Abstract
Symmetry is an organizational principle that is ubiquitous throughout the visual world. However, this property can also be detected through non-visual modalities such as touch. The role of prior visual experience on detecting tactile patterns containing symmetry remains unclear. We compared the behavioral performance of early blind and sighted (blindfolded) controls on a tactile symmetry detection task. The tactile patterns used were similar in design and complexity as in previous visual perceptual studies. The neural correlates associated with this behavioral task were identified with functional magnetic resonance imaging (fMRI). In line with growing evidence demonstrating enhanced tactile processing abilities in the blind, we found that early blind individuals showed significantly superior performance in detecting tactile symmetric patterns compared to sighted controls. Furthermore, comparing patterns of activation between these two groups identified common areas of activation (e.g. superior parietal cortex) but key differences also emerged. In particular, tactile symmetry detection in the early blind was also associated with activation that included peri-calcarine cortex, lateral occipital (LO), and middle temporal cortex, as well as inferior temporal and fusiform cortex. These results contribute to the growing evidence supporting superior behavioral abilities in the blind, and the neural correlates associated with crossmodal neuroplasticity following visual deprivation.
Keywords: Blind, lateral occipital cortex, symmetry, striate cortex, extrastriate cortex, haptic, tactile, crossmodal, plasticity
1.0 Introduction
Bilateral symmetry represents an organizational principle that characterizes many biological shapes and forms such as faces, animals and plants, as well as man-made objects like buildings and tools (see Treder 2010 for review). Thus, it is not surprising that the human visual system has developed an exceptional ability to detect the presence of symmetry, even with very brief presentation times on the order of milliseconds (Carmody, Nodine et al. 1977; Wagemans 1995; Huang, Pashler et al. 2004; Machilsen, Pauwels et al. 2009). The ability to detect symmetry may be related to our need to quickly recognize objects irrespective of their position or orientation in the visual field (Enquist and Arak 1994). Indeed, the presence of symmetry has been shown to mediate figure-ground segregation and perceptual grouping, thereby facilitating object detection and recognition (Labonte, Shapira et al. 1995; Machilsen, Pauwels et al. 2009). Furthermore, the salience of symmetry in organizing visually perceived information extends to complex object recognition and memory studies. For example, the presence of symmetry facilitates the recognition of faces (Little and Jones 2006) and visuo-spatial working memory (Rossi-Arnaud, Pieroni et al. 2006; Rossi-Arnaud, Pieroni et al. 2012). Moreover, from an evolutionary perspective, it has been suggested that the exquisite sensitivity of the visual system to symmetry may have vital survival value in supporting crucial functions such as detecting predators, locating food, and even the selection of suitable mates (Moller 1992; Enquist and Arak 1994).
Given the importance of symmetry in visual perception, it is perhaps not surprising that studies have focused within this domain. However, symmetry has also been the subject of a number of tactile/haptic investigations. Early work by investigators such as Davidson (1972) (Davidson 1972) and also Locher and Simmons (1978) explored the interaction between configural properties of shapes (e.g. curvature and symmetry) and haptic scanning strategies as they relate to performance in object detection and memory recognition. Specifically, in sighted (blindfolded) participants, the haptic identification of plastic nonrepresentational shapes with symmetrical configurations was found to be more difficult (quantified by increased number of identification errors and longer times for detection) than asymmetrical ones (Locher and Simmons 1978). These observations were attributed to the serial nature of tactile/haptic exploration and encoding processes necessary for integrating global shape information (see also Loomis, Klatzky et al. 1991). More recent behavioral studies by Ballesteros and collaborators revealed that tactile patterns containing bilateral symmetry showed facilitatory effects during haptic exploration for the discrimination of shape as well as in memory tasks. Specifically (and contrary to earlier reports), judgments of symmetric raised line shapes and unfamiliar three dimensional (3D) objects were found to be more accurate than asymmetric ones (Ballesteros, Manga et al. 1997; Ballesteros and Reales 2004).
A number of studies have also investigated the effect of prior visual experience on the tactile/haptic detection of shapes and tactile picture recognition by comparing performance in blind and sighted (blindfolded) individuals. For example, Heller (1989) reported similar performance in the congenitally blind and sighted, while tactile object recognition was more accurate in late-blind individuals (Heller 1989). Other studies found contradictory findings, whereby congenitally blind showed superior performance (e.g. Lederman, Klatzky et al. 1990; Heller, Calcaterra et al. 1996) suggesting that the role of prior visual experience on tactile object recognition remains unclear. To address the role of prior visual experience on tactile symmetry specifically, Cattaneo and colleagues carried out behavioral tests assessing the detection of tactile patterns in blind and sighted (blindfolded) individuals. Instead of using raised line drawings or 3D shapes, the authors employed a tactile memory matrix task for haptic exploration of tactile patterns and showed that the presence of symmetry facilitated memory retrieval in congenitally and late blind individuals as well as normally sighted controls (Cattaneo, Fantino et al. 2010; Cattaneo, Vecchi et al. 2013). Thus, as has been reported for vision, the presence of symmetry may indeed have a facilitatory effect on the detection of patterns in the tactile domain. However, prior visual experience along with stimulus complexity, the exploratory strategies employed, and the nature of the task demands all appear to be important factors relating to overall performance.
The neural correlates associated with the detection of symmetry have been investigated in the visual domain using functional magnetic resonance imaging (fMRI) in humans (Sasaki, Vanduffel et al. 2005; Tyler, Baseler et al. 2005) as well as macaque monkeys (Sasaki, Vanduffel et al. 2005). Using visual stimuli comprised of random dot patterns with different axes of symmetry, these studies reported that symmetry perception activates extrastriate retinotopic areas including V3, V4A, V7, and in particular, the lateral occipital (LO) cortex (Sasaki, Vanduffel et al. 2005; Tyler, Baseler et al. 2005). By comparison, early visual cortical areas (i.e. V1/V2) showed minimal activation with this same task (Sasaki, Vanduffel et al. 2005; Tyler, Baseler et al. 2005). This distributed pattern of cortical recruitment is consistent with the notion that the detection of visual patterns containing symmetry requires the integration of object features over a large visual field (Tyler, Baseler et al. 2005). Finally, a more recent study using noninvasive brain stimulation (i.e. repetitive transcranial magnetic brain stimulation; rTMS) has provided causal support for the role of a targeted extrastriate visual area in visual symmetry detection. Using fMRI-guided rTMS (on-line,10Hz) to disrupt local cortical activity, stimulation delivered to either the left or right LO (but not striate) cortex was shown to impair visual symmetry detection in normally sighted humans (Bona, Herbert et al. 2014).
It has yet to be clearly elucidated whether the same neural correlates are responsible for detecting the presence of symmetry in the tactile domain, particularly while employing random dot patterns similar to what have been used previously in visual perceptual studies (e.g. Sasaki, Vanduffel et al. 2005). This would allow for an initial comparison to be made regarding associated neural processing mechanisms across these sensory modalities. Furthermore, comparing performance between sighted (blindfolded) and early blind individuals would allow for the role of prior visual experience on the ability to be explored. This issue is also relevant within the context of numerous reports documenting superior sensory abilities in the blind, as well as investigating the underlying compensatory and crossmodal neuroplastic changes associated with non-visual sensory processing (e.g. Van Boven, Hamilton et al. 2000; Goldreich and Kanics 2003; see also Merabet and Pascual-Leone 2010; Sathian and Stilla 2010; Kupers and Ptito 2013 for reviews on this topic).
The experimental aims for this study were two-fold. First, we conducted a tactile behavioral experiment in early blind and sighted (blindfolded) controls to compare performance in detecting the presence of symmetry utilizing tactile patterns that were similar in design and spatial complexity as used previously in visual symmetry detection studies. Second (using fMRI), we identified the neural correlates associated with detecting tactile symmetry patterns while participants completed this task in the scanning environment. We further compared the patterns of task-related activation between the two groups and reasoned that activation implicating similar brain regions may be indicative of common underlying neural processing mechanisms irrespective of prior visual experience. In contrast, any observed differences may be related to compensatory neural mechanisms related to early visual deprivation.
2.0 Materials and Methods
2.1 Study Participants
Eight early blind (6 males, mean age: 33.37 years ± 7.21 S.D.) and seven sighted controls (5 males, mean age 30.6 years ± 5.06 S.D.) matched for education level were recruited for this study. Participants in the early blind group were all experienced Braille readers (either grade I or II level Braille) and had documented blindness prior to the age of three due to ocular and/or pre-geniculate cause (see Table 1). For the purposes of this study, early blindness was defined as documented residual visual function no greater than light perception and/or hand motion acquired prior to, or by the age of three (i.e. before the development of high level language function and the retention of vivid visual memories). While the majority of the participants had diagnoses that could be considered of “congenital” cause, we relied on documented evidence of profound blindness based on clinical visual functional assessment. Sighted controls all had normal, or corrected-to-normal, visual acuity. All sighted controls were right handed while early blind participants were equally divided between left and right handed dominant (based on self-report). All participants used their dominant hand (i.e. preferred reading hand in the case of the blind subjects) to explore the tactile patterns. Apart from blindness, all participants had no documented cognitive or neurological abnormalities. Written informed consent was obtained from all subjects prior to participation and experimental procedures were approved by the Institutional Review Board at the Massachusetts Eye and Ear Infirmary, Boston, MA, USA.
