Abstract
The scarcity of human cadaveric pancreata limits large-scale application of islet transplantation for patients with diabetes. Islets isolated from pathogen-free pigs provide an economical and abundant alternative source assuming immunological barriers are appropriate. Membrane receptors involved in insulin secretion that also have potential as imaging targets were investigated in isolated porcine islets. Quantitative (q)PCR revealed that porcine islets express mRNA transcripts for sulfonylurea receptor 1 (Sur1), inward rectifying potassium channel (Kir6.2, associated with Sur1), glucagon-like peptide 1 receptor (GLP1R), and adrenergic receptor alpha 2A (ADRα2A). Receptor function was assessed in static incubations with stimulatory glucose concentrations, and in the presence of receptor agonists. Glibenclamide, an anti-diabetic sulfonylurea, and Exendin-4, a GLP-1 mimetic, potentiated glucose-stimulated insulin secretion >2-fold. Conversely, epinephrine maximally reduced insulin secretion 72±9% (p < 0.05) and had a half maximal inhibitory concentration of 60nM in porcine islets (95% confidence interval of 45–830nM). The epinephrine action was inhibited by the ADRα2A antagonist yohimbine. Our findings demonstrate that porcine islets express and are responsive to both stimulatory and inhibitory membrane localized receptors, which can be used as imaging targets after transplantation or to modify insulin secretion.
Keywords: Porcine islet, insulin secretion, glibenclamide, epinephrine, exendin-4
Introduction
Islet transplantation (ITx) is emerging as a promising treatment option for diabetes. Widespread application of ITx is currently restricted due to the limited supply of human cadaveric pancreas donors and the need for major systemic immunosuppression. A more cost-effective and widespread application of ITx may be possible using porcine islets; which are available in essentially unlimited quantities. Encapsulation technologies provide an attractive avenue to create immune-privileged transplant devices that can facilitate xenotransplantation (1). Porcine islet use in these applications is already in the clinic (2). Thus methods that enable non-invasive longitudinal functional monitoring of the transplant milieu as well as methods to augment fractional insulin secretion responsiveness are essential. To achieve this, membrane receptors enriched on pancreatic β-cells of other species were evaluated and include sulfonylurea receptor-1 (Sur1), glucagon-like peptide 1 receptor (GLP1R), and adrenergic receptor alpha, isoform 2A (ADRα2A)(3). These receptors provide the basis for targeting with a combination of multivalent ligands for enhancing specificity of contrast agents for imaging. Recently, multivalent ligands designed for Sur1/GLP1R showed high affinity binding in vitro, while ligands to GLP-1R/ADRα2 showed β-cell specific binding in rodents in vivo (4–6).
The presence of these receptors in isolated porcine islets will have tangible effects on islet function. Thus characterizing the response of intact porcine islets to natural ligands and pharmaceuticals used to target these receptors is valuable in understanding how the graft can be modulated after transplant - potentially increasing the effectiveness or lifetime of the transplant. Upon activation, these receptors have physiological roles in the β-cell, including modifying insulin secretion. Glucose entry into β-cells elevates the ATP/ADP ratio, binds a heteromultimeric pore consisting of inward rectifying K+ channel (Kir6.2) and a high affinity Sulfonylurea receptor-1 (Sur1) (7). The Sur1/Kir6.2 complex is essential for stimulus-secretion coupling, highly abundant and specific to pancreatic β-cells. Sulfonylurea drugs, used to activate Sur1, are readily available, FDA approved, and relevant for therapeutic applications in transplant recipients. GLP1R and ADRα2A are G protein coupled receptors that have expansive roles in stimulus-secretion coupling. Activation of GLP1R enhances the production of cAMP and PKA signaling, which leads to mobilization of cytosolic Ca2+, modulation of secretory granule pH and inhibition of ATP-sensitive and voltage-dependent K+ channels to potentiate GSIS (8–10). Conversely, activation of ADRα2A by epinephrine or norepinephrine can negatively modulate insulin release at both early and late stages of stimulus-secretion coupling (11,12).
The abundance of Sur1/Kir6.2, GLP1R, and ADRα2A on the porcine β-cell compared to other islet cell types, may provide useful for more precise imaging of insulin producing cells during graft assessment (13). The present aim was to characterize these receptors in isolated porcine islets available for xenotransplantation. The importance of characterizing these receptors on porcine islets available for xenotransplantation is two fold; the first, to verify they can be targeted by ligands and xenobiotics and secondly, to establish if activation of these receptors has tangible effects on β-cell function.
