Abstract
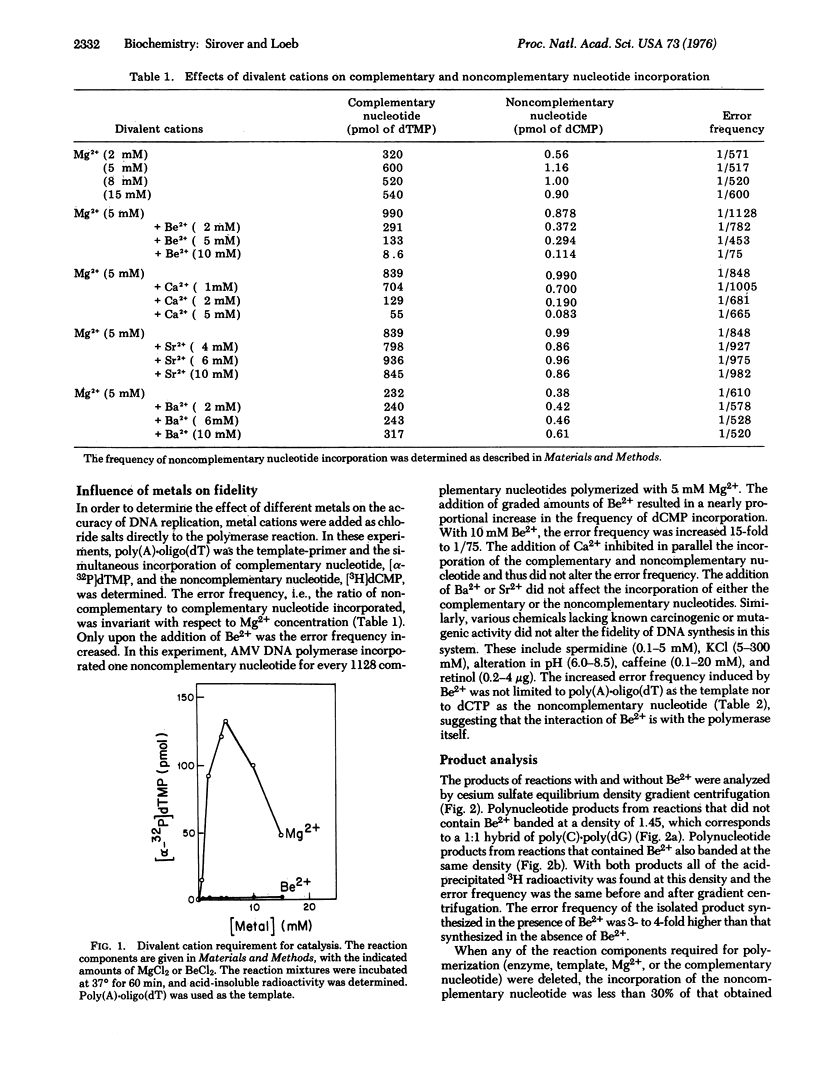
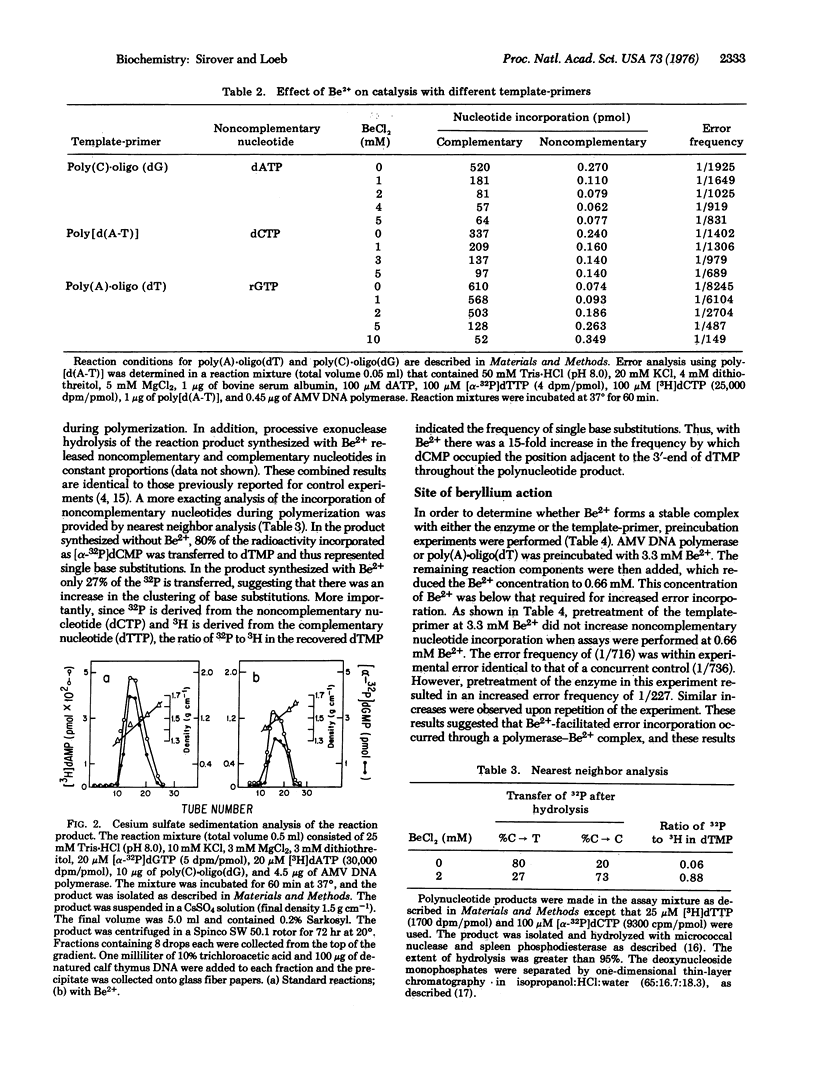
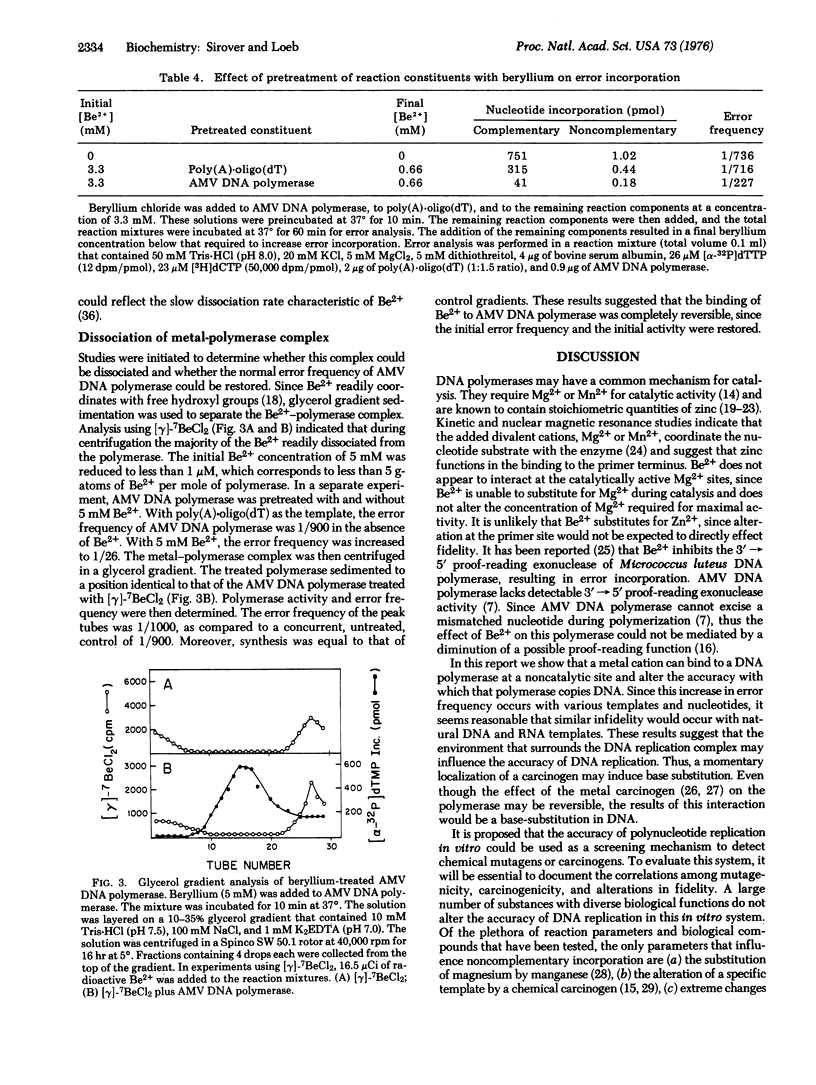
The effect of several divalent cations on the accuracy of DNA replication in vitro has been examined. Only Be2+ altered the accuracy of DNA synthesis using purified DNA polymerase (DNA nucleotidyltransferase; deoxynucleosidetriphosphate:DNA deoxynucleotidyltransferase; EC 2.7.7.7) from avian myeloblastosis virus. The Be2+-induced base substitutions occurred with all templates and with all nucleotides tested. Analysis of the product by equilibrium density centrifugation and processive hydrolysis with snake venom phosphodiesterase suggested that the noncomplementary nucleotides were present in phosphodiester linkage. Nearest neighbor studies indicated that many of the Be2+-induced errors were present as single base substitutions. The enhancement of error frequency could be duplicated by the pretreatment of the enzyme, but not the template, with Be2+. Glycerol gradient centrifugation dissociated the Be2+-DNA polymerase complex and restored the initial error frequency of the polymerase. Thus, the weak binding of a metal cation to a DNA polymerase could alter the accuracy with which that polymerase copied DNA. Beryllium is a known carcinogen. The potential use of this system as a screening technique to detect chemical mutagens and carcinogens is considered.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Durston W. E., Yamasaki E., Lee F. D. Carcinogens are mutagens: a simple test system combining liver homogenates for activation and bacteria for detection. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2281–2285. doi: 10.1073/pnas.70.8.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld D. S., Kawaguchi H., Livingston D. M., Vallee B. L. Zinc reverse transcriptases from mammalian RNA type C viruses. Biochem Biophys Res Commun. 1975 Jan 20;62(2):296–302. doi: 10.1016/s0006-291x(75)80137-9. [DOI] [PubMed] [Google Scholar]