Table 1.
Participant Demographics
| subject | age | gender | hand used | Braille reader | blindness onset* | residual visual function | diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | 32 | M | right | yes | birth | LP | retinitis pigmentosa |
| 2 | 26 | M | left | yes | birth | NLP | anophthalmia |
| 3 | 38 | M | right | yes | birth | LP/hand motion | Leber's congenital amaurosis |
| 4 | 43 | M | right | yes | birth | NLP | congenital optic nerve damage |
| 5 | 41 | M | left | yes | birth | NLP | Leber's congenital amaurosis |
| 6 | 36 | M | right | yes | 3 y.o. | LP | glaucoma, retinitis pimentosa |
| 7 | 24 | F | left | yes | birth | LP | Leber's congenital amaurosis |
| 8 | 27 | F | left | yes | 3 y.o. | NLP | unknown ocular cause |
Abbreviations: LP = light perception, NLP = no light perception.
Blindness onset based on self-report
2.2 Tactile Stimuli and Tasks
Tactile stimuli were presented using a custom-made, fMRI compatible, refreshable tactile Braille display (KGS Corporation, Japan). The display consisted of a 32 by 36 pin array (inter-pin spacing of 2.4 mm) covering an area of 8.5 by 7.5 cm (Figure 1A). Each pin could be controlled independently and presented in either a fully raised or retracted position. Stimulus parameters (such as the pattern to be displayed, presentation time, and number of stimuli) were controlled using a custom designed software developed by the manufacturer. Two-dimensional random dot tactile patterns (inspired by the visual random dot patterns used in previous studies e.g. Sasaki, Vanduffel et al. 2005) were first generated using a commercial photo editing software and the corresponding .bmp files were then presented with the tactile display. For the purposes of this experiment, all tactile patterns comprised of 50 dots (i.e. tactile pins). For symmetrical patterns, pins were arranged such that exactly 30% of the pins were located within the central 3 positions along the mid-axis of the display. The pattern was then reflected about the vertical (i.e. vertically symmetric, Figure 1A) or horizontal (i.e. horizontally symmetric, Figure 1B) axis. For random patterns, a completely asymmetric distribution of pins was used (Figure 1C). A total of 30 tactile patterns (i.e. 10 individual patterns for vertically symmetric, horizontally symmetric and random) were used. Finally, as a control stimulus, a tactile pattern comprised of all the pins in the raised position was also used (see motor control condition below).
Figure 1.
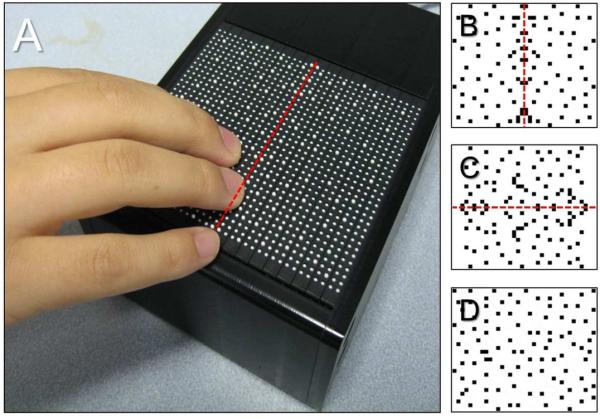
(A) Tactile stimuli were presented using an fMRI compatible, refreshable tactile Braille display consisting of a 32 by 36 pin array (KGS Corporation, Japan). Examples of a vertical (B) and horizontal (C) symmetric and random dot pattern (D) used in this study. The red line indicates the plane of symmetry of a given pattern (for illustrative purposes only).
Behavioral testing was carried out in the fMRI scanner. A pseudo-randomized block-design was used to present periods of the symmetry detection task (composed of either random, vertically symmetric, or horizontally symmetric patterns) and a motor control task interleaved with blocks of rest. During the symmetry detection block, each tactile pattern was presented for five seconds followed by a one-second response window in which participants were required to indicate (using the opposite hand) whether they perceived the pattern to be symmetric or random (i.e. indicating “yes” or “no” using a two-button response box). A combination of either three symmetric and two asymmetric patterns or, four symmetric and one asymmetric pattern could be presented for a total of 30 seconds per symmetry detection task block. For the motor control block, a full pattern of dots was presented in the same fashion as the symmetry detection block. This stimulus was designed to create a sense of maximal tactile sensation without any symmetrical pattern. Subjects were instructed to explore the full tactile display pattern in the same manner as in the symmetry detection task and provide random responses using the response box after each stimulus presentation. For the rest period (duration 30 seconds), subjects were instructed to lie motionless. The start of each task block was signaled with a briefly presented and unique tactile pattern cue corresponding to the task block to be carried out. Each run was comprised of four symmetry detection and four motor control blocks with intervening rest periods, for a run total of eight minutes. Four runs (with a counterbalanced block order) per scanning session were acquired for each participant.
Prior to the scan, a 30 to 60 minute training session was carried out (using different tactile patterns) to ensure that participants were familiar with the task and cues. All participants were required to achieve a minimum criterion performance of 80% correct in detecting the practice symmetric and random patterns prior to starting the scanning session. All participants were blindfolded throughout the training and scanning sessions.
2.3 Behavioral analysis
Behavioral performance on the symmetry detection task was scored following the scanning session using the acquired response data. A Welch's two sample t-test was carried out to compare performance between early blind and sighted controls. As a secondary analysis, a two-way ANOVA was carried out to compare symmetry detection performance between the two groups and the effect of axis of symmetry (i.e. vertical vs. horizontal patterns). All data were analyzed using SPSS statistical software (v. 21).
2.4 fMRI acquisition and analysis
Imaging data were acquired using a 3T Philips Achieva System and an 8 channel phased array head coil. Structural T1-weighted anatomical scans were collected using a turbo spin echo sequence (TE = 3.1 ms, TR = 6.8 ms, flip angle = 9°, 1 mm isotropic voxel size) and whole brain functional data were collected using a single-shot EPI sequence (TE = 28 ms, TR = 2000 ms, 0 mm slice gap, 3 mm isotropic voxel size). A B0 field map was also acquired to control for field inhomogeneities.
Image processing and analysis of functional data were performed using standard whole-brain analysis procedures in FSL version 5.0.5 (Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB), Oxford University, Oxford, U.K., http://ww9w2.fmrib.ox.ac.uk/fsl). Preprocessing included head motion correction, B0 unwarping, brain extraction, intensity normalization, high pass temporal filtering with a frequency cutoff of 120 sec, and Gaussian spatial smoothing (6.0 mm FWHM). Registration was performed with FLIRT (FMRIB's Linear Image Registration Tool). Each functional image was registered to the representative T1-weighted anatomical image (using a 6 degree of freedom boundary based linear registration) and to the MNI 152 template (using a 12 parameter nonlinear affine transformation with a warp resolution of 10 mm).
Individual time series analysis was carried out using a general linear model (GLM). Both active (motor and symmetry tasks) and passive (rest) conditions were convolved with a Gaussian haemodynamic response function and their temporal derivatives were used to model the data. The primary lower level contrasts were conducted for symmetry detection vs. motor control (primary contrast of interest), as well as symmetry detection vs. rest. As a next level of analysis, a separate GLM examined the effects of separating symmetric vs. asymmetric (i.e. random) pattern detection within the symmetry detection task blocks. By separating symmetric vs. asymmetric runs, we attempted to isolate activation patterns related to the detection of tactile symmetry specifically, and thereby also controlling for task related attention processing mechanisms (potentially not present during the exploration of the control pattern stimulus).
Higher level analyses (whole brain) were carried out using FMRIB's Local Analysis of Mixed Effects (FLAME 1+2). The first GLM was used to test for main effects of symmetry detection compared to the control motor task between the two subject groups, while the second GLM was used to test for the effects of symmetric vs. asymmetric pattern detection. For each of the GLMs, unequal variance among the two groups was assumed. In both lower and higher level analyses, z-statistic images were corrected for multiple comparisons with clusters determined by z > 2.3 voxel-wise thresholding and a family wise-error (FWE) corrected cluster significance threshold of p < 0.05 was applied to the supra-threshold clusters. Finally as an exploratory analysis, a third GLM examined the possibility of a correlation between patterns of brain activation and individual tactile symmetry detection performance. Specifically, whole brain analyses were used to test for a group by task (i.e. symmetry detection accuracy scores) interaction, as well as a within group effect of symmetry detection accuracy on brain activation. The accuracy scores were mean centered across all subjects, however the effects were tested separately within each group.