Material and Methods
Islet Source & Culture
Islet procurement was accomplished by an experienced team at The Schulze Diabetes Institute at the University of Minnesota, under the approval of the Institutional Animal Care and Use Committee. Briefly, adult Landrace sows were sacrificed, pancreata were immediately removed, and islets were isolated using a modified Ricordi method (14). Isolated islets were shipped and maintained at room temperature in gas-permeable culture devices (G-Rex100, Wilson-Wolf Manufacturing, New Brighton, MN) (15,16). Islets were cultured free-floating in ME199 culture medium (Corning Inc, Corning, NY), supplemented with 10% heat-inactivated porcine serum (Gibco, Auckland, NZ), L-glutamine (Corning Inc) and heparin (10U/mL) in G-Rex100 at 37 °C, in humidified air without added CO2. Islet samples were counted after dithizone stain (Sigma-Aldrich, St. Louis, MO) to assess purity. Viability throughout culture was assessed by oxygen consumption rate (OCR) measurements, using a fiber optic oxygen-sensing device (Instech, Plymouth Meeting, PA), as described previously (17). OCR was normalized to DNA content, measured by Quant-iT Picogreen dsDNA (Molecular Probes Inc. Eugene, OR) nucleic acid stain run in quadruplicate.
Insulin Secretion
For all insulin secretion assays, islets were handpicked into 1.5 ml reaction vessels (10 islets/vessel, 4 vessels/condition). Healthy, intact islets were chosen at random. The coefficient of variation was calculated from the total DNA present in each replicate, and was < 20% within a single treatment group and < 25% between experiments. To determine fractional insulin release, islets were challenged with 0, 2.8, 7.5, or 16.7mM glucose KRB for 1hr. Islets were then pelleted by centrifugation (800 × g for 3 min), media aspirated then frozen, and intra-islet insulin extracted with acid-ethanol (1mol/L HCl/ 70% ethanol). To determine response to ligands, islets were incubated in 0mM glucose KRB for 1 hour, then 2.8mM glucose KRB for 1 hour, before stimulating with 11mM glucose alone or supplemented with Exendin-4 (Sigma; 50 nM) or Glibenclamide (Sigma; 50 nM). Epinephrine (Sigma; 0–100µM) was assessed in 16.7mM glucose supplemented with 10µM forskolin, which maximally stimulated glucose potentiated insulin release in porcine islets. A portion of the incubation media was taken after 5 minutes to account for insulin release initiated by mechanical stimulation. Insulin concentrations were measured with an enzyme-linked immunosorbent assay (ELISA) for porcine insulin (Mercodia, Uppsala, Sweden). Coefficient of variation was 11.3 ± 0.6% for samples run on different plates (n=18).
RNA Quantification
RNA was extracted from purified porcine islets using RNeasy Micro Kit (Qiagen, Valencia, CA). RNA quality and concentration was assessed using absorbance at 260 and 280 nm with a NanoDrop ND-1000 Spectrophotometer (NanoDrop, Wilmington, DE). Synthetic oligonucleotide primers were designed against coding sequences of published Sus scrofa genome (Table 1). PCR products for receptors of interest were amplified from mRNA by reverse-transcription-PCR using Superscript III reverse transcriptase and Taq DNA polymerase (Qiagen) according to the manufacturer’s instructions. The PCR products of the correct size were inserted into the TOPO TA cloning expression vector pCRII and transformed into One Shot Mach T1 Phage-Resistant Chemically Competent E. coli (Invitrogen Life Technologies). Plasmids were prepared for nucleotide sequencing with a QIAprep Spin Miniprep Kit (Qiagen) and sequenced at the University of Arizona DNA sequencing service.
Table 1.
Primer sequences, product size, and accession number of published sequence literature.
| Gene | Primer Sequence (5’ to 3’) | Product Size |
Accession # | |
|---|---|---|---|---|
| ADRa2A | F | CGAGCTGGAATGGGACAGAG | 294 | NM_214400.1 |
| R | AGATCTCACACCACGCCTTG | |||
| GLP1R | F | CACAGGCTTGTTCTGCAACC | 414 | NM_001256594.1 |
| R | AAGACGGACAGTGCTCGAAG | |||
| Kir6.2 | F | GTGACCATTGGTTTTGGCGG | 336 | EU655630.1 |
| R | CATGGGGATGTCCACTTGGT | |||
| Sur1 | F | ACATCGAGACGTCCAACTTCC | 115 | EU655631 |
| R | AAGCCGATAGCATGGTCGTAG | |||
| S15 | F | AGAGCAGAAGAAGAGGCGGA | 245 | NM_214334.1 |
| R | GGATGATCATGTCTCGCAGGT | |||
The relative mRNA expression for each receptor was determined by quantitative PCR using SYBR Green (Qiagen) in an iQ5 Real-Time PCR Detection System (Bio-Rad Laboratories, Irvine, CA). Initial denaturation was accomplished by incubation of samples at 95°C for 15 min. After initial denaturation, all reactions went through 45 cycles of 96 °C (30sec), annealing temperature (30sec), and 72 °C (10sec) at which fluorescence was measurement. Optimal annealing temperatures (54–62 °C) for primer sets were determined. Melt curve analysis, starting at 60 °C with an increase of 0.2 °C every 6 seconds to 96 °C, was performed at the end of the amplification to confirm product homogeneity. PCR efficiency was determined with gene-specific plasmid DNA, for which threshold cycles (Ct) were linear over six orders of magnitude. Samples were run in triplicate for each qPCR reaction. Standard curves for each gene product were run concurrently to determine the absolute starting quantity mass by linear regression analysis (18,19). Starting quantities were normalized to the amount of RNA in each reaction and then to the reference gene, ribosomal protein s15.
