Abstract
AIM: To study the effect of indomethacin (IN) on human colon cancer cell line SW480 with p53 mutant and SW480 transfected wild-type p53 (wtp53/SW480) in vitro and investigate molecular mechanism of anti-tumor effect of IN on colon cancer.
METHODS: SW480 cells and wtp53/SW480 cells were treated with different concentrations of IN respectively, the expressions of CDK2, CDK4 and p21WAF1/CIP1 protein were detected by Western blotting.
RESULTS: IN gradually down-regulated the expression of CDK2, CDK4 protein of wtp53/SW480 cells in a dose-dependent manner, and inhibitory effect reached the maximum level at 600 μmol/L; IN up-regulated the expression of p21WAF1/CIP1 protein in a dose-dependent manner at a certain concentration range, and the expression reached the maximum level at 400 μmol/L, and returned to the base level at 600 μmol/L. The expression of CDK2, CDK4 and p21WAF1/CIP1 protein of SW480 cells did not change.
CONCLUSION: IN exerts antitumor effect partly through down regulation of the expression of CDK2, CDK4 protein and up regulation of the expression of p21WAF1/PIC1.
Keywords: CDK2, CDK4, p21WAF1/CIP1, SW480, Colon cancer
INTRODUCTION
Nonsteroidal anti-inflammatory drugs (NSAIDs) have been shown to reduce the incidence and mortality from colorectal cancer[1,2]. Indomethacin (IN), a NSAID, is related with the decrease of incidence rate and mortality rate of colon cancer when administered on a long-term basis. Previous studies demonstrated that IN inhibit growth and metastasis of tumor by non-selectively inhibiting cyclooxygenase (COX)[3-5]. It is found in recent years that inhibiting cell proliferation and inducing cell apoptosis is another mechanism by which IN inhibit tumor[6-8]. In this study, Western blot analysis was employed to examine the effect of IN on the expression of cyclin-dependent kinase (CDK) CDK2, CDK4 and cyclin-dependent kinase inhibitor (CKI) p21WAF1/CIP1 in wild-type p53 transfected colon cancer cell line SW480 (wtp53/SW480), so as to provide experimental evidence to elucidate the anti-tumor mechanism of IN.
MATERIALS AND METHODS
Reagents
p53-Mutated human colon cancer SW480 cell line was purchased from ATCC. wtp53/SW480 was SW480 transfected with wild-type p53 allele according to Bampoe and Shetty ascribed previously[9,10], wtp53/PLXSN recombinant plasmid was kindly provided by Dr. Zhou Xiao, wtp53/PLXSN plasmid was transfected into SW480 cells. Eight-hundred milligrams per liter of G418 was added 48 h after transfection and kept at 200 mg/L concentration for G418 resistance screening. G418-resistant clones of wtp53/SW480 cells were selected randomly. The cells were amplified in media with 200 mg/L G418. The blank plasmid PLXSN was transfected into SW480 cells for the control. Total RNA was extracted from wtp53/SW480 cells, SW480 cells and blank plasmid transfected SW480 cells using Trizol isolation kit according to the manufacturer’s instructions. cDNA was synthesized from total RNA in 20 μL reaction mixture using a RT-PCR kit (GIBICO-BRL), including 25 mmol/L MgCl2 24 μL, 10×RT buffer 2 μL, 10 mmol/L dNTP 2 μL, Rnasin 40 U, AMV 200 U, Oligo(dT) 151 μL. The mixture was incubated at 42 °C for 60 min, 95 °C for 5 min. Fifty microliters of PCR reaction mixture contained 5 μL product of the reverse transcription as a template, 10×PCR buffer 5 μL, sense and anti-sense primers 50 pmol, 10 mmol dNTP 2 μL, Taq DNA polymerase 2 U. The PCR condition was 94 °C for 3 min pre-denaturing, then 30 cycles at 94 °C for 50 s, 53 °C for 30 s, and 72 °C for 40 s, and further extension at 72 °C for 7 min, PCR products were resolved on 1% agarose gels and visualized by ethidium bromide staining. RPMI-1640 was purchased from GIBICO-BRL, IN was dissolved in DMSO (Sigma) to make 200 mmol/L stock solution and kept at -20 °C. Rabbit anti-CDK2, CDK4, and p21WAF1/CIP polyclonal antibodies were purchased from Santa Cruz; anti-β-actin antibody was kindly provided by Dr. Zhi-Ming He from the Cancer Research Institute, Xiangya Medical College, horseradish peroxidase-conjugated goat anti-rabbit and goat anti-mouse second antibodies, NC membranes were from Huamei Biotech Company (Luo Yan, China); BCATM Protein Assay Reagent Kit was from Pierce Chemical Co. (Rockford, IL), ECL chemiluminescence kit was from NENTM life science.
