascular diseases
| Genes | Expression cells | Major phenotypes | References |
|---|---|---|---|
|
Apolipoproteins:
| |||
| Apo(a) | Liver | Atherogenic | (Fan, Shimoyamada, et al., 2001; Ichikawa, et al., 2002; Kitajima, et al., 2007; Rouy, et al., 1998; Sun, et al., 2002) |
| ApoA-I | Liver | Athero-protective | (Duverger, Kruth, et al., 1996; Duverger, Viglietta, et al., 1996) |
| ApoA-II | Liver | Athero-protective | (Koike, Kitajima, Yu, Li, et al., 2009; Y. Wang, M. Niimi, et al., 2013) |
| ApoB-100 | Liver | LDL↑, HDL↓ | (Fan, et al., 1995b) |
| ApoCIII | Liver | VLDL↑ | (Ding, et al., 2011) |
| ApoE2 | Liver | Atherogenic | (Huang, et al., 1997) |
| ApoE3 | Liver | Atherogenic | (Fan, et al., 1998; Huang, et al., 1999) |
| Enzymes: | |||
| Hepatic lipase | Liver | Athero-protective | (Fan, et al., 1994a) |
| apoB mRNA editing protein | Liver | LDL↓ | (Yamanaka, et al., 1995a) |
| LCAT | Liver | Athero-protective | (J.M. Hoeg, et al., 1998; J. M. Hoeg, et al., 1996) |
| Lipoprotein lipase | Universal | Athero-protective | (Fan, Unoki, et al., 2001) |
| PLTP | Universal | Atherogenic | (Masson, et al., 2011) |
| Vascular factors: | |||
| Lipoprotein lipase | Macrophage | Atherogenic | (Ichikawa, et al., 2005) |
| MMP-12 | Macrophage | Atherogenic | (Liang, et al., 2006; Yamada, et al., 2008) |
| C-reactive protein | Liver | Thrombogenic | (Kondo, et al., 2009; Matsuda, et al., 2011) |
| 15-lypooxygenase | Macrophage | Athero-protective hemangiomas and impaired glomerular functions | (Shen, et al., 1996) |
| VEGF | Liver | (Kitajima, et al., 2005; Liu, et al., 2007) | |
ND: not done. LCAT, lecithin:cholesterol acyltransferase; PLTP, phospholipid transfer protein; MMP, matrix metalloproteinase; VEGF, vascular endothelial cell growth factor.
Table 4.
Comparison of rabbit and mouse heart characteristics
| Human | Rabbit | Mouse | References | |
|---|---|---|---|---|
| Heart beats (times/min) | 75 (60~100) | 205 (123~304) | 625 (470~780) | (James, et al., 2002; King, et al., 2010; Peng, 2012; Pogwizd & Bers, 2008; Tanaka, et al., 2008) |
| Major sarcomeric protein | β-MHC | β-MHC | α-MHC | |
| Ion channels | Ikr and IKs | Ikr and IKs | Ito and IK, slow | |
| Action potential duration | Long | Long | Extremely short |
The basic method for the generation of Tg rabbits is still pronuclear microinjection (Duranthon, et al., 2012) and its principle and practical protocols were extensively reviewed and discussed in previous reviews (Fan, Challah, & Watanabe, 1999; Fan & Watanabe, 2003). Because the efficiency of Tg rabbits produced by pronuclear microinjection of DNA is rather low (~10%), other methods have been attempted and developed. One promising approach is transposon-mediated transgenesis (called Sleeping Beauty transposon system) (Mates, et al., 2009) by which high-efficiency of Tg rabbits was achieved. Recently, the details of transposon transgenesis methods have been reported (Bosze, et al., 2012; Ivics, et al., 2014).
KO rabbits
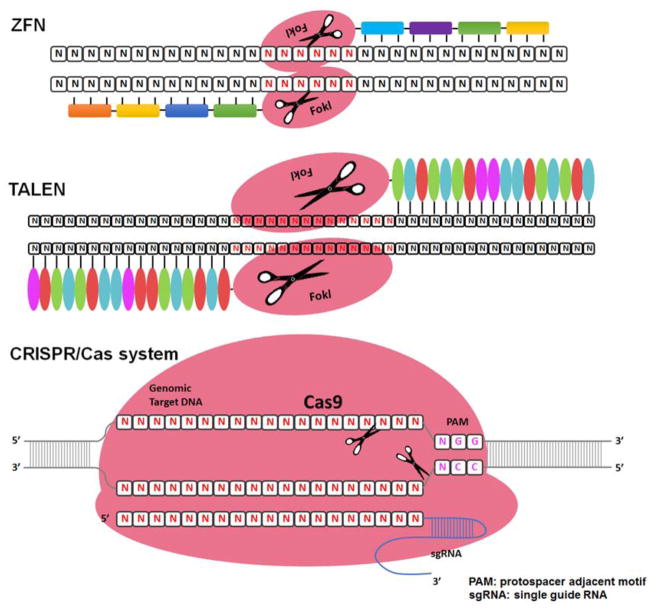
Owing to the lack of embryonic stem (ES) cells and genome information in rabbits, for a long time, it has been impossible to generate knock out (KO) rabbits using homologous recombination-based genomic manipulation in ES cells as in mice. Lack of KO rabbits also constitutes another barrier for researchers to use gene deficiency rabbits for human disease study. We attempted to adopt somatic cell nuclear transfer for gene targeting in rabbits since the first cloned rabbit was reported 12 years ago (Chesne, et al., 2002). However, after tremendous efforts, we realized that the generation of KO rabbits by somatic cell nuclear transfer is still far away from being achieved. It is impractical to use this nuclear transfer technique as a research tool because of the extremely low efficiency of gene transfer into somatic cells and the difficulty in generating cloned rabbits. However, during the past five years, there have been breakthroughs that have made it possible to generate KO rabbits. In 2009, a draft of the rabbit genome sequence (7x coverage) was initially completed by the Broad Institute (http://www.broadinstitute.org/models/europeanrabbit). We have now launched the rabbit genome sequencing project aiming at completion of genomes of all experimental rabbits along with RNA sequences of those tissues which are directly associated with human atherosclerosis and metabolic diseases. Additionally, emergence of gene-editing technologies, as illustrated in Fig. 12 and Table 5, has made it possible to generate KO rabbits.
Fig. 12.
Schematic illustration of zinc finger, TALE and RNA-guided nucleases for genome editing.
Table 5.
