Central venous catheters are associated with preventable bloodstream infections, leading to increased morbidity, length of stay, and costs. Quality improvement interventions aim to improve professional adherence to prevention measures. This meta-analysis provides evidence that quality improvement interventions decrease infection rates.
Keywords: central line–associated bloodstream infection, catheter-related bloodstream infection, quality improvement intervention, meta-analysis
Abstract
This systematic review and meta-analysis examines the impact of quality improvement interventions on central line–associated bloodstream infections in adult intensive care units. Studies were identified through Medline and manual searches (1995–June 2012). Random-effects meta-analysis obtained pooled odds ratios (ORs) and 95% confidence intervals (CIs). Meta-regression assessed the impact of bundle/checklist interventions and high baseline rates on intervention effect. Forty-one before–after studies identified an infection rate decrease (OR, 0.39 [95% CI, .33–.46]; P < .001). This effect was more pronounced for trials implementing a bundle or checklist approach (P = .03). Furthermore, meta-analysis of 6 interrupted time series studies revealed an infection rate reduction 3 months postintervention (OR, 0.30 [95% CI, .10–.88]; P = .03). There was no difference in infection rates between studies with low or high baseline rates (P = .18). These results suggest that quality improvement interventions contribute to the prevention of central line–associated bloodstream infections. Implementation of care bundles and checklists appears to yield stronger risk reductions.
Central venous catheters are indispensable devices in the intensive care unit (ICU), necessary for infusion of medication, fluid, or blood products; hemodialysis; blood withdrawal; or hemodynamic monitoring. However, these invasive devices predispose patients to preventable central line–associated bloodstream infections (CLABSIs), defined as bloodstream infections in patients with a central line 48 hours before infection onset, not related to another site (Table 1). CLABSIs are associated with increased morbidity, leading to increased length of hospitalization and resource use [3, 4], and might impact mortality and compromise patient prognosis [5–7].
Table 1.
Centers for Disease Control and Prevention Definitions for Central Line–Associated Bloodstream Infection Terminology
| Terminology | Definition |
|---|---|
| CLABSI | An LCBI where a central line was in place for >2 calendar days and a central line was in place on the date of event or the day before. |
| LCBI | To be defined as LCBI, it must meet 1 of the following criteria: |
| (1) Patient has a recognized pathogen cultured from 1 or more blood cultures, and organism cultured from blood is not related to an infection at another site; | |
| (2) Patient has at least 1 of the following signs or symptoms: fever (>38°C), chills, or hypotension, and positive laboratory results are not related to an infection at another site and the same common commensal is cultured from 2 or more blood cultures drawn on separate occasions. | |
| Central line days | A daily count of the number of patients with a central line in the patient care location during a time period. A patient with multiple central lines for a day only counts as 1 central line day. |
| Patient-days | A daily count of the number of patients in the patient care location during a time period. |
| Device utilization ratio | Central line utilization ratio is calculated by dividing the number of central line days by the number of patient-days. |
Infection prevention measures during central line insertion or maintenance, such as hand hygiene, maximal sterile barriers during catheter insertion, chlorhexidine skin disinfection, optimal catheter site selection, and daily review of line necessity with prompt removal of unnecessary lines, are known to decrease CLABSI risk [8, 9]. The Institute for Healthcare Improvement (IHI) recommends use of aforementioned items, in a central line care bundle, to decrease CLABSI occurrence. Despite the availability of evidence-based interventions summarized in guidelines [10, 11], CLABSI remains a substantial threat for hospitalized patients, with pooled estimated mean occurrence rates of 4.4 CLABSIs per 100 devices inserted (95% confidence interval [CI], 4.1–4.9) and 2.7 CLABSIs per 1000 catheter-days (95% CI, 2.6–2.9) [12].
In recent years, it has become clear that the limiting factor to infection prevention resides in the implementation of published recommendations [13]. Introducing prevention measures may be hampered by factors such as lack of problem awareness, poor familiarity or nonagreement with guidelines, low self-efficacy, inability to change practice, or lack of resources [14, 15]. Quality improvement interventions such as personnel education or catheter care bundles and checklists aim to decrease CLABSIs by improving adherence to prevention measures [16]. However, efficacy of these interventions has not been fully assessed.
This study examined whether quality improvement interventions reduce CLABSI rates in adult ICUs. Subgroup analysis assessed whether bundle/checklist interventions, high study power, or high baseline CLABSI rates influenced the intervention effect.
METHODS
Search Strategy
Medline was systematically searched (1995–June 2012) through a combination of search terms: catheter-related infections/prevention and control; catheterization, central venous/adverse effects; catheters, indwelling/adverse effects; infection control/methods; infection control/standards; intensive care units; quality control; quality of healthcare; and bundle (Supplementary Appendix 1). Extra studies were identified via reference lists, manually and through Ovid and ScienceDirect databases.
Study Selection
Eligible studies used before–after, interrupted time series (ITS), controlled before–after, nonrandomized controlled trial, or randomized controlled trial study designs that complied with the Cochrane Effective Practice and Organisation of Care Group methodological criteria. ITS studies report at least 3 data points before and after a defined point in time in which the intervention is implemented. Participants consisted of adult ICU patients with central line catheters. Trials implemented quality improvement interventions aimed at increasing professional adherence to evidence-based infection prevention processes. The primary outcome measure was the number of CLABSIs per catheter-days pre- and postintervention. Only English-language papers were included. Medline search results were screened by title and abstract. Selected papers underwent a full-text assessment, and eligibility issues were resolved between authors.
Data Extraction
Extracted data included author and year of publication, settings and study populations, study designs and periods, quality improvement and preventive interventions implemented in the baseline and intervention periods, compliance measures, number of CLABSI and catheter-days, and applied CLABSI definitions. Study authors were not contacted for additional data. To obtain effect sizes for ITS studies, infection rate data were extracted from study figures using the program Plot Digitizer. Results reported as a mix from both included and excluded study participants were included. Quality improvement interventions were classified under general headers (Table 2), and only preventive interventions described by Centers for Disease Control and Prevention (CDC) guidelines [10] and applicable to the majority of ICU patients were noted.
Table 2.
