Abstract
Watch any crowded intersection, and you will see how adept people are at reading the subtle movements of one another. While adults can readily discriminate small differences in the direction of a moving person, it is unclear if this sensitivity is in place early in development. Here, we present evidence that 4-year-old children are sensitive to small differences in a person's direction of walking (~7°) far beyond what has been previously shown. This sensitivity only occurred for perception of an upright walker, consistent with the recruitment of high-level visual areas. Even at 4 years of age, children's sensitivity approached that of adults’. This suggests that the sophisticated mechanisms adults use to perceive a person's direction of movement are in place and developing early in childhood. Although the neural mechanisms for perceiving biological motion develop slowly, they are refined enough by age 4 to support subtle perceptual judgments of heading. These judgments may be useful for predicting a person's future location or even their intentions and goals.
Keywords: biological motion, perceptual development, direction, extrapolation, predictive judgment
Watch a dance floor, a basketball game, or just any crowded intersection, and you will see how remarkably adept people are at reading subtle signals from the movements of others. Within milliseconds, people can “read” what another person is about to do and coordinate their own actions with the movements of others. People can make these judgments even with point-light walkers—images created by placing lights at human joints and filming in the dark (Johansson, 1973). These images harness the global movements of points of light to convey human form and complex social information, like a person's direction of walking. Perceiving an approaching person may be especially important, because oncoming biological motion is a good indicator that a social interaction is about to occur. Adults are strikingly sensitive to an oncoming person's heading (Brooks et al., 2008; Gurnsey, Roddy, & Troje, 2010; Schouten, Troje, & Verfaillie, 2011; Sweeny, Haroz, & Whitney, 2012a), presumably because the visual system devotes increased resources for this discrimination (Sweeny, Haroz, & Whitney, 2012b).
Is this fine discrimination of direction the result of experience and expertise, or is it in place early in development? Sensitivity to coarser biological motion cues appears early in life. For example, infants can distinguish between biological and nonbiological motion (Bertenthal, Proffitt, & Kramer, 1987; Fox & McDaniel, 1982; Simion, Regolin, & Bulf, 2008), and they can discriminate very large differences in heading (e.g., they can tell whether a person in a full profile view is walking leftward or rightward; Kuhlmeier, Troje, & Lee, 2010). However, adult-like sensitivity to more subtle and complex biological motion cues develops slowly (Freire, Lewis, Maurer, & Blake, 2006; Pavlova, Krageloh-Mann, Sokolov, & Birbaumer, 2001; Pavlova & Sokolov, 2000), and specialized neural mechanisms for biological motion perception come online only late in childhood (Lichtensteiger, Loenneker, Bucher, Martin, & Klaver, 2008) and continue to develop even into adolescence (Carter & Pelphrey, 2006; Hirai, Watanabe, Honda, & Kakigi, 2009). It is not clear if these slowly developing mechanisms are sensitive enough to allow young children to make fine-grained discriminations of motion direction.
Here, we tested the hypothesis that 4-year-old children could perceive subtle differences in a person's oncoming direction of walking. We tested a range of headings much narrower than has been previously tested in children. We tested children who were 4 years old because this age falls within the developmental range for perceiving other complex biological motions from point-light animations (e.g., discriminating dogs and birds from people; Pavlova et al., 2001), biological motion in noise (Freire et al., 2006), and much coarser heading differences in noise (Jordan, Reiss, Hoff man, & Landau, 2002). Based on these previous investigations and the gradual development of specialized biological motion mechanisms, we expected that children would have sensitivity to heading well beyond simple leftward versus rightward discrimination, but that this sensitivity would not be fully mature.
Method
Observers
Twelve children (M = 4.51 years, SD = 0.27 years; seven girls and five boys), and 12 adults (M = 29.1 years, SD = 3.25 years; five women and seven men) participated in the experiment.