Comparison of ZFNs, TALENs and RGENs
| ZFNs | TALENs | RGENs | |
|---|---|---|---|
| Binding mode | Protein-DNA | Protein–DNA | RNA-DNA |
| Plasmids needed | ZF pools | > 70 RVD plasmids | 2 Plasmids |
| Arms | Left and right arms | Left and right arms | Single arm |
| DNA specificity | Depends on the number of zinc fingers | Depends on the number of RVDs | Fixed length 5′-N(13)-NGG-3′ 5′-GN20GG-3′ 5′-GG-N18-NGG-3′ |
| ENDs | 5′ overhangs | 5′ overhangs | Blunt ends |
| Assembly methods | Complicated (specialized by companies) | Complicated (multistep subcloning or PCR) | Most readily customized (oligo annealing) |
| Mutiplex gene editing | Multiple pairs of ZFN needed | Multiple pairs of TALEN pairs needed | Multiple spacers in one step |
In 2011, using the zinc finger nuclease (ZFN)-mediated genome-editing method, Flisikowska et al. reported the first KO rabbits in which the immunoglobulin M locus was targeted in an attempt to produce humanized antibodies (Flisikowska, et al., 2011). ZFNs are engineered DNA-cleaving enzymes made by fusing a tailor-made DNA-binding domain to the DNA cleavage domain of Fok1, a type II restriction enzyme. ZFNs induce site-specific double-strand breaks in the DNA at user-defined locations, which leads to targeted modification of the genome. Our laboratory successfully made KO rabbits which are deficient in apoCIII (D. Yang, et al., 2013).
In addition to ZFNs described above, other techniques are also emerging and have been successfully used for gene targeting in mammals (Carlson, et al., 2012; Mussolino & Cathomen, 2013). Transcription activator-like (TAL) effector nucleases (TALENs) are artificial restriction enzymes created by fusing an engineered TAL DNA binding domain (which targets a desired sequence) with the DNA cleavage domain of Fok1. TALEN DNA binding domain contains a repeated highly conserved 33–34-amino-acid sequence with the exception of the 12th and 13th amino acids. These two locations are highly variable (repeat variable diresidue, RVD) and show a strong correlation with specific nucleotide recognition (Boch, et al., 2009; Moscou & Bogdanove, 2009). The simple relationship between amino acid sequence and DNA recognition has made it easy to design and relatively practical compared with ZFN technology. Using the TALEN technique, Song et al. recently generated KO rabbits deficient in recombination activating genes (Song, et al., 2013).
More recently, RNA-guided endonucleases (RGENs) have become another novel and programmable genome engineering tool that were developed from the bacterial adaptive immune machinery that serves to identify and destroy foreign DNA. These have been proven to be the most efficient way to establish gene-targeted animal models in many species (Cong, et al., 2013; H. Wang, et al., 2013). In this system, the clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated (Cas) immune response-the protein Cas9 forms a sequence-specific endonuclease when complexed with two RNAs, one of which guides target selection. Likewise, RGENs consist of constant components (Cas9 and tracrRNA) and a target-specific RNA (crRNA). RGENs utilize a short guide RNA (gRNA) to recognize DNA, bind the Cas9 endonuclease and induce site-specific cleavage to achieve RNA-guided genome engineering. To establish a solid and practical method for generation of KO rabbits, we successfully generated apo-CIII KO rabbits using the ZFN (D. Yang, et al., 2013) and then apoE, CD36 receptor, ryanodine receptor 2, cystic fibrosis transmembrane conductance receptor KO rabbits using the Cas9 method (D. S. Yang, et al., 2014). Among these three methods (ZFN, TALENs and Cas9), we found that the Cas9 method is highly efficient and easy to use. For example, the efficient rate (referring to successful gene targeting in the rabbit embryos) is in the range of 10 to 100% (average 50%) within 9 genes we examined (D. S. Yang, et al., 2014). Interestingly, among nine genes targeted by the Cas9 method, we identified four bi-allelic mutations (with alleles of single gene targeted at the same time. In another word, it is possible for one to generate homozygous KO rabbits at one step. Therefore, these approaches are not only highly efficient for gene targeting in rabbits, but also technically much easier and superior to traditional pronuclear microinjection because specific gene targeting can be achieved by microinjection of gene editing reagents into the embryo’s cytoplasm with much higher efficiency (>30%). Therefore, one can predict that, in the next few years, through these novel technologies, considerably more KO rabbits will be generated and used in translational studies of cardiovascular diseases.
Considerations and limitations
Although rabbits are a valuable experimental model in cardiovascular research, they should not be considered as a simple substitute of rodents, as discussed above. Rabbits should only be used for those experiments aimed at answering questions or elucidating mechanisms that cannot be properly addressed in other animal models, such as translational research for the development of lipid-lowering drugs or diagnostic methods. Without these considerations, there may be less advantage of using rabbits than using rodents, which have shorter life spans, shorter gestation periods, lower inter-individual variability, lower cost and a broad availability of reagents as benefits compared with the rabbit. Therefore, rabbits should be used to bridge the gaps between small animal models (mice and rats), which are perhaps the best for the elucidation of gene expression and functions, and larger animal models (pigs and monkeys), which are often required for pre-clinical translational research. Compared with those larger animals, rabbits (medium size) are relatively inexpensive to purchase, house and can be maintained in the setting of laboratories. They are easy to breed and handle and are a well-established model in terms of being recognized by the scientific and regulatory communities. In addition, rabbits are phylogenetically closer to primates than rodents and further offer a more diverse genetic background than inbred and outbred rodent strains, which makes the model a better overall approximation to humans. Despite these advantages, there are some common concerns that should be taken into consideration whenever rabbits are used for the study of cholesterol-induced atherosclerosis.
Most laboratory rabbits are commercially available but they are not inbred (although inbred rabbits are indeed present in some facilities). Therefore, the inter-individual variations (plasma cholesterol levels and lesion size in response to a cholesterol diet) can be problematic. To minimize the variations in rabbit colonies, we recommend using rabbits provided by the same breeders in addition to age-, sex- and strain-matches. Furthermore, rabbits may respond to a cholesterol diet differently and some rabbits do not show marked hypercholesterolemia even on a cholesterol-rich diet. A partially inbred line of cholesterol-resistant rabbits was reported twenty years ago (Overturf, et al., 1989). These cholesterol-resistant rabbits are also called hypo-responders and do not develop hypercholesterolemia and atherosclerosis in response to cholesterol, possibly due to enhanced production and secretion of bile acids mediated by high expression of 7α-hydroxylase (Poorman, Buck, Smith, Overturf, & Loose-Mitchell, 1993). In our experience, this happens in less than 20% of JW rabbits. To avoid these problems, it is sometimes better to perform pre-screening for a week to exclude those non-responder rabbits when using this model for drug development studies. To do so, all rabbits are fed a 0.5% cholesterol diet for a week and their plasma cholesterol levels are measured (Chen, et al., 2013; Niimi, et al., 2013). In this way, one can exclude those hypo-responder rabbits before the experiment.
For breeding Tg and KO rabbits, the best way to reduce these variations is to collect semen from one male rabbit and perform artificial insemination with multiple females so that researchers can obtain all littermates with a similar genetic background in a large number within a short time, which is important to reduce the time and cost of rabbit experiments. From our own experience, the semen collected from one ejaculation of a male JW rabbit is sufficient for conducting artificial insemination into up to 20 females.