Classification of Quality Improvement Interventions and Number of Studies
| Quality Improvement Intervention(No. of Studies) | Definition and Examples |
|---|---|
| Education (n = 33) | Teaching lectures transmitting theoretical knowledge concerning CLABSI |
| |
| Training (n = 4) | Training sessions for practical skills associated with CVC care and maintenance |
| |
| Feedback (n = 20) | Reporting of CLABSI or care item compliance rates to ICU personnel |
| |
| Clinical reminders (n = 15) | Reminders of optimal clinical practice strategically placed to improve awareness or application of prevention measures |
|
|
| Bundle (n = 11) | A short list of at least 2 IHI prevention measures to be used during CVC insertion and/or maintenance |
| |
| Checklist (n = 18) | Checklist of bundled care item prevention measures to increase adherence to evidence-based infection prevention practices |
| |
| Empowerment to stop procedure (n = 10) | Nurses are empowered to halt and restart CVC insertion care or maintenance when a prevention measure is not implemented correctly to ensure optimal catheter care |
| Surveillance: compliance monitoring (n = 12) | Nurses intermittently or continually supervise CVC insertion or maintenance prevention measures, with/without use of a bundle/checklist |
| Leader designation (n = 11) | A leader is designated to facilitate implementation of quality intervention processes by planning activities to improve awareness or introduction of bundled care items |
| Prepackaging of CVC materials (n = 16) | Use of a CVC cart or kit stocked with all necessary supplies to insert or maintain a central line |
| Infrastructure changes (n = 2) | Changes to hospital infrastructure to facilitate adherence to prevention measures |
| |
| Organizational changes (n = 4) | Organizational changes in personnel staffing or duties to improve adherence to prevention measures |
|
Abbreviations: CLABSI, central line–associated bloodstream infection; CVC, central venous catheter; ICU, intensive care unit; IHI, Institute for Healthcare Improvement.
Quality Assessment
The Downs and Black checklist ascertained study methodological risk of bias [17]. It consists of 27 questions that evaluate the reporting, external validity, internal validity, and power of nonrandomized studies of healthcare interventions. Studies were scored based on these item criteria, adapted for CLABSI prevention research.
Statistical Analysis
A random-effects meta-analysis using the DerSimonian-Laird estimator obtained odds ratios (ORs) and 95% CIs for CLABSI rate reductions. The Higgins I2 test was predefined to quantify heterogeneity (I2 ≤ 25% for low, 25% < I2 < 50% for moderate, and I2 ≥ 50% for high), and funnel plots assessed publication bias. Subgroup analysis through meta-regression for before–after study designs compared studies with or without bundle/checklist interventions, baseline rates above or below 4.0 CLABSIs per 1000 catheter-days, and power scores above or below 0.75. Univariate analysis calculated changes in device utilization rates. Sensitivity analysis identified heterogeneous studies that influenced the meta-analysis.
Monthly ITS data were standardized for meta-analysis by dividing the outcome and standard error (SE) by the standard deviation (SD) of the preintervention trend. One study reported annual data points, which were used for the 12- and 24-month follow-up analyses [18]. SPSS version 22 calculated the intervention effect using segmented time series regression analysis, adjusting for time trend and autocorrelation. A negative change in level or slope indicated an infection rate reduction [19]. A P value <.05 was considered statistically significant.
RESULTS
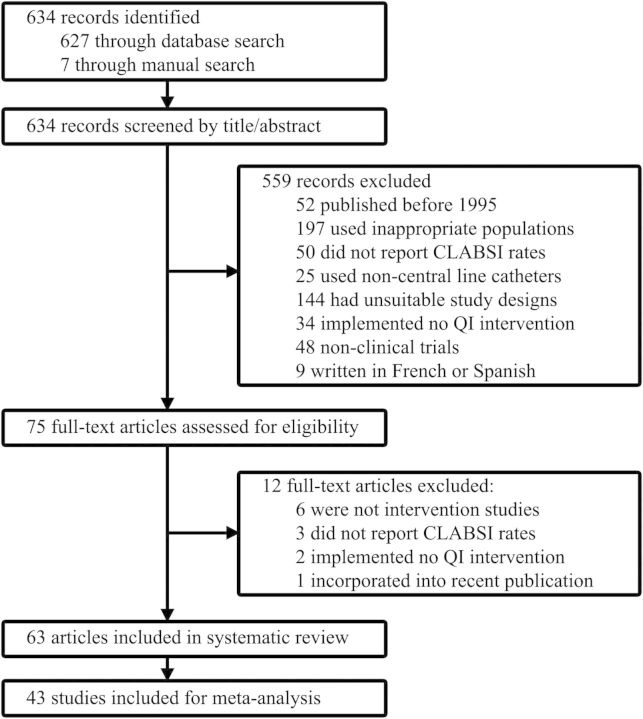
The search algorithm identified 634 records (627 in PubMed and 7 in Ovid and ScienceDirect). Forty-three studies, published in English between January 1995 and June 2012 involving 584 ICUs, were included for meta-analysis (Figure 1). Two studies [20, 21] continued their quality improvement initiatives and republished old data with new results [9, 22]. The older study by Coopersmith et al [20] was included for ITS analysis, and the article by Pronovost et al [21] was accessed to supplement information. One trial was not included for subgroup analysis because, although pre- and postintervention initiatives were qualitatively different, no new intervention types were implemented [23]. Another study included multiple data sets, of which the set with the longest follow-up period was chosen [24]. Eleven studies could not be included for ITS analysis because they implemented interventions in a stepwise manner [22, 23, 25–33].
Figure 1.
Study selection flow diagram. Abbreviations: CLABSI, central line–associated bloodstream infection; QI, quality improvement.
The 43 studies involved primarily medical-surgical ICUs, implemented quality improvement interventions without simultaneously introducing novel prevention measures, and applied CDC methods and definitions for CLABSI diagnosis (Supplementary Appendix 2).
The 584 included ICUs consisted of 564 adult, 11 pediatric [24, 34, 35], and 9 neonatal units [24]. Four studies reported the number of adult ICUs studied, but did not specify the ICU type (n = 270) [35–38]. The remaining 294 adult ICUs involved medical-surgical (n = 135), medical (n = 51), and surgical (n = 61).