Stimuli
Point-light walkers were composed of configurations of 13 white dots (each dot: 0.08° × 0.08°; 149 cd/m2) presented against a black background (0.36 cd/m2). The dots were placed at the locations of the major joints and the head such that the overall configuration would be perceived as a human body (Johansson, 1973). We generated “videos” from sets of 20 static frames (interpolated as every third frame from a set of coordinates provided in a freely available stimulus set; Vanrie & Verfaillie, 2004). The local position of each dot changed from frame to frame in a manner consistent with a natural human gait, and the gait appeared to be smooth. Each frame was presented for 48 ms, and each gait cycle (i.e., one step by each foot) lasted 960 ms. The walker was visible (and the sequence of frames looped) until the observer made his or her response (see Procedure section). The application to generate the videos was written in C# and interfaced with OpenGL via the Open Toolkit Library (http://www.opentk.com). We multiplied the three-dimensional motion vector (after ortho-graphic projection) of each dot by a rotation matrix to produce 10 different headings (–18°, –15°, –12°, –9°, or –6° leftward from straight ahead, or 6°, 9°, 12°, 15°, or 18° rightward from straight ahead). On average, dot configurations subtended 0.81° × 1.91° of visual angle.
Point-light walkers did not include any depth cues; the size and surface illumination of each dot remained uniform and overlapping dots did not provide occlusion cues. As such, these walkers could have been interpreted as either walking toward or away from the observers. Although there have been no studies indicating whether children are like adults in exhibiting a “facing bias” when viewing a person's ambiguous direction of walking (e.g., Schouten et al., 2011; Vanrie, Dekeyser, & Verfaillie, 2004), the contextual cues (discussed later) and anecdotal evidence (only one child of the 12 indicated the contrary) suggest that the children perceived the walkers as oncoming.
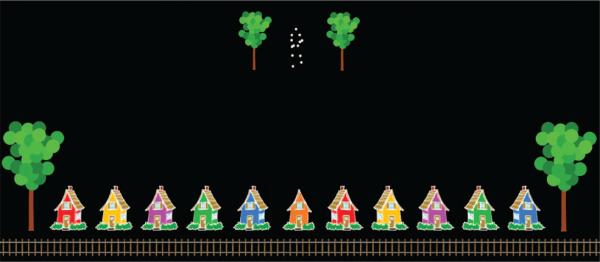
To evaluate heading sensitivity, we asked observers to make predictive judgments about a walker's heading. We created a cartoon scene that included a row of 11 equally spaced cartoon “train stations” and railroad tracks along the bottom of the screen along with linear perspective cues (trees with receding size) that created the impression of depth (see Figure 1). Train stations (1.71° × 2.01°) were identical cartoon images of a house, with the exception that the stations were given different colors. Stations to the left and right of the center station were mirror-image-reversed profile views so as to give the impression of perspective. The middle station had no perspective, so as to appear straight ahead. The cartoon train (1.1° × 1.1°) had a smiling expression and a head-on view, such that no cars were visible behind it. The train always appeared on top of the tracks and remained under the center station until the experimenter directed it to the selected station.
Figure 1.
Example of a point-light walker (with a –18° heading) and response options. For display purposes, the dimensions of the stimuli are close to but do not exactly match the dimensions from the actual experiment.
One pair of cartoon trees (each tree: 2.4° × 4.11°) flanked the stations and another pair (each tree: 1.11° × 2.11°) flanked the walker near the center of the screen. The decreasing size of the trees produced the impression of linear perspective and made the walker appear to be far away but headed toward the stations. Although the size and placement of the houses, trees, and walker were not consistent with perfect linear perspective, they gave the impression of depth adequately for our purposes.
Procedure
Each child was seated approximately 57 cm in front of a laptop computer and was familiarized with the stimuli and procedure before testing. Each trial began with the presentation of the cartoon scene. Next, a point-light walker appeared at the center of the screen with one of 10 different headings so as to appear headed toward one of the stations. The walker moved as if on a treadmill and thus had no net motion across space. On each trial, the experimenter explained that the walker was trying to get to one of the stations and that a cartoon train needed the child's help to travel to the correct station and pick the walker up. The child was asked to point to the station toward which he or she perceived the walker to be headed. We included this narrative so that the children would interpret the task as a fun game and cooperate throughout the duration of the experiment (Berger, Jones, Rothbart, & Posner, 2000), which lasted approximately 15 min.
A brief training session preceded the main experiment. During this training, the experimenter presented three different walkers with distinct headings (–15°, –6°, and 12°), explained that each walker was headed to a station, showed the child how to point to the station toward which the walker appeared to move, and gave the child feedback about his or her response.
In the main experiment, we presented each walker heading twice, in random order, for a total of 20 trials. A privacy filter was placed over the screen so that the experimenter could not see the heading of the walker but could see the station toward which the child pointed. This procedure ensured that the experimenter was blind to whether the child made an appropriate response and thus could not give the child feedback. The stations had different colors so that each child could name the color of the station if his or her direction of pointing was unclear to the experimenter. The experimenter initiated each trial by pressing the space bar. As in the training period, the walker remained on the screen until the child pointed to a station. The experimenter then pressed the arrow keys on the keypad to move the cartoon train, accompanied by the sound of a train whistle, to the child's selected station.