Analytical methods to evaluate the aortic atherosclerotic lesions of both cholesterol-fed JW and WHHL rabbits have been described in our previous publications (Koike, Kitajima, Yu, Nishijima, et al., 2009; Koike, et al., 2005; Wang, Zhao, Fan, & Liu, 2013; Zhang, et al., 2010). We recommend performing Sudan IV staining of the whole aortas first (Fig. 4). Sudanophilic area (lesions) can be easily measured using image analysis software. After that, all sections should be routinely stained with hematoxylin and eosin (HE) and elastica van Gieson (EVG) for evaluation of the lesion quality and quantity under a light microscope (S. Li, et al., 2014). For examination of lesion cellular components, RAM11 and HHF35 antibodies are routinely used for detecting and quantifying lesion macrophages and smooth muscle cells. Because of the large size of the rabbit aorta, it is also possible to obtain a piece of aorta and to analyze the gene and protein expression using Western blotting and real-time RT-PCR methods in the same rabbits while the lesions are evaluated (Liang, et al., 2006; Yu, et al., 2008). In this way, one can compare the lesion changes with the gene expression in one animal, which is impossible in mouse models.
Development of coronary atherosclerosis is another important feature of both cholesterol-fed (Besterman, 1970) and WHHL rabbits (Shiomi & Fan, 2008; Shiomi, et al., 2003). Compared with that of aortic atherosclerosis, however, the quantitative analysis of coronary lesions is complicated because the lesions can only be observed microscopically (Liang, et al., 2006). Many factors should be taken into considerations for accurate analysis of coronary atherosclerosis, such as methods of sectioning rabbit heart, numbers of sections to be quantified, skill of the researchers and the purpose of each experiment (e.g. more sections are required if one wants to search for ruptured coronary lesions). In this review, we have proposed a practical protocol for quantifying rabbit coronary atherosclerosis based on our experiments using more than one hundred rabbits (see Supplemental materials and S-Figs. 1–3).
Conclusions
The rabbit is an important model for the study of human atherosclerosis and lipoprotein metabolism. Transgenic and KO rabbits will provide novel means not only for the elucidation of molecular mechanisms but also for translational research. We can expect that these novel models will provide new insights into the understanding of atherosclerosis and expand the power of the rabbit model for translational research in cardiovascular disease. In order to enhance the use of rabbit models in future, it is essential to refine the rabbit genome information and establish an international rabbit bio-resource community (such as Rabbit Bioscience Association in Japan and the Lagomorph Genomics Consortium in Europe) that will make it easy for all researchers to use, produce (Tg and KO rabbits) and share these rabbit models.
Supplementary Material
Acknowledgments
We would like thank Drs. Shiomi M., Niimi M., Li S., Koike T., Yu Y., Liang J., Waqar BA, Ning B., Nishijima K. for their contributions to this project.
Sources of funding
This work was supported in part by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan (71790514, 19390099, 21659078, and 22390068 to JF), the National Institutes of Health (HL105114, HL088391, NS066652, and HL068878 to Y.E.C), and the American Heart Association (National Scientist Development 0835237N to J.Z.).
Abbreviations
- Apo
apolipoprotein
- Cas9
CRISPR-associated (Cas) immune response-the protein
- CETP
cholesteryl ester transfer protein
- EVG
elastica van Gieson
- FPLC
Fast protein liquid chromatography
- HDL
high density lipoporoteins
- HE
hematoxylin eosin
- RGENs
RNA-guided endonucleases
- KO
knock-out
- JW
Japanese White
- LCAT
lecithin:cholesterol acyltransferase
- LDL
low density lipoproteins
- Lp(a)
lipoprotein (a)
- NZW
New Zealand White
- PLTP
phospholipid transfer protein
- RVD
repeat variable diresidue
- TAL
transcription activator-like
- TALEN
transcription activator-like effector nucleases
- Tg
transgenic
- VLDL
very low density lipoproteins
- WHHL
Watanabe heritable hyperlipidemic
- ZFN
zinc finger nuclease
Footnotes
DISCLOSURES
NONE
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aikawa M, Voglic SJ, Sugiyama S, Rabkin E, Taubman MB, Fallon JT, Libby P. Dietary lipid lowering reduces tissue factor expression in rabbit atheroma. Circulation. 1999;100:1215–1222. doi: 10.1161/01.cir.100.11.1215. [DOI] [PubMed] [Google Scholar]
- Badimon JJ, Badimon L, Fuster V. Regression of atherosclerotic lesions by high density lipoprotein plasma fraction in the cholesterol-fed rabbit. J Clin Invest. 1990;85:1234–1241. doi: 10.1172/JCI114558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badimon JJ, Badimon L, Galvez A, Dische R, Fuster V. High density lipoprotein plasma fractions inhibit aortic fatty streaks in cholesterol-fed rabbits. Lab Invest. 1989;60:455–461. [PubMed] [Google Scholar]
- Besterman EM. Experimental coronary atherosclerosis in rabbits. Atherosclerosis. 1970;12:75–83. doi: 10.1016/0021-9150(70)90085-7. [DOI] [PubMed] [Google Scholar]
- Bocan TM, Mueller SB, Mazur MJ, Uhlendorf PD, Brown EQ, Kieft KA. The relationship between the degree of dietary-induced hypercholesterolemia in the rabbit and atherosclerotic lesion formation. Atherosclerosis. 1993;102:9–22. doi: 10.1016/0021-9150(93)90080-e. [DOI] [PubMed] [Google Scholar]
- Boch J, Scholze H, Schornack S, Landgraf A, Hahn S, Kay S, Lahaye T, Nickstadt A, Bonas U. Breaking the code of DNA binding specificity of TAL-type III effectors. Science. 2009;326:1509–1512. doi: 10.1126/science.1178811. [DOI] [PubMed] [Google Scholar]