Statistical Analysis
Values are expressed as the mean ± SEM and significant differences were accepted at P<0.05. An ANOVA was performed on the OCR/DNA with the repeated measure as day with MIXED procedures (SAS Institute, Cary NC, USA). The dose response curve was generated using the log-dose model with standard slope (Hill coefficient=-1.0) in GraphPad Prism (San Diego, CA).
Results
Islet Characterization
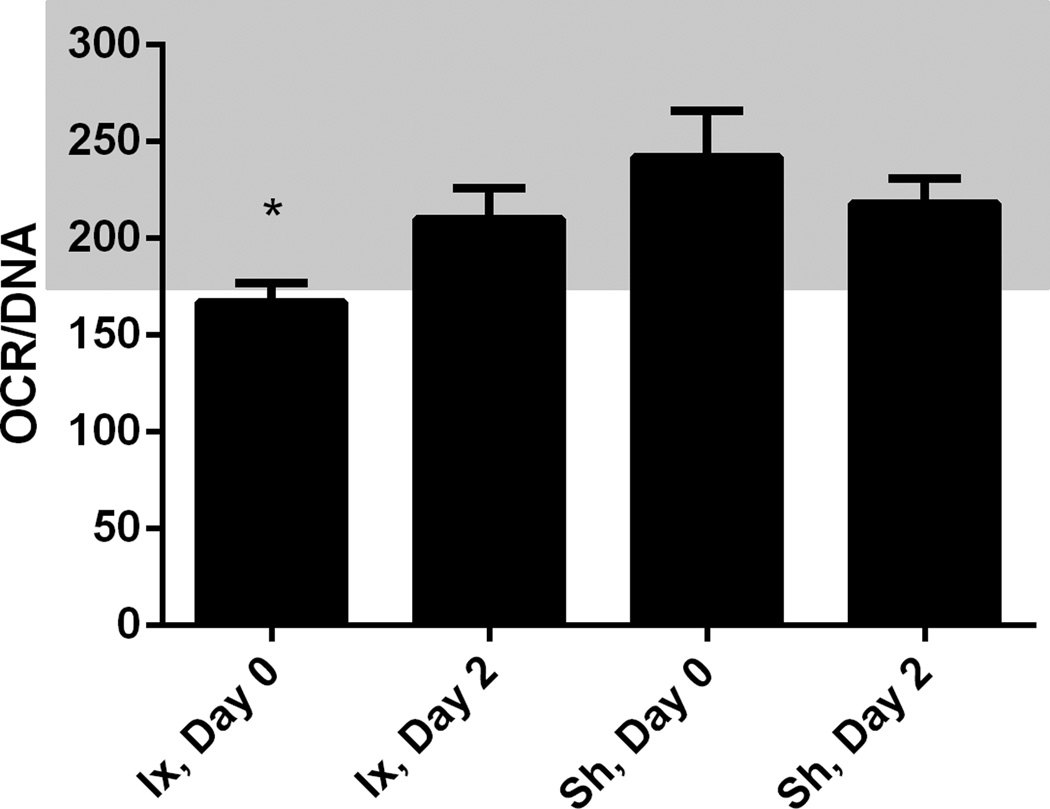
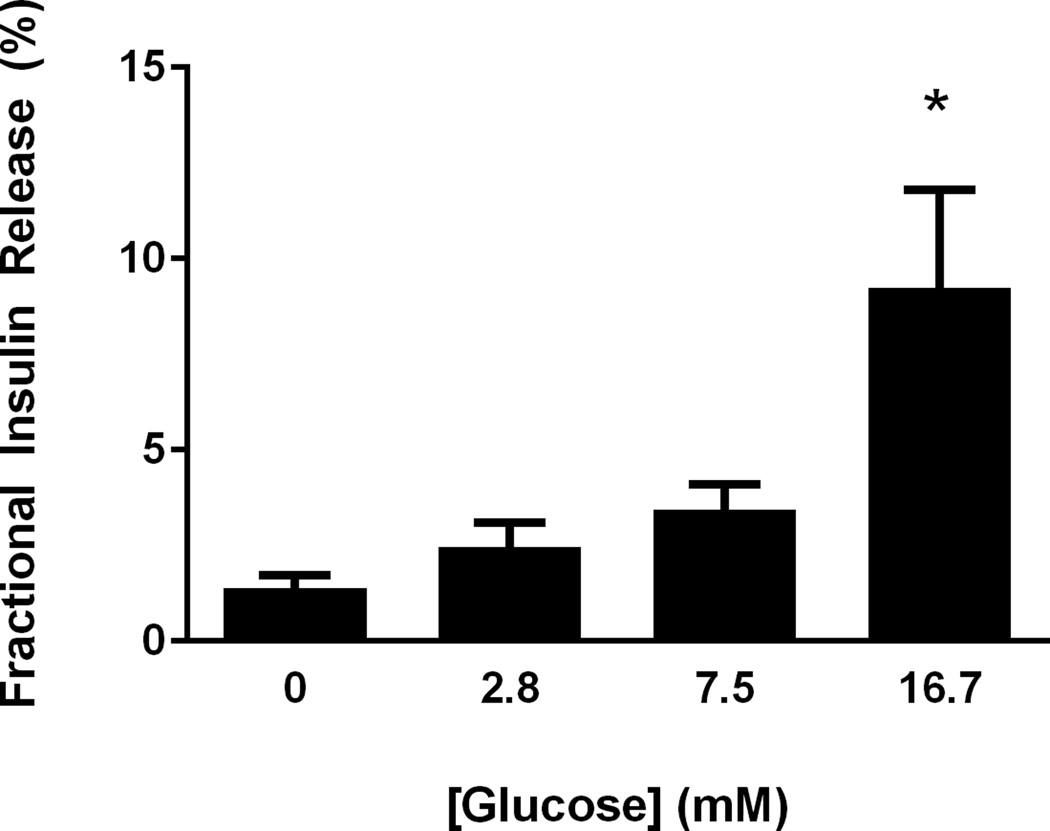
Six shipments (n=6) of islets were received following five independent porcine isolations at the University of Minnesota. Each islet shipment was heterogeneous for day in culture (mean, 26±11 days) and animal source, as independent animal isolations were mixed before shipment. The isolation group scored islets > 95% pure, which is consistent with our evaluation after shipment using dithizone stain. Islet viability was determined by OCR/DNA measurement, assessed immediately after isolation (166±10 nmolO2 *mmHg/ng DNA) and 2 days after isolation (210±16 nmolO2 *mmHg/ng DNA) at the University of Minnesota (Figure 1). OCR/DNA was also assessed immediately after shipment, on the pooled islets, and again 2 days after shipment (Figure 2). There was an improvement in OCR/DNA between day 0 and day 2 after isolation; evidence a recovery period is prudent (Figure 1). There was no significant loss in viability as a result of combining isolations, shipment conditions, or duration in culture (Figure 1). Islets responded to glucose in a dose dependent manner, secreting 9.2±2.5% of their total insulin content in 16.7mM glucose incubations, which was 3.7±0.22 fold greater than the insulin release at 2.8mM glucose (Figure 2).