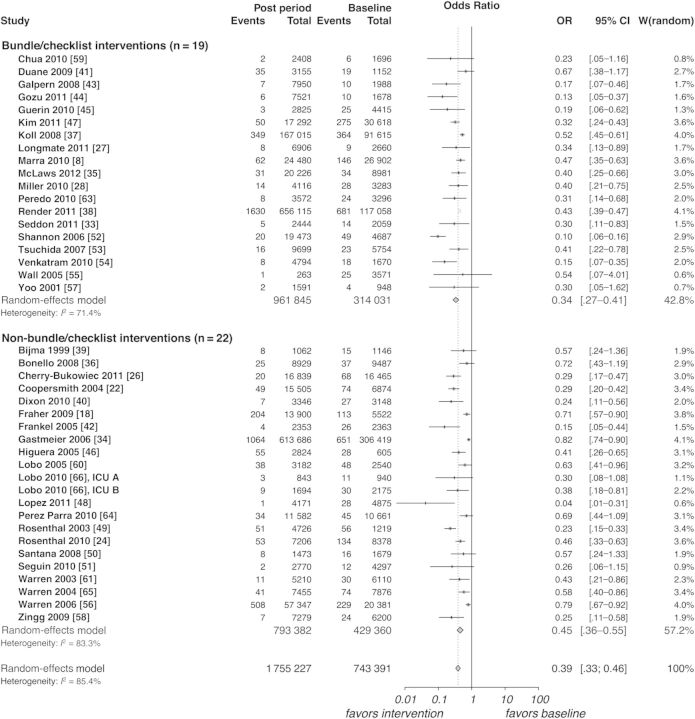
The meta-analysis consisted of 35 before–after [8, 22, 24, 26–28, 33–61], 7 ITS [18, 20, 40, 62, 63, 64, 65], and 1 controlled before–after study [66]. Five ITS studies were included in the meta-analysis of before–after study designs [18, 40, 63–65]. Duration of study periods ranged from 9 months [58] to 180 months [18], with a mean length of 26.75 months.
Up to 14 different types of interventions were reported. Studies introduced multiple quality improvement interventions in different combinations, usually implementing 1–5 interventions (n = 34). Four studies implemented initiatives through improvement systems such as plan-do-study-act, Six Sigma, and root cause analysis [36, 38, 42, 52].
Quality improvement interventions, details of their description, methods used to apply them, and compliance measure reporting varied. Educational interventions consisted of single, monthly, quarterly, or yearly sessions. Feedback reporting of infection or compliance rates occurred at monthly or quarterly intervals. Surveillance of compliance with preventive interventions was implemented daily, periodically, or at random intervals. Likewise, studies reported compliance with different items or only during the intervention period.
Twenty-eight studies reported before–after device utilization rates (n = 10) [8, 27, 28, 34, 38, 44, 45, 54, 56, 57], catheterization duration (n = 11) [22, 24, 27, 39, 43, 51, 53, 57, 58, 61, 63], or prevention measure compliance (n = 18) [22, 24, 27, 33, 35, 36, 45, 46, 49, 53, 55–60, 63, 66]. Some studies reduced [24, 51] or increased duration of catheterization [27, 58], yet most improved compliance (n = 10) [24, 27, 35, 36, 45, 46, 49, 56, 60, 66]. Analysis of 7 studies revealed device utilization rate increases [38, 45, 57] and decreases (Supplementary Appendix 3) [8, 54, 56].
Half of trials implemented bundles or checklists (n = 20). Trials either introduced bundles without checklists (n = 2) [8, 45], only checklists because bundles were used during baseline (n = 9) [38, 44, 52, 53, 55, 57, 59, 62, 63], or both bundle and checklist interventions (n = 9) [27, 28, 33, 35, 37, 41, 43, 47, 54].
Differing amounts of preventive care items were grouped together to form a bundle or checklist. Two trials [52, 59] did not report which items their bundle comprised, and 1 trial used a checklist a sole item [53]. Other trials used all 5 (n = 7) [8, 27, 37, 38, 43, 54, 63], 4 (n = 5) [28, 33, 41, 47, 62], 3 (n = 3) [35, 44, 55], or 2 (n = 2) [45, 57] IHI items in their bundle or checklist. The items “optimal catheter site selection” and “daily review of line necessity” were included least (Figure 2).
Figure 2.
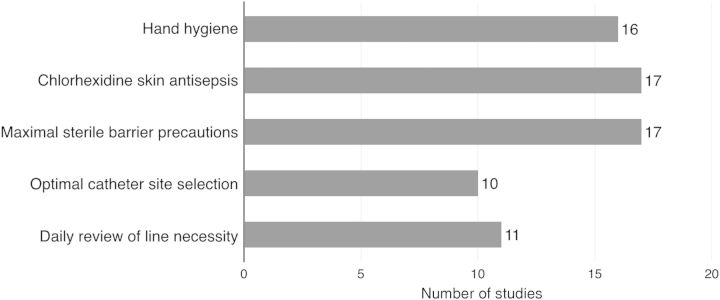
Frequency of included care items in bundles and checklists. Eighteen trials implementing bundle or checklist interventions reported which care items were implemented during the intervention period. One study used povidone-iodine in their bundle instead of chlorhexidine [57]. Hand hygiene: personnel practiced antiseptic hand hygiene before/after procedure; chlorhexidine skin antisepsis: skin disinfection before catheter insertion; maximal sterile barrier precautions: personnel wore sterile coat and gloves, mask, and hat during insertion, optimal catheter site selection: personnel strived to insert catheters in the subclavian vein, daily review of line necessity: catheter need was assessed daily with prompt removal of unnecessary central lines.
Four studies targeted other healthcare-associated infections such as ventilator-associated pneumonia (VAP) [36], both VAP and catheter-associated urinary tract infections [28, 59], or VAP and surgical site infections [34]. Eight studies initiated new prevention measures alongside quality improvement interventions [26, 39, 40, 42, 43, 48, 52, 53].
The baseline CLABSI incidence varied; rates ranged from 2.1 [34] to 46.3 CLABSIs per 1000 catheter-days [46]. Trials reported baseline rates <5 [26, 27, 34–37, 43, 51–53, 57–59, 61, 62, 64] and >15 CLABSIs per 1000 catheter-days [18, 24, 41, 46, 49, 60].