Because we presented a point-light walker with no actual form or overall motion across the screen, children had to integrate the local trajectories of individual points in order to perceive a human form and discriminate each walker's heading. To confirm that they had, we included a control condition in which the walker was inverted, with similar motion but with a disrupted appearance of humanness (Troje & Westhoff, 2006) and reduced recruitment of high-level visual areas (Grossman & Blake, 2001; Reid, Hoehl, & Striano, 2006). This control tested whether children used a high-level percept of a human, and not just the motion of a few select points, to perceive heading and extrapolate the future destination of the walker. Testing with the inverted condition occurred in a separate block, which included the same number of trials and a training session as in the upright block. Testing with the inverted condition was always conducted second and on a different day so that predicted poor performance would occur despite greater familiarity with the task.
Adults were tested in a single block of trials that only included an upright walker. We included these adults as a control group for assessing the development of children's heading sensitivity. The experimenter gave each adult the same set of instructions that were given to the children, once, at the beginning of the block. Adults used the keypad to make their responses without the help of the experimenter.
Results
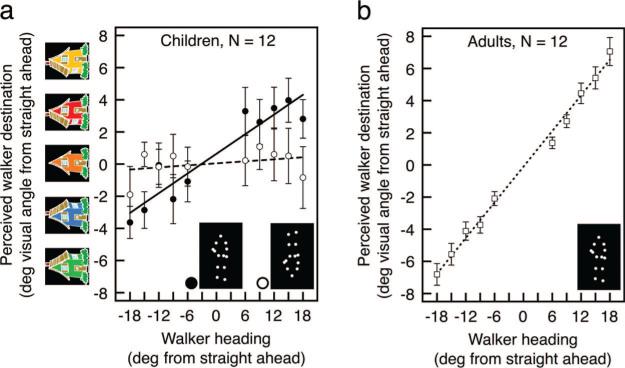
For each child and adult, we plotted the relationship between the walker's heading (measured in degrees of rotation angle) and the perception of the walker's final destination (measured as the physical location of the selected station in degrees of visual angle from the middle station). Positive slopes would indicate, at the very least, the ability to correctly categorize the headings within our narrow range (36°) as leftward and rightward. Children were able to discriminate between the leftward and rightward headings, as indicated by positive slopes for 11 of 12 children (M = 0.324, SD = 0.444), t(11) = 2.52, p < .05, d = 0.73 (see Figure 2A), and a significant linear pattern overall (R2 = .878, Fisher's z = 1.71, p < .01).
Figure 2.
(a) Children's perception of an upright (filled black circles and black line) and inverted (open circles and dashed line) walker's heading. Positive slopes and a good linear fit indicate that children discriminated between the leftward and rightward headings. For example, children perceived the –18° walker in Figure 1 as headed approximately toward the green station on the left. Note that because the x and y axes contain different units of measurement, a response need not have matching values on the axes to reflect accurate perception of heading. Overall, children could discriminate heading differences up to 7.34°. (b) Adults’ perception of an upright walker's heading. Overall, adults could discriminate heading differences up to 1.42°. Error bars in both panels reflect ± standard error of the mean (adjusted for within-observer comparisons); in both panels, deg = degrees.
Exactly how sensitive are children to the direction of a person's movement? To estimate the minimum difference in heading that children could perceive, on average, we generated bootstrapped linear fits to the average of the children's data (sampling with replacement 1,000 times; Manly, 2007) and calculated the 95% confidence interval of the bootstrapped distribution of x-intercept values. The width of this interval provided our estimate of heading sensitivity. Because we sampled with replacement, data neatly (or loosely) organized around the linear fit would produce a narrow (or wide) distribution of x-intercepts and a concomitant estimate of precise (or imprecise) sensitivity. Children showed precise heading sensitivity (7.36°) well beyond anything previously reported (e.g., 90°).
Adults were more sensitive than the children at discriminating heading. Adults perceived changes in heading with great precision (R2 = .958, Fisher's z = 2.27, p < .01), and with greater slopes than the children, t(22) = 2.42, p < .05, d = 1.03 (Figure 2B). Based on our bootstrapping analysis, adults were sensitive to a heading difference of 1.42°. This value is strikingly similar to estimates from a previous investigation (Gurnsey et al., 2010).
