Abstract
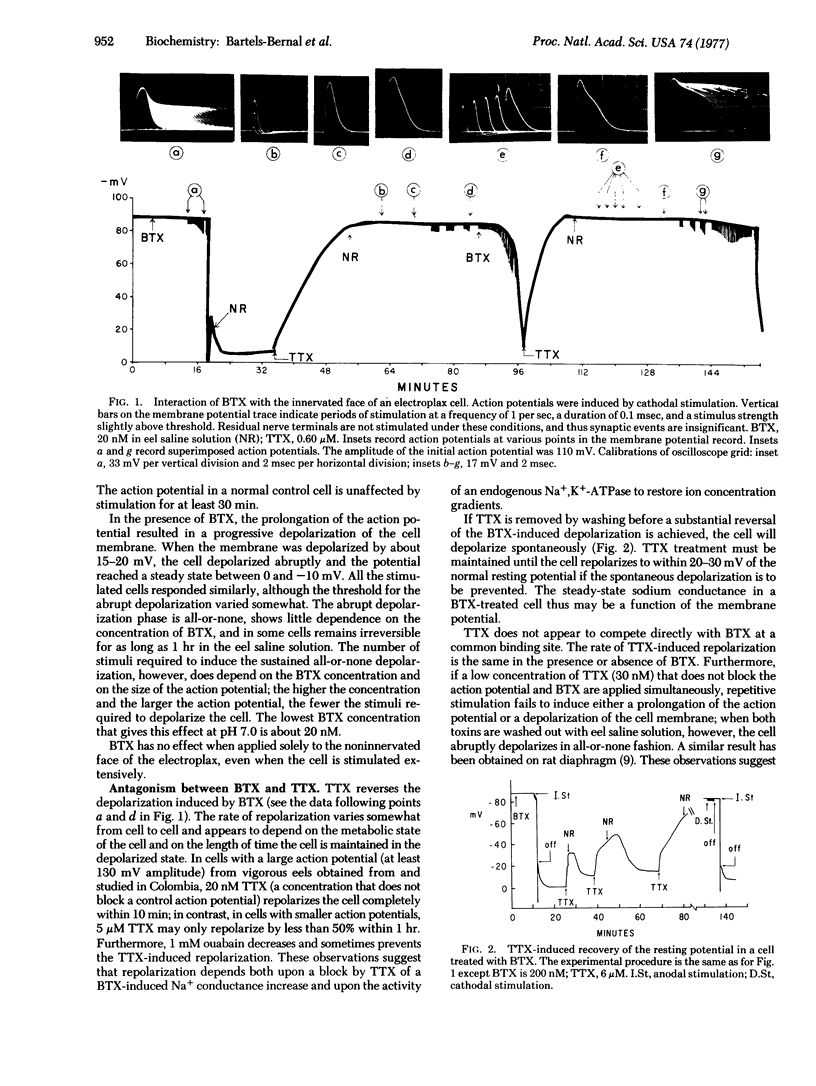
Batrachotoxin under certain conditions has a strong depolarizing effect on the innervated membrane of the monocellular electroplax preparation from the electric eel, El-ectrophorus electricus. No effect is observed when the toxin (50-200 nM) is applied to the resting membrane for periods up to 1 hr. However, if the membrane is exposed to batrachotoxin and the cell is subjected to stimulation at a stimulus voltage slightly above the threshold for action potential firing, a progressive prolongation of the action potential and concomitant progressive depolarization of the innervated membrane is observed. When the membrane is depolarized by 15-20 mV, a further abrupt all-or-none depolarization occurs, and the potential attains a steady-state value between 0 and -10 mV. Brief stimulation of a cell in the presence of batrachotoxin is sufficient to define a batrachotoxin-treated cell, even though negligible depolarization occurs. If depolarizing agents such as carbamoylcholine or potassium chloride are introduced to such a cell in concentrations that normally produce a 20-30 mV depolarization, the abrupt all-or-none depolarization immediately occurs. All-or-none depolarizations arising from either electrical stimulation or depolarizing agents are unaffected by d-tubocurarine but are completely reversed by tetrodotoxin. Batrachotoxin thus appears to activate only the action potential sodium channels. In the batrachotoxin-treated membrane, these channels can attain stable steady states in either a closed configuration at the normal resting potential or in an open configuration after complete depolarization. A striking hysteresis cycle thus can be generated, which is strongly indicative of a voltage-dependent interaction of the toxin with the action potential sodium channels.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALTAMIRANO M., COATES C. W., GRUNDFEST H. Mechanisms of direct and neural excitability in electroplaques of electric eel. J Gen Physiol. 1955 Jan 20;38(3):319–360. doi: 10.1085/jgp.38.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque E. X., Daly J. W., Witkop B. Batrachotoxin: chemistry and pharmacology. Science. 1971 Jun 4;172(3987):995–1002. doi: 10.1126/science.172.3987.995. [DOI] [PubMed] [Google Scholar]
- Albuquerque E. X., Warnick J. E., Sansone F. M. The pharmacology of batrachotoxin. II. Effect on electrical properties of the mammalian nerve and skeletal muscle membranes. J Pharmacol Exp Ther. 1971 Mar;176(3):511–528. [PubMed] [Google Scholar]