- Bosze Z, Hiripi L, Hoffmann OI, Gocza E, Kvell K, Mates L, Izsvak Z. Creation and characterization of second generation transgenic rabbit models. Transgenic Research. 2012;21:902–903. [Google Scholar]
- Brem G, Brenig B, Goodman HM, Selden RC, Graf F, Kruff B, Springman K, Hondele J, Meyer J, Winnacker EL, Kraublich H. Production of transgenic mice, rabbits, and pigs by microinjection into pronuclei. Zychthygience. 1985;20:251–252. [Google Scholar]
- Brown MS, Goldstein JL. A tribute to Akira Endo, discoverer of a “Penicillin” for cholesterol. Atheroscler Suppl. 2004;5:13–16. [Google Scholar]
- Brunner M, Peng X, Liu GX, Ren XQ, Ziv O, Choi BR, Mathur R, Hajjiri M, Odening KE, Steinberg E, Folco EJ, Pringa E, Centracchio J, Macharzina RR, Donahay T, Schofield L, Rana N, Kirk M, Mitchell GF, Poppas A, Zehender M, Koren G. Mechanisms of cardiac arrhythmias and sudden death in transgenic rabbits with long QT syndrome. J Clin Invest. 2008;118:2246–2259. doi: 10.1172/JCI33578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson DF, Tan W, Lillico SG, Stverakova D, Proudfoot C, Christian M, Voytas DF, Long CR, Whitelaw CB, Fahrenkrug SC. Efficient TALEN-mediated gene knockout in livestock. Proc Natl Acad Sci U S A. 2012;109:17382–17387. doi: 10.1073/pnas.1211446109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhao S, Huang B, Wang Y, Li Y, Waqar AB, Liu R, Bai L, Fan J, Liu E. Probucol and cilostazol exert a combinatorial anti-atherogenic effect in cholesterol-fed rabbits. Thromb Res. 2013;132:565–571. doi: 10.1016/j.thromres.2013.09.007. [DOI] [PubMed] [Google Scholar]
- Chesne P, Adenot PG, Viglietta C, Baratte M, Boulanger L, Renard JP. Cloned rabbits produced by nuclear transfer from adult somatic cells. Nat Biotechnol. 2002;20:366–369. doi: 10.1038/nbt0402-366. [DOI] [PubMed] [Google Scholar]
- Chiesa G, Hobbs HH, Koschinsky ML, Lawn RM, Maika SD, Hammer RE. Reconstitution of lipoprotein(a) by infusion of human low density lipoprotein into transgenic mice expressing human apolipoprotein(a) J Biol Chem. 1992;267:24369–24374. [PubMed] [Google Scholar]
- Cong L, Ran FA, Cox D, Lin S, Barretto R, Habib N, Hsu PD, Wu X, Jiang W, Marraffini LA, Zhang F. Multiplex genome engineering using CRISPR/Cas systems. Science. 2013;339:819–823. doi: 10.1126/science.1231143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti R, Osende J, Hutter R, Viles-Gonzalez JF, Zafar U, Valdivieso C, Mizsei G, Fallon JT, Fuster V, Badimon JJ. Fenofibrate induces plaque regression in hypercholesterolemic atherosclerotic rabbits: in vivo demonstration by high-resolution MRI. Atherosclerosis. 2007;190:106–113. doi: 10.1016/j.atherosclerosis.2006.02.036. [DOI] [PubMed] [Google Scholar]
- Cybulsky MI, Gimbrone MA., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991;251:788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- Daugherty A, Zweifel BS, Schonfeld G. Probucol attenuates the development of aortic atherosclerosis in cholesterol-fed rabbits. Br J Pharmacol. 1989;98:612–618. doi: 10.1111/j.1476-5381.1989.tb12635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Wang Y, Zhu H, Fan J, Yu L, Liu G, Liu E. Hypertriglyceridemia and delayed clearance of fat load in transgenic rabbits expressing human apolipoprotein CIII. Transgenic Res. 2011;20:867–875. doi: 10.1007/s11248-010-9467-5. [DOI] [PubMed] [Google Scholar]
- Duff GL, Mc MG. The effect of alloxan diabetes on experimental cholesterol atherosclerosis in the rabbit. J Exp Med. 1949;89:611–630. doi: 10.1084/jem.89.6.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duranthon V, Beaujean N, Brunner M, Odening KE, Santos AN, Kacskovics I, Hiripi L, Weinstein EJ, Bosze Z. On the emerging role of rabbit as human disease model and the instrumental role of novel transgenic tools. Transgenic Research. 2012;21:699–713. doi: 10.1007/s11248-012-9599-x. [DOI] [PubMed] [Google Scholar]
- Duverger N, Kruth H, Emmanuel F, Caillaud JM, Viglietta C, Castro G, Tailleux A, Fievet C, Fruchart JC, Houdebine LM, Denefle P. Inhibition of atherosclerosis development in cholesterol-fed human apolipoprotein A-I-transgenic rabbits. Circulation. 1996;94:713–717. doi: 10.1161/01.cir.94.4.713. [DOI] [PubMed] [Google Scholar]
- Duverger N, Viglietta C, Berthou L, Emmanuel F, Tailleux A, Parmentier-Nihoul L, Laine B, Fievet C, Castro G, Fruchart JC, Houbebine LM, Denefie P. Transgenic rabbits expressing human apolipoprotein A-I in the liver. Arterioscl Thromb Vasc Biol. 1996;16:1424–1429. doi: 10.1161/01.atv.16.12.1424. [DOI] [PubMed] [Google Scholar]
- Endo A. Regulation of cholesterol synthesis, as focused on the regulation of HMG-CoA reductase. Seikagaku. 1980;52:1033–1049. [PubMed] [Google Scholar]
- Fan J, Araki M, Wu L, Challah M, Shimoyamada H, Lawn MR, Kakuta H, Shikama H, Watanabe T. Assembly of lipoprotein (a) in transgenic rabbits expressing human apolipoprotein (a) Biochem Biophy Res Commun. 1999;255:639–644. doi: 10.1006/bbrc.1999.0242. [DOI] [PubMed] [Google Scholar]
- Fan J, Challah M, Watanabe T. Transgenic rabbit models for biomedical research: Current status, basic methods and future perspectives. Pathol Int. 1999;49:583–594. doi: 10.1046/j.1440-1827.1999.00923.x. [DOI] [PubMed] [Google Scholar]
- Fan J, Ji ZS, Huang Y, de Silva H, Sanan D, Mahley R, Innerarity T, Taylor J. Increased expression of apolioprotein E in transgenic rabbits results in reduced levels of very low density lipoproteins and an accumulation of low density lipoproteins in plasma. J Clin Invest. 1998;101:2151–2164. doi: 10.1172/JCI1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, McCormick SP, Krauss RM, Taylor S, Quan R, Taylor JM, Young SG. Overexpression of human apolipoprotein B-100 in transgenic rabbits results in increased levels of LDL and decreased levels of HDL. Arterioscl, Thromb Vascu Biol. 1995a;15:1889–1899. doi: 10.1161/01.atv.15.11.1889. [DOI] [PubMed] [Google Scholar]