Figure 1.
Islet viability determined with oxygen consumption rates throughout in vitro culture. Measurements taken on single animal preparations after isolation are denoted: Ix, Day 0 and Ix, Day 2. Measurements taken after shipment on mixed islet preparations are denoted; Sh, Day 0 and Sh, Day 2. Averages ± SEM (n=5) are presented normalized to total DNA content (nmolO2/min*mg DNA). The general acceptable range for transplantable islets is 175 – 350 mmHg/min*mg DNA, which is highlighted. Islet OCR was acceptable during the duration of culture, except immediately following isolation (P<0.05) is denoted with *.
Figure 2.
Glucose stimulates insulin secretion in porcine islets. Fractional insulin secretion expressed as the proportion of insulin secretion relative to the total insulin content is shown for static incubations with glucose concentration listed on the x-axis. The data represent means from three independent isolations and significance (P<0.05) is denoted with *.
Receptor Expression
RT-PCR indicates porcine islets expressed all three receptors and Kir6.2. Using quantitative RT-PCR, it was found that Kir6.2 and Sur1 were expressed (n=5) 9.9±2.0 and 5.6±0.8 fg/pg S15 respectively. GLP1R and ADRα2A were expressed (n=5) 0.64±0.14 and 0.41±0.08 fg/pg S15 respectively.