In contrast to performance with an upright walker, children were not sensitive to an inverted walker's heading. Overall, slopes were not significantly different from zero (M = 0.017, SD = 0.363), t(11) = 0.164, ns, d = 0.045, and were reduced compared with those with an upright walker, t(11) = 2.52, p < .05, d = 0.728 for the interaction (Figure 2A). This suggests that children used complex biological motion, which routinely conveys social information, rather than simple motion cues to discriminate a walker's heading.
Discussion
We showed, for the first time, that young children are sensitive to subtle differences in a person's direction of walking. Our findings complement coarser categorical demonstrations of heading perception with infants (Kuhlmeier et al., 2010), and they exceed sensitivity previously shown with 6-year-old children (Jordan et al., 2002). This sensitivity was not available for discriminating an inverted person's heading, suggesting that children used high-level visual mechanisms that integrate low-level motion cues with a person's global human configuration. This interpretation is consistent with several demonstrations that infants and children process upright and inverted point-light walkers differently (e.g., Fox & McDaniel, 1982; Moore, Goodwin, George, Axelsson, & Braddick, 2007; Reid et al., 2006). The upright selectivity of our results suggests that even though specialized neural mechanisms for perceiving biological motion develop slowly (Carter & Pelphrey, 2006; Freire et al., 2006; Hirai et al., 2009; Lichtensteiger et al., 2008), they are refined enough by the time children reach the age of 4 years to support subtle perceptual judgments about another person's direction of walking. Children's sensitivity was close to but not as refined as adults’ sensitivity, which indicates that the precise heading perception mechanisms that are operative in adults (Brooks et al., 2008; Gurnsey et al., 2010; Schouten et al., 2011; Sweeny et al., 2012a, 2012b) are in place and developing at the age of 4.
Our findings also demonstrate a level of perceptual sophistication that could be used for making predictive spatiotemporal judgments about the future location of a person. To measure heading sensitivity, we instructed children to extrapolate a person's direction of walking across space and indicate his or her future destination. It is plausible (and even likely) that children actually made these predictive judgments rather than simply associating certain headings with certain response options. The age of the children in our investigation is within the range of typical development of spatiotemporal extrapolation, which begins within the first year of life for simple translational motion cues (Hespos, Gredebäck, von Hofsten, & Spelke, 2009; von Hofsten, 1980) and is refined enough at age 3–4 years to extrapolate the direction of a person's gaze (Lee, Eskritt, Symons, & Muir, 1998)—a social cue similar to biological motion in complexity and recruitment of high-level visual processing (Calder et al., 2007; Hoffman & Haxby, 2000; Perrett et al., 1985).
Overall, our results provide the first evidence that young children utilize developing high-level biological motion mechanisms to perceive subtle differences in a person's direction of walking. By the age of 4, children possess the requisite sensitivity that could be used to make complex predictions about a person's future location, or more generally, for understanding a person's bodily movement in terms of intentions and goals (e.g., Gleissner, Meltzoff, & Bekkering, 2000; Woodward, 2009).
Acknowledgments
This research was supported in part by National Institutes of Health Grants T32 EY007043 and R01 EY018216, and National Science Foundation Grant NSF 0748689. We thank Ann Wakeley, Faraz Farzin, Steve Haroz, and Sophie Bridgers for their help.
Contributor Information
Timothy D. Sweeny, Vision Science Group and Department of Psychology, University of California, Berkeley
Nicole Wurnitsch, Department of Psychology, University of California, Berkeley.
Alison Gopnik, Department of Psychology and Department of Philosophy, University of California, Berkeley.
David Whitney, Vision Science Group and Department of Psychology, University of California, Berkeley..