3.0 Results
3.1 Symmetry Detection – Behavioral
Both early blind and sighted (blindfolded) controls were able to successfully carry out the symmetry detection task in the scanner environment. For the early blind group, mean symmetry detection accuracy was significantly higher (88.37% correct ± 9.06 S.D.) compared to sighted controls (74.14% correct ± 9.93 S.D.) [Welch's two sample t-test: t(13)= 2.70, p= 0.018; see Figure 2]. A repeated-measures ANOVA with symmetry axis (vertical vs. horizontal) as the within-subjects variable and group (blind vs. sighted) as a between subjects variable revealed no significant main effect of axis [F(1,13)<1, p= 0.73, np2= 0.01], a significant main effect of group [F(1,13)= 11.97, p= 0.004, np2=.48], and no significant interaction axis by group [F(1,13)<1, p= 0.94, np2=.001] (individual group data: early blind: horizontal 89.12% correct ± 9.53 S.D., vertical 88.5% correct ± 6.04 S.D.; sighted control: horizontal 74.71% ± 12.8 S.D., vertical 73.71% ± 7.65 S.D.).
Figure 2.
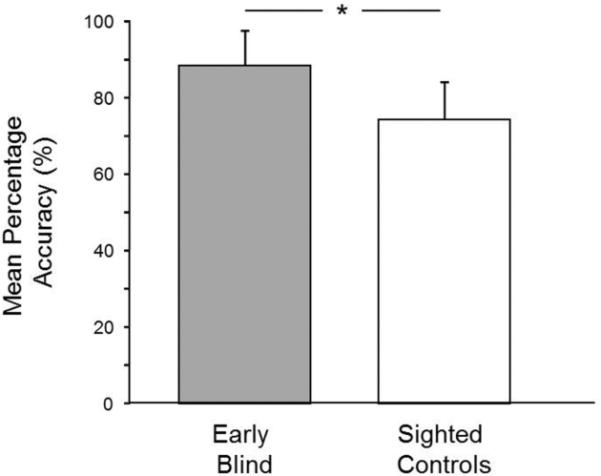
Comparison of mean tactile symmetry detection accuracy in early blind and sighted controls (vertical and horizontal patterns collapsed). Early blind subjects were significantly better in detecting the presence of symmetry in a tactile pattern (*= p<0.005; error bars represent S.D.)
3.2 fMRI
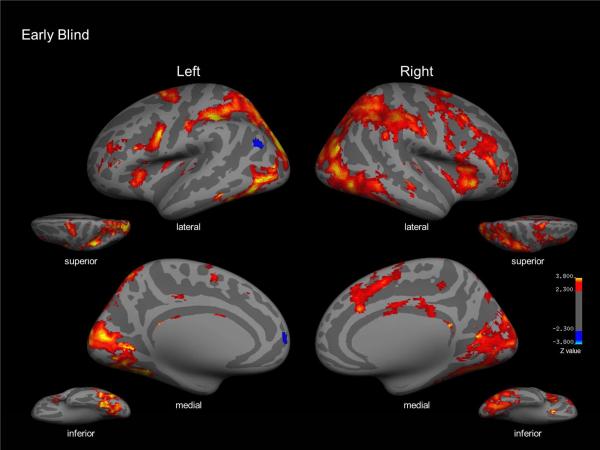
In early blind subjects, tactile symmetry detection contrasted with the motor control condition was associated with activation within a large network of cortical areas. In particular, robust activation was observed within right superior frontal cortex, as well as bilateral superior parietal, inferior temporal, and occipital cortices. Activation within occipital cortex included peri-calcarine areas, lateral occipital (LO) cortex, middle temporal cortices, as well as broad activation encompassing fusiform cortices. The motor control condition contrasted with tactile symmetry detection did not reveal any significant areas of activation (Figure 3; specific coordinates and expanded details can be found in Table 2).
Figure 3.
Early blind group map for tactile symmetry detection vs. motor control contrast (red). Inflated lateral, medial, superior, and inferior views of left and right hemispheres reveal activation within right superior frontal cortex as well as superior parietal cortex and occipital cortex bilaterally. Activation within occipital cortex included peri-calcarine areas, lateral occipital cortex, middle temporal cortices, as well as broad activation encompassing inferior temporal and fusiform cortices.
Table 2.
Loci and Coordinates of fMRI Activations
| Early Blind (symmetry > motor) | ||||||
|---|---|---|---|---|---|---|
| Number of voxels | p | Z value | MNI Coordinates | Anatomical Region | ||
| X | Y | Z | ||||
| 76362 | <0.001 | 12.1 | 46 | 10 | 50 | Right Middle Frontal Gyrus |
| <0.001 | 11.1 | −24 | −64 | 64 | Left Lateral Occipital Cortex, Left Superior Parietal Lobule | |
| <0.001 | 9.19 | 36 | −72 | −18 | Right Occipital Fusiform Gyrus, Right Lateral Occipital Cortex | |
| <0.001 | 8.32 | −56 | −30 | 44 | Left Supramarginal Gyrus, Left Postcentral Gyrus | |
| <0.001 | 8.26 | −42 | −66 | −4 | Left Inferior Temporal Gyrus | |
| <0.001 | 7.69 | −36 | −64 | −6 | Left Occipital Fusiform Gyrus, Left Temporal Occipital Fusiform Cortex | |
| Sighted Controls (symmetry > motor) | ||||||
|---|---|---|---|---|---|---|
| Number of voxels | p | Z value | MNI Coordinates | Anatomical Region | ||
| X | Y | Z | ||||
| 9436 | <0.001 | 11.6 | 2 | −80 | −12 | Right Lingual Gyrus |
| <0.001 | 8.53 | −28 | 28 | 4 | Left Insular Cortex, Left Frontal Orbital Cortex | |
| <0.001 | 7.8 | −30 | 26 | −8 | Left Frontal Orbital Cortex | |
| 4930 | <0.001 | 4.74 | 30 | −6 | 42 | Right Precentral Gyrus, Right Middle Frontal Gyrus |
| 4322 | <0.001 | 7.1 | −12 | −80 | 50 | Left Lateral Occipital Cortex |
| 3506 | <0.001 | 5.75 | 54 | −18 | 28 | Right Postcentral Gyrus |
| 2238 | <0.001 | 7 | −6 | 16 | 50 | Left Superior Frontal Gyrus, Left Supplementary Motor Cortex |
| 1822 | <0.001 | 4.59 | 8 | −48 | 74 | Right Superior Parietal Lobule, Right Postcentral Gyrus |
| Sighted Controls (motor > symmetry) | ||||||
|---|---|---|---|---|---|---|
| Number of voxels | p | Z value | MNI Coordinates | Anatomical Region | ||
| X | Y | Z | ||||
| 23912 | <0.001 | 10.4 | 24 | −34 | −18 | Right Parahippocampal Gyrus |
| <0.001 | 10.1 | −36 | −72 | 30 | Left Lateral Occipital Cortex | |
| <0.001 | 8.57 | 30 | −30 | −18 | Right Temporal Fusiform Cortex | |
| <0.001 | 8.53 | −26 | 6 | −24 | Left Frontal Orbital Cortex, Left Parahippocampal Gyrus | |
| 5808 | <0.001 | 8.08 | −56 | −44 | −8 | Left Middle Temporal Gyrus |
| 3514 | <0.001 | 6.94 | −40 | 12 | 44 | Left Middle Frontal Gyrus |
| 23912 | <0.001 | 5.58 | 22 | −86 | 28 | Right Lateral Occipital Cortex, Right Occipital Pole |
| Early Bind > Sighted Controls (symmetry > motor) | ||||||
|---|---|---|---|---|---|---|
| Number of voxels | p | Z value | MNI Coordinates | Anatomical Region | ||
| X | Y | Z | ||||
| 23742 | <0.001 | 4.29 | 42 | −76 | −16 | Right Occipital Fusiform Gyrus |
| <0.001 | 4.24 | −16 | −92 | 14 | Left Occipital Pole, Left Cuneal Cortex, Left Lateral Occipital Cortex | |
| <0.001 | 4.1 | 40 | −78 | 20 | Right Lateral Occipital Cortex | |
| <0.001 | 4.1 | −22 | −66 | −12 | Left Occipital Fusiform Gyrus, Left Lingual Gyrus | |
| <0.001 | 4.05 | −22 | −60 | −2 | Left Lingual Gyrus, Left Occipital Fusiform Gyrus | |
| <0.001 | 4 | −18 | −86 | 22 | Left Lateral Occipital Cortex, Left Occipital Pole | |
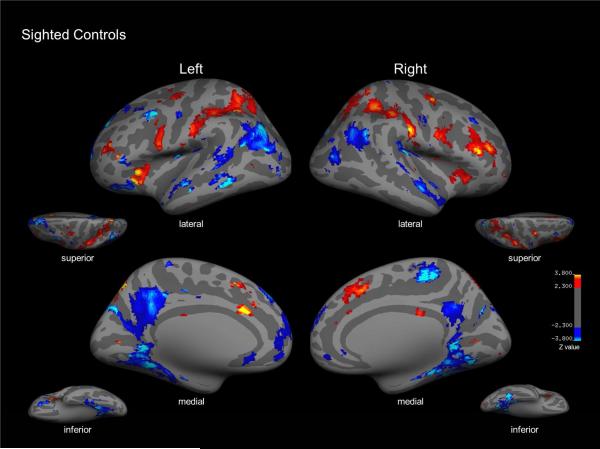
For sighted (blindfolded) controls, tactile symmetry detection contrasted with the motor control condition was associated with activation within right superior frontal cortex, superior parietal cortex, and the operculum (Figure 4). Activation was also evident within left lateral occipital (LO) cortex; however, overall, activation within occipital cortical areas was not as robust as compared to early blind subjects. In addition, the contrast of motor control condition compared to symmetry task revealed bilateral activation in regions including precuneus, inferior parietal cortex, and parahippocampal regions (specific coordinates and expanded details can be found in Table 2).