Downs and Black quality assessment scores ranged from 15 [59] to 26 [22, 24, 49], with a mean of 21.2 (Supplementary Appendix 4). The checklist revealed that 2 studies did not describe CLABSI definitions [40, 59], 9 did not sufficiently describe their quality improvement interventions [24, 33, 43, 46, 53, 56, 57, 59, 65], and 34 measured prevention measure compliance [8, 22, 24, 27, 28, 33–41, 43–46, 48–51, 53–61, 63, 66]. Twenty-eight studies reported confounding factors such as device utilization rates, catheterization duration, patient characteristics, or injury severity [8, 20, 22, 24, 26–28, 34, 38, 39, 41, 43–46, 49–54, 56–58, 61, 63, 66], which were comparable between baseline and postintervention in 18 trials [20, 22, 24, 27, 28, 39, 46, 49, 50, 52–54, 57, 58, 61, 63, 66]. Two trials corrected for these measured differences in patient characteristics [41, 51]. Studies tended to have either low (n = 25) or high (n = 14) power scores [8, 18, 20, 22, 24, 26, 34, 37, 38, 47, 49, 52, 56, 65].
Ten before–after trials did not demonstrate CLABSI rate decreases [36, 39, 41, 50, 51, 55, 57, 59, 64, 66]. Two studies revealed nonsignificant results for neurosurgical, neurological, cardiothoracic, and coronary care units, yet decreased their total CLABSI rate [46, 47]. ITS analysis demonstrated beneficial changes in infection rate slope [18] and levels at 3 [62], 6 [40], 12 [18, 40], and 24 months postintervention [18].
Meta-analysis was performed on 41 before–after and 7 ITS study designs to assess the impact of quality improvement interventions on the occurrence of CLABSIs. Before–after trials showed reductions in the CLABSI rate (OR, 0.39 [95% CI, .33–.46]; P < .0001, Figure 3) with high statistical heterogeneity (I2 = 85.4%). Analysis of 6 ITS studies, involving 11 ICUs, identified a change in level for the CLABSI rate at 3 months postintervention (OR, 0.30 [95% CI, .10–.88]; P = .028, Figure 4) with low heterogeneity (I2 = 24.5%). Changes in infection rate slope (OR, 0.81 [95% CI, .59–1.13]; P = .216) and levels at 6 (OR, 0.36 [95% CI, .11–1.19]; P = .094), 12 (OR, 0.17 [95% CI, .02–1.27]; P = .084), and 24 months postintervention (OR, 0.052 [95% CI, .003–1.02]; P = .051) trended toward reductions, yet were not significant (Supplementary Appendix 5).
Figure 3.
Meta-analysis and subgroup analysis of before-after studies. Bundle/checklist interventions: studies implementing a bundle and/or checklist. Non–bundle/checklist interventions: studies implementing neither a bundle nor checklist. Baseline period: before intervention implementation. Post period: after start of intervention implementation. Events: total number of central line–associated bloodstream infections. Total: total number of central line days. W, weight assigned per study. Abbreviations: CI, confidence interval; OR, odds ration.
Figure 4.
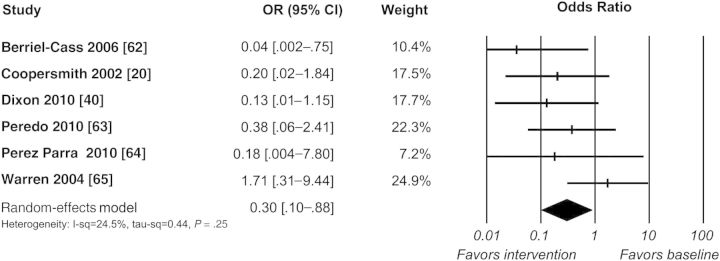
Meta-analysis of interrupted time series studies: change in central line-associated bloodstream infection rate level at 3 months postintervention. Abbreviations: CI, confidence interval; OR, odds ratio.
Subgroup analysis of before–after trials revealed that the CLABSI risk reduction was significantly stronger (P = .026; Figure 3) in trials with care bundles or checklists (OR, 0.34 [95% CI, .27–.41]) than in those without them (OR, 0.45 [95% CI, .36–.55]). Further analysis revealed that studies with baseline rates >4.0 CLABSIs per 1000 catheter-days (OR, 0.37 [95% CI, .33–.46]) did not demonstrate more pronounced risk reductions (P = .18) compared with studies below this baseline infection rate (OR, 0.49 [95% CI, .37–.66]). Low-power (OR, 0.33 [95% CI, .26–.42]) and high-power studies (OR, 0.44 [95% CI, .36–.54]) exhibited near-different rate reductions (P = .06).
Funnel plots displayed an asymmetrical pattern for before–after, but not ITS, study designs (Supplementary Appendix 6). The results of the sensitivity analysis of before-after study designs suggest that 2 studies contribute to residual heterogeneity; removing them from the meta-analysis would reduce variability between studies [49, 52]. However, because this did not affect the results, these studies were retained (Supplementary Appendix 7).
DISCUSSION
This meta-analysis of 43 studies, involving 584 ICUs, provides evidence that quality improvement interventions reduce CLABSI rates in adult ICUs. The effect size of 41 studies was significant yet highly heterogeneous. This infection rate decrease was more pronounced in studies using bundles or checklists, suggesting that their implementation alongside other initiatives leads to stronger rate reductions. The change in infection rate level for 6 studies at 3 months postintervention also demonstrates the beneficial impact of quality improvement interventions, with low heterogeneity. However, only 1 of these studies showed significant rate decreases [62], and the overall intervention effect was not sustained over longer follow-up periods. These findings may reflect the presence of the Hawthorne effect and need for CLABSI awareness promotion through continuous stepwise, multifaceted quality improvement interventions.
This study offers a broad look on the state of current research and applicable interventions, and applies a novel classification system to synthesize evidence for quality improvement initiatives. The meta-analysis is the first to include before–after studies and identify an additive preventive effect associated with bundle and checklist interventions. Two previous systematic reviews were unable to conclude which quality improvement interventions should be recommended for widespread implementation [16, 67]. Another recommended the use of educational programs and multidisciplinary teams [68]. A meta-analysis of ITS studies likewise demonstrated effect sizes with broad confidence intervals; however, they used different population criteria and studies, calculated rate reductions per quarter-year, reported mixed effects with small effect sizes, and did not investigate compliance measures. Additionally, the exclusion of before–after study designs discards much observational evidence, negatively impacting the external validity of the results [19]. Comparable points of criticism were the low quality of included studies due to high baseline infection rates, inadequate reporting of multiple CLABSI data points, compliance measurements, and intervention details.