- Battula N., Dube D., Loeb L. A. Avian myeloblastosis virus DNA polymerase. Kinetic studies on the incorporation of noncomplementary nucleotides. J Biol Chem. 1975 Nov 10;250(21):8404–8408. [PubMed] [Google Scholar]
- Battula N., Loeb L. A. On the fidelity of DNA replication. Characterization of polynucleotides with errors in base-pairing synthesized by avian myeloblastosis virus deoxyribonucleic acid polymerase. J Biol Chem. 1975 Jun 25;250(12):4405–4409. [PubMed] [Google Scholar]
- Battula N., Loeb L. A. On the fidelity of DNA replication. Lack of exodeoxyribonuclease activity and error-correcting function in avian myeloblastosis virus DNA polymerase. J Biol Chem. 1976 Feb 25;251(4):982–986. [PubMed] [Google Scholar]
- Battula N., Loeb L. A. The infidelity of avian myeloblastosis virus deoxyribonucleic acid polymerase in polynucleotide replication. J Biol Chem. 1974 Jul 10;249(13):4086–4093. [PubMed] [Google Scholar]
- Brutlag D., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. 36. A proofreading function for the 3' leads to 5' exonuclease activity in deoxyribonucleic acid polymerases. J Biol Chem. 1972 Jan 10;247(1):241–248. [PubMed] [Google Scholar]
- Dube D. K., Loeb L. A. Manganese as a mutagenic agent during in vitro DNA synthesis. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1041–1046. doi: 10.1016/0006-291x(75)90779-2. [DOI] [PubMed] [Google Scholar]
- HUEPER W. C. Experimental studies in metal cancerigenesis. IX. Pulmonary lesions in guinea pigs and rats exposed to prolonged inhalation of powdered metallic nickel. AMA Arch Pathol. 1958 Jun;65(6):600–607. [PubMed] [Google Scholar]