Receptor Function
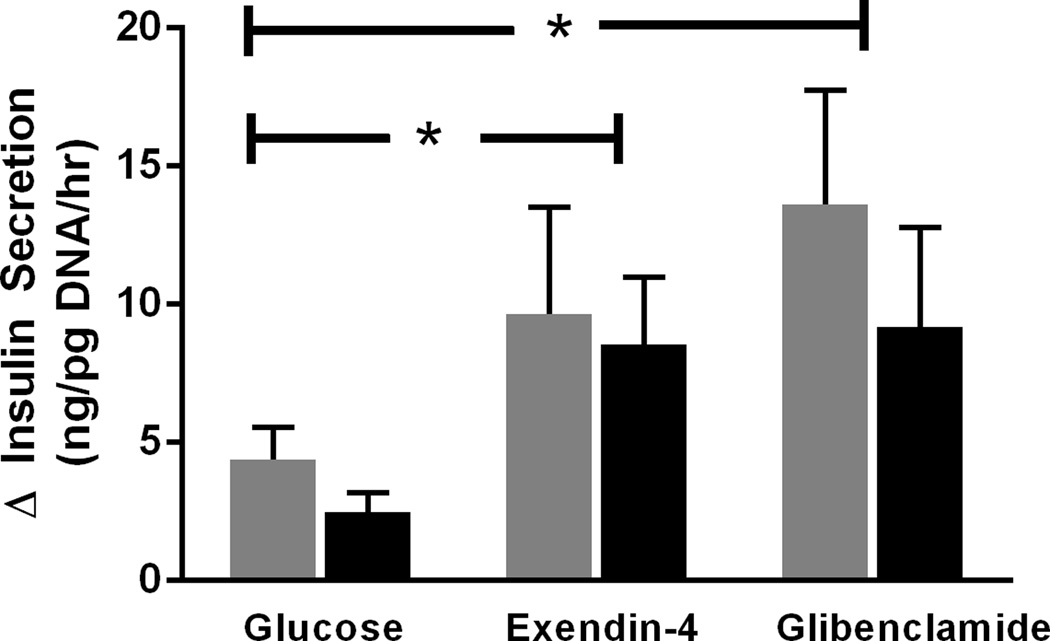
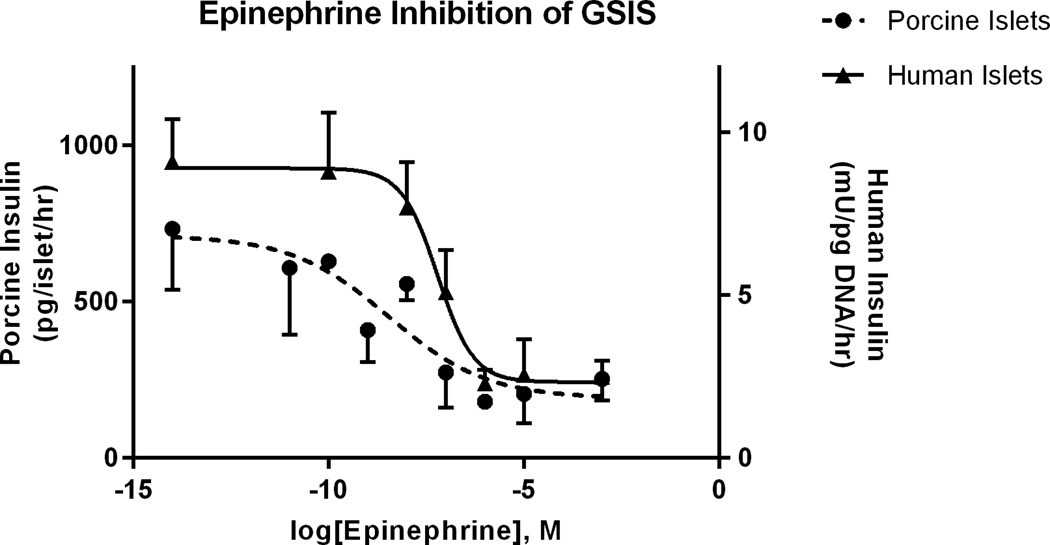
Porcine islets (n=2, 5 replicates) challenged with 11mM glucose had 3.8±0.9 fold greater insulin secretion compared to islets incubated in the presence of 2.8mM glucose. Insulin release with 50nM Exendin-4 was potentiated 3.6±1.0 fold compared to 11mM glucose and 10.6±2.4 fold compared to 2.8mM glucose condition (Figure 3). Similarly, 50nM Glibenclamide potentiated insulin secretion 2.6±0.47 fold compared to 11mM glucose and 9.0±2.5 fold compared to 2.8mM glucose condition (Figure 3). ADRα2A inhibited insulin secretion in a dose dependent manner (Figure 4). The half maximal inhibitory concentration (IC50) for epinephrine was 193nM in porcine islets (95% confidence interval of 4.2–862nM, n=4 independent isolations). Suppression of GSIS by epinephrine was blocked in the presence of 10µM Yohimbine, proving specificity to the α2A adrenergic receptor.
Figure 3.
Glibenclamide and Exendin-4 potentiated insulin secretion. Porcine islets were incubated in 11mM glucose, 50nM glibenclamide+11mM glucose, or 50nM exendin-4+11mM glucose (5 replicates/condition). Data from 2 independent islet isolations are presented separately as indicated by either a gray or black bar. Replicate average (± SEM) change in insulin secretion from 2.8mM to 11mM glucose are presented. (P<0.05) is denoted with *.
Figure 4.
Epinephrine dose-dependent suppression of porcine islet insulin secretion. The epinephrine dose-response was determined under maximal stimulatory conditions for glucose (16.7mM). Mean insulin concentrations (± SEM, pg/islet/h) for the various epinephrine concentrations (x-axis) are presented for four independent islet shipments. Non-linear regression in a log-dose model determined the IC50 to be 60nM.