Although interventions implemented in settings with higher baseline rates would appear more likely to be successful, no difference (P = .18) was found between studies with baseline infection rates above or below a suboptimal rate of 4.0 CLABSI per 1000 catheter-days. Furthermore, high-power studies demonstrated CLABSI rate decreases not significantly different from low-power studies (P = .06). Noteworthy is that the study with the lowest baseline rate (2.1 CLABSI per 1000 catheter-days) still achieved a significant rate reduction by providing feedback of biannual infection rates [34].
Strengths of this study include the comprehensive search strategy encompassing various quality improvement interventions, the methodological quality assessment of trials, and the random-effects model analysis with multiple studies and ITS study designs. It is, however, hampered by certain limitations: a lack of randomized or controlled study designs, inconsistent reporting of prevention measure compliance, and heterogeneity. Before–after studies run a higher risk of bias due to their liberal study design, as they hamper the ability to recognize phenomena that influence the CLABSI rate such as virulent epidemic outbreaks or spontaneous regression to the mean [16]. There is some evidence to suggest that the effects of quality improvement interventions are overestimated when based on before–after studies. Time series designs limit this risk of bias by detecting whether an intervention had an effect significantly greater than the underlying baseline trend [69]. However, because these designs require initiatives to begin at a well-defined point in time, 11 studies with multifaceted stepwise intervention implementation had to be excluded. This limitation could lead to an underestimation of the effect, as there is evidence for the effectiveness of gradual intervention introduction [70].
There are several issues related to the meta-analysis of before–after studies. All quality improvement interventions were considered to have an equal impact, yet this assumption may not be fair. Assuming interventions take months to implement, those introduced in a later study period could have less effect compared with earlier initiatives. Inclusion of studies from identical authors can lead to bias [24, 49, 56, 60, 61, 65, 66]. Two of these studies were performed in the same hospital, which could overestimate the intervention effect due to hospital experience in intervention implementation [60, 66]. The forest plot of before–after studies revealed a lack of smaller studies with less drastic infection rate decreases, suggesting publication bias; however, subgroup analysis of high-power studies revealed CLABSI decreases. Nevertheless, analysis of ITS studies aims to avert these barriers, and there was little evidence of publication bias among those studies.
Interventions to change risk exposure confound results. Although statistically equivalent, a catheter-day from days 1–2 contains less infection risk than days 14–15 due to microbial biofilm development and accumulating gaps in prevention measure adherence. Studies that reduce device utilization rates with increased average catheterization duration, reflecting a cohort of patients no longer managed with short-term central line usage, could underestimate intervention effects and vice versa [27]. This impact is unclear, as studies with significant changes in device utilization rates and duration of catheterization reported mixed effects. Analysis of catheterization duration was not feasible because CLABSI definitions do not account for usage of multiple catheters per patient.
Clinical and methodological heterogeneity stemmed from the use of differing intervention strategies, study designs, population characteristics, and baseline standards of care. No distinction was made between interventions applied as part of a general program or introduced to solve a specific recurring problem. For example, one study formed a team of nurses to evaluate care processes related to an infection rate increase. By applying a comparable yet distinct multifaceted quality improvement strategy, they decreased their rate from 1.5 to 0 CLABSIs per 1000 catheter-days [23]. Differing standards of care hinder comparison through meta-analysis. The effect of implemented quality improvement interventions is dependent on the efficacy or amount of baseline prevention measures. Simultaneous introduction of daily chlorhexidine bathing alongside a quality improvement initiative may have influenced one ITS study's intervention effect [40]. Last, this review did not aim to identify strategies that lead to optimal uptake of quality improvement initiatives.
In conclusion, the results of this meta-analysis provide evidence that quality improvement interventions reduce CLABSI in adult ICUs. Forty-one before–after studies demonstrated consistent, beneficial results, which appeared to be more pronounced among studies implementing bundle and checklist interventions. Quality improvement interventions appeared equally effective in studies with low and high power or baseline CLABSI rate settings. The CLABSI rate reduction appears to be confirmed by the methodologically more robust interrupted time series studies. Further research should assess requirements for successful adaptation of quality improvement interventions, for example, through improvement systems, over longer follow- up periods. Studies should report before–after compliance measures, device utilization rates, and catheterization duration. These latter 2 items are necessary to assess confounding factors, because increased catheter use for shorter durations leads to intervention effect overestimations. To properly address these issues, studies need to account for the number of catheters per patient. Finally, studies should apply ITS study designs and, when introducing stepwise initiatives, enough time should be spaced between interventions to facilitate ITS analysis.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Author contributions. K. B. conceived of and designed the study; performed the search of published work, literature search, data acquisition, interpretation and synthesis, and statistical analysis; and wrote the paper. J. B. performed the statistical analysis, contributed to data interpretation, and revised the statistical portions of the report. D. Vo. substantially contributed to data analysis and interpretation and critically revised the final manuscript. S. B. designed the study; substantially contributed to the search of published work, data interpretation and synthesis; and critically revised the final manuscript. D. Va. conceived of and designed the study; substantially contributed to data interpretation and synthesis; and critically revised the final manuscript. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Financial support. S. B. holds a research mandate of the Specific Research Fund at Ghent University.
Potential conflicts of interest. D. Vo. has received an institutional grant for work under consideration for publication from Pfizer, and has been a consultant for Astellas, Pfizer, and Tibotec. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Centers for Disease Control and Prevention. Central line–associated bloodstream infection (CLABSI) event protocol. 2014. pp. 1–10. Available at: http://www.cdc.gov. Accessed 9 February 2014.