- Fan J, McCormick SP, Krauss RM, Taylor S, Quan R, Taylor JM, Young SG. Overexpression of human apolipoprotein B-100 in transgenic rabbits results in increased levels of LDL and decreased levels of HDL. Arterioscler Thromb Vasc Biol. 1995b;15:1889–1899. doi: 10.1161/01.atv.15.11.1889. [DOI] [PubMed] [Google Scholar]
- Fan J, Shimoyamada H, Sun H, Marcovina S, Honda K, Watanabe T. Transgenic rabbits expressing human apolipoprotein(a) develop more extensive atherosclerotic lesions in response to a cholesterol-rich diet. Arterioscler Thromb Vasc Biol. 2001;21:88–94. doi: 10.1161/01.atv.21.1.88. [DOI] [PubMed] [Google Scholar]
- Fan J, Unoki H, Kojima N, Sun H, Shimoyamada H, Deng H, Okazaki M, Shikama H, Yamada N, Watanabe T. Overexpression of lipoprotein lipase in transgenic rabbits inhibits diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem. 2001;276:40071–40079. doi: 10.1074/jbc.M105456200. [DOI] [PubMed] [Google Scholar]
- Fan J, Wang J, Bensadoun A, Lauer SJ, Dang Q, Mahley RW, Taylor JM. Overexpression of hepatic lipase in transgenic rabbits leads to a marked reduction of plasma high density lipoproteins and intermediate density lipoproteins. Proc Natl Acad Sci U S A. 1994a;91:8724–8728. doi: 10.1073/pnas.91.18.8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Wang J, Bensadoun A, Lauer SJ, Dang Q, Mahley RW, Taylor JM. Overexpression of hepatic lipase in transgenic rabbits leads to a marked reduction of plasma high density lipoproteins and intermediate density lipoproteins. Proc Natl Acad Sci USA. 1994b;91:8724–8728. doi: 10.1073/pnas.91.18.8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Watanabe T. Cholesterol-fed and transgenic rabbit models for the study of atherosclerosis. J Atheroscler Thromb. 2000;7:26–32. doi: 10.5551/jat1994.7.26. [DOI] [PubMed] [Google Scholar]
- Fan J, Watanabe T. Transgenic rabbits as therapeutic protein bioreactors and human disease models. Pharmacol Ther. 2003;99:261–282. doi: 10.1016/s0163-7258(03)00069-x. [DOI] [PubMed] [Google Scholar]
- Flisikowska T, Thorey IS, Offner S, Ros F, Lifke V, Zeitler B, Rottmann O, Vincent A, Zhang L, Jenkins S, Niersbach H, Kind AJ, Gregory PD, Schnieke AE, Platzer J. Efficient immunoglobulin gene disruption and targeted replacement in rabbit using zinc finger nucleases. PLoS One. 2011;6:e21045. doi: 10.1371/journal.pone.0021045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getz GS, Reardon CA. Animal models of atherosclerosis. Arterioscler Thromb Vasc Biol. 2012;32:1104–1115. doi: 10.1161/ATVBAHA.111.237693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Kita T, Brown MS. Defective lipoprotein receptors and atherosclerosis. Lessons from an animal counterpart of familial hypercholesterolemia. N Engl J Med. 1983;309:288–296. doi: 10.1056/NEJM198308043090507. [DOI] [PubMed] [Google Scholar]
- Hammer RE, Pursel VG, Rexroad CE, Jr, Wall RJ, Bolt DJ, Ebert KM, Palmiter RD, Brinster RL. Production of transgenic rabbits, sheep and pigs by microinjection. Nature. 1985;315:680–683. doi: 10.1038/315680a0. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Seifert PS, Olsson G, Bondjers G. Immunohistochemical detection of macrophages and T lymphocytes in atherosclerotic lesions of cholesterol-fed rabbits. Arterioscler Thromb. 1991;11:745–750. doi: 10.1161/01.atv.11.3.745. [DOI] [PubMed] [Google Scholar]
- Hirata M, Watanabe T. Regression of atherosclerosis in normotensive and hypertensive rabbits. A quantitative analysis of cholesterol-induced aortic and coronary lesions with an image-processing system. Acta Pathol Jpn. 1988;38:559–575. [PubMed] [Google Scholar]
- Hoeg JM, Kauffman RD, Herderick E, Demonsky SJ, Jr, Evans W, Brousseau M. Lecithin:cholesterol acyl transferase requires functional LDL receptors to prevent atherosclerosis. Circulation. 1998;98(Supl):I-464. Abstract. [Google Scholar]
- Hoeg JM, Santamarina-Fojo S, Berard AM, Cornhill JF, Herderick EE, Feldman SH, Haudenschild CC, Vaisman BL, Hoyt RF, Jr, Demosky SJ, Jr, Kauffman RD, Hazel CM, Marcovina SM, Brewer HB., Jr Overexpression of lecithin:cholesterol acyltransferase in transgenic rabbits prevents diet-induced atherosclerosis. Proc Natl Acad Sci USA. 1996;93:11448–11453. doi: 10.1073/pnas.93.21.11448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Ji ZS, Brecht WJ, Rall SC, Jr, Taylor JM, Mahley RW. Overexpression of apolipoprotein E3 in transgenic rabbits causes combined hyperlipidemia by stimulating hepatic VLDL production and impairing VLDL lipolysis. Arterioscler Thromb Vasc Biol. 1999;19:2952–2959. doi: 10.1161/01.atv.19.12.2952. [DOI] [PubMed] [Google Scholar]
- Huang Y, Schwendner SW, Rall SCJ, Sanan DA, Mahley RW. Apolipoprotein E2 transgenic rabbits. J Biol Chem. 1997;272:22685–22694. doi: 10.1074/jbc.272.36.22685. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Liang J, Kitajima S, Koike T, Wang X, Sun H, Morimoto M, Shikama H, Watanabe T, Yamada N, Fan J. Macrophage-derived lipoprotein lipase increases aortic atherosclerosis in cholesterol-fed Tg rabbits. Atherosclerosis. 2005;179:87–95. doi: 10.1016/j.atherosclerosis.2004.10.044. [DOI] [PubMed] [Google Scholar]
- Ichikawa T, Unoki H, Sun H, Shimoyamada H, Marcovina S, Shikama H, Watanabe T, Fan J. Lipoprotein(a) promotes smooth muscle cell proliferation and dedifferentiation in atherosclerotic lesions of human apo(a) transgenic rabbits. Am J Pathol. 2002;160:227–236. doi: 10.1016/S0002-9440(10)64366-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowski AC. Influence of animal food on the organism of rabbits. S Peterb Izviest Imp Voyenno-Med Akad. 1908;16:154–173. [Google Scholar]
- Ivics Z, Hiripi L, Hoffmann OI, Mates L, Yau TY, Bashir S, Zidek V, Landa V, Geurts A, Pravenec M, Rulicke T, Bosze Z, Izsvak Z. Germline transgenesis in rabbits by pronuclear microinjection of Sleeping Beauty transposons. Nat Protoc. 2014;9:794–809. doi: 10.1038/nprot.2014.009. [DOI] [PubMed] [Google Scholar]