- Bartels E., Rosenberry T. L. Modification of electroplax excitability by veratridine. Biochim Biophys Acta. 1973 Apr 16;298(4):973–985. doi: 10.1016/0005-2736(73)90401-x. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Activation of the action potential Na+ ionophore of cultured neuroblastoma cells by veratridine and batrachotoxin. J Biol Chem. 1975 Jun 10;250(11):4053–4059. [PubMed] [Google Scholar]
- Catterall W. A., Ray R., Morrow C. S. Membrane potential dependent binding of scorpion toxin to action potential Na+ ionophore. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2682–2686. doi: 10.1073/pnas.73.8.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khodorov B. I., Peganov E. M., Revenko S. V., Shishkova L. D. Sodium currents in voltage clamped nerve fiber of frog under the combined action of batrachotoxin and procaine. Brain Res. 1975 Feb 14;84(3):541–546. doi: 10.1016/0006-8993(75)90771-4. [DOI] [PubMed] [Google Scholar]
- Narahashi T., Albuquerque E. X., Deguchi T. Effects of batrachotoxin on membrane potential and conductance of squid giant axons. J Gen Physiol. 1971 Jul;58(1):54–70. doi: 10.1085/jgp.58.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi W. D., Rosenberry T. L. Veratridine enhancement of agonist-induced synaptic membrane depolarization in electroplax. Life Sci. 1976 Sep 1;19(5):707–718. doi: 10.1016/0024-3205(76)90168-5. [DOI] [PubMed] [Google Scholar]
- SCHOFFENIELS E., NACHMANSOHN D. An isolated single electroplax preparation. I. New data on the effect of acetylcholine and related compounds. Biochim Biophys Acta. 1957 Oct;26(1):1–15. doi: 10.1016/0006-3002(57)90047-1. [DOI] [PubMed] [Google Scholar]
- Tokuyama T., Daly J., Witkop B. The structure of batrachotoxin, a steroidal alkaloid from the Colombian arrow poison frog, Phyllobates aurotaenia, and partial synthesis of batrachotoxin and its analogs and homologs. J Am Chem Soc. 1969 Jul 2;91(14):3931–3938. doi: 10.1021/ja01042a042. [DOI] [PubMed] [Google Scholar]
- Ulbricht W. The effect of veratridine on excitable membranes of nerve and muscle. Ergeb Physiol. 1969;61:18–71. doi: 10.1007/BFb0111446. [DOI] [PubMed] [Google Scholar]
- Villegas J., Sevcik C., Barnola F. V., Villegas R. Grayanotoxin, veratrine, and tetrodotoxin-sensitive sodium pathways in the Schwann cell membrane of squid nerve fibers. J Gen Physiol. 1976 Mar;67(3):369–380. doi: 10.1085/jgp.67.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnick J. E., Albuquerque E. X., Onur R., Jansson S. E., Daly J., Tokuyama T., Witkop B. The pharmacology of batrachotoxin. VII. Structure-activity relationships and the effects of pH. J Pharmacol Exp Ther. 1975 Apr;193(1):232–245. [PubMed] [Google Scholar]