- 2.Centers for Disease Control and Prevention. Atlanta, GA: CDC; 2014. NHSN key terms. pp. 1–16. Available at: http://www.cdc.gov. Accessed 22 March 2014. [Google Scholar]
- 3.Blot SI, Depuydt P, Annemans L, et al. Clinical and economic outcomes in critically ill patients with nosocomial catheter-related bloodstream infections. Clin Infect Dis. 2005;41:1591–8. doi: 10.1086/497833. [DOI] [PubMed] [Google Scholar]
- 4.Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ. Estimating the proportion of healthcare-associated infections that are reasonably preventable and the related mortality and costs. Infect Control Hosp Epidemiol. 2011;32:101–14. doi: 10.1086/657912. [DOI] [PubMed] [Google Scholar]
- 5.Rosenthal VD. Central line–associated bloodstream infections in limited-resource countries: a review of the literature. Clin Infect Dis. 2009;49:1899–907. doi: 10.1086/648439. [DOI] [PubMed] [Google Scholar]
- 6.Olaechea PM, Palomar M, Álvarez-Lerma F, et al. Morbidity and mortality associated with primary and catheter-related bloodstream infections in critically ill patients. Rev Esp Quimioter. 2013;26:21–9. [PubMed] [Google Scholar]
- 7.Januel JM, Harbarth S, Allard R, et al. Estimating attributable mortality due to nosocomial infections acquired in intensive care units. Infect Control Hosp Epidemiol. 2010;31:388–94. doi: 10.1086/650754. [DOI] [PubMed] [Google Scholar]
- 8.Marra AR, Cal RG, Durao MS, et al. Impact of a program to prevent central line-associated bloodstream infection in the zero tolerance era. Am J Infect Control. 2010;38:434–9. doi: 10.1016/j.ajic.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Pronovost PJ, Goeschel CA, Colantuoni E, et al. Sustaining reductions in catheter related bloodstream infections in Michigan intensive care units: observational study. BMJ. 2010;340:c309. doi: 10.1136/bmj.c309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Grady NP, Alexander M, Burns LA, et al. Guidelines for the prevention of intravascular catheter-related infections. Clin Infect Dis. 2011;52:e162–93. doi: 10.1093/cid/cir257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yokoe DS, Mermel LA, Anderson DJ, et al. Executive summary: a compendium of strategies to prevent healthcare-associated infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29:S12–21. doi: 10.1086/591060. [DOI] [PubMed] [Google Scholar]
- 12.Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81:1159–71. doi: 10.4065/81.9.1159. [DOI] [PubMed] [Google Scholar]
- 13.Blot S, Vandijck D, Vogelaers D, Labeau S. Bridging the gap between theory and practice. ICU Management. 2011;11:40–11. [Google Scholar]
- 14.Labeau SO, Vandijck DM, Rello J, et al. Centers for Disease Control and Prevention guidelines for preventing central venous catheter-related infection: results of a knowledge test among 3405 European intensive care nurses. Crit Care Med. 2009;37:320–23. doi: 10.1097/CCM.0b013e3181926489. [DOI] [PubMed] [Google Scholar]
- 15.Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA. 1999;282:1458–65. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 16.Ranji SR, Shetty K, Posley KA, et al. Rockville, MD: Agency for Healthcare Research and Quality; 2007. Closing the quality gap: a critical analysis of quality improvement strategies. Vol 6: Prevention of healthcare–associated infections. [PubMed] [Google Scholar]
- 17.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–84. doi: 10.1136/jech.52.6.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraher MH, Collins CJ, Bourke J, Phelan D, Lynch M. Cost-effectiveness of employing a total parenteral nutrition surveillance nurse for the prevention of catheter-related bloodstream infections. J Hosp Infect. 2009;73:129–34. doi: 10.1016/j.jhin.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Flodgren G, Conterno LO, Mayhew A, Omar O, Pereira CR, Shepperd S. Interventions to improve professional adherence to guidelines for prevention of device-related infections. Cochrane Database Syst Rev. 2013 doi: 10.1002/14651858.CD006559.pub2. doi:10.1002/14651858.CD006559.pub2. [DOI] [PubMed] [Google Scholar]
- 20.Coopersmith CM, Rebmann TL, Zack JE, et al. Effect of an education program on decreasing catheter-related bloodstream infections in the surgical intensive care unit. Crit Care Med. 2002;30:59–64. doi: 10.1097/00003246-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–32. doi: 10.1056/NEJMoa061115. [DOI] [PubMed] [Google Scholar]
- 22.Coopersmith CM, Zack JE, Ward MR, et al. The impact of bedside behavior on catheter-related bacteremia in the intensive care unit. Arch Surg. 2004;139:131–6. doi: 10.1001/archsurg.139.2.131. [DOI] [PubMed] [Google Scholar]
- 23.Richardson J, Tjoelker R. Beyond the central line-associated bloodstream infection bundle: the value of the clinical nurse specialist in continuing evidence-based practice changes. Clin Nurse Spec. 2012;26:205–11. doi: 10.1097/NUR.0b013e31825aebab. [DOI] [PubMed] [Google Scholar]
- 24.Rosenthal VD, Maki DG, Rodrigues C, et al. Impact of International Nosocomial Infection Control Consortium (INICC) strategy on central line–associated bloodstream infection rates in the intensive care units of 15 developing countries. Infect Control Hosp Epidemiol. 2010;31:1264–72. doi: 10.1086/657140. [DOI] [PubMed] [Google Scholar]
- 25.Berenholtz SM, Pronovost PJ, Lipsett PA, et al. Eliminating catheter-related bloodstream infections in the intensive care unit. Crit Care Med. 2004;32:2014–20. doi: 10.1097/01.ccm.0000142399.70913.2f. [DOI] [PubMed] [Google Scholar]
- 26.Cherry-Bukowiec JR, Denchev K, Dickinson S, et al. Prevention of catheter-related blood stream infection: back to basics? Surg Infect (Larchmt) 2011;12:27–32. doi: 10.1089/sur.2009.082. [DOI] [PubMed] [Google Scholar]
- 27.Longmate AG, Ellis KS, Boyle L, et al. Elimination of central-venous-catheter-related bloodstream infections from the intensive care unit. BMJ Qual Saf. 2011;20:174–80. doi: 10.1136/bmjqs.2009.037200. [DOI] [PubMed] [Google Scholar]
