Abstract
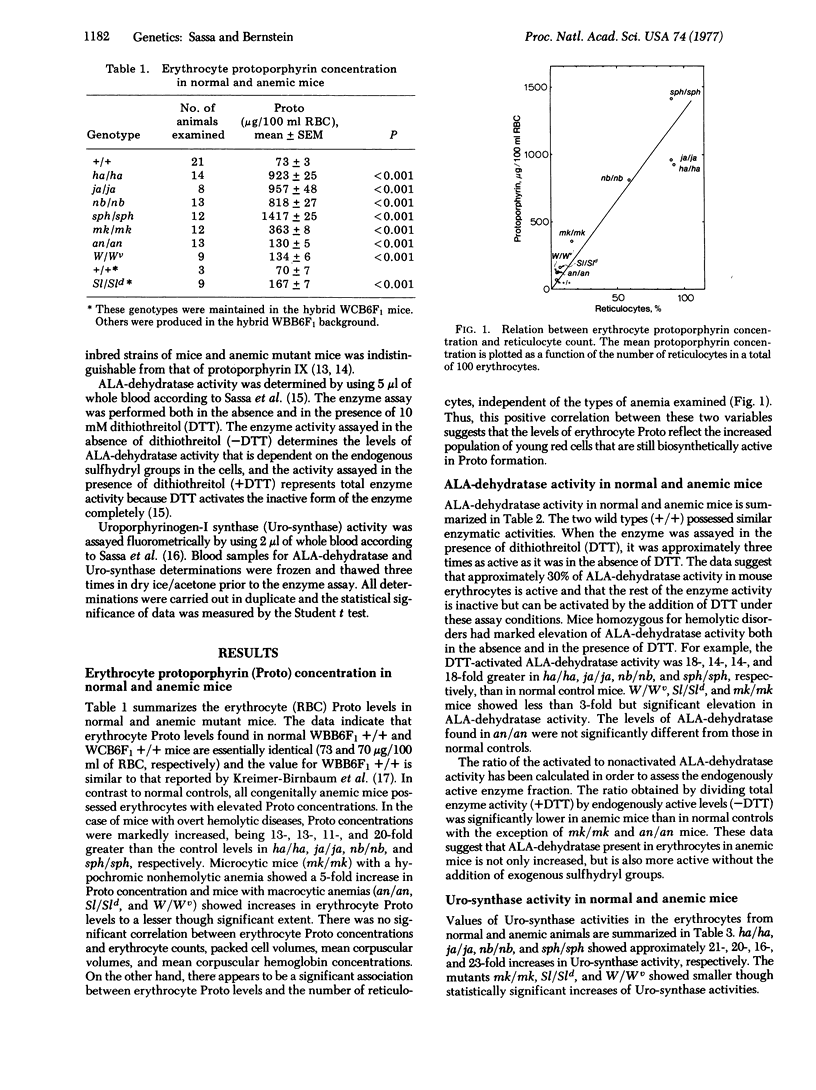
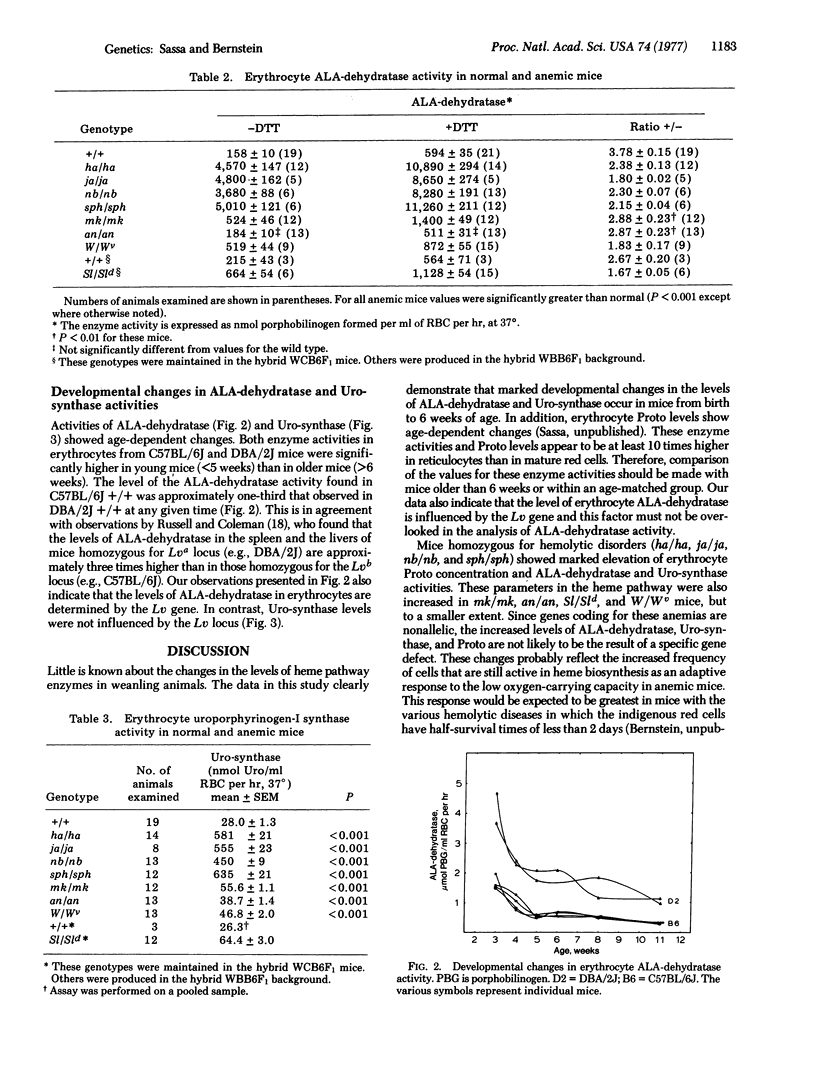
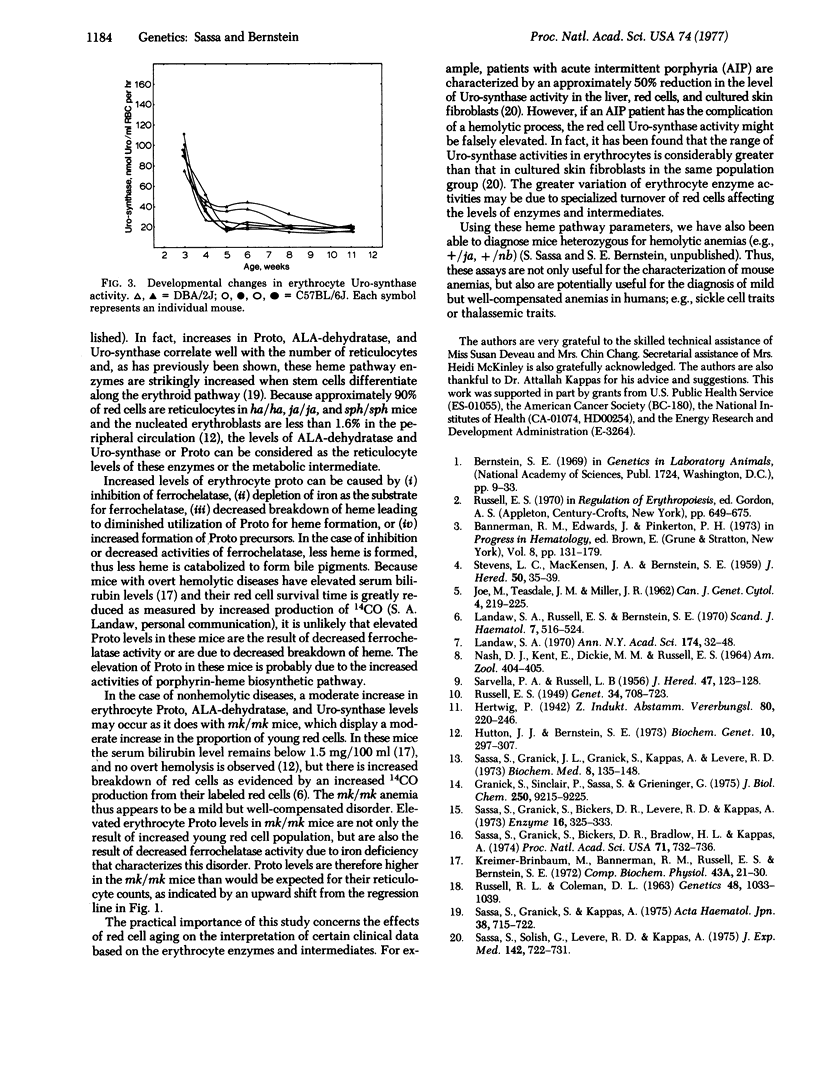
Levels of erythrocyte delta-aminolevulinate dehydratase [ALA-dehydratase; porphobilinogen synthase; 5-aminolevulinate hydro-lyase (adding 5-aminolevulinate and cyclizing), EC 4.2.1.24], UROPORPHYRINOGEN-I synthase [Uro-synthase; porphobilinogen ammonia-lyase (polymerizing), EC 4.3;1.8], AND PROTOPORPHYRIN IX (Proto) were measured by sensitive semimicroassays using 2-5 mul of whole blood obtained from normal and anemic mutant mice. The levels of erythrocyte ALA-dehydratase and Uro-synthase showed marked developmental changes and ALA-dehydratase was influenced by the Lv gene. Mice with overt hemolytic diseases (ja/ja, sph/sph, nb/nb, ha/ha) had 10- to 20-fold increases in ALA-dehydratase, Uro-synthase, and Proto compared with their normal controls. Mice with an iron deficiency (mk/mk) and mice with hypoplastic anemias (W/Wv, Sl/Sld, an/an) had mild to moderate increases in these parameters. Elevated enzyme activities and Proto correlated well with the number of reticulocytes. Because all mice with anemias possessed elevated levels of ALA-dehydratase, Uro-synthase, and Proto independent of differences in their genotypes, the increase in these parameters is not likely to be the result of a specific gene defect. The increased enzyme activities and Proto concentration probably reflect increased frequency of young red cells that are still active in heme biosynthesis.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Granick S., Sinclair P., Sassa S., Grieninger G. Effects by heme, insulin, and serum albumin on heme and protein synthesis in chick embryo liver cells cultured in a chemically defined medium, and a spectrofluorometric assay for porphyrin composition. J Biol Chem. 1975 Dec 25;250(24):9215–9225. [PubMed] [Google Scholar]
