Abstract
Adult humans have a substantial amount of inducible-brown (or beige) fat, which is associated with increased energy expenditure and reduced weight gain via thermogenesis. Despite the identification of key regulators of beige adipogenesis, impacts of dietary factors on adaptive thermogenesis are largely unknown, partly due to a lack of validated human cell models. Bone morphogenetic protein 7 (BMP7) is known to promote brown adipogenesis in rodent and human progenitor cells. However, controversy still surrounds the cellular identity in BMP7-mediated transition of white to brown adipocytes. The aim of this study is to confirm BMP7-derived human adipocytes as a relevant in vitro model of human beige adipocyte by verifying the cellular lineage and metabolic activity. In this study, we hypothesized that pre-exposure of stromal vascular (SV) fraction of primary human adipogenic precursor cells (hASC) to BMP7 would convert metabolically active brown adipocytes. Our results showed that exposure of hASC to human BMP7 was associated with significant escalation of 1) UCP1 gene expression, a signature gene of brown adipocytes, 2) beige specific marker gene expression (i.e., CD137 and TMEM26), 3) glucose and fatty acid uptake, and 4) basal and cAMP-stimulated oxygen consumption rate compared to white adipocyte control. Taken together, we demonstrated that BMP7 mediates conversion of hASC into metabolically active beige adipocytes. By confirming the cellular identity and metabolic activity, this BMP7-induced human beige adipocytes from hASC should aid in the discovery and assessment of bioactive molecules to promote adaptive thermogenesis.
Keywords: brown adipose tissue, beige adipogenesis, Bone morphogenetic protein 7, UCP1, human adipose-derived stem cells
Introduction
Humans possess two types of fats with opposite functions, white (WAT) and brown adipose tissue (BAT). WAT stores energy in the form of triglyceride (TAG), while BAT dissipates energy in the form of heat via uncoupling protein 1 (UCP1)[1]. WAT is the predominant fat type in adult humans and the role of BAT in energy metabolism has long been disregarded due to its assumed low abundance. However, research over the past 5 years has revealed that adult humans have a substantial amount of metabolically active BAT [2–4]. In addition, the amount of detectable BAT has been shown to be inversely associated with adiposity and insulin resistance, suggesting that BAT-mediated adaptive thermogenesis counteracts obesity [5,6]. At least two metabolically distinct BATs are found in adult humans near the neck and clavicle area, classical brown and beige adipocytes [7–9].The classical brown adipocytes possess the molecular attributes of interscapular BAT (iBAT) of rodents based on constitutive UCP1 expression, homogeneous multilocular morphology, and a myogenic origin (Myf 5+) [10,11]. Conversely, beige adipocytes (also known as brite or brown-like adipocytes), which heterogeneously arise within WAT, are inducible in response to environmental stimuli such as cold temperature [12,13] and physical activity [14]. It has been shown that beige adipocytes are differentiated from the non-myogenic lineage progenitors (Myf5−), but controversy still surrounds the cellular identity, anatomical location, and trans-differentiation between white and beige adipocytes [15,16]. Although classical brown adipocytes possess higher thermogenic potential, their physiological importance is diluted due to the paucity in humans. In comparison, the metabolic significance of beige adipocytes has been highlighted both in rodents and humans [7,17] suggesting that formation of new beige adipocytes (beige adipogenesis) and/or activation of beige adipocytes (beigeing) is a promising strategy to combat obesity and its associated metabolic complications.
There are several ways to obtain murine brown adipocyte cultures by utilizing embryonic stem cell lines or iBAT-derived brown precursor cells in conjunction with external stimuli such as bone morphogenetic protein 7 (BMP7) [18], brain derived neurotrophic factor (BDNF) [19], fibronectin type III domain containing 5 (FNDC5, the precursor of irisin) and fibroblast growth factor 21 (FGF21) [20,21]. However, less effort has been employed to differentiate hASC into brown adipocytes. Human adipose-derived stem cells (hASC) are multi-potent cells that can be differentiated into several different lineage cells [22,23]. Hitherto, preparation of human brown adipocytes from hASC has been described by several groups along with BMP7 and BMP4 [24,25]. However, cellular identity and metabolic activity of BMP-derived adipocytes as metabolically acid brown adipocytes has not been completely understood yet. The aim of this study is to confirm in vitro model of human brown adipocytes from unsorted hASC via human BMP7 treatment. Here, we have demonstrated that hASC is a reliable source for metabolically active beige adipocytes via continuous exposure of BMP7.