- James J, Zhang Y, Wright K, Witt S, Glascock E, Osinska H, Klevitsky R, Martin L, Yager K, Sanbe A, Robbins J. Transgenic Rabbits Expressing Mutant Essential Light Chain do not Develop Hypertrophic Cardiomyopathy. J Mol Cell Cardiol. 2002;34:873–882. doi: 10.1006/jmcc.2002.2025. [DOI] [PubMed] [Google Scholar]
- Kato H, Tokunaga O, Watanabe T, Sunaga T. Experimental cerebral atherosclerosis in the rabbit. Scanning electron microscopic study of the initial lesion site. Pathol Res Pract. 1991;187:797–805. doi: 10.1016/S0344-0338(11)80575-3. [DOI] [PubMed] [Google Scholar]
- King VL, Hatch NW, Chan HW, de Beer MC, de Beer FC, Tannock LR. A murine model of obesity with accelerated atherosclerosis. Obesity (Silver Spring) 2010;18:35–41. doi: 10.1038/oby.2009.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T, Nagano Y, Yokode M, Ishii K, Kume N, Ooshima A, Yoshida H, Kawai C. Probucol prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbit, an animal model for familial hypercholesterolemia. Proc Natl Acad Sci U S A. 1987;84:5928–5931. doi: 10.1073/pnas.84.16.5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima S, Jin Y, Koike T, Yu Y, Liu E, Shiomi M, Marcovina SM, Morimoto M, Watanabe T, Fan J. Lp(a) enhances coronary atherosclerosis in transgenic Watanabe heritable hyperlipidemic rabbits. Atherosclerosis. 2007;193:269–276. doi: 10.1016/j.atherosclerosis.2006.08.056. [DOI] [PubMed] [Google Scholar]
- Kitajima S, Liu E, Morimoto M, Koike T, Yu Y, Watanabe T, Imagawa S, Fan J. Transgenic rabbits with increased VEGF expression develop hemangiomas in the liver: a new model for Kasabach-Merritt syndrome. Lab Invest. 2005;85:1517–1527. doi: 10.1038/labinvest.3700346. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ito T, Shiomi M. Roles of the WHHL rabbit in translational research on hypercholesterolemia and cardiovascular diseases. J Biomed Biotechnol. 2011;2011:406473. doi: 10.1155/2011/406473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike T, Kitajima S, Yu Y, Li Y, Nishijima K, Liu E, Sun H, Waqar AB, Shibata N, Inoue T, Wang Y, Zhang B, Kobayashi J, Morimoto M, Saku K, Watanabe T, Fan J. Expression of human apoAII in transgenic rabbits leads to dyslipidemia: a new model for combined hyperlipidemia. Arterioscler Thromb Vasc Biol. 2009;29:2047–2053. doi: 10.1161/ATVBAHA.109.190264. [DOI] [PubMed] [Google Scholar]
- Koike T, Kitajima S, Yu Y, Nishijima K, Zhang J, Ozaki Y, Morimoto M, Watanabe T, Bhakdi S, Asada Y, Chen YE, Fan J. Human C-reactive protein does not promote atherosclerosis in transgenic rabbits. Circulation. 2009;120:2088–2094. doi: 10.1161/CIRCULATIONAHA.109.872796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike T, Liang J, Wang X, Ichikawa T, Shiomi M, Sun H, Watanabe T, Liu G, Fan J. Enhanced aortic atherosclerosis in transgenic Watanabe heritable hyperlipidemic rabbits expressing lipoprotein lipase. Cardiovasc Res. 2005;65:524–534. doi: 10.1016/j.cardiores.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Kondo M, Sakai T, Komeima K, Kurimoto Y, Ueno S, Nishizawa Y, Usukura J, Fujikado T, Tano Y, Terasaki H. Generation of a transgenic rabbit model of retinal degeneration. Invest Ophthalmol Vis Sci. 2009;50:1371–1377. doi: 10.1167/iovs.08-2863. [DOI] [PubMed] [Google Scholar]
- Kritchevsky D. Role of cholesterol vehicle in experimental atherosclerosis. Am J Clin Nutr. 1970;23:1105–1110. doi: 10.1093/ajcn/23.8.1105. [DOI] [PubMed] [Google Scholar]
- Li H, Cybulsky MI, Gimbrone MA, Jr, Libby P. Inducible expression of vascular cell adhesion molecule-1 by vascular smooth muscle cells in vitro and within rabbit atheroma. Am J Pathol. 1993;143:1551–1559. [PMC free article] [PubMed] [Google Scholar]
- Li S, Liang J, Niimi M, Waqar AB, Kang D, Koike T, Shiomi M, Fan J. Probucol suppresses macrophage infiltration and MMP expression in atherosclerotic plaques of WHHL rabbits. J Atheroscl Thromb. 2014 doi: 10.5551/jat.21600. In press. [DOI] [PubMed] [Google Scholar]
- Li X, Catalina F, Grundy SM, Patel S. Method to measure apolipoprotein B-48 and B-100 secretion rates in an individual mouse: evidence for a very rapid turnover of VLDL and preferential removal of B-48- relative to B-100-containing lipoproteins. J Lipid Res. 1996;37:210–220. [PubMed] [Google Scholar]
- Liang J, Liu E, Yu Y, Kitajima S, Koike T, Jin Y, Morimoto M, Hatakeyama K, Asada Y, Watanabe T, Sasaguri Y, Watanabe S, Fan J. Macrophage metalloelastase accelerates the progression of atherosclerosis in transgenic rabbits. Circulation. 2006;113:1993–2001. doi: 10.1161/CIRCULATIONAHA.105.596031. [DOI] [PubMed] [Google Scholar]
- Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- Liu E, Morimoto M, Kitajima S, Koike T, Yu Y, Shiiki H, Nagata M, Watanabe T, Fan J. Increased expression of vascular endothelial growth factor in kidney leads to progressive impairment of glomerular functions. J Am Soc Nephrol. 2007;18:2094–2104. doi: 10.1681/ASN.2006010075. [DOI] [PubMed] [Google Scholar]
- Mahley RW, Innerarity TL, Brown MS, Ho YK, Goldstein JL. Cholesteryl ester synthesis in macrophages: stimulation by beta-very low density lipoproteins from cholesterol-fed animals of several species. J Lipid Res. 1980;21:970–980. [PubMed] [Google Scholar]
- Marian AJ, Wu Y, Lim DS, McCluggage M, Youker K, Yu QT, Brugada R, DeMayo F, Quinones M, Roberts R. A transgenic rabbit model for human hypertrophic cardiomyopathy. J Clin Invest. 1999;104:1683–1692. doi: 10.1172/JCI7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson D, Deckert V, Gautier T, Klein A, Desrumaux C, Viglietta C, Pais de Barros JP, Le Guern N, Grober J, Labbe J, Menetrier F, Ripoll PJ, Leroux-Coyau M, Jolivet G, Houdebine LM, Lagrost L. Worsening of diet-induced atherosclerosis in a new model of transgenic rabbit expressing the human plasma phospholipid transfer protein. Arterioscler Thromb Vasc Biol. 2011;31:766–774. doi: 10.1161/ATVBAHA.110.215756. [DOI] [PubMed] [Google Scholar]
