Abstract
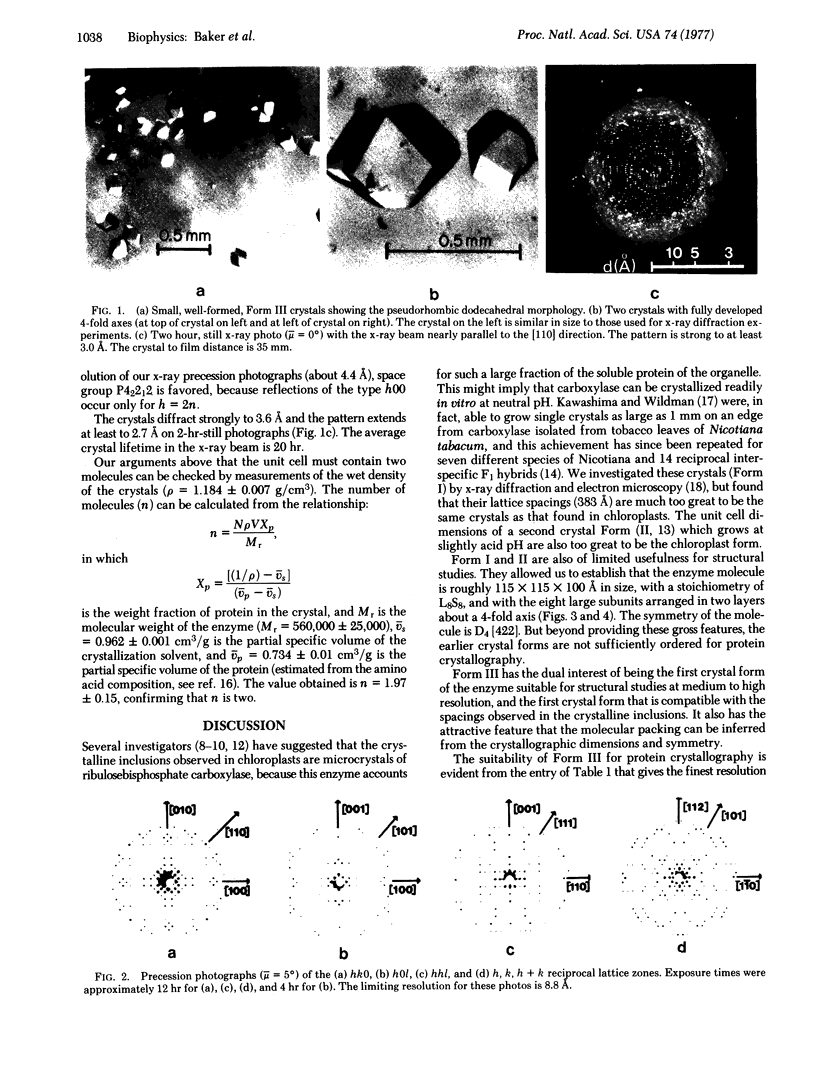
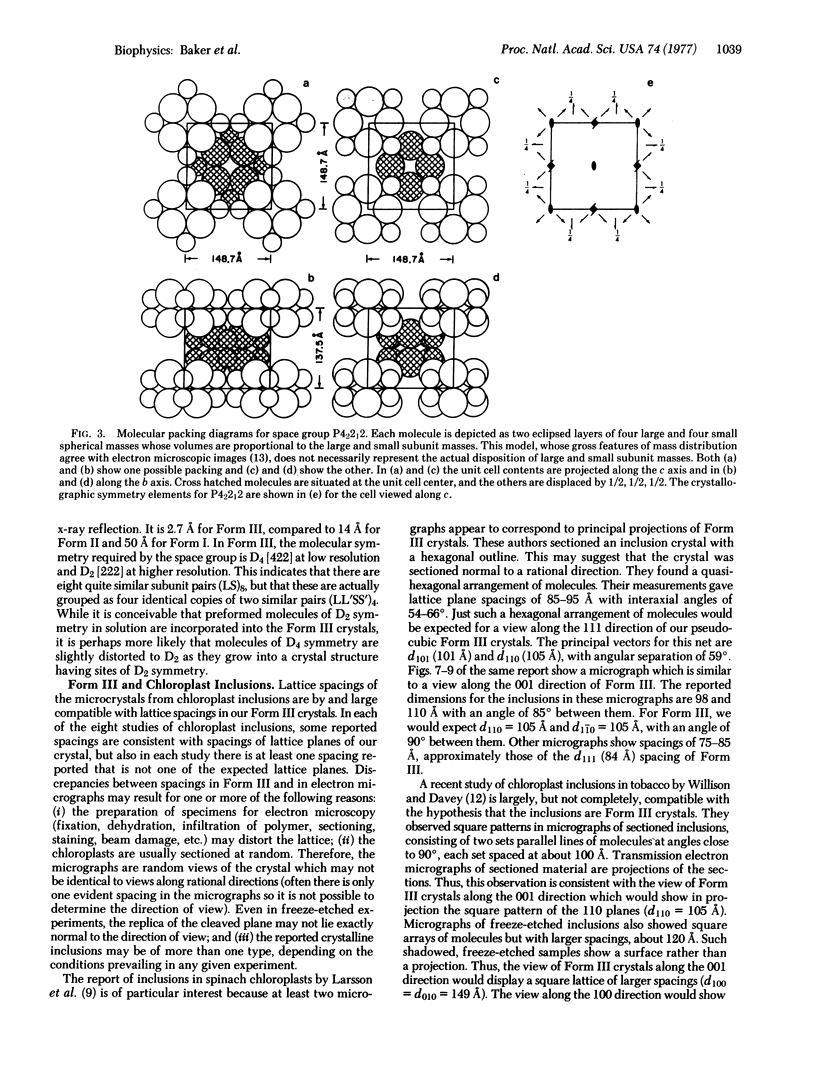
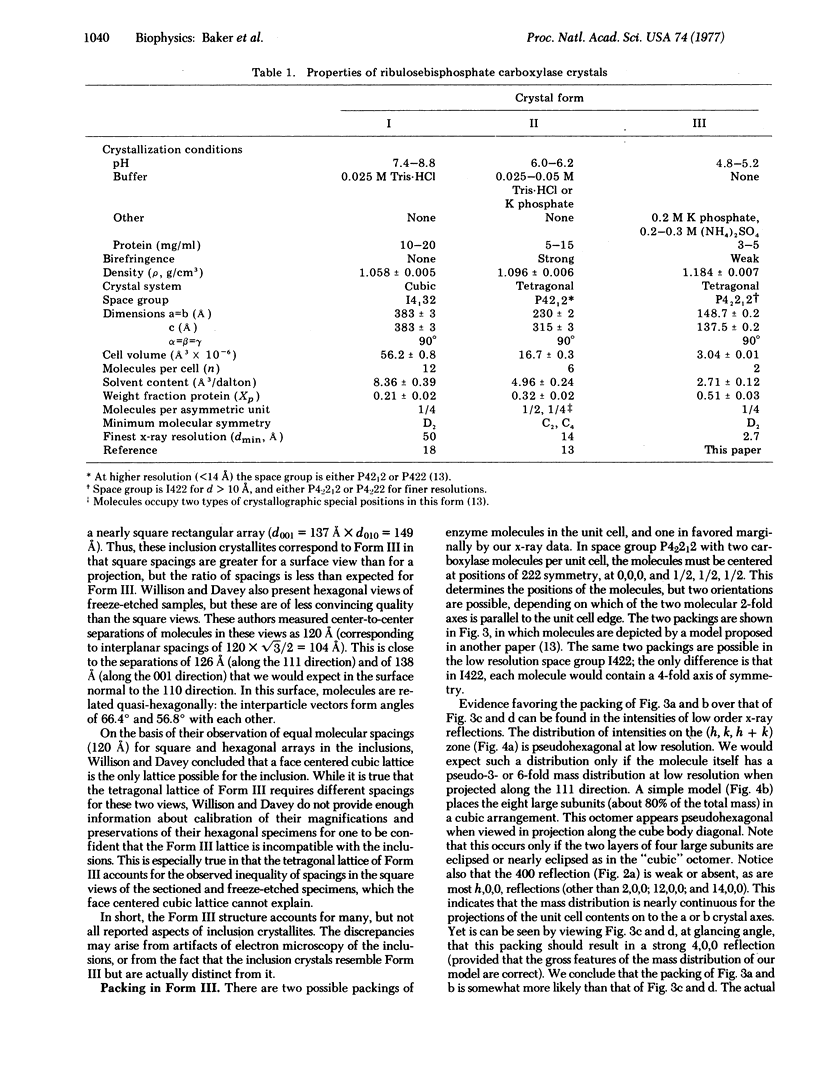
A new crystal form (III) of tobacco leaf ribulosebisphosphate carboxylase [3-phospho-D-glycerate carboxy-lyase (dimerizing), EC 4.1.1.39] has been grown by dialysis procedures, and is suitable for structural studies at near atomic resolution. The crystals exhibit birefringence, grow as pseudo-regular rhombic dodecahedrons, and belong to the tetragonal space group P42212 with a = b = 149 Å, c = 138 Å, and V = 3.04 × 106 A3. Each unit cell contains two molecules, with two large and two small subunits per asymmetric unit. At low resolution (>10 Å) the crystal structure is body centered belonging to space group 1422 with one large/small pair in the asymmetric unit. Thus, at low resolution the molecular symmetry is D4, the highest possible symmetry for an oligomer of stoichiometry large8small8. Form III crystals may be identical to crystalline inclusions found in chloroplasts.
Keywords: protein structure, x-ray diffraction, molecular symmetry, chloroplast crystals