Materials and Methods
Preparation of BMP7 conditioned medium and adipocyte differentiation
Abdominal adipose tissue (subcutaneous fat) was obtained from adult females with a body mass index (BMI) ~30 during liposuction or abdominal plastic surgeries. Adipose-derived stem cells (hASC) were isolated and cultured as we described previously [26]. hASC from 4–5 different human subjects were pooled to reduce the individual variation. In all experiments passage 1 or 2 cells were seeded in confluent density and induced to differentiate at the next day. All protocols and procedures were approved by the Institutional Review Board (IRB) at the University of Florida and University of Nebraska. To obtain human BMP7, we infected adenoviral BMP7 (MOI 0.1-5) to hASC, and collected conditioned medium. Ad-BMP7 was a generous gift from Tong Chuan He at University of Chicago Medical Center. The titration of recombinant adenovirus was performed using a commercial titration kit (Adeno-XTM Rapid Titer kit, Clonetech). The secretion of BMP7 to the medium was quantified by ELISA following the manufacturer’s instructions (R&D Systems) and BMP7 protein secretion was confirmed by western blot analysis (Novus). Before adipogenic stimulation, hASC were grown with or without conditioned-medium containing ~100 ng/mL of BMP7. After 3 days of BMP7 incubation (~100 ng/mL) , hASC were induced to differentiate by adding differentiation cocktail containing 0.25 mmol/L isobutylmethylxanthine, 125 nmol/L indomethacin, 1 mmol/L dexamethasone, 0.1 mmol/L insulin, 1 nmol/L T3, and 1 μmol/L rosiglitazone (BRL). Throughout the whole adipocyte differentiation program, ~100 ng/mL of BMP7 was maintained by adding fresh conditioned-medium every three days. To prepare the control conditioned-medium, adenovirus-GFP was infected to the hASC.
Collection of adipose tissue from nonhuman primates
Male African green monkeys (Chlorocebus aethiops) were housed at a temperature range of 22.2–24.4 °C and fed a diet containing 35 % of energy as fat for 10 weeks (n=5) at the Wake Forest University Medical Center. At the end of the diet period, BAT was collected from the supraclavicular region (distinguished by brownish color), and subcutaneous WAT (subQ) was collected from the lower abdominal region of monkey. BAT and WAT samples were transferred to the University of Florida for analysis. All procedures were approved by the Wake Forest University Health Sciences and the University of Florida Animal Care and Use Committees.
qPCR Analysis
Total RNA was extracted from adipocyte cultures using Trizol (Invitrogen) according to the manufacturer’s instructions. To remove potential genomic DNA contamination, RNA was treated with DNase (Mediatech) before it was reverse transcribed to cDNA. A total of 1 μg of RNA was reverse transcribed to cDNA in a total volume of 20 μl (iScript™ cDNA synthesis kit, Bio-Rad). Gene expression was determined by real-time qPCR (CFX96, Bio-Rad), and relative gene expression was normalized with 36B4. The primer sequences used are listed in supplemental Table1. For positive controls, ~100 mg of WAT and BAT from nonhuman primates was homogenized by using a polytron and total RNA was extracted from the tissue using Trizol (Invitrogen).
[3H] 2-deoxy-glucose uptake
To determine basal and insulin-stimulated glucose uptake, adipocyte cultures grown with or without BMP7 were incubated with 1 mL serum free basal medium containing 1,000 mg/L d-glucose and 20 pmol/L human insulin. After 24 hours in serum-free media, culture media was removed and replaced with 1 mL of HBSS buffer containing 100 nmol/L human insulin for 10 minutes, followed by addition of [3H]-2DOG (Perkin Elmer, final concentration was 0.1 μCi/mL) for 90 minutes at 37 °C. Glucose uptake was terminated by adding 1 mL of stop buffer (ice-cold Krebs-Ringers bicarbonate (KRBC) buffer supplemented with 25 mmol/L d-glucose). After washing cells with KRBC buffer three times to reduce background radioactivity, cells were lysed in 0.1 % SDS. [3H]-2DOG uptake was determined by liquid scintillation counting [27] .