- Mates L, Chuah MK, Belay E, Jerchow B, Manoj N, Acosta-Sanchez A, Grzela DP, Schmitt A, Becker K, Matrai J, Ma L, Samara-Kuko E, Gysemans C, Pryputniewicz D, Miskey C, Fletcher B, Vanden Driessche T, Ivics Z, Izsvak Z. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat Genet. 2009;41:753–761. doi: 10.1038/ng.343. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Yamashita A, Sato Y, Kitajima S, Koike T, Sugita C, Moriguchi-Goto S, Hatakeyama K, Takahashi M, Koshimoto C, Matsuura Y, Iwakiri T, Chen YE, Fan J, Asada Y. Human C-reactive protein enhances thrombus formation after neointimal balloon injury in transgenic rabbits. J Thromb Haemost. 2011;9:201–208. doi: 10.1111/j.1538-7836.2010.04086.x. [DOI] [PubMed] [Google Scholar]
- Moghadasian MH. Experimental atherosclerosis: a historical overview. Life Sci. 2002;70:855–865. doi: 10.1016/s0024-3205(01)01479-5. [DOI] [PubMed] [Google Scholar]
- Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscou MJ, Bogdanove AJ. A simple cipher governs DNA recognition by TAL effectors. Science. 2009;326:1501. doi: 10.1126/science.1178817. [DOI] [PubMed] [Google Scholar]
- Mussolino C, Cathomen T. RNA guides genome engineering. Nature Biotechnology. 2013;31:208–209. doi: 10.1038/nbt.2527. [DOI] [PubMed] [Google Scholar]
- Names N. Three obstackes which confronted the development of compactin. Atheroscler Suppl. 2004;5:21. [Google Scholar]
- Niimi M, Keyamura Y, Nozako M, Koyama T, Kohashi M, Yasufuku R, Yoshikawa T, Fan J. Probucol inhibits the initiation of atherosclerosis in cholesterol-fed rabbits. Lipids Health Dis. 2013;12:166. doi: 10.1186/1476-511X-12-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordestgaard BG, Zilversmit DB. Large lipoproteins are excluded from the arterial wall in diabetic cholesterol-fed rabbits. J Lipid Res. 1988;29:1491–1500. [PubMed] [Google Scholar]
- Overturf ML, Smith SA, Hewett-Emmett D, Loose-Mitchell DS, Soma MR, Gotto AM, Jr, Morrisett JD. Development and partial metabolic characterization of a dietary cholesterol-resistant colony of rabbits. J Lipid Res. 1989;30:263–273. [PubMed] [Google Scholar]
- Peng X. Transgenic rabbit models for studying human cardiovascular diseases. Comp Med. 2012;62:472–479. [PMC free article] [PubMed] [Google Scholar]
- Pogwizd SM, Bers DM. Rabbit models of heart disease. Drug Discovery Today, Disease Models. 2008;5:185–193. doi: 10.1016/j.ddmod.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poorman JA, Buck RA, Smith SA, Overturf ML, Loose-Mitchell DS. Bile acid excretion and cholesterol 7 alpha-hydroxylase expression in hypercholesterolemia-resistant rabbits. J Lipid Res. 1993;34:1675–1685. [PubMed] [Google Scholar]
- Roberts DCK, West CE, Redgrave TG, Smith JB. Plasma cholesterol concentration in normal and cholesterol-fed rabbits. Atherosclerosis. 1974;19:369–380. doi: 10.1016/s0021-9150(74)80002-x. [DOI] [PubMed] [Google Scholar]
- Rosenfeld ME, Yla-Herttuala S, Lipton BA, Ord VA, Witztum JL, Steinberg D. Macrophage colony-stimulating factor mRNA and protein in atherosclerotic lesions of rabbits and humans. Am J Pathol. 1992;140:291–300. [PMC free article] [PubMed] [Google Scholar]
- Rouy D, Duverger N, Lin SD, Emmanuel F, Houdebine LM, Denefle P, Viglietta C, Gong E, Rubin EM, Hughes SD. Apolipoprotein(a) yeast artificial chromosome transgenic rabbits. Lipoprotein(a) assembly with human and rabbit apolipoprotein B. J Biol Chem. 1998;273:1247–1251. doi: 10.1074/jbc.273.2.1247. [DOI] [PubMed] [Google Scholar]
- Shen J, Herderick E, Cornhill JF, Zsigmond E, Kim HS, Kuhn H, Guevara NV, Chan L. Macrophage-mediated 15-lipoxygenase expression protects against atherosclerosis development. J Clin Invest. 1996;98:2201–2208. doi: 10.1172/JCI119029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiomi M, Fan J. Unstable coronary plaques and cardiac events in myocardial infarction-prone Watanabe heritable hyperlipidemic rabbits: questions and quandaries. Curr Opin Lipidol. 2008;19:631–636. doi: 10.1097/MOL.0b013e3283189c18. [DOI] [PubMed] [Google Scholar]
- Shiomi M, Ito T. The Watanabe heritable hyperlipidemic (WHHL) rabbit, its characteristics and history of development: a tribute to the late Dr. Yoshio Watanabe. Atherosclerosis. 2009;207:1–7. doi: 10.1016/j.atherosclerosis.2009.03.024. [DOI] [PubMed] [Google Scholar]
- Shiomi M, Ito T, Yamada S, Kawashima S, Fan J. Development of an animal model for spontaneous myocardial infarction (WHHLMI rabbits) Arterioscler Thromb Vasc Biol. 2003;23:1239–1244. doi: 10.1161/01.ATV.0000075947.28567.50. [DOI] [PubMed] [Google Scholar]
- Shiomi M, Koike T, Ito T. Contribution of the WHHL rabbit, an animal model of familial hypercholesterolemia, to elucidation of the anti-atherosclerotic effects of statins. Atherosclerosis. 2013;231:39–47. doi: 10.1016/j.atherosclerosis.2013.08.030. [DOI] [PubMed] [Google Scholar]
- Song J, Zhong J, Guo X, Chen Y, Zou Q, Huang J, Li X, Zhang Q, Jiang Z, Tang C, Yang H, Liu T, Li P, Pei D, Lai L. Generation of RAG 1- and 2-deficient rabbits by embryo microinjection of TALENs. Cell Res. 2013;23:1059–1062. doi: 10.1038/cr.2013.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy: part I. J Lipid Res. 2004;45:1583–1593. doi: 10.1194/jlr.R400003-JLR200. [DOI] [PubMed] [Google Scholar]