Cell culture
SW480 and wtp53/SW480 were maintained in RPMI-1640 medium containing 10% inactive fetal bovine serum, 50 IU/L penicillin and 50 μg/L streptomycin at 37 °C under an atmosphere of 5 mL/L CO2.
Drug treatment
In experimental group, the cells were treated with IN at final concentration of 100, 200, 400 and 600 μmol/L; in the control group, the cells were treated only with medium or DMSO. The cells were harvested in 24 h treatment.
Protein extraction
The cells were washed twice with PBS, followed by 5000 r/min centrifugation at 4 °C for 10 min, lysed with lysis buffer (50 mmol/L Tris-Cl pH 6.8, 1 mmol/L EDTA, 2%SDS, 5 mmol/L DTT, 10 mmol/L PMSF) on ice, denaturation at boiling water, renaturation on ice, disruption with ultrasound for 30 s, 12000 g centrifugation again for 30 min and discard supernatant. The protein concentration was analyzed with Protein Assay Reagent Kit, and aliquots of cell lysates were stocked at -70 °C.
Western blotting
Aliquots of cell lysates containing 80 μg of total proteins were separated by SDS-polyacrylamide gel for 2-3 h, and transferred to nitrocellulose filters. The filters were blocked with PBS-T buffer containing 5% skimmed milk for 4-6 h, incubated with rabbit anti-CDK2 (1:200), anti-CDK4 (1:200), anti-p21WAF1/CIP1 (1:500) polyclonal antibodies respectively for 1 h, then the filters washed with PBS-T for 4 min×15 min and followed by the addition of horseradish peroxidase-linked goat anti-rabbit IgG and ECL visualization of the bands.
For internal control, the same filters were washed with PBS-T for 30 min×3 min, followed by incubation with mouse anti-β-actin in 5% skimmed milk (1:10000 dilution) for 1 h, incubation with horseradish peroxidase-linked goat anti-mouse IgG for 1 h, and ECL visualization of the bands.
RESULTS
Effect of IN on CDK2, CDK4 and p21WAF1/CIP1 protein expression in SW480 cells
After 24 h of action of IN at different concentration, it was found by Western blot analysis that the protein expression of not only CDK2 and CDK4 but also p21WAF1/CIP1 were not obviously changed in a certain range of IN concentration (Figure 1).
Figure 1.
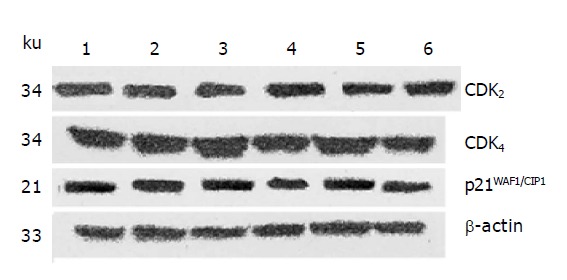
The effect of IN on CDK2, CDK4 and p21WAF1/CIP1 protein expression in SW480 cells. Lane 1: Control group; lane 2: DMSO group; lane 3: IN 100 μmol/L; lane 4: IN 200 μmol/L; lane 5: IN 400 μmol/L; lane 6: IN 600 μmol/L.
Effect of IN on CDK2, CDK4 and p21WAF1/CIP1 protein expression in wtp53/SW480 cells
After 24 h of action of IN at different concentration, it was found by Western blot analysis that the expression of CDK2 and CDK4 was decreased with the increase in IN concentration in certain range. Figure 2 shows that IN can significantly down-regulate the protein expression of CDK2 and CDK4 in a dose-dependent manner in a certain range, and the inhibitory effect was most evident at a IN concentration of 400 and 600 μmol/L; CDK4 seems to be more effective. Meanwhile, the protein expression of p21WAF1/CIPI was increased with the augment of IN concentration in a dose-dependent manner in certain range, most evidently at a IN concentration of 400 μmol/L, and reduced to basic level at a IN concentration of 600 μmol/L.
Figure 2.