- 28.Miller RS, Norris PR, Jenkins JM, et al. Systems initiatives reduce healthcare-associated infections: a study of 22,928 device days in a single trauma unit. J Trauma. 2010;68:23–31. doi: 10.1097/TA.0b013e3181c82678. [DOI] [PubMed] [Google Scholar]
- 29.Munoz-Price LS, Dezfulian C, Wyckoff M, et al. Effectiveness of stepwise interventions targeted to decrease central catheter-associated bloodstream infections. Crit Care Med. 2012;40:1464–9. doi: 10.1097/CCM.0b013e31823e9f5b. [DOI] [PubMed] [Google Scholar]
- 30.Ong A, Dysert K, Herbert C, et al. Trends in central line–associated bloodstream infections in a trauma-surgical intensive care unit. Arch Surg. 2011;146:302–7. doi: 10.1001/archsurg.2011.9. [DOI] [PubMed] [Google Scholar]
- 31.Ramos ER, Reitzel R, Jiang Y, et al. Clinical effectiveness and risk of emerging resistance associated with prolonged use of antibiotic-impregnated catheters: more than 0.5 million catheter days and 7 years of clinical experience. Crit Care Med. 2011;39:245–51. doi: 10.1097/CCM.0b013e3181feb83e. [DOI] [PubMed] [Google Scholar]
- 32.Royer T. Implementing a better bundle to achieve and sustain a zero central line-associated bloodstream infection rate. J Infus Nurs. 2010;33:398–406. doi: 10.1097/NAN.0b013e3181f8586b. [DOI] [PubMed] [Google Scholar]
- 33.Seddon ME, Hocking CJ, Mead P, Simpson C. Aiming for zero: decreasing central line associated bacteraemia in the intensive care unit. N Z Med J. 2011;124:9–21. [PubMed] [Google Scholar]
- 34.Gastmeier P, Geffers C, Brandt C, et al. Effectiveness of a nationwide nosocomial infection surveillance system for reducing nosocomial infections. J Hosp Infect. 2006;64:16–22. doi: 10.1016/j.jhin.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 35.McLaws ML, Burrell AR. Zero risk for central line-associated bloodstream infection: are we there yet? Crit Care Med. 2012;40:388–93. doi: 10.1097/CCM.0b013e318232e4f3. [DOI] [PubMed] [Google Scholar]
- 36.Bonello RS, Fletcher CE, Becker WK, et al. An intensive care unit quality improvement collaborative in nine Department of Veterans Affairs hospitals: reducing ventilator-associated pneumonia and catheter-related bloodstream infection rates. Jt Comm J Qual Patient Saf. 2008;34:639–45. doi: 10.1016/s1553-7250(08)34081-1. [DOI] [PubMed] [Google Scholar]
- 37.Koll BS, Straub TA, Jalon HS, Block R, Heller KS, Ruiz RE. The CLABs collaborative: a regionwide effort to improve the quality of care in hospitals. Jt Comm J Qual Patient Saf. 2008;34:713–23. doi: 10.1016/s1553-7250(08)34094-x. [DOI] [PubMed] [Google Scholar]
- 38.Render ML, Hasselbeck R, Freyberg RW, Hofer TP, Sales AE, Almenoff PL. Reduction of central line infections in Veterans Administration intensive care units: an observational cohort using a central infrastructure to support learning and improvement. BMJ Qual Saf. 2011;20:725–32. doi: 10.1136/bmjqs.2010.048462. [DOI] [PubMed] [Google Scholar]
- 39.Bijma R, Girbes AR, Kleijer DJ, Zwaveling JH. Preventing central venous catheter-related infection in a surgical intensive-care unit. Infect Control Hosp Epidemiol. 1999;20:618–20. doi: 10.1086/501682. [DOI] [PubMed] [Google Scholar]
- 40.Dixon JM, Carver RL. Daily chlorohexidine gluconate bathing with impregnated cloths results in statistically significant reduction in central line-associated bloodstream infections. Am J Infect Control. 2010;38:817–21. doi: 10.1016/j.ajic.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Duane TM, Brown H, Borchers CT, et al. A central venous line protocol decreases bloodstream infections and length of stay in a trauma intensive care unit population. Am Surg. 2009;75:1166–70. [PubMed] [Google Scholar]
- 42.Frankel HL, Crede WB, Topal JE, Roumanis SA, Devlin MW, Foley AB. Use of corporate Six Sigma performance-improvement strategies to reduce incidence of catheter-related bloodstream infections in a surgical ICU. J Am Coll Surg. 2005;201:349–58. doi: 10.1016/j.jamcollsurg.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 43.Galpern D, Guerrero A, Tu A, Fahoum B, Wise L. Effectiveness of a central line bundle campaign on line-associated infections in the intensive care unit. Surgery. 2008;144:492–5. doi: 10.1016/j.surg.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Gozu A, Clay C, Younus F. Hospital-wide reduction in central line-associated bloodstream infections: a tale of two small community hospitals. Infect Control Hosp Epidemiol. 2011;32:619–22. doi: 10.1086/660098. [DOI] [PubMed] [Google Scholar]
- 45.Guerin K, Wagner J, Rains K, Bessesen M. Reduction in central line-associated bloodstream infections by implementation of a postinsertion care bundle. Am J Infect Control. 2010;38:430–3. doi: 10.1016/j.ajic.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Higuera F, Rosenthal VD, Duarte P, Ruiz J, Franco G, Safdar N. The effect of process control on the incidence of central venous catheter-associated bloodstream infections and mortality in intensive care units in Mexico. Crit Care Med. 2005;33:2022–7. doi: 10.1097/01.ccm.0000178190.89663.e5. [DOI] [PubMed] [Google Scholar]
- 47.Kim JS, Holtom P, Vigen C. Reduction of catheter-related bloodstream infections through the use of a central venous line bundle: epidemiologic and economic consequences. Am J Infect Control. 2011;39:640–6. doi: 10.1016/j.ajic.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Lopez AC. A quality improvement program combining maximal barrier precaution compliance monitoring and daily chlorhexidine gluconate baths resulting in decreased central line bloodstream infections. Dimens Crit Care Nurs. 2011;30:293–8. doi: 10.1097/DCC.0b013e318227767f. [DOI] [PubMed] [Google Scholar]
- 49.Rosenthal VD, Guzman S, Pezzotto SM, Crnich CJ. Effect of an infection control program using education and performance feedback on rates of intravascular device-associated bloodstream infections in intensive care units in Argentina. Am J Infect Control. 2003;31:405–9. doi: 10.1067/mic.2003.52. [DOI] [PubMed] [Google Scholar]