- Sun H, Unoki H, Wang X, Liang J, Ichikawa T, Arai Y, Shiomi M, Marcovina SM, Watanabe T, Fan J. Lipoprotein(a) enhances advanced atherosclerosis and vascular calcification in WHHL transgenic rabbits expressing human apolipoprotein(a) J Biol Chem. 2002;277:47486–47492. doi: 10.1074/jbc.M205814200. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Palmer BM, James J, Wang Y, Chen Z, VanBuren P, Maughan DW, Robbins J, LeWinter MM. Effects of cardiac myosin isoform variation on myofilament function and crossbridge kinetics in transgenic rabbits. Circ Heart Fail. 2009;2:334–341. doi: 10.1161/CIRCHEARTFAILURE.108.802298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S, Ito T, Zenimaru Y, Suzuki J, Miyamori I, Takahashi M, Ishida T, Hirata K, Yamamoto TT, Iwasaki T, Hattori H, Shiomi M. Species differences of macrophage very low-density-lipoprotein (VLDL) receptor protein expression. Biochem Biophys Res Commun. 2011;407:656–662. doi: 10.1016/j.bbrc.2011.03.069. [DOI] [PubMed] [Google Scholar]
- Tall AR. Plasma cholesteryl ester transfer protein. J Lipid Res. 1986;27:361–367. [PubMed] [Google Scholar]
- Tanaka Y, Aleksunes LM, Yeager RL, Gyamfi MA, Esterly N, Guo GL, Klaassen CD. NF-E2-related factor 2 inhibits lipid accumulation and oxidative stress in mice fed a high-fat diet. J Pharmacol Exp Ther. 2008;325:655–664. doi: 10.1124/jpet.107.135822. [DOI] [PubMed] [Google Scholar]
- Taylor JM, Fan J. Transgenic rabbit models for the study of atherosclerosis. Front Biosci. 1997;2:298–308. doi: 10.2741/a192. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Fan J. Transgenic rabbit models for the study of atherosclerosis. Front Biosci. 1997;2:298–308. doi: 10.2741/a192. [DOI] [PubMed] [Google Scholar]
- Territo MC, Berliner JA, Almada L, Ramirez R, Fogelman AM. Beta-very low density lipoprotein pretreatment of endothelial monolayers increases monocyte adhesion. Arteriosclerosis. 1989;9:824–828. doi: 10.1161/01.atv.9.6.824. [DOI] [PubMed] [Google Scholar]
- Vesselinovitch D. Animal models and the study of atherosclerosis. Arch Pathol Lab Med. 1988;112:1011–1017. [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Niimi M, Nishijima K, Yu Y, Koike T, Kitajima K, Inoue T, Waqar AB, Liu E, Kohashi M, Ketamura Y, Yoshikawa T, Zhang J, Ma L, Zha X, Watanabe T, Asada Y, Chen EY, Fan J. Human apolipoprotein AII protects against diet-induced atherosclerosis in transgenic rabbits. Arterioscler Thromb Vas Biol. 2013;33:224–231. doi: 10.1161/ATVBAHA.112.300445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhao S, Fan J, Liu E. Expression Systems and Species Used for Transgenic Animal Bioreactors. Biomed Res Internat, 2013. 2013:Article ID 580463. doi: 10.1155/2013/580463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren RJ, Ebert DL, Mitchell A, Barter PJ. Rabbit hepatic lipase cDNA sequence: low activity is associated with low messenger RNA levels. J Lipid Res. 1991;32:1333–1339. [PubMed] [Google Scholar]
- Watanabe T, Hirata M, Yoshikawa Y, Nagafuchi Y, Toyoshima H. Role of macrophages in atherosclerosis. Sequential observations of cholesterol-induced rabbit aortic lesion by the immunoperoxidase technique using monoclonal antimacrophage antibody. Lab Invest. 1985;53:80–90. [PubMed] [Google Scholar]
- Watanabe Y. Serial inbreeding of rabbits with hereditary hyperlipidemia (WHHL-rabbit) Atherosclerosis. 1980;36:261–268. doi: 10.1016/0021-9150(80)90234-8. [DOI] [PubMed] [Google Scholar]
- Yamada S, Wang KY, Tanimoto A, Fan J, Shimajiri S, Kitajima S, Morimoto M, Tsutsui M, Watanabe T, Yasumoto K, Sasaguri Y. Matrix metalloproteinase 12 accelerates the initiation of atherosclerosis and stimulates the progression of fatty streaks to fibrous plaques in transgenic rabbits. Am J Pathol. 2008;172:1419–1429. doi: 10.2353/ajpath.2008.070604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Bishop RW, Brown MS, Goldstein JL, Russell DW. Deletion in cysteine-rich region of LDL receptor impedes transport to cell surface in WHHL rabbit. Science. 1986;232:1230–1237. doi: 10.1126/science.3010466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S, Balestra ME, Ferrell LD, Fan J, Arnold KS, Taylor S, Taylor JM, Innerarity TL. Apolipoprotein B mRNA-editing protein induces hepatocellular carcinoma and dysplasia in transgenic animals. Proc Natl Acad Sci U S A. 1995a;92:8483–8487. doi: 10.1073/pnas.92.18.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S, Balestra ME, Ferrell LD, Fan J, Arnold KS, Taylor S, Taylor JM, Innerarity TL. Apolipoprotein B mRNA-editing protein induces hepatocellular carcinoma and dysplasia in transgenic animals. Proc Natl Acad Sci USA. 1995b;92:8483–8487. doi: 10.1073/pnas.92.18.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Zhang J, Xu J, Zhu T, Fan Y, Fan J, Chen YE. Effective gene targeting in rabbits using RNA-guided Cas9 nucleases. J Vis Exp. 2013 doi: 10.3791/50957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang DS, Xu J, Zhu TQ, Fan JL, Lai LX, Zhang JF, Chen YE. Effective gene targeting in rabbits using RNA-guided Cas9 nucleases. Journal of Molecular Cell Biology. 2014;6:97–99. doi: 10.1093/jmcb/mjt047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yla-Herttuala S, Lipton BA, Rosenfeld ME, Sarkioja T, Yoshimura T, Leonard EJ, Witztum JL, Steinberg D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc Natl Acad Sci U S A. 1991;88:5252–5256. doi: 10.1073/pnas.88.12.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Koike T, Kitajima S, Liu E, Morimoto M, Shiomi M, Hatakeyama K, Asada Y, Wang KY, Sasaguri Y, Watanabe T, Fan J. Temporal and quantitative analysis of expression of metalloproteinases (MMPs) and their endogenous inhibitors in atherosclerotic lesions. Histol Histopathol. 2008;23:1503–1516. doi: 10.14670/HH-23.1503. [DOI] [PubMed] [Google Scholar]
- Zhang C, Zheng H, Yu Q, Yang P, Li Y, Cheng F, Fan J, Liu E. A practical method for quantifying atherosclerotic lesions in rabbits. J Comp Pathol. 2010;142:122–128. doi: 10.1016/j.jcpa.2009.08.159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.