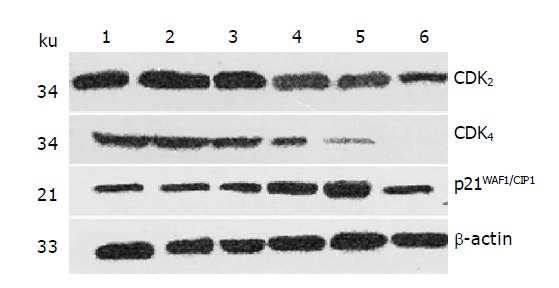
The effect of IN on CDK2, CDK4 and p21WAF1/CIP1 protein expression in wtp53/SW480 cells. Lane 1: Control group; lane 2: DMSO group; lane 3: IN 100 μmol/L; lane 4: IN 200 μmol/L; lane 5 IN 400 μmol/L; lane 6: IN 600 μmol/L.
DISCUSSION
Inhibition of tumor cells proliferation and induction of tumor cells apoptosis are associated with the blocking of the programmed progression of cell cycle[11-13]. NSAIDs inhibit proliferation and induce apoptosis in human colorectal cancer cells in vitro[14]. Our previous studies have shown that IN inhibit proliferation of colon cancer cells by increasing the ratio of cells in G0/G1 phase in tumor cells, reducing the ratio of cells in S phase and G2/M phase and thus lead to cell apoptosis, western blot analysis was employed to investigate the effect of IN on colon cancer cell line HCT116 contained with wild-type p53 and found it could down-regulate the expression of CDK2 and CDK4, and up-regulate p21WAF1/CIP1 expression.
For its instability in solution and a short half-life of only few minutes, p53 protein is difficult to be detected by western blot. In this study, Western blot analysis was employed to detect the expression of CDK2, CDK4 and p21WAF1/CIP1 protein. To eliminate the error caused by the volume of protein, β-actin protein was used as control, for its stable expression and abundant content in the same class of cells which could ensure the reliability of objective protein and β-actin protein.
The results of the study showed that: IN down-regulated the expression of CDK2 and CDK4 protein, especially caused dramatic decreases in Cdk4 activities, which leads to a G1 phase cell cycle arrest, in the meantime, up-regulated p21WAF1/CIP1 protein in wtp53/SW480 cells, in a dose-dependent manner within a certain range of concentration; while it had no effect on the expression of CDK2, CDK4 and p21WAF1/CIP1 protein in SW480 cells, suggesting that the existence of wild-type p53 was required for the down-regulation of CDK2 and CDK4 expression by IN[15]. Therefore, we can speculate that up-regulation of p21WAF1/CIP1 protein expression inhibits CDK2 and CDK4 expression as well as their activity, p21WAF1/CIP1 being an important physiological regulator of CdK4 complexes[16], it increased with the augment of IN concentration, most evidently at 400 μmol/L, and reduced to a basic level at 600 μmol/L, the most effective being 400 μmol/L; the drop of CDK2 and CDK4 activity in turn decreases the activity of CDK2 or CDK4 complex and induces cell cycle arrest in G2 phase, indicating the effect of IN on colon cancer cells transfected with wild-type p53, namely wtp53/SW480 cells, being to induce cell apoptosis depending on p53 in a dose-dependent manner. Based on the results of previous studies and current research, we conclude IN induces cell cycle arrest in G2 phase in a p53-dependent way and inhibits cell proliferation in a p53-p21WAF1/CIP1 dependent way.
