Abstract
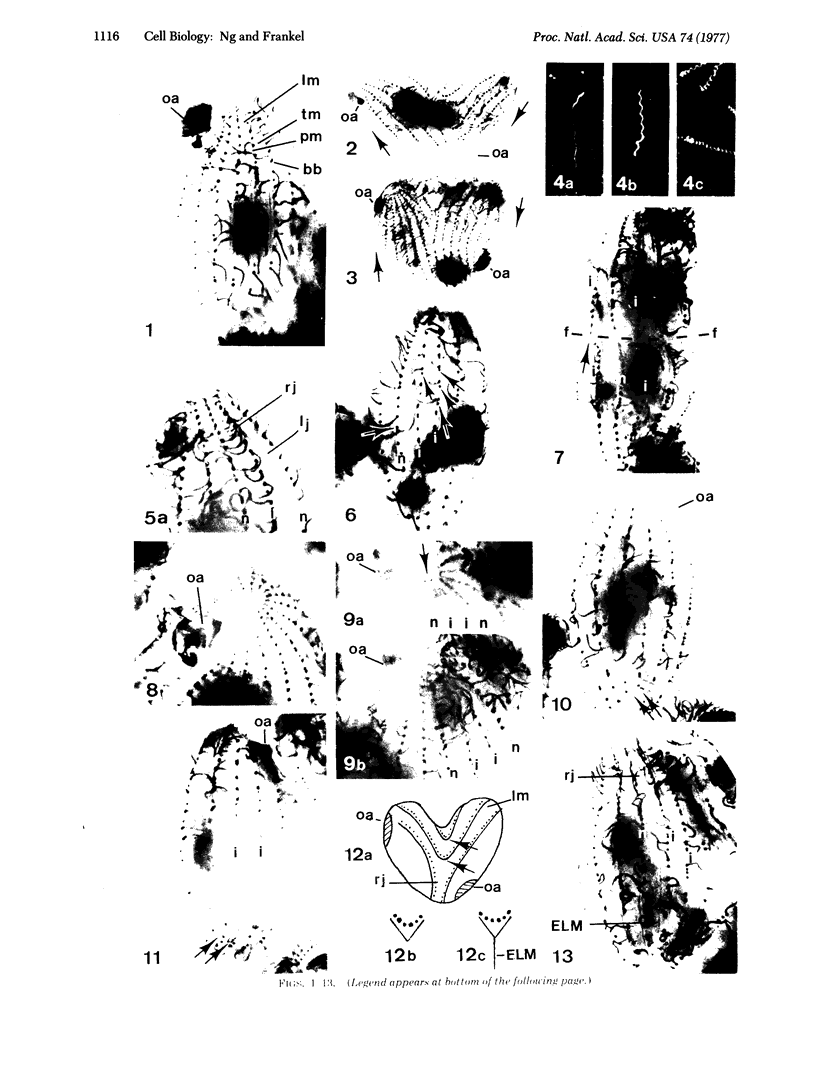
With quasi-surgical techniques, longitudinal somatic ciliary rows in Tetrahymena pyriformis have been rotated 180 degrees. New structures formed in the rotated ciliary rows during growth and reproduction are disposed 180 degrees opposite to their normal positions or orientations, confirming the earlier findings of Beisson and Sonneborn on Paramecium. However, during cell fission the rotated ciliary rows exhibit abnormality in orientation along the fission zone; the configuration of these rows near the anterior end of the posterior product of fission is consequently affected. Rotated ciliary rows have been employed as a tool in the analysis of morphogenetic problems: (a) The contractile vacuole pore is normally located on the left side of a ciliary row; but it is on the right of inverted rows. Hence, the morphogenetic properties of the two sides of the ciliary row associated with the contractile vacuole pore are different and this difference is the sole determinative factor as to the side of the ciliary row on which the contractile vacuole pore is located. (b) The process that generates the rotated ciliary rows frequently also brings about the implantation of an extra band of longitudinal microtubules at a specific site on the cell surface. This extra structure is inheritable, which opens up opportunities for the study of microtubular assembly in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. D. Fine structure, reconstruction and possible functions of components of the cortex of Tetrahymena pyriformis. J Protozool. 1967 Nov;14(4):553–565. doi: 10.1111/j.1550-7408.1967.tb02042.x. [DOI] [PubMed] [Google Scholar]
- BEISSON J., SONNEBORN T. M. CYTOPLASMIC INHERITANCE OF THE ORGANIZATION OF THE CELL CORTEX IN PARAMECIUM AURELIA. Proc Natl Acad Sci U S A. 1965 Feb;53:275–282. doi: 10.1073/pnas.53.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel J., Jenkins L. M., Doerder F. P., Nelsen E. M. Mutations affecting cell division in Tetrahymena pyriformis. I. Selection and genetic analysis. Genetics. 1976 Jul;83(3 PT2):489–506. [PMC free article] [PubMed] [Google Scholar]
- Nanney D. L. Patterns of basal body addition in ciliary rows in Tetrahymena. J Cell Biol. 1975 Jun;65(3):503–512. doi: 10.1083/jcb.65.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONNEBORN T. M. THE DETERMINANTS AND EVOLUTION OF LIFE. THE DIFFERENTIATION OF CELLS. Proc Natl Acad Sci U S A. 1964 May;51:915–929. doi: 10.1073/pnas.51.5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneborn T. M. Gene action in development. Proc R Soc Lond B Biol Sci. 1970 Dec 1;176(1044):347–366. doi: 10.1098/rspb.1970.0054. [DOI] [PubMed] [Google Scholar]
- Tamm S. L., Sonneborn T. M., Dippell R. V. The role of cortical orientation in the control of the direction of ciliary beat in Paramecium. J Cell Biol. 1975 Jan;64(1):98–112. doi: 10.1083/jcb.64.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]