- Leis J. P., Hurwitz J. RNA-dependent DNA polymerase activity of RNA tumor viruses. II. Directing influence of RNA in the reaction. J Virol. 1972 Jan;9(1):130–142. doi: 10.1128/jvi.9.1.130-142.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke M. Z., Hamilton L., Hollocher T. C. Beryllium-induced misincorporation by a DNA polymerase: a possible factor in beryllium toxicity. Biochem Biophys Res Commun. 1975 Jan 20;62(2):497–501. doi: 10.1016/s0006-291x(75)80166-5. [DOI] [PubMed] [Google Scholar]
- McCann J., Spingarn N. E., Kobori J., Ames B. N. Detection of carcinogens as mutagens: bacterial tester strains with R factor plasmids. Proc Natl Acad Sci U S A. 1975 Mar;72(3):979–983. doi: 10.1073/pnas.72.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgel A., Orgel L. E. Induction of mutations in bacteriophage T4 with divalent manganese. J Mol Biol. 1965 Dec;14(2):453–457. doi: 10.1016/s0022-2836(65)80195-4. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Battula N., Loeb L. A. Zinc in reverse transcriptase. Biochem Biophys Res Commun. 1974 Feb 27;56(4):959–964. doi: 10.1016/s0006-291x(74)80282-2. [DOI] [PubMed] [Google Scholar]
- Poiesz B. J., Seal G., Loeb L. A. Reverse transcriptase: correlation of zinc content with activity. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4892–4896. doi: 10.1073/pnas.71.12.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADDING C. M., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. XIII. Kinetics of primed and de novo synthesis of deoxynucleotide polymers. J Biol Chem. 1962 Sep;237:2877–2882. [PubMed] [Google Scholar]
- Reeves A. L., Deitch D., Vorwald A. J. Beryllium carcinogenesis. I. Inhalation exposure of rats to beryllium sulfate aerosol. Cancer Res. 1967 Mar;27(3):439–445. [PubMed] [Google Scholar]
- Reeves A. L., Vorwald A. J. Beryllium carcinogenesis. II. Pulmonary deposition and clearance of inhaled beryllium sulfate in the rat. Cancer Res. 1967 Mar;27(3):446–451. [PubMed] [Google Scholar]