As it is well known, cell cycle is dominated by a regulatory network. A cell proliferation-regulating system composed of cyclins, CDKs and CKIs, can accelerate the progression of cell cycle and thus promote cell proliferation. The main regulatory point to start cell cycle is in G1 phase, the core in cell cycle regulation are cyclin and CDK[17], and the formation of CyclinD/CDK4 and CyclinE/CDK2 is the rate-limiting step for progression of G1 phase. Activation of CDK2 and CDK4 can yield many cell cycle related proteins, thus prompting progression from G1 phase to S phase[18,19]; p21WAE1/CIP1, a major member of CIKs family, which is closely related with growth and differentiation of tumor cells, is an extensive inhibitor for CDK, it can bind and block Cyclin/CDK complex and thus inhibit the activity of CDK2 and CDK4, stop the progression of cell cycle and induce G2 phase arrest[11]. Wild-type p53 and p21WAF1/CIP1, which are functionally related, are the inhibitors for CDKs-cyclin complex[20,21], so abnormality in the p53 or p21WAF1/CIP1 gene will lead to abnormal cell proliferation[22-24]. There is a high frequency of p53 mutation in colon cancer, and mutant p53 can neither stop cell growth nor induce cell apoptosis, therefore, colon cancer is resistant to conventional chemotherapy drugs and not sensitive to assistant treatment of DNA damage. Transfection of wild-type p53 together with chemotherapy and radiotherapy can evidently induce apoptosis, reduce the dosage of chemotherapy and radiotherapy and decrease side effects[25,26]. Thus, it is held by some that for many tumors with p53 mutation or deletion which has a very poor sensitivity to radiotherapy and chemotherapy, gene transfection should be considered to enhance the sensitivity[27-29]. So the tumor-suppressing protein p53 mediate chemotherapy drugs induced apoptosis through the regulatory effect of p21 protein on cell cycle progression[30,31]. In vitro experiments have proved that IN inhibits cell proliferation, alters cell cycle and arrests G1 phase possibly through down-regulating CDK2 and CDK4 protein expression and up-regulating p21WAF1/CIP1 protein, giving elementary experimental evidence to elucidate the mechanism by which IN inhibits colon cancer, but much needs to be done to elucidate the anti-tumor mechanism of IN in detail[32] and on a network basis.
Footnotes
Supported by the Ministry of Health, Natural Science Foundation of China, No. 98-2-088
Co-first-author: Gui-Ying Zhang
Science Editor Li WZ Language Editor Elsevier HK
References
- 1.Gołab J, Kozar K, Kamiński R, Czajka A, Marczak M, Switaj T, Giermasz A, Stokłosa T, Lasek W, Zagozdzon R, et al. Interleukin 12 and indomethacin exert a synergistic, angiogenesis-dependent antitumor activity in mice. Life Sci. 2000;66:1223–1230. doi: 10.1016/s0024-3205(00)00427-6. [DOI] [PubMed] [Google Scholar]
- 2.Pozzi A, Yan X, Macias-Perez I, Wei S, Hata AN, Breyer RM, Morrow JD, Capdevila JH. Colon carcinoma cell growth is associated with prostaglandin E2/EP4 receptor-evoked ERK activation. J Biol Chem. 2004;279:29797–29804. doi: 10.1074/jbc.M313989200. [DOI] [PubMed] [Google Scholar]
- 3.Kokoska ER, Smith GS, Wolff AB, Deshpande Y, Miller TA. Nonsteroidal anti-inflammatory drugs attenuate epidermal growth factor-induced proliferation independent of prostaglandin synthesis inhibition. J Surg Res. 1999;84:186–192. doi: 10.1006/jsre.1999.5640. [DOI] [PubMed] [Google Scholar]
- 4.Yoshimi N, Shimizu M, Matsunaga K, Yamada Y, Fujii K, Hara A, Mori H. Chemopreventive effect of N-(2-cyclohexyloxy-4-nitrophenyl)methane sulfonamide (NS-398), a selective cyclooxygenase-2 inhibitor, in rat colon carcinogenesis induced by azoxymethane. Jpn J Cancer Res. 1999;90:406–412. doi: 10.1111/j.1349-7006.1999.tb00762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassano G, Gasparre G, Susca F, Lippe C, Guanti G. Lack of effect by prostaglandin F2alpha on the proliferation of the HCT-8 and HT-29 human adenocarcinoma cell lines. Oncol Rep. 2000;7:183–186. doi: 10.3892/or.7.1.183. [DOI] [PubMed] [Google Scholar]