- 50.Santana SL, Furtado GH, Wey SB, Medeiros EA. Impact of an education program on the incidence of central line-associated bloodstream infection in 2 medical-surgical intensive care units in Brazil. Infect Control Hosp Epidemiol. 2008;29:1171–3. doi: 10.1086/591862. [DOI] [PubMed] [Google Scholar]
- 51.Seguin P, Laviolle B, Isslame S, Coue A, Malledant Y. Effectiveness of simple daily sensitization of physicians to the duration of central venous and urinary tract catheterization. Intensive Care Med. 2010;36:1202–6. doi: 10.1007/s00134-010-1829-1. [DOI] [PubMed] [Google Scholar]
- 52.Shannon RP, Frndak D, Grunden N, et al. Using real-time problem solving to eliminate central line infections. Jt Comm J Qual Patient Saf. 2006;32:479–87. doi: 10.1016/s1553-7250(06)32063-6. [DOI] [PubMed] [Google Scholar]
- 53.Tsuchida T, Makimoto K, Toki M, Sakai K, Onaka E, Otani Y. The effectiveness of a nurse-initiated intervention to reduce catheter-associated bloodstream infections in an urban acute hospital: an intervention study with before and after comparison. Int J Nurs Stud. 2007;44:1324–33. doi: 10.1016/j.ijnurstu.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 54.Venkatram S, Rachmale S, Kanna B. Study of device use adjusted rates in health care-associated infections after implementation of ‘bundles’ in a closed-model medical intensive care unit. J Crit Care. 2010;25:174.e11–18. doi: 10.1016/j.jcrc.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 55.Wall RJ, Ely EW, Elasy TA, et al. Using real time process measurements to reduce catheter related bloodstream infections in the intensive care unit. Qual Saf Health Care. 2005;14:295–302. doi: 10.1136/qshc.2004.013516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Warren DK, Cosgrove SE, Diekema DJ, et al. A multicenter intervention to prevent catheter-associated bloodstream infections. Infect Control Hosp Epidemiol. 2006;27:662–9. doi: 10.1086/506184. [DOI] [PubMed] [Google Scholar]
- 57.Yoo S, Ha M, Choi D, Pai H. Effectiveness of surveillance of central catheter-related bloodstream infection in an ICU in korea. Infect Control Hosp Epidemiol. 2001;22:433–6. doi: 10.1086/501930. [DOI] [PubMed] [Google Scholar]
- 58.Zingg W, Imhof A, Maggiorini M, Stocker R, Keller E, Ruef C. Impact of a prevention strategy targeting hand hygiene and catheter care on the incidence of catheter-related bloodstream infections. Crit Care Med. 2009;37:2167–80. doi: 10.1097/CCM.0b013e3181a02d8f. [DOI] [PubMed] [Google Scholar]
- 59.Chua C, Wisniewski T, Ramos A, Schlepp M, Fildes JJ, Kuhls DA. Multidisciplinary trauma intensive care unit checklist: impact on infection rates. J Trauma Nurs. 2010;17:163–6. doi: 10.1097/JTN.0b013e3181fb38a6. [DOI] [PubMed] [Google Scholar]
- 60.Lobo RD, Levin AS, Gomes LM, et al. Impact of an educational program and policy changes on decreasing catheter-associated bloodstream infections in a medical intensive care unit in Brazil. Am J Infect Control. 2005;33:83–7. doi: 10.1016/j.ajic.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Warren DK, Zack JE, Cox MJ, Cohen MM, Fraser VJ. An educational intervention to prevent catheter-associated bloodstream infections in a nonteaching, community medical center. Crit Care Med. 2003;31:1959–63. doi: 10.1097/01.CCM.0000069513.15417.1C. [DOI] [PubMed] [Google Scholar]
- 62.Berriel-Cass D, Adkins FW, Jones P, Fakih MG. Eliminating nosocomial infections at Ascension Health. Jt Comm J Qual Patient Saf. 2006;32:612–20. doi: 10.1016/s1553-7250(06)32079-x. [DOI] [PubMed] [Google Scholar]
- 63.Peredo R, Sabatier C, Villagra A, et al. Reduction in catheter-related bloodstream infections in critically ill patients through a multiple system intervention. Eur J Clin Microbiol Infect Dis. 2010;29:1173–7. doi: 10.1007/s10096-010-0971-6. [DOI] [PubMed] [Google Scholar]
- 64.Perez Parra A, Cruz Menarguez M, Perez Granda MJ, Tomey MJ, Padilla B, Bouza E. A simple educational intervention to decrease incidence of central line-associated bloodstream infection (CLABSI) in intensive care units with low baseline incidence of CLABSI. Infect Control Hosp Epidemiol. 2010;31:964–7. doi: 10.1086/655841. [DOI] [PubMed] [Google Scholar]
- 65.Warren DK, Zack JE, Mayfield JL, et al. The effect of an education program on the incidence of central venous catheter-associated bloodstream infection in a medical ICU. Chest. 2004;126:1612–8. doi: 10.1378/chest.126.5.1612. [DOI] [PubMed] [Google Scholar]
- 66.Lobo RD, Levin AS, Oliveira MS, et al. Evaluation of interventions to reduce catheter-associated bloodstream infection: continuous tailored education versus one basic lecture. Am J Infect Control. 2010;38:440–8. doi: 10.1016/j.ajic.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 67.Safdar N, Abad C. Educational interventions for prevention of healthcare-associated infection: a systematic review. Crit Care Med. 2008;36:933–40. doi: 10.1097/CCM.0B013E318165FAF3. [DOI] [PubMed] [Google Scholar]
- 68.Aboelela SW, Stone PW, Larson EL. Effectiveness of bundled behavioural interventions to control healthcare-associated infections: a systematic review of the literature. J Hosp Infect. 2007;66:101–8. doi: 10.1016/j.jhin.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 69.Eccles M, Grimshaw J, Campbell M, Ramsay C. Research designs for studies evaluating the effectiveness of change and improvement strategies. Qual Saf Health Care. 2003;12:47–52. doi: 10.1136/qhc.12.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Prior M, Guerin M, Grimmer-Somers K. The effectiveness of clinical guideline implementation strategies—a synthesis of systematic review findings. J Eval Clin Pract. 2008;14:888–97. doi: 10.1111/j.1365-2753.2008.01014.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.