References
- Berger A, Jones L, Rothbart MK, Posner MJ. Computerized games to study the development of attention in childhood. Behavior Research Methods, Instruments & Computers. 2000;32:297–303. doi: 10.3758/bf03207798. doi:10.3758/BF03207798. [DOI] [PubMed] [Google Scholar]
- Bertenthal BI, Proffitt DR, Kramer SJ. Perception of biomechanical motions by infants: Implementation of various processing constraints. Journal of Experimental Psychology: Human Perception and Performance. 1987;13:577–585. doi: 10.1037//0096-1523.13.4.577. doi:10.1037/0096-1523.13.4.577. [DOI] [PubMed] [Google Scholar]
- Brooks A, Schouten B, Troje NF, Verfaillie K, Blanke O, van der Zwan R. Correlated changes in perceptions of the gender and orientation of ambiguous biological motion figures. Current Biology. 2008;18:R728–R729. doi: 10.1016/j.cub.2008.06.054. doi:10.1016/j.cub.2008.06.054. [DOI] [PubMed] [Google Scholar]
- Calder AJ, Beaver JD, Winston JS, Dolan RJ, Jenkins R, Eger E, Henson RNA. Separate coding of different gaze directions in the superior temporal sulcus and inferior parietal lobule. Current Biology. 2007;17:396–411. doi: 10.1016/j.cub.2006.10.052. doi:10.1016/j.cub.2006.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter EJ, Pelphrey KA. School-aged children exhibit domain-specific responses to biological motion. Social Neuroscience. 2006;1:396–411. doi: 10.1080/17470910601041382. doi:10.1080/17470910601041382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox R, McDaniel C. The perception of biological motion by human infants. Science. 1982 Oct 29;218:486–487. doi: 10.1126/science.7123249. doi:10.1126/science.7123249. [DOI] [PubMed] [Google Scholar]
- Freire A, Lewis TL, Maurer D, Blake R. The development of sensitivity to biological motion in noise. Perception. 2006;35:647–657. doi: 10.1068/p5403. doi:10.1068/p5403. [DOI] [PubMed] [Google Scholar]
- Gleissner B, Meltzoff AN, Bekkering H. Children’s coding of human action: Cognitive factors influencing imitation in 3-yearold-s. Developmental Science. 2000;3:405–414. doi: 10.1111/1467-7687.00135. doi:10.1111/1467-7687.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman ED, Blake R. Brain activity evoked by inverted and imagined biological motion. Vision Research. 2001;41:1475–1482. doi: 10.1016/s0042-6989(00)00317-5. doi: 10.1016/S0042-6989(00)00317-5. [DOI] [PubMed] [Google Scholar]
- Gurnsey R, Roddy G, Troje NF. Limits of peripheral direction discrimination of point-light walkers. Journal of Vision. 2010;10:15, 11–17. doi: 10.1167/10.2.15. doi:10.1167/10.2.15. [DOI] [PubMed] [Google Scholar]
- Hespos S, Gredebäck G, von Hofsten C, Spelke ES. Occlusion is hard: Comparing predictive reaching for visible and hidden objects in infants and adults. Cognitive Science. 2009;33:1483–1502. doi: 10.1111/j.1551-6709.2009.01051.x. doi: 10.1111/j.1551-6709.2009.01051.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai M, Watanabe S, Honda Y, Kakigi R. Developmental changes in point-light walker processing during childhood and adolescence: An event-related potential study. Neuroscience. 2009;161:311–325. doi: 10.1016/j.neuroscience.2009.03.026. doi:10.1016/j.neuroscience.2009.03.026. [DOI] [PubMed] [Google Scholar]
- Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nature Neuroscience. 2000;3:80–84. doi: 10.1038/71152. doi:10.1038/71152. [DOI] [PubMed] [Google Scholar]
- Johansson G. Visual perception of biological motion and a model for its analysis. Perception & Psychophysics. 1973;14:201–211. doi:10.3758/BF03212378. [Google Scholar]
- Jordan H, Reiss JE, Hoffman JE, Landau B. Intact perception of biological motion in the face of profound spatial deficits: Williams syndrome. Psychological Science. 2002;13:162–167. doi: 10.1111/1467-9280.00429. doi:10.1111/1467-9280.00429. [DOI] [PubMed] [Google Scholar]
- Kuhlmeier VA, Troje NF, Lee V. Young infants detect the direction of biological motion in point-light displays. Infancy. 2010;15:83–93. doi: 10.1111/j.1532-7078.2009.00003.x. doi:10.1111/j.1532-7078.2009.00003.x. [DOI] [PubMed] [Google Scholar]