- 6.Zhu GH, Wong BC, Ching CK, Lai KC, Lam SK. Differential apoptosis by indomethacin in gastric epithelial cells through the constitutive expression of wild-type p53 and/or up-regulation of c-myc. Biochem Pharmacol. 1999;58:193–200. doi: 10.1016/s0006-2952(99)00058-1. [DOI] [PubMed] [Google Scholar]
- 7.Zhu GH, Wong BC, Eggo MC, Ching CK, Yuen ST, Chan EY, Lai KC, Lam SK. Non-steroidal anti-inflammatory drug-induced apoptosis in gastric cancer cells is blocked by protein kinase C activation through inhibition of c-myc. Br J Cancer. 1999;79:393–400. doi: 10.1038/sj.bjc.6690062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kralj M, Kapitanović S, Kovacević D, Lukac J, Spaventi S, Pavelić K. Effect of the nonsteroidal anti-inflammatory drug indomethacin on proliferation and apoptosis of colon carcinoma cells. J Cancer Res Clin Oncol. 2001;127:173–179. doi: 10.1007/s004320000196. [DOI] [PubMed] [Google Scholar]
- 9.Bampoe J, Glen J, Hubbard SL, Salhia B, Shannon P, Rutka J, Bernstein M. Adenoviral vector-mediated gene transfer: timing of wild-type p53 gene expression in vivo and effect of tumor transduction on survival in a rat glioma brachytherapy model. J Neurooncol. 2000;49:27–39. doi: 10.1023/a:1006476608036. [DOI] [PubMed] [Google Scholar]
- 10.Shetty S, Taylor AC, Harris LC. Selective chemosensitization of rhabdomyosarcoma cell lines following wild-type p53 adenoviral transduction. Anticancer Drugs. 2002;13:881–889. doi: 10.1097/00001813-200209000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Zhang G, Tu C, Zhang G, Zhou G, Zheng W. Indomethacin induces apoptosis and inhibits proliferation in chronic myeloid leukemia cells. Leuk Res. 2000;24:385–392. doi: 10.1016/s0145-2126(99)00198-8. [DOI] [PubMed] [Google Scholar]
- 12.Bukholm IK, Nesland JM. Protein expression of p53, p21 (WAF1/CIP1), bcl-2, Bax, cyclin D1 and pRb in human colon carcinomas. Virchows Arch. 2000;436:224–228. doi: 10.1007/s004280050034. [DOI] [PubMed] [Google Scholar]
- 13.Pan MH, Chen WJ, Lin-Shiau SY, Ho CT, Lin JK. Tangeretin induces cell-cycle G1 arrest through inhibiting cyclin-dependent kinases 2 and 4 activities as well as elevating Cdk inhibitors p21 and p27 in human colorectal carcinoma cells. Carcinogenesis. 2002;23:1677–1684. doi: 10.1093/carcin/23.10.1677. [DOI] [PubMed] [Google Scholar]
- 14.Smith ML, Hawcroft G, Hull MA. The effect of non-steroidal anti-inflammatory drugs on human colorectal cancer cells: evidence of different mechanisms of action. Eur J Cancer. 2000;36:664–674. doi: 10.1016/s0959-8049(99)00333-0. [DOI] [PubMed] [Google Scholar]
- 15.Kim OH, Lim JH, Woo KJ, Kim YH, Jin IN, Han ST, Park JW, Kwon TK. Influence of p53 and p21Waf1 expression on G2/M phase arrest of colorectal carcinoma HCT116 cells to proteasome inhibitors. Int J Oncol. 2004;24:935–941. [PubMed] [Google Scholar]
- 16.Skildum AJ, Mukherjee S, Conrad SE. The cyclin-dependent kinase inhibitor p21WAF1/Cip1 is an antiestrogen-regulated inhibitor of Cdk4 in human breast cancer cells. J Biol Chem. 2002;277:5145–5152. doi: 10.1074/jbc.M109179200. [DOI] [PubMed] [Google Scholar]
- 17.Pohl G, Rudas M, Taucher S, Stranzl T, Steger GG, Jakesz R, Pirker R, Filipits M. Expression of cell cycle regulatory proteins in breast carcinomas before and after preoperative chemotherapy. Breast Cancer Res Treat. 2003;78:97–103. doi: 10.1023/a:1022165715043. [DOI] [PubMed] [Google Scholar]
- 18.Yu B, Lane ME, Wadler S. SU9516, a cyclin-dependent kinase 2 inhibitor, promotes accumulation of high molecular weight E2F complexes in human colon carcinoma cells. Biochem Pharmacol. 2002;64:1091–1100. doi: 10.1016/s0006-2952(02)01264-9. [DOI] [PubMed] [Google Scholar]