Discussion
We demonstrate the presence and function of Kir6.2/Sur1, GLP1R, and ADRα2A in intact porcine islets available for xenotransplantation studies. The receptors were specifically selected because they are enriched on the pancreatic β-cell of other species and represent useful targets for therapeutics and delivery of image contrast agents (3,4,20). Activation of these receptors positively or negatively influences insulin secretion. We demonstrate responsiveness of porcine islets to synthetic ligands at pharmacologic doses used in the treatment of human β-cell dysfunction. We also demonstrate responsiveness to a natural ligand at relevant, physiological concentrations (21,22).
Targeting GLP1R and Sur1 for improving insulin secretion in Type 2 diabetics is a common therapeutic approach, and activation of GLP1R has recently improved human islet engraftment in single donor islet transplants (23,24). To our knowledge, our work demonstrates the first use of the sulfonylurea drug Glibenclamide to enhance insulin secretion in intact, isolated porcine islets. Results from this study are consistent with those in dispersed porcine endocrine cells where Glibenclamide stimulated insulin release 2-fold (25). In isolated human islets, 1nM Glibenclamide was sufficient to activate SUR1 and 10uM is commonly used to potentiate insulin secretion (26,27). Previous work in porcine islets had already shown that, Liraglutide, a GLP-1 mimetic, prolonged porcine islet culture in vitro before auto-transplant and enhanced insulin secretion stimulation post-transplant (28). Similarly, GLP-1 potentiated GSIS in dispersed porcine islet cells at 10 and 15mM glucose concentrations but not at low glucose (29). We have extended this work to include Exendin-4 (or Exenatide, marketed as BYETTA by Amylin Pharmaceuticals) in vitro in porcine islets. Intravenous infusion of Exendin-4 (40 pM/kg) into live pigs increased serum insulin concentrations nearly 3-fold while marginally improving blood glucose clearance rates (30). Exendin-4 has been shown to reduce oxidative stress in islets and recently was used to improve islet transplant outcome in both hypoxic and normal porcine islets (31). These improvements are partly described by recent findings demonstrating sustained GLP1R activation increased levels of vascular endothelial growth factor (VEGF) in transplanted porcine islets (32).
Alpha adrenergic receptors are expressed in islets of other species and their activation through ligand binding is known to suppress insulin secretion, primarily through the isoform α2A. We established porcine islets express ADRα2A mRNA and 193nM epinephrine suppressed insulin secretion 50%. This logarithmic dose response was not seen in previous work using dispersed pancreatic endocrine cells, indicating a divergence between intact islet and single cell studies (25). Additionally, our preliminary work in isolated human islets indicates only 0.6nM of epinephrine is required to suppress insulin secretion 50% in culture and 100-fold more epinephrine is required to suppress oxygen consumption rates compared to rat and sheep islets (unpublished data). Therefore, porcine islets appear to exhibit decreased responsiveness to epinephrine and may indicate reduced membrane expression of ADRα2A. Use of porcine islets may therefore be advantageous because even in the absence of receptor stimulation, ADRα2A negatively regulates GSIS. Overexpression due to polymorphisms in the ADRα2A loci have recently been associated with the development of Type 2 Diabetes (33–35).
In conclusion, this work further characterizes porcine islets and justifies their use as a model islet system for a range of applications, including as ‘xenoislets’. As development of bio-artificial pancreata progress, non-invasive strategies aimed at assessing β-cell mass will depend on greater understanding of receptors as potential imaging agents (4,5,20,36,37). We also established that sulfonylureas and incretins used in therapy for non-insulin dependent diabetics can be used to potentiate insulin secretion in porcine islets. This may be beneficial as adult porcine islets secrete less insulin than human islets in response to stimulus yet contain similar quantities of total insulin content (38,39). The ability to improve insulin secretion while possibly initiating protective mechanisms through incretin and adrenergic signaling improves the feasibility of porcine islet xenotransplantation.
Acknowledgements
The authors would like to acknowledge the isolation core at the University of Minnesota for technical expertise in porcine islet isolations.