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker T. S., Eisenberg D., Eiserling F. A., Weissman L. The structure of form I crystals of D-ribulose-1,5-diphosphate carboxylase. J Mol Biol. 1975 Feb 5;91(4):391–399. doi: 10.1016/0022-2836(75)90267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan P. H., Sakano K., Singh S., Wildman S. G. Crystalline fraction I protein: preparation in large yield. Science. 1972 Jun 9;176(4039):1145–1146. doi: 10.1126/science.176.4039.1145. [DOI] [PubMed] [Google Scholar]
- DORNER R. W., KAHN A., WILDMAN S. G. The proteins of green leaves. VII. Synthesis and decay of the cytoplasmic proteins during the life of the tobacco leaf. J Biol Chem. 1957 Dec;229(2):945–952. [PubMed] [Google Scholar]
- Esau K. Crystalline inclusion in thylakoids of spinach chloroplasts. J Ultrastruct Res. 1975 Nov;53(2):235–243. doi: 10.1016/s0022-5320(75)80140-7. [DOI] [PubMed] [Google Scholar]
- Kawashima N., Wildman S. G. Studies on fraction-I protein. I. Effect of crystallization of fraction-I protein from tobacco leaves on ribulose diphosphate carboxylase activity. Biochim Biophys Acta. 1971 Jan 19;229(1):240–249. [PubMed] [Google Scholar]
- Larsson C., Collin C., Albertsson P. A. The fine structure of chloroplast stroma crystals. J Ultrastruct Res. 1973 Oct;45(1):50–58. doi: 10.1016/s0022-5320(73)90032-4. [DOI] [PubMed] [Google Scholar]
- Miller K. R., Bloodgood R. A., Staehelin L. A. Crystals within thylakoids: a structural analysis. J Ultrastruct Res. 1976 Jan;54(1):29–36. doi: 10.1016/s0022-5320(76)80005-6. [DOI] [PubMed] [Google Scholar]
- Nishimura M., Takabe T., Sugiyama T., Akazawa T. Structure and function of chloroplast proteins. XIX. Dissociation of spinach leaf ribulose-1,5-diphosphate carboxylase by p-mercuribenzoate. J Biochem. 1973 Nov;74(5):945–954. [PubMed] [Google Scholar]
- Willison J. H., Davey M. R. Fraction 1 protein crystals in chloroplasts of isolated tobacco leaf protoplasts: a thin-section and freeze-etch morphological study. J Ultrastruct Res. 1976 Jun;55(3):303–311. doi: 10.1016/s0022-5320(76)80088-3. [DOI] [PubMed] [Google Scholar]