Figure 4.
Sighted control group map for tactile symmetry detection vs. motor control contrast (red). Inflated lateral, medial, superior, and inferior views of left and right hemispheres reveal activation within right superior frontal cortex and bilateral activation within frontal areas, superior parietal cortex as well as the operculum. The contrast of motor control vs. symmetry detection (blue) showed significantly greater bilateral activation in regions including inferior parietal cortex, precuneus and along parahippocampal regions.
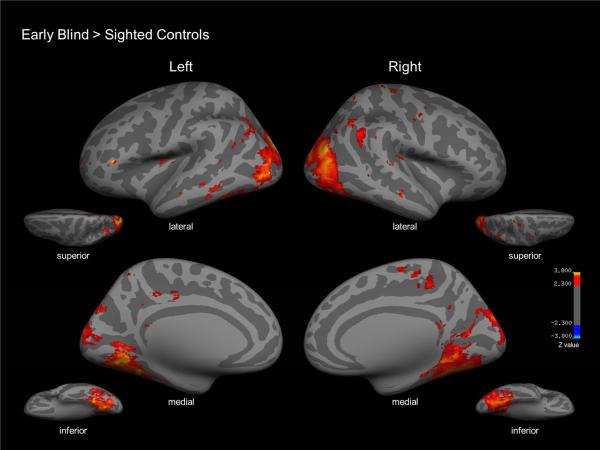
Comparing the neural correlates associated with tactile symmetry detection (contrasted with the motor control condition) in early blind and sighted (blindfolded) controls, revealed common areas of activation primarily within frontal and parietal regions bilaterally including the superior parietal lobule and intraparietal sulcus, as well as left frontal orbital cortex and right inferior frontal gyrus. Significant clusters of activation including the LO cortex were also identified (see supplemental data table 1 for specific coordinates of the conjunction analysis). In contrast, a between group comparison (i.e. early blind vs. sighted controls) for the symmetry detection task contrasted with the motor control condition identified numerous key differences. Specifically, in the early blind, bilateral activation was also observed within the occipital pole including the lingual, middle temporal, and lateral occipital cortices, as well as areas encompassing inferior temporal and fusiform cortices. The motor control condition contrasted with tactile symmetry detection did not reveal significant areas of activation (Figure 5; specific coordinates and expanded details can be found in Table 2).
Figure 5.
Between group comparison (i.e. early blind > sighted controls) for the symmetry detection task contrasted with the motor control condition (red). Bilateral activation was evident within the occipital pole including the lateral occipital cortex, middle temporal and lingual cortices, as well as activation encompassing inferior temporal and fusiform cortices.
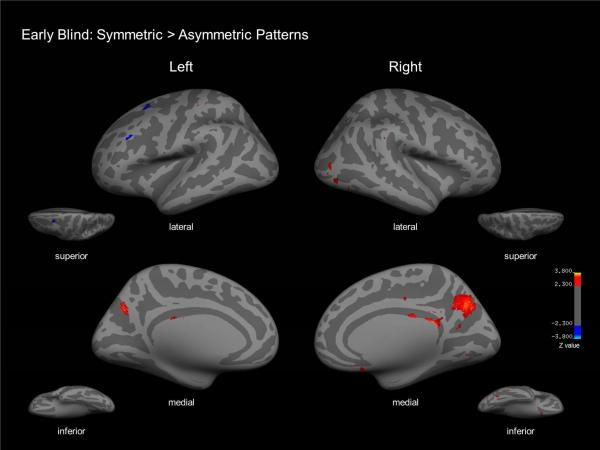
To isolate neural correlates associated with the detection of symmetric tactile patterns specifically, we conducted a secondary level analysis contrasting activation related to the detection of symmetric vs. asymmetric (i.e. random) patterns within the symmetry detection task blocks. In the early blind group, this contrast confirmed activation within the right cuneus region and precuneus areas bilaterally, as well as right LO cortex (Figure 6; specific coordinates and expanded details can be found in Table 3). The same contrast in sighted controls did not reveal significant areas of activation. The interaction analysis of early blind and sighted controls for the contrast of symmetric vs. asymmetric pattern detection confirmed activation with the right LO and right precuneus cortices (see supplemental table 2 for specific coordinates).
Figure 6.
Early blind group map for tactile symmetry detection (detection of symmetric vs. asymmetric patterns). Inflated lateral, medial, superior, and inferior views of left and right hemispheres reveal activation within cuneus and calcarine areas bilaterally as well as right LO cortex. The same contrast in sighted controls did not reveal significant areas of activation.
Table 3.
Loci and Coordinates of fMRI Activations (Symmetric vs Asymmetric Patterns)
| Early Blind (symmetric > asymmetric) | ||||||
|---|---|---|---|---|---|---|
| cluster volume (mm3) | p | Z value | MNI Coordinates | Anatomical Region | ||
| X | Y | Z | ||||
| 1656 | p<0.001 | 3.66 | 8 | −70 | 3 | Right Precuneus Cortex |
| p<0.001 | 3.66 | 40 | −80 | 4 | Right Lateral Occipital Cortex | |
| p<0.001 | 3.63 | 12 | −70 | 3 | Right Cuneal Cortex | |
| p<0.001 | 3.34 | 4 | −42 | 3 | Right Cingulate Gyrus | |
| p<0.001 | 3.16 | −12 | −68 | 3 | Left Precuneus Cortex | |
Finally, an exploratory analysis correlating individual tactile symmetry detection performance with whole brain activation did not reveal any significant areas of activation in either the early blind or sighted (blindfolded) group.
4.0 Discussion
In a behavioral task requiring the detection of tactile symmetrical patterns, early blind participants performed significantly better than sighted (blindfolded) controls. In both groups, no significant difference in behavioral performance was observed when considering the axis of symmetry (i.e. vertical or horizontal) of the tactile pattern. Comparing the neural correlates associated with tactile symmetry detection revealed similar bilateral activation within frontal and superior parietal cortices. Activation within the LO cortex was evident in both groups however, early blind subjects showed more robust bilateral activation, while it appeared only left lateralized in sighted controls. While common areas of activation between the two groups were evident, notable differences were also observed. In early blind, the detection of tactile symmetric patterns was associated with bilateral activation within a broad area encompassing the occipital cortex. Specifically, this included peri-calcarine cortex, LO cortex, as well as middle temporal cortex and areas of the inferior temporal and fusiform cortex. Apart from activation localized within left LO cortex, this pattern of activation implicating occipital and inferior temporal areas was comparatively absent in sighted (blindfolded) controls. Furthermore, in the early blind group, isolating the neural correlates associated with the detection of symmetric tactile patterns revealed activation primarily within the cuneus region and calcarine areas bilaterally as well as right LO cortex. Finally, no correlation between individual tactile symmetry detection performance and whole brain activation was observed.
4.1 Enhanced Tactile Performance in the Early Blind
To our knowledge, this is the first demonstration of superior tactile symmetry detection in the early blind (compared to the normally sighted controls) using random dot patterns similar in design as used previously in visual symmetry perception studies.