Abbreviations
- Sur1
Sulfonylurea receptor 1
- Kir6.2
inward rectifying potassium channel
- GLP1R
glucagon like peptide receptor 1
- ADRα2A
adrenergic receptor alpha 2A
- GSIS
glucose stimulated insulin secretion
- ITx
Islet transplantation
References
- 1.Veriter S, Mergen J, Goebbels RM, et al. In vivo selection of biocompatible alginates for islet encapsulation and subcutaneous transplantation. Tissue Eng. Part A. 2010;16(5):1503–1513. doi: 10.1089/ten.TEA.2009.0286. [DOI] [PubMed] [Google Scholar]
- 2.Kitzmann JP, Law L, Shome A, et al. Real-time assessment of encapsulated neonatal porcine islets prior to clinical xenotransplantation. Xenotransplantation. 2012;19(6):333–336. doi: 10.1111/xen.12005. [DOI] [PubMed] [Google Scholar]
- 3.Ahren B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nat. Rev. Drug Discov. 2009;8(5):369–385. doi: 10.1038/nrd2782. [DOI] [PubMed] [Google Scholar]
- 4.Hart NJ, Chung WJ, Weber C, et al. Hetero-bivalent GLP-1/Glibenclamide for Targeting Pancreatic beta-Cells. Chembiochem. 2013 doi: 10.1002/cbic.201300375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wess J. More is not always better: alpha2A-adrenoceptor expression in type 2 diabetes. Cell. Metab. 2010;11(1):3–5. doi: 10.1016/j.cmet.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Penrod L, Anderson M, Ananthakrishnan K, et al. A Heterobivalent Ligand Comprised of GLP-1 and Yohimbine Specifically Targets Pancreatic β-cells In Vivo. Diabetes. 2013;62(suppl 1) (73rd Scientific Sessions). [Google Scholar]
- 7.Seino S. ATP-sensitive potassium channels: a model of heteromultimeric potassium channel/receptor assemblies. Annu. Rev. Physiol. 1999;61:337. doi: 10.1146/annurev.physiol.61.1.337. [DOI] [PubMed] [Google Scholar]
- 8.Holz GG, Kuhtreiber WM, Habener JF. Pancreatic beta-cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1(7–37) Nature. 1993;361(6410):362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JH, Kim SJ, Park SH, et al. Glucagon-like peptide-1 enhances glucokinase activity in pancreatic beta-cells through the association of Epac2 with Rim2 and Rab3A. Endocrinology. 2012;153(2):574–582. doi: 10.1210/en.2011-0259. [DOI] [PubMed] [Google Scholar]
- 10.Tompkins LS, Nullmeyer KD, Murphy SM, Weber CS, Lynch RM. Regulation of secretory granule pH in insulin-secreting cells. Am. J. Physiol. Cell. Physiol. 2002;283(2):C429–C437. doi: 10.1152/ajpcell.01066.2000. [DOI] [PubMed] [Google Scholar]
- 11.Sharp GW. Mechanisms of inhibition of insulin release. Am. J. Physiol. 1996;271(6 Pt 1):C1781–C1799. doi: 10.1152/ajpcell.1996.271.6.C1781. [DOI] [PubMed] [Google Scholar]
- 12.Zhao Y, Fang Q, Straub SG, Lindau M, Sharp GW. Noradrenaline inhibits exocytosis via the G protein betagamma subunit and refilling of the readily releasable granule pool via the alpha(i1/2) subunit. J. Physiol. 2010;588(Pt 18):3485–3498. doi: 10.1113/jphysiol.2010.190090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stutzer I, Esterhazy D, Stoffel M. The pancreatic beta cell surface proteome. Diabetologia. 2012;55(7):1877–1889. doi: 10.1007/s00125-012-2531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hering BJ, Wijkstrom M, Graham ML, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat. Med. 2006;12(3):301. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 15.Rozak PR, Weegman BP, Avgoustiniatos ES, et al. Devices and methods for maintenance of temperature and pressure during islet shipment. Transplant. Proc. 2008;40(2):407–410. doi: 10.1016/j.transproceed.2008.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papas KK, Avgoustiniatos ES, Tempelman LA, et al. High-density culture of human islets on top of silicone rubber membranes. Transplant. Proc. 2005;37(8):3412–3414. doi: 10.1016/j.transproceed.2005.09.086. [DOI] [PubMed] [Google Scholar]
- 17.Papas KK, Pisania A, Wu H, Weir GC, Colton CK. A stirred microchamber for oxygen consumption rate measurements with pancreatic islets. Biotechnol. Bioeng. 2007;98(5):1071–1082. doi: 10.1002/bit.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limesand SW, Rozance PJ, Smith D, Hay WWJR. Increased insulin sensitivity and maintenance of glucose utilization rates in fetal sheep with placental insufficiency and intrauterine growth restriction. Am. J. Physiol. Endocrinol. Metab. 2007;293(6):E1716–E1725. doi: 10.1152/ajpendo.00459.2007. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Green AS, Macko AR, Yates DT, Kelly AC, Limesand SW. Enhanced Insulin Responsiveness and Islet Adrenergic Desensitization after Discontinuing Chronic Norepinephrine Suppression in Fetal Sheep. Am. J. Physiol. Endocrinol. Metab. 2013 doi: 10.1152/ajpendo.00517.2013. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cline GW, Zhao X, Jakowski AB, Soeller WC, Treadway JL. Islet-selectivity of G-protein coupled receptor ligands evaluated for PET imaging of pancreatic beta-cell mass. Biochem. Biophys. Res. Commun. 2011;412(3):413–418. doi: 10.1016/j.bbrc.2011.07.077. [DOI] [PubMed] [Google Scholar]