- Hutton J. J., Bernstein S. E. Metabolic properties of erythrocytes of normal and genetically anemic mice. Biochem Genet. 1973 Nov;10(3):297–307. doi: 10.1007/BF00485707. [DOI] [PubMed] [Google Scholar]
- JOE M., TEASDALE J. M., MILLER J. R. A new mutation (sph) causing neonatal jaundice in the house mouse. Can J Genet Cytol. 1962 Jun;4:219–225. doi: 10.1139/g62-026. [DOI] [PubMed] [Google Scholar]
- Kreimer-Birnbaum M., Bannerman R. M., Russell E. S., Bernstein S. E. Pyrrole pigments in normal and congenitally anaemic mice (+:+, W-W v , ha-ha, nb-nb, mk-mk, f-f and sla-Y). Comp Biochem Physiol A Comp Physiol. 1972 Sep 1;43(1):21–30. doi: 10.1016/0300-9629(72)90464-1. [DOI] [PubMed] [Google Scholar]
- Landaw S. A. Kinetic aspects of endogenous carbon monoxide production in experimental animals. Ann N Y Acad Sci. 1970 Oct 5;174(1):32–48. doi: 10.1111/j.1749-6632.1970.tb49770.x. [DOI] [PubMed] [Google Scholar]
- Landaw S. A., Russell E. S., Bernstein S. E. Splenic destruction of newly-formed red blood cells and shortened erythrocyte survival in mice with congenital microcytosis. Scand J Haematol. 1970;7(6):516–524. doi: 10.1111/j.1600-0609.1970.tb01940.x. [DOI] [PubMed] [Google Scholar]
- RUSSELL E. S. Analysis of pleiotropism at the W-locus in the mouse; relationship between the effects of W and Wv substitution on hair pigmentation and on erythrocytes. Genetics. 1949 Nov;34(6):708–723. doi: 10.1093/genetics/34.6.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSSELL R. L., COLEMAN D. L. GENETIC CONTROL OF HEPATIC DELTA-AMINOLEVULINATE DEHYDRATASE IN MICE. Genetics. 