- 19.Bardon S, Foussard V, Fournel S, Loubat A. Monoterpenes inhibit proliferation of human colon cancer cells by modulating cell cycle-related protein expression. Cancer Lett. 2002;181:187–194. doi: 10.1016/s0304-3835(02)00047-2. [DOI] [PubMed] [Google Scholar]
- 20.Wolter F, Akoglu B, Clausnitzer A, Stein J. Downregulation of the cyclin D1/Cdk4 complex occurs during resveratrol-induced cell cycle arrest in colon cancer cell lines. J Nutr. 2001;131:2197–2203. doi: 10.1093/jn/131.8.2197. [DOI] [PubMed] [Google Scholar]
- 21.Saegusa M, Hashimura M, Kuwata T, Hamano M, Okayasu I. Beta-catenin simultaneously induces activation of the p53-p21WAF1 pathway and overexpression of cyclin D1 during squamous differentiation of endometrial carcinoma cells. Am J Pathol. 2004;164:1739–1749. doi: 10.1016/s0002-9440(10)63732-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu WG, Hileman T, Ke Y, Wang P, Lu S, Duan W, Dai Z, Tong T, Villalona-Calero MA, Plass C, et al. 5-aza-2'-deoxycytidine activates the p53/p21Waf1/Cip1 pathway to inhibit cell proliferation. J Biol Chem. 2004;279:15161–15166. doi: 10.1074/jbc.M311703200. [DOI] [PubMed] [Google Scholar]
- 23.Wu Y, Yan CH, Jin Y, Zhang GY, Li P, Fu SB. Overexpression of p21WAF1 and p53 in human lung adenocarcinoma cell line. Zhongguo YiXue KeXueYuan XueBao. 2003;25:149–152. [PubMed] [Google Scholar]
- 24.Park YN, Chae KJ, Kwon KW, Oh BK, Lee KS, Lee WJ, Park C. p53 and p21WAF1/CIP1 in hepatitis B virus related hepatocarcinogenesis. Hepatogastroenterology. 2003;50:1292–1296. [PubMed] [Google Scholar]
- 25.Kourea HP, Koutras AK, Scopa CD, Marangos MN, Tzoracoeleftherakis E, Koukouras D, Kalofonos HP. Expression of the cell cycle regulatory proteins p34cdc2, p21waf1, and p53 in node negative invasive ductal breast carcinoma. Mol Pathol. 2003;56:328–335. doi: 10.1136/mp.56.6.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasaki Y, Morimoto I, Ishida S, Yamashita T, Imai K, Tokino T. Adenovirus-mediated transfer of the p53 family genes, p73 and p51/p63 induces cell cycle arrest and apoptosis in colorectal cancer cell lines: potential application to gene therapy of colorectal cancer. Gene Ther. 2001;8:1401–1408. doi: 10.1038/sj.gt.3301538. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi A, Ohnishi K, Ota I, Asakawa I, Tamamoto T, Furusawa Y, Matsumoto H, Ohnishi T. p53-dependent thermal enhancement of cellular sensitivity in human squamous cell carcinomas in relation to LET. Int J Radiat Biol. 2001;77:1043–1051. doi: 10.1080/09553000110066095. [DOI] [PubMed] [Google Scholar]
- 28.Gao N, Hu YD, Cao XY, Zhou J, Cao SL. The exogenous wild-type p14ARF gene induces growth arrest and promotes radiosensitivity in human lung cancer cell lines. J Cancer Res Clin Oncol. 2001;127:359–367. doi: 10.1007/s004320000184. [DOI] [PubMed] [Google Scholar]
- 29.Riva CM. Restoration of wild-type p53 activity enhances the sensitivity of pleural metastasis to cisplatin through an apoptotic mechanism. Anticancer Res. 2000;20:4463–4471. [PubMed] [Google Scholar]
- 30.Bampoe J, Glen J, Hubbard SL, Salhia B, Shannon P, Rutka J, Bernstein M. Adenoviral vector-mediated gene transfer: timing of wild-type p53 gene expression in vivo and effect of tumor transduction on survival in a rat glioma brachytherapy model. J Neurooncol. 2000;49:27–39. doi: 10.1023/a:1006476608036. [DOI] [PubMed] [Google Scholar]
- 31.Badie C, Bourhis J, Sobczak-Thépot J, Haddada H, Chiron M, Janicot M, Janot F, Tursz T, Vassal G. p53-dependent G2 arrest associated with a decrease in cyclins A2 and B1 levels in a human carcinoma cell line. Br J Cancer. 2000;82:642–650. doi: 10.1054/bjoc.1999.0976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chu EC, Chai J, Tarnawski AS. NSAIDs activate PTEN and other phosphatases in human colon cancer cells: novel mechanism for chemopreventive action of NSAIDs. Biochem Biophys Res Commun. 2004;320:875–879. doi: 10.1016/j.bbrc.2004.06.036. [DOI] [PubMed] [Google Scholar]