- 21.Lake CR, Chernow B, Goldstein DS, Glass DG, Coleman M, Ziegler MG. Plasma catecholamine levels in normal subjects and in patients with secondary hypertension. Fed. Proc. 1984;43(1):52. [PubMed] [Google Scholar]
- 22.Nyholm B, Walker M, Gravholt CH, et al. Twenty-four-hour insulin secretion rates, circulating concentrations of fuel substrates and gut incretin hormones in healthy offspring of Type II (non-insulin-dependent) diabetic parents: evidence of several aberrations. Diabetologia. 1999;42(11):1314–1323. doi: 10.1007/s001250051444. [DOI] [PubMed] [Google Scholar]
- 23.Ghofaili KA, Fung M, Ao Z, et al. Effect of exenatide on beta cell function after islet transplantation in type 1 diabetes. Transplantation. 2007;83(1):24. doi: 10.1097/01.tp.0000251379.46596.2d. [DOI] [PubMed] [Google Scholar]
- 24.Faradji RN, Tharavanij T, Messinger S, et al. Long-term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation. 2008;86(12):1658. doi: 10.1097/TP.0b013e31818fe448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Niwa T, Fukasawa T, Yu W, et al. Characterization of secretory and morphologic properties of primary cultured endocrine cells from porcine pancreata. Pancreas. 2001;22(2):135. doi: 10.1097/00006676-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Giannaccini G, Lupi R, Trincavelli ML, et al. Characterization of sulfonylurea receptors in isolated human pancreatic islets. J. Cell. Biochem. 1998;71(2):182. doi: 10.1002/(sici)1097-4644(19981101)71:2<182::aid-jcb4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 27.Del Guerra S, Marselli L, Lupi R, et al. Effects of prolonged in vitro exposure to sulphonylureas on the function and survival of human islets. J. Diabetes Complications. 2005;19(1):60. doi: 10.1016/j.jdiacomp.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Emamaullee JA, Merani S, Toso C, et al. Porcine marginal mass islet autografts resist metabolic failure over time and are enhanced by early treatment with liraglutide. Endocrinology. 2009;150(5):2145–2152. doi: 10.1210/en.2008-1116. [DOI] [PubMed] [Google Scholar]
- 29.Davalli AM, Bertuzzi F, Meoni C, et al. Insulin and intracellular calcium responsiveness to glucagon-like peptide-1 and pituitary adenylate cyclase-activating peptide by dispersed adult porcine islet cells. Transplantation. 1999;67(1):174–176. doi: 10.1097/00007890-199901150-00028. [DOI] [PubMed] [Google Scholar]
- 30.Renner S, Fehlings C, Herbach N, et al. Glucose intolerance and reduced proliferation of pancreatic beta-cells in transgenic pigs with impaired glucose-dependent insulinotropic polypeptide function. Diabetes. 2010;59(5):1228. doi: 10.2337/db09-0519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukai E, Fujimoto S, Sato H, et al. Exendin-4 suppresses SRC activation and reactive oxygen species production in diabetic Goto-Kakizaki rat islets in an Epac-dependent manner. Diabetes. 2011;60(1):218. doi: 10.2337/db10-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Samikannu B, Chen C, Lingwal N, et al. Dipeptidyl Peptidase IV Inhibition Activates CREB and Improves Islet Vascularization through VEGF-A/VEGFR-2 Signaling Pathway. PLoS One. 2013;8(12):e82639. doi: 10.1371/journal.pone.0082639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosengren AH, Jokubka R, Tojjar D, et al. Overexpression of alpha2A-adrenergic receptors contributes to type 2 diabetes. Science. 2010;327(5962):217–220. doi: 10.1126/science.1176827. [DOI] [PubMed] [Google Scholar]
- 34.Boesgaard TW, Grarup N, Jorgensen T, et al. Variants at DGKB/TMEM195, ADRA2A, GLIS3 and C2CD4B loci are associated with reduced glucose-stimulated beta cell function in middle-aged Danish people. Diabetologia. 2010;53(8):1647–1655. doi: 10.1007/s00125-010-1753-5. [DOI] [PubMed] [Google Scholar]
- 35.Dupuis J, Langenberg C, Prokopenko I, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 2010;42(2):105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gotthardt M, Lalyko G, van Eerd-Vismale J, et al. A new technique for in vivo imaging of specific GLP-1 binding sites: first results in small rodents. Regul. Pept. 2006;137(3):162–167. doi: 10.1016/j.regpep.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Selvaraju RK, Velikyan I, Johansson L, et al. In vivo imaging of the glucagon like peptide 1 receptor in the pancreas with 68Ga-labeled DO3A-exendin-4. J. Nucl. Med. 2013;54(8):1458–1463. doi: 10.2967/jnumed.112.114066. [DOI] [PubMed] [Google Scholar]
- 38.Graham ML, Bellin MD, Papas KK, Hering BJ, Schuurman HJ. Species incompatibilities in the pig-to-macaque islet xenotransplant model affect transplant outcome: a comparison with allotransplantation. Xenotransplantation. 2011;18(6):328. doi: 10.1111/j.1399-3089.2011.00676.x. [DOI] [PubMed] [Google Scholar]
- 39.Mueller KR, Balamurugan AN, Cline GW, et al. Differences in glucose-stimulated insulin secretion in vitro of islets from human, nonhuman primate, and porcine origin. Xenotransplantation. 2013;20(2):75. doi: 10.1111/xen.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]