1963 Aug;48:1033–1039. doi: 10.1093/genetics/48.8.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Granick J. L., Granick S., Kappas A., Levere R. D. Studies in lead poisoning. I. Microanalysis of erythrocyte protoporphyrin levels by spectrophotometry in the detection of chronic lead intoxication in the subclinical range. Biochem Med. 1973 Aug;8(1):135–148. doi: 10.1016/0006-2944(73)90017-3. [DOI] [PubMed] [Google Scholar]
- Sassa S., Granick S., Bickers D. R., Bradlow H. L., Kappas A. A microassay for uroporphyrinogen I synthase, one of three abnormal enzyme activities in acute intermittent porphyria, and its application to the study of the genetics of this disease. Proc Natl Acad Sci U S A. 1974 Mar;71(3):732–736. doi: 10.1073/pnas.71.3.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassa S., Granick S., Bickers D. R., Levere R. D., Kappas A. Studies on the inheritance of human erythrocyte delta-aminolevulinate dehydratase and uroporphyrinogen synthetase. Enzyme. 1973;16(1):326–333. doi: 10.1159/000459397. [DOI] [PubMed] [Google Scholar]
- Sassa S., Granick S., Kappas A. Sequential induction of enzymes in the heme biosynthetic pathway during erythroid differentiation. Nihon Ketsueki Gakkai Zasshi. 1975 Dec;38(6):715–722. [PubMed] [Google Scholar]
- Sassa S., Solish G., Levere R. D., Kappas A. Studies in porphyria. IV. Expression of the gene defect of acute intermittent porphyria in cultured human skin fibroblasts and amniotic cells: prenatal diagnosis of the porphyric trait. J Exp Med. 1975 Sep 1;142(3):722–731. doi: 10.1084/jem.142.3.722. [DOI] [PMC free article] [PubMed] [Google Scholar]