- Sirover M. A., Loeb L. A. Erroneous base-pairing induced by a chemical carcinogen during DNA synthesis. Nature. 1974 Nov 29;252(5482):414–416. doi: 10.1038/252414a0. [DOI] [PubMed] [Google Scholar]
- Sirover M. A., Loeb L. A. Infidelity of DNA synthesis: a general property of RNA tumor viruses. Biochem Biophys Res Commun. 1974 Nov 27;61(2):410–414. doi: 10.1016/0006-291x(74)90972-3. [DOI] [PubMed] [Google Scholar]
- Sirover M. A., Loeb L. A. Restriction of carcinogen-induced error incorporation during in vitro DNA synthesis. Cancer Res. 1976 Feb;36(2 Pt 1):516–523. [PubMed] [Google Scholar]
- Slater J. P., Mildvan A. S., Loeb L. A. Zinc in DNA polymerases. Biochem Biophys Res Commun. 1971 Jul 2;44(1):37–43. doi: 10.1016/s0006-291x(71)80155-9. [DOI] [PubMed] [Google Scholar]
- Springgate C. F., Loeb L. A. Mutagenic DNA polymerase in human leukemic cells. Proc Natl Acad Sci U S A. 1973 Jan;70(1):245–249. doi: 10.1073/pnas.70.1.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springgate C. F., Loeb L. A. On the fidelity of transcription by Escherichia coli ribonucleic acid polymerase. J Mol Biol. 1975 Oct 5;97(4):577–591. doi: 10.1016/s0022-2836(75)80060-x. [DOI] [PubMed] [Google Scholar]
- Springgate C. F., Mildvan A. S., Abramson R., Engle J. L., Loeb L. A. Escherichia coli deoxyribonucleic acid polymerase I, a zinc metalloenzyme. Nuclear quadrupolar relaxation studies of the role of bound zinc. J Biol Chem. 1973 Sep 10;248(17):5987–5993. [PubMed] [Google Scholar]
- TRAUTNER T. A., SWARTZ M. N., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. X. Influence of bromouracil substitutions on replication. Proc Natl Acad Sci U S A. 1962 Mar 15;48:449–455. doi: 10.1073/pnas.48.3.449. [DOI] [PMC free article] [PubMed] [Google Scholar]