On a behavioral level, numerous studies have reported superior performance in blind individuals on a variety of sensory and cognitive processing tasks including tactile/haptic, auditory, olfactory, and verbal memory recall (e.g. Lessard, Pare et al. 1998; Goldreich and Kanics 2003; Cuevas, Plaza et al. 2009; Pasqualotto, Lam et al. 2013, for a recent review on this subject, see Kupers and Ptito 2013). Focusing on the tactile domain, Van Boven and colleagues (2000) reported evidence of enhanced tactile spatial acuity in early blind proficient Braille readers (compared to sighted controls) using a tactile grating orientation discrimination task. This enhanced tactile performance was evident for the fingers of the dominant hand as well as the index finger used for Braille reading (Van Boven, Hamilton et al. 2000). Replicating this experiment with a larger study sample, Goldreich and Kanics (2003) compared performance on a similar tactile grating orientation task in congenitally and late blind participants as well as sighted controls. Testing the dominant Braille reading finger confirmed evidence of enhanced tactile acuity in congenitally and late blind participants (Goldreich and Kanics 2003). Beyond Braille reading and Braille related tactile stimuli, a number of studies have also provided evidence of superior behavioral performance in the blind compared to sighted peers. This includes 3D shape discrimination (Norman and Bartholomew 2011), tactile acuity assessed by grating orientation discrimination (Norman and Bartholomew 2011), as well as using a 2D angle discrimination task (Alary, Duquette et al. 2009) and virbrotactile perception (Wan, Wood et al. 2010). Furthermore, it has been found that unlike their sighted counterparts, blind Braille readers retain high tactile acuity (measured with specially designed tactile acuity charts requiring active tactile exploration) without an age-related decline (Legge, Madison et al. 2008). However, it is also worth noting that other studies failed to find evidence of enhanced performance in the blind when testing other parameters related to tactile discriminations (e.g. Heller 1989; Stevens and Cruz 1996; Grant, Thiagarajah et al. 2000; Stilla, Hanna et al. 2008). As such, the overall generalizability and potential origin of these tactile behavioral enhancements have been the source of debate leaving open the issue as to the true nature of the cognitive process implicated in these observed behaviors as well as the impact of prior sensory and task experience (see Sathian and Stilla 2010 for a review on this topic). A more recent study however by Wong and colleagues (2011) was carried out to specifically address the question of whether enhanced tactile acuity in the blind was more likely related to tactile experience or rather, due to visual deprivation itself. Crucially, the blind individuals recruited to participate in this aforementioned study had varying degrees of Braille reading experience. Using a force controlled grating orientation discrimination task, these authors tested tactile spatial acuity on the fingers of the dominant and non-dominant reading hand of blind and sighted individuals (using the lip surface as a control site). A number of important observations resulted from this investigation. First, the investigators found that as a group, blind participants outperformed sighted controls in terms of tactile spatial acuity on all fingers tested (but not on the lip surface). Secondly, proficient Braille readers outperformed non-Braille readers when their preferred reading finger (i.e. index) was tested specifically. Finally, and consistent with the hypothesis of experience-dependent enhancement, this observed superior tactile spatial acuity was correlated with Braille reading experience (as indexed by weekly reading time) (Wong, Gnanakumaran et al. 2011). In relation to the findings presented here, our evidence of superior performance in the detection of tactile symmetric patterns is in line with previous reports of enhanced tactile discrimination abilities in the blind. Furthermore, as all our early blind subjects were also experienced and proficient Braille readers, these same individuals may have possessed greater familiarity with exploring tactile patterns in general (e.g. use of tactile maps). Thus, in line with work by Wong and colleagues (2011), the effect of tactile experience may have contributed to the enhanced performance observed in our early blind group.
With regards to tactile symmetry tasks, previous work carried out by co-authors of this study demonstrated that the presence of tactile symmetry also facilitates memory retrieval (i.e. improved recall) of haptically-explored tactile pattern configurations (Cattaneo, Fantino et al. 2010; Cattaneo, Vecchi et al. 2013). Furthermore, there appeared to be an advantage for recalling the spatial layout of tactile patterns containing a vertical (compared to horizontal) axis of symmetry. However, this vertical advantage was not observed in congenitally blind subjects and evident only in late blind and sighted blindfolded subjects. Specifically, the vertical advantage was present when sighted subjects explored patterns in both the frontal and horizontal plane, while the vertical advantage was only present in the frontal plane for the late blind (Cattaneo, Fantino et al. 2010; Cattaneo, Vecchi et al. 2013). These latter observations suggest that the role of prior visual experience may influence spatial encoding abilities related to symmetry when explored through touch; at least when patterns are presented in the frontal external reference frame. In the present study, we did not observe a performance advantage in either early blind or sighted control subjects in detecting tactile patterns with a vertical axis of symmetry. Methodological differences may account for this discrepancy in the results. In the studies of Cattaneo and colleagues (2010, 2013), participants were allowed to explore the tactile patterns using both hands and using an unconstrained scanning strategy. Previous research carried out in sighted participants suggests that symmetry is less salient with touch under uni-manual compared to bimanual exploration (e.g. Ballesteros, Millar et al. 1998; Ballesteros and Reales 2004). Moreover, even under bimanual exploration, this greater salience for patterns containing a vertical axis has not always been consistently reported (e.g. Ballesteros, Millar et al. 1998). In our study, only uni-manual exploration was possible and the patterns explored were presented while participants were lying supine (as opposed to a frontal reference frame). All of these factors may have altered the use of the body vertical midline as reference frame which appears necessary in determining a vertical axis advantage in symmetry appraisal in the tactile modality.
4.2 Neural Correlates Associated with Tactile Symmetry Detection
Comparing the neural correlates associated with detecting tactile symmetrical patterns revealed common patterns of activation in both early blind and sighted control subjects. In particular, robust bilateral activation was found within regions of the frontal and superior parietal cortex. The role of frontal and parietal cortical areas in spatial attention is well established (see Macaluso 2010; Bisley 2011). In the tactile domain, a number of neuroimaging studies have demonstrated activation within frontal and parietal areas in response to the exploration of tactile objects and spatial tasks in both sighted (Reed, Klatzky et al. 2005; Merabet, Swisher et al. 2007; Peltier, Stilla et al. 2007; Renier, Anurova et al. 2009) as well as blind (e.g. Burton, Sinclair et al. 2004; Bonino, Ricciardi et al. 2008; Stilla, Hanna et al. 2008; Leo, Bernardi et al. 2012) individuals. This common activation of frontal and superior parietal areas in both visual and tactile tasks suggests that these regions may implicate neural processing mechanisms common across both modalities. Indeed, it has been shown that superior (as well as posterior) parietal regions mediate the conversion of visual inputs into a common coordinate system, allowing for multimodal spatial processing (Goodale and Milner 1992; Creem and Proffitt 2001). Finally, using careful retinotopic mapping, Swisher and colleagues (2007) were able to characterize a number of distinct retinotopic areas within the medial bank of the intraparietal sulcus (IPS) in sighted subjects. Notably, this group found that exploration of tactile stimuli in the same individuals undergoing retinotopic mapping generated complementary activation patterns immediately adjacent to these identified visual field maps, thus providing a potential structural basis for the multi-modal and functional organization of this area (Swisher, Halko et al. 2007).
In early blind participants, we also observed robust activation within bilateral regions of the occipital cortex. While activation within left lateral occipital (LO) cortex in the sighted was evident, this pattern of activation within extrastriate areas was much more robust in the early blind. Specifically, we found bilateral activation within regions corresponding to extrastriate cortical areas including lateral (LO) cortex, as well as activation encompassing inferior temporal, middle temporal, and fusiform cortices. On a first level, this finding is of interest given that it is largely in agreement with the pattern of activation observed in sighted subjects carrying out symmetry perceptual tasks in the visual domain (see Sasaki, Vanduffel et al. 2005; Tyler, Baseler et al. 2005 reporting activation of areas including V3, V4A, V7, and lateral occipital cortex). In other words, it appears that in the absence of visual experience, early blind individuals may recruit similar occipital cortical areas for the detection of tactile symmetry as those identified in sighted individuals for the purposes of detecting visual symmetry. However, there was an important difference between our findings and the aforementioned studies. Specifically, in our early blind subjects, we also observed activation within peri-calcarine areas (corresponding to striate cortex) that not observed in sighted controls. The implications of this activation may be related to the processing demands of this tactile task in the blind. In the visual domain, it has been suggested that the rapid detection of symmetry may depend on low level visual spatial filtering mechanisms akin to texture processing (Dakin and Hess 1997; Dakin and Herbert 1998). However, in their visual symmetry detection study, Tyler and colleagues (2005) reasoned that the pattern of activation implicating higher order visual areas (and corresponding lack of activation within early visual cortical areas; i.e. V1/V2) is consistent with the notion that visual symmetry detection requires the integration of object features over a large area within the visual field (Tyler, Baseler et al. 2005). Neurons possessing larger receptive fields within extrastriate regions (as opposed to striate cortex) appear more ideally suited to carry out this task for the purposes of integrating scene features over a large area (Tyler, Baseler et al. 2005). In the case of vision, key information that defines a symmetrical pattern is revealed by comparing information across relatively large distances rather than through local point by point, serial comparisons (Tyler, Hardage et al. 1995; Dakin and Herbert 1998). However, in the early blind, we argue that the tactile detection of symmetrical patterns requires spatial integration of both global features over an entire surface (i.e. the overall pattern) as well as local area features (e.g. elements near the axis of symmetry). This notion is consistent with studies suggesting that initially, the discrimination of Braille and Braille-like patterns depends on dot-density differences rather than global shape coding (Millar 1981). Thus, the involvement of peri-calcarine areas may be crucial for this purpose and somehow related to the superior performance observed in the early blind group (see further discussion below).
4.3 Crossmodal Sensory Processing as it Relates to Blindness and Tactile Processing
The recruitment of occipital cortex in the blind as it relates to non-visual sensory processing has been reported extensively in the scientific literature and in particular, within the context of compensatory behaviors resulting from visual deprivation. With regards to task-related activation associated with tactile processing in the blind, a number of studies have reported the involvement of striate and extrastriate cortical areas in Braille reading (Sadato, Pascual-Leone et al. 1996; Buchel, Price et al. 1998; Burton, Snyder et al. 2002) as well as exploring non-Braille shapes (e.g. Burton, Sinclair et al. 2004). In the blind, the functional significance of this activation related to tactile processing has been supported by evidence through experimental manipulation of brain activity. Specifically, repetitive TMS (rTMS) delivered to the occipital cortex has been shown to disrupt the reading of Braille letters and text (e.g. Cohen, Celnik et al. 1997; Hamilton and Pascual-Leone 1998).