- Lee K, Eskritt M, Symons LA, Muir D. Children’s use of triadic eye gaze information for “mind reading.”. Developmental Psychology. 1998;34:525–539. doi: 10.1037//0012-1649.34.3.525. doi:10.1037/0012-1649.34.3.525. [DOI] [PubMed] [Google Scholar]
- Lichtensteiger J, Loenneker T, Bucher K, Martin E, Klaver P. Role of dorsal and ventral stream development in biological motion perception. NeuroReport. 2008;19:1763–1767. doi: 10.1097/WNR.0b013e328318ede3. doi:10.1097/WNR .0b013e328318ede3. [DOI] [PubMed] [Google Scholar]
- Manly BFJ. Randomization, bootstrap, and Monte Carlo methods in biology. Chapman & Hall/CRC.; Boca Raton, FL: 2007. [Google Scholar]
- Moore DG, Goodwin JE, George R, Axelsson EL, Braddick FMB. Infants perceive human point-light displays as solid forms. Cognition. 2007;104:377–396. doi: 10.1016/j.cognition.2006.07.007. doi:10.1016/j.cognition.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Pavlova M, Krageloh-Mann I, Sokolov A, Birbaumer N. Recognition of point-light biological motion displays by young children. Perception. 2001;30:925–933. doi: 10.1068/p3157. doi:10.1068/p3157. [DOI] [PubMed] [Google Scholar]
- Pavlova M, Sokolov A. Orientation specificity in biological motion perception. Perception & Psychophysics. 2000;62:889–899. doi: 10.3758/bf03212075. doi: 10.3758/BF03212075. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Smith PAJ, Potter DD, Mistlin AJ, Head AS, Milner AD, Jeeves MA. Visual cells in the temporal cortex sensitive to face view and gaze direction. Proceedings of the Royal Society of London, B: Biological Sciences. 1985;223:293–317. doi: 10.1098/rspb.1985.0003. doi: 10.1098/rspb.1985.0003. [DOI] [PubMed] [Google Scholar]
- Reid VM, Hoehl S, Striano T. The perception of biological motion by infants: An event-related potential study. Neuroscience Letters. 2006;395:211–214. doi: 10.1016/j.neulet.2005.10.080. doi:10.1016/j.neulet.2005.10.080. [DOI] [PubMed] [Google Scholar]
- Schouten B, Troje NF, Verfaillie K. The facing bias in biological motion perception: Structure, kinematics, and body parts. Attention, Perception, & Psychophysics. 2011;73:130–143. doi: 10.3758/s13414-010-0018-1. doi:10.3758/ s13414-010-0018-1. [DOI] [PubMed] [Google Scholar]
- Simion F, Regolin L, Bulf H. A predisposition for biological motion in the newborn baby. PNAS: Proceedings of the National Academy of Sciences of the United States of America. 2008;105:809–813. doi: 10.1073/pnas.0707021105. doi: 10.1073/pnas.0707021105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeny TD, Haroz S, Whitney D. Perceiving group behavior: Sensitive ensemble coding mechanisms for biological motion of human crowds. Journal of Experimental Psychology: Human Perception and Performance. 2012a doi: 10.1037/a0028712. doi:10.1037/a0028712. [DOI] [PubMed] [Google Scholar]
- Sweeny TD, Haroz S, Whitney D. Reference repulsion in the categorical perception of biological motion. Vision Research. 2012b;64:26–34. doi: 10.1016/j.visres.2012.05.008. doi:10.1016/j.visres.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troje NF, Westhoff C. The inversion effect in biological motion perception: Evidence for a “life detector”? Current Biology. 2006;16:821–824. doi: 10.1016/j.cub.2006.03.022. doi:10.1016/j.cub.2006.03.022. [DOI] [PubMed] [Google Scholar]
- Vanrie J, Dekeyser M, Verfaillie K. Bistability and biasing effects in the perception of ambiguous point-light walkers. Perception. 2004;33:547–560. doi: 10.1068/p5004. doi:10.1068/p5004. [DOI] [PubMed] [Google Scholar]
- Vanrie J, Verfaillie K. Perception of biological motion: A stimulus set of human point-light actions. Behavior Research Methods, Instruments & Computers. 2004;36:625–629. doi: 10.3758/bf03206542. doi:10.3758/BF03206542. [DOI] [PubMed] [Google Scholar]
- von Hofsten C. Predictive reaching for moving objects by human infants. Journal of Experimental Child Psychology. 1980;30:369–382. doi: 10.1016/0022-0965(80)90043-0. doi: 10.1016/0022-0965(80)90043-0. [DOI] [PubMed] [Google Scholar]
- Woodward AL. Infants’ grasp of others’ intentions. Current Directions in Psychological Science. 2009;18:53–57. doi: 10.1111/j.1467-8721.2009.01605.x. doi:10.1111/j.1467-8721.2009.01605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]