Along with its purported role in visual symmetry detection (Sasaki, Vanduffel et al. 2005; Tyler, Baseler et al. 2005; Bona, Herbert et al. 2014), the lateral occipital (LO) cortex appears to represent a key region in shape and object detection that is modality independent (Malach, Reppas et al. 1995). Support for this view comes from a number of neuroimaging studies investigating the role of this area in blind and sighted subjects (Amedi, Malach et al. 2001; Grill-Spector, Kourtzi et al. 2001; Amedi, Jacobson et al. 2002; Pietrini, Furey et al. 2004; Zhang, Weisser et al. 2004; James et al., 2002; Peltier, Stilla et al. 2007; Stilla and Sathian 2008). For its part, human middle temporal (MT) complex is also activated during moving tactile pattern perception tasks in both sighted (Hagen, Franzen et al. 2002; Blake, Sobel et al. 2004) and blind subjects (Sani, Ricciardi et al. 2010).
Finally, the role of fusiform cortical areas in crossmodal sensory processing has been less clear. While this area has been largely associated with the perception of faces in sighted individuals, activation in this area has also been seen in the tactile exploration of faces with basic facial expressions in late blind subjects (Goyal, Hansen et al. 2006). In a recent study, Kitada and colleagues (2013) reported activation within middle temporal and inferior frontal gyri while early blind participants haptically explored face mask figures with difference facial expressions (Kitada, Okamoto et al. 2013). Indeed, the perception of faces and symmetry are intimately related. For example, It has been shown that face likeness enhances symmetry perception (Jones, Victor et al. 2012) and the right fusiform face area (FFA) is sensitive to symmetrical compared to asymmetrical patterns (Caldara and Seghier 2009). The role of FFA in the specific case of early blindness and the detection of tactile symmetry requires more careful study. However, the notion that complementary visual and haptic/tactile tasks engage similar extrastriate cortical areas (and further, in a manner that is feature specific), supports the notion of common neural representation for the perception of particular shapes, objects, and forms (see Sathian and Stilla 2010 for review).
Along this direction of identifying brain networks related to somatosensory processing, Stilla and colleagues (2007 and 2008) used fMRI to identify cortical activation while subjects carried out a tactile micro spatial discrimination task. In this task, participants were required to discriminate the direction of offset of the central dot presented in a 3 dot pattern. By contrasting activation related to temporal control task (i.e. without a spatial offset), the authors were able to isolate activation related to this purely spatial task. In sighted (blindfolded) subjects, activation associated with this task was localized within a distributed fronto-parietal network. Notably, activation within object selective areas (such as LO cortex) and retinotopic organized visual cortex was largely absent (Stilla, Deshpande et al. 2007). A follow-up study in blind participants (early and late blind) using the same experimental protocol confirmed similar spatial selective network activation implicating fronto-parietal areas (Stilla, Hanna et al. 2008). However (and in line with the results presented here), spatial tactile performance on this same task was also associated with activation within right LO cortex, bilateral foci with the fusiform gyrus, as well as right V3A. Interestingly, behavioral performance on the task was not statistically different between sighted and blind participants; however, the authors did find that individual tactile acuity thresholds correlated with activation magnitudes within right posteromedial parietal cortex (in sighted) and primary somatosensory and visual cortical foci (in blind). These latter two observations appear at odds with the findings of our study. Specifically, we found that early blind participants outperformed sighted controls in the detection of tactile symmetrical patterns and furthermore, no correlation between individual performance and brain activation was observed. While the cause of these discrepancies is not clear at this time, there is a marked similarity in the task-related brain networks identified; specifically, frontal-parietal areas (in both blind and sighted) as well as retinotopic and object selective areas (in the blind). The results of these aforementioned studies appear to support our findings that certain tactile spatial processing tasks in the blind depend on a more distributed network of activation implicating the involvement of retinotopic and object selective areas.
4.4 Crossmodal Tactile Processing in the Sighted
In sighted subjects, investigating the role of occipital visual areas in tactile processing has been carried out using combined neuroimaging and TMS approaches. First reported by Sathian and colleagues (1997), activation within a parieto-occipital area likely corresponding to area V6 (revealed with PET imaging), was associated with the detection of tactile grating orientation (Sathian, Zangaladze et al. 1997). In a follow up study, Zangaladze and colleagues (1999) provided causal support for the functional role of this area by demonstrating that reversible disruption of this same area (using TMS) impaired tactile grating orientation discrimination (Zangaladze, Epstein et al. 1999). Given the selective disruptive effect observed on grating orientation (compared to grating groove width determinations), the authors interpreted their findings to suggest that this targeted visual cortical area was involved in the detection of large scale (“macrogeometric”) tactile features (Zangaladze, Epstein et al. 1999). A later set of experiments by Merabet and colleagues (2004) showed a double dissociation effect for the determination of tactile roughness and linear inter-dot distance judgments following rTMS delivered to parietal and occipital cortex, respectively (Merabet, Thut et al. 2004). A follow up study using fMRI and individual visual retinotopic mapping revealed activation during the same tactile roughness and distance task within primary visual cortex (V1) and deactivation of extrastriate visual areas including V3, V3A, and hV4 (Merabet, Swisher et al. 2007). However, the localization of pattern of activation did not appear to be task-specific, as suggested by the prior TMS study. Furthermore, it is not clear at this juncture why tactile activation of raised dot patterns in the study of Merabet et al. (2004) was associated with activation within striate cortex (i.e. V1) while a similar pattern of was not observed in our sighted controls.
4.5 Potential Study Limitations
On a first level, it is important to realize that only early comparisons can be drawn from the results presented here and previous studies investigating the neural correlates associated with symmetry detection in the visual domain. Again, by design, the random dot tactile stimuli we employed were similar to the visual stimuli used of previous studies. However, to make a truly accurate comparison across visual and tactile modalities would require identifying the neural correlates associated with carrying out both the visual and tactile correlates of the symmetry detection task in the same group of sighted control participants. Further, by matching performance more closing with early blind subjects, a more accurate comparison can be drawn regarding the brain networks related to somatosensory processing and, in particular, the supramodal nature of spatial and object related sensory representations.
Finally, it is important to note that disentangling the role of striate versus extrastriate occipital visual areas in tactile processing (in either blind or sighted individuals) remains a difficult task to resolve. In neuroimaging studies, it is crucial to incorporate appropriate control tasks (as opposed to simply contrasting against rest conditions) in order to best isolate cognitive, attention, and other related components of the behavioral task (see Sathian and Stilla 2010). While this may not always be entirely feasible depending on the task being investigated, careful study design would help in terms of understanding the role of these areas. Certainly, providing causal support for specific regional cortical function using noninvasive brain stimulation techniques such as TMS can also prove very helpful (e.g. Cohen, Celnik et al. 1997). However, there are still inherent limitations in the functional resolution with this technique that pose challenges (e.g. the ability to selective disrupt primary visual areas/striate cortex compared to extratstriate targets). These issues notwithstanding, it is reasonable to speculate that activation within occipital and inferior temporal cortical areas in the early blind maybe important for the detection of tactile symmetrical patterns. However, causal demonstration of this hypothesis awaits appropriate and confirmatory studies. Finally, replicating this experiment in blind subjects with varying levels of Braille reading experience (see Wong, Gnanakumaran et al. 2011) may further help to uncover the effect of tactile experience on our observed superior performance in detecting tactile symmetrical patterns.
5.0 Conclusions
Overall, this study confirms that early blind individuals appear to exhibit enhanced abilities to detect symmetrical tactile patterns compared to sighted (blindfolded) controls. This suggests that prior visual experience may not be a crucial prerequisite for this ability. Secondly, while the neural correlates associated with this task reveal similar patterns of activation in both early blind and sighted controls, they are also marked by key differences. Particularly, in the early blind, we found that tactile detection of symmetry implicates robust activation within inferior temporal as well as occipital and cortices including peri-calcarine and extrastriate areas. Given the importance that symmetry perception plays in the visual domain, our findings could be perhaps even considered counterintuitive. However, framed within the context of compensatory behaviors and crossmodal plasticity, these results contribute to current notions regarding enhanced abilities in the blind with respect to prolonged and early onset profound visual deprivation.
Supplementary Material
Highlights.
- Symmetry is ubiquitous throughout the visual world
- The role of early visual deprivation on tactile symmetry detection remains unclear
- Early blind outperform sighted controls in detecting tactile symmetry patterns
- Functional neuroimaging reveals key similarities and differences in blind and sighted
Acknowledgements
This work was supported by an NIH/NEI RO1 GRANT EY019924 (L.B.M.).
Abbreviations
- EPI
echo planar imaging
- IPS
intraparietal sulcus
- FFA
fusiform face area
- FLAME
FMRIB's Local Analysis of Mixed Effects
- fMRI
functional magnetic resonance imaging
- FWE
family-wise error
- FWHM
full width at half maximum
- GLM
general linear model
- LO
lateral occipital
- MT
middle temporal
- TE
echo time
- TR
repetition time
- rTMS
repetitive transcranial magnetic stimulation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alary F, Duquette M, et al. Tactile acuity in the blind: a closer look reveals superiority over the sighted in some but not all cutaneous tasks. Neuropsychologia. 2009;47(10):2037–2043. doi: 10.1016/j.neuropsychologia.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Amedi A, Jacobson G, et al. Convergence of visual and tactile shape processing in the human lateral occipital complex. Cerebral cortex. 2002;12(11):1202–1212. doi: 10.1093/cercor/12.11.1202. [DOI] [PubMed] [Google Scholar]
- Amedi A, Malach R, et al. Visuo-haptic object-related activation in the ventral visual pathway. Nature neuroscience. 2001;4(3):324–330. doi: 10.1038/85201. [DOI] [PubMed] [Google Scholar]
- Ballesteros S, Manga D, et al. Haptic discrimination of bilateral symmetry in 2-dimensional and 3-dimensional unfamiliar displays. Perception & psychophysics. 1997;59(1):37–50. doi: 10.3758/bf03206846. [DOI] [PubMed] [Google Scholar]
- Ballesteros S, Millar S, et al. Symmetry in haptic and in visual shape perception. Perception & psychophysics. 1998;60(3):389–404. doi: 10.3758/bf03206862. [DOI] [PubMed] [Google Scholar]
- Ballesteros S, Reales JM. Visual and haptic discrimination of symmetry in unfamiliar displays extended in the z-axis. Perception. 2004;33(3):315–327. doi: 10.1068/p5017. [DOI] [PubMed] [Google Scholar]
- Bisley JW. The neural basis of visual attention. The Journal of physiology. 2011;589(Pt 1):49–57. doi: 10.1113/jphysiol.2010.192666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake R, Sobel KV, et al. Neural synergy between kinetic vision and touch. Psychological science. 2004;15(6):397–402. doi: 10.1111/j.0956-7976.2004.00691.x. [DOI] [PubMed] [Google Scholar]
- Bona S, Herbert A, et al. The causal role of the lateral occipital complex in visual mirror symmetry detection and grouping: An fMRI-guided TMS study. Cortex; a journal devoted to the study of the nervous system and behavior. 2014;51:46–55. doi: 10.1016/j.cortex.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Bonino D, Ricciardi E, et al. Tactile spatial working memory activates the dorsal extrastriate cortical pathway in congenitally blind individuals. Archives italiennes de biologie. 2008;146(3-4):133–146. [PubMed] [Google Scholar]
- Buchel C, Price C, et al. Different activation patterns in the visual cortex of late and congenitally blind subjects. Brain : a journal of neurology. 1998;121(Pt 3):409–419. doi: 10.1093/brain/121.3.409. [DOI] [PubMed] [Google Scholar]
- Burton H, Sinclair RJ, et al. Cortical activity to vibrotactile stimulation: an fMRI study in blind and sighted individuals. Human brain mapping. 2004;23(4):210–228. doi: 10.1002/hbm.20064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton H, Snyder AZ, et al. Adaptive changes in early and late blind: a fMRI study of Braille reading. Journal of neurophysiology. 2002;87(1):589–607. doi: 10.1152/jn.00285.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldara R, Seghier ML. The Fusiform Face Area responds automatically to statistical regularities optimal for face categorization. Human brain mapping. 2009;30(5):1615–1625. doi: 10.1002/hbm.20626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmody DP, Nodine CF, et al. Global detection of symmetry. Perceptual and motor skills. 1977;45(3 Pt 2):1267–1273. doi: 10.2466/pms.1977.45.3f.1267. [DOI] [PubMed] [Google Scholar]
- Cattaneo Z, Fantino M, et al. Symmetry perception in the blind. Acta psychologica. 2010;134(3):398–402. doi: 10.1016/j.actpsy.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Cattaneo Z, Vecchi T, et al. The effect of vertical and horizontal symmetry on memory for tactile patterns in late blind individuals. Attention, perception & psychophysics. 2013;75(2):375–382. doi: 10.3758/s13414-012-0393-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LG, Celnik P, et al. Functional relevance of cross-modal plasticity in blind humans. Nature. 1997;389(6647):180–183. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- Creem SH, Proffitt DR. Defining the cortical visual systems: “what”, “where”, and “how”. Acta psychologica. 2001;107(1-3):43–68. doi: 10.1016/s0001-6918(01)00021-x. [DOI] [PubMed] [Google Scholar]
- Cuevas I, Plaza P, et al. Odour discrimination and identification are improved in early blindness. Neuropsychologia. 2009;47(14):3079–3083. doi: 10.1016/j.neuropsychologia.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Dakin SC, Herbert AM. The spatial region of integration for visual symmetry detection. Proceedings. Biological sciences / The Royal Society. 1998;265(1397):659–664. doi: 10.1098/rspb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakin SC, Hess RF. The spatial mechanisms mediating symmetry perception. Vision research. 1997;37(20):2915–2930. doi: 10.1016/s0042-6989(97)00031-x. [DOI] [PubMed] [Google Scholar]
- Davidson PW. Haptic judgments of curvature by blind and sighted humans. Journal of experimental psychology. 1972;93(1):43–55. doi: 10.1037/h0032632. [DOI] [PubMed] [Google Scholar]
- Enquist M, Arak A. Symmetry, beauty and evolution. Nature. 1994;372(6502):169–172. doi: 10.1038/372169a0. [DOI] [PubMed] [Google Scholar]
- Goldreich D, Kanics IM. Tactile acuity is enhanced in blindness. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23(8):3439–3445. doi: 10.1523/JNEUROSCI.23-08-03439.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodale MA, Milner AD. Separate visual pathways for perception and action. Trends in neurosciences. 1992;15(1):20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- Goyal MS, Hansen PJ, et al. Tactile perception recruits functionally related visual areas in the late-blind. Neuroreport. 2006;17(13):1381–1384. doi: 10.1097/01.wnr.0000227990.23046.fe. [DOI] [PubMed] [Google Scholar]
- Grant AC, Thiagarajah MC, et al. Tactile perception in blind Braille readers: a psychophysical study of acuity and hyperacuity using gratings and dot patterns. Perception & psychophysics. 2000;62(2):301–312. doi: 10.3758/bf03205550. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Kourtzi Z, et al. The lateral occipital complex and its role in object recognition. Vision research. 2001;41(10-11):1409–1422. doi: 10.1016/s0042-6989(01)00073-6. [DOI] [PubMed] [Google Scholar]
- Hagen MC, Franzen O, et al. Tactile motion activates the human middle temporal/V5 (MT/V5) complex. The European journal of neuroscience. 2002;16(5):957–964. doi: 10.1046/j.1460-9568.2002.02139.x. [DOI] [PubMed] [Google Scholar]
- Hamilton RH, Pascual-Leone A. Cortical plasticity associated with Braille learning. Trends in cognitive sciences. 1998;2(5):168–174. doi: 10.1016/s1364-6613(98)01172-3. [DOI] [PubMed] [Google Scholar]
- Heller MA. Picture and pattern perception in the sighted and the blind: the advantage of the late blind. Perception. 1989;18(3):379–389. doi: 10.1068/p180379. [DOI] [PubMed] [Google Scholar]
- Heller MA. Tactile memory in sighted and blind observers: the influence of orientation and rate of presentation. Perception. 1989;18(1):121–133. doi: 10.1068/p180121. [DOI] [PubMed] [Google Scholar]
- Heller MA, Calcaterra JA, et al. Tactual picture identification by blind and sighted people: effects of providing categorical information. Perception & psychophysics. 1996;58(2):310–323. doi: 10.3758/bf03211884. [DOI] [PubMed] [Google Scholar]
- Huang L, Pashler H, et al. Are there capacity limitations in symmetry perception? Psychonomic bulletin & review. 2004;11(5):862–869. doi: 10.3758/bf03196713. [DOI] [PubMed] [Google Scholar]
- James TW, Humphrey GK, Gati JS, Servos P, Menon RS, Goodale MA. Haptic study of three-dimensional objects activates extrastriate visual areas. Neuropsychologia. 40(10):1706–14. doi: 10.1016/s0028-3932(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Jones RM, Victor JD, et al. Detecting symmetry and faces: separating the tasks and identifying their interactions. Attention, perception & psychophysics. 2012;74(5):988–1000. doi: 10.3758/s13414-012-0273-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada R, Okamoto Y, et al. Early visual experience and the recognition of basic facial expressions: involvement of the middle temporal and inferior frontal gyri during haptic identification by the early blind. Frontiers in human neuroscience. 2013;7:7. doi: 10.3389/fnhum.2013.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupers R, Ptito M. Compensatory plasticity and cross-modal reorganization following early visual deprivation. Neuroscience and biobehavioral reviews. 2013 doi: 10.1016/j.neubiorev.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Labonte F, Shapira Y, et al. A model for global symmetry detection in dense images. Spatial vision. 1995;9(1):33–55. doi: 10.1163/156856895x00106. [DOI] [PubMed] [Google Scholar]
- Lederman SJ, Klatzky RL, et al. Visual mediation and the haptic recognition of two-dimensional pictures of common objects. Perception & psychophysics. 1990;47(1):54–64. doi: 10.3758/bf03208164. [DOI] [PubMed] [Google Scholar]
- Legge GE, Madison C, et al. Retention of high tactile acuity throughout the life span in blindness. Perception & psychophysics. 2008;70(8):1471–1488. doi: 10.3758/PP.70.8.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo A, Bernardi G, et al. Increased BOLD variability in the parietal cortex and enhanced parieto-occipital connectivity during tactile perception in congenitally blind individuals. Neural plasticity. 2012;2012:720278. doi: 10.1155/2012/720278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessard N, Pare M, et al. Early-blind human subjects localize sound sources better than sighted subjects. Nature. 1998;395(6699):278–280. doi: 10.1038/26228. [DOI] [PubMed] [Google Scholar]
- Little AC, Jones BC. Attraction independent of detection suggests special mechanisms for symmetry preferences in human face perception. Proceedings. Biological sciences / The Royal Society. 2006;273(1605):3093–3099. doi: 10.1098/rspb.2006.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher PJ, Simmons RW. Influence of stimulus symmetry and complexity upon haptic scanning strategies during detection, learning, and recognition tasks. Perception & psychophysics. 1978;23(2):110–116. doi: 10.3758/bf03208290. [DOI] [PubMed] [Google Scholar]
- Loomis JM, Klatzky RL, et al. Similarity of tactual and visual picture recognition with limited field of view. Perception. 1991;20(2):167–177. doi: 10.1068/p200167. [DOI] [PubMed] [Google Scholar]
- Macaluso E. Orienting of spatial attention and the interplay between the senses. Cortex; a journal devoted to the study of the nervous system and behavior. 2010;46(3):282–297. doi: 10.1016/j.cortex.2009.05.010. [DOI] [PubMed] [Google Scholar]
- Machilsen B, Pauwels M, et al. The role of vertical mirror symmetry in visual shape detection. Journal of vision. 2009;9(12):11, 11–11. doi: 10.1167/9.12.11. [DOI] [PubMed] [Google Scholar]
- Malach R, Reppas JB, et al. Object-related activity revealed by functional magnetic resonance imaging in human occipital cortex. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(18):8135–8139. doi: 10.1073/pnas.92.18.8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet L, Thut G, et al. Feeling by sight or seeing by touch? Neuron. 2004;42(1):173–179. doi: 10.1016/s0896-6273(04)00147-3. [DOI] [PubMed] [Google Scholar]
- Merabet LB, Pascual-Leone A. Neural reorganization following sensory loss: the opportunity of change. Nature reviews. Neuroscience. 2010;11(1):44–52. doi: 10.1038/nrn2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merabet LB, Swisher JD, et al. Combined activation and deactivation of visual cortex during tactile sensory processing. Journal of neurophysiology. 2007;97(2):1633–1641. doi: 10.1152/jn.00806.2006. [DOI] [PubMed] [Google Scholar]
- Millar S. Self-referent and movement cues in coding spatial location by blind and sighted children. Perception. 1981;10(3):255–264. doi: 10.1068/p100255. [DOI] [PubMed] [Google Scholar]
- Moller AP. Female swallow preference for symmetrical male sexual ornaments. Nature. 1992;357(6375):238–240. doi: 10.1038/357238a0. [DOI] [PubMed] [Google Scholar]
- Norman JF, Bartholomew AN. Blindness enhances tactile acuity and haptic 3-D shape discrimination. Attention, perception & psychophysics. 2011;73(7):2323–2331. doi: 10.3758/s13414-011-0160-4. [DOI] [PubMed] [Google Scholar]
- Pasqualotto A, Lam JS, et al. Congenital blindness improves semantic and episodic memory. Behavioural brain research. 2013;244:162–165. doi: 10.1016/j.bbr.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Peltier S, Stilla R, et al. Activity and effective connectivity of parietal and occipital cortical regions during haptic shape perception. Neuropsychologia. 2007;45(3):476–483. doi: 10.1016/j.neuropsychologia.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Pietrini P, Furey ML, et al. Beyond sensory images: Object-based representation in the human ventral pathway. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(15):5658–5663. doi: 10.1073/pnas.0400707101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed CL, Klatzky RL, et al. What vs. where in touch: an fMRI study. NeuroImage. 2005;25(3):718–726. doi: 10.1016/j.neuroimage.2004.11.044. [DOI] [PubMed] [Google Scholar]
- Renier LA, Anurova I, et al. Multisensory integration of sounds and vibrotactile stimuli in processing streams for “what” and “where”. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(35):10950–10960. doi: 10.1523/JNEUROSCI.0910-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi-Arnaud C, Pieroni L, et al. Symmetry and binding in visuo-spatial working memory. Neuroscience. 2006;139(1):393–400. doi: 10.1016/j.neuroscience.2005.10.048. [DOI] [PubMed] [Google Scholar]
- Rossi-Arnaud C, Pieroni L, et al. Working memory and individual differences in the encoding of vertical, horizontal and diagonal symmetry. Acta psychologica. 2012;141(1):122–132. doi: 10.1016/j.actpsy.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Sadato N, Pascual-Leone A, et al. Activation of the primary visual cortex by Braille reading in blind subjects. Nature. 1996;380(6574):526–528. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- Sani L, Ricciardi E, et al. Effects of Visual Experience on the Human MT+ Functional Connectivity Networks: An fMRI Study of Motion Perception in Sighted and Congenitally Blind Individuals. Frontiers in systems neuroscience. 2010;4:159. doi: 10.3389/fnsys.2010.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Vanduffel W, et al. Symmetry activates extrastriate visual cortex in human and nonhuman primates. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(8):3159–3163. doi: 10.1073/pnas.0500319102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathian K, Stilla R. Cross-modal plasticity of tactile perception in blindness. Restorative neurology and neuroscience. 2010;28(2):271–281. doi: 10.3233/RNN-2010-0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathian K, Zangaladze A, et al. Tactile spatial acuity and roughness discrimination: impairments due to aging and Parkinson's disease. Neurology. 1997;49(1):168–177. doi: 10.1212/wnl.49.1.168. [DOI] [PubMed] [Google Scholar]
- Stevens JC, Cruz LA. Spatial acuity of touch: ubiquitous decline with aging revealed by repeated threshold testing. Somatosensory & motor research. 1996;13(1):1–10. doi: 10.3109/08990229609028907. [DOI] [PubMed] [Google Scholar]
- Stilla R, Deshpande G, et al. Posteromedial parietal cortical activity and inputs predict tactile spatial acuity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(41):11091–11102. doi: 10.1523/JNEUROSCI.1808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilla R, Hanna R, et al. Neural processing underlying tactile microspatial discrimination in the blind: a functional magnetic resonance imaging study. Journal of vision. 2008;8(10):13, 11–19. doi: 10.1167/8.10.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stilla R, Sathian K. Selective visuo-haptic processing of shape and texture. Human brain mapping. 2008;29(10):1123–1138. doi: 10.1002/hbm.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swisher JD, Halko MA, et al. Visual topography of human intraparietal sulcus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(20):5326–5337. doi: 10.1523/JNEUROSCI.0991-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treder MS. Behind the looking glass: A review on human symmetry perception. Symmetry. 2010;2:510–543. [Google Scholar]
- Tyler CW, Baseler HA, et al. Predominantly extra-retinotopic cortical response to pattern symmetry. NeuroImage. 2005;24(2):306–314. doi: 10.1016/j.neuroimage.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Tyler CW, Hardage L, et al. Multiple mechanisms for the detection of mirror symmetry. Spatial vision. 1995;9(1):79–100. doi: 10.1163/156856895x00124. [DOI] [PubMed] [Google Scholar]
- Van Boven RW, Hamilton RH, et al. Tactile spatial resolution in blind braille readers. Neurology. 2000;54(12):2230–2236. doi: 10.1212/wnl.54.12.2230. [DOI] [PubMed] [Google Scholar]
- Wagemans J. Detection of visual symmetries. Spatial vision. 1995;9(1):9–32. doi: 10.1163/156856895x00098. [DOI] [PubMed] [Google Scholar]
- Wan CY, Wood AG, et al. Congenital blindness leads to enhanced vibrotactile perception. Neuropsychologia. 2010;48(2):631–635. doi: 10.1016/j.neuropsychologia.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Wong M, Gnanakumaran V, et al. Tactile spatial acuity enhancement in blindness: evidence for experience-dependent mechanisms. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31(19):7028–7037. doi: 10.1523/JNEUROSCI.6461-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangaladze A, Epstein CM, et al. Involvement of visual cortex in tactile discrimination of orientation. Nature. 1999;401(6753):587–590. doi: 10.1038/44139. [DOI] [PubMed] [Google Scholar]
- Zhang M, Weisser VD, et al. Multisensory cortical processing of object shape and its relation to mental imagery. Cognitive, affective & behavioral neuroscience. 2004;4(2):251–259. doi: 10.3758/cabn.4.2.251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.