[14C]-oleate uptake and release to the media
To determine fatty acid uptake rate, we followed the previously published methods by Chung et al. [28]. [14C]-oleic acid (OLA) (Perkin Elmer, final concentration of 0.5 μCi/mL, specific activity 50mCi/mmol) was complexed with fatty acid-free BSA, then added to adipocyte cultures treated with or BMP7 for 1, 2, and 4 hours. At each time point, medium containing unincorporated isotope was removed, and cells were thoroughly washed 3 times with PBS. The total cellular [14C] radioactivity was determined by lipid scintillation counting. For basal lipolysis determination, adipocyte cultures incubated with or without BMP7 were incubated with [14C]-OLA overnight. Unincorporated radioactivity was removed by washing (3 times with PBS) and then fresh medium (no radioactivity) was added to the cultures. After 2 hours, medium and cells were collected to determine [14C] radioactivity. Basal lipolysis was expressed as % [14C] in medium relative to the total cellular radioactivity.
Oxygen consumption rate (OCR)
Adipocytes grown with or without BMP7 were seeded into a 96-well clear bottom black polystyrene sterile plate (Corning). To determine the stimulated levels of oxygen consumption, cells were incubated with 1 mM of Bt2-cAMP for 12 hours. Oxygen consumption rate (OCR) was determined by using MitoXpress® (Cayman Chemical) according to the manufacturer’sprotocol. Briefly, an increase of phosphorescent signal from the oxygen-sensitive probe in the medium, was measured every three minutes over 5 hours using a Synergy H1 multi-mode microplate reader (BioTek).
Statistics
The results are presented as means ± SEM. Data were statistically analyzed using student's t-test or one-way ANOVA with Tukey’s multiple comparison tests. Gene expression profile during the adipocyte differentiation with or without BMP7 was analyzed by two-way ANOVA with multiple comparison between groups at each 0, 4, 7 and 10 days post-differentiation (alpha=0.05). All statistical analyses were conducted by GraphPad Prism 6 (Version 6.02).
Results
Exposure of hASC to BMP7 promoted gene expression signatures of brown adipocytes
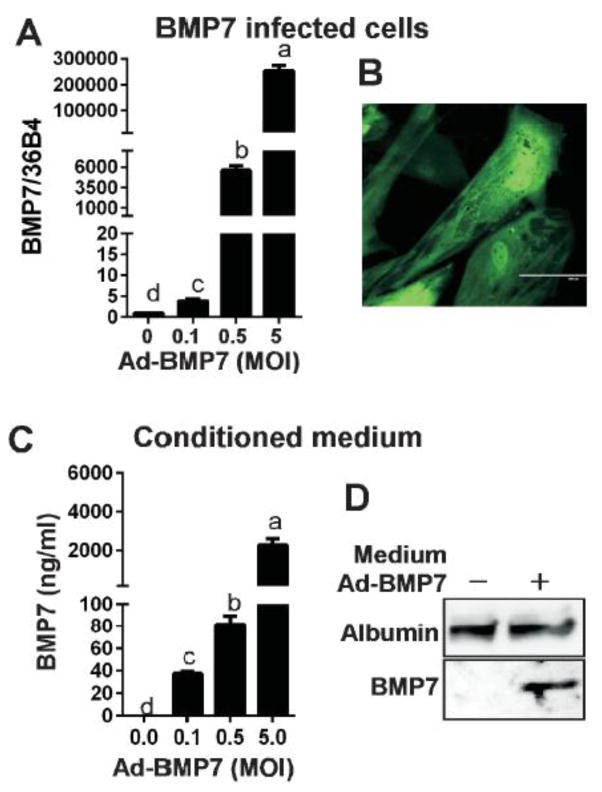
The fundamental question has been raised as to whether primary cultures of human brown adipocytes could be prepared in a reliable and reproducible manner using the unsorted hASC, the multi-potent stromal vascular fraction isolated from human subQ. To answer this question, BMP7 was delivered to hASC cells based on previous research showing human BMP7 effectively converts stem cells from adipose tissue or muscle into brown precursor cells [18,24,25]. BMP7-conditioned medium was prepared in a separated set of undifferentiated hASC by infecting adenoviral human BMP7 for 72 hr. Infection of Ad-BMP7-GFP within 0.1–5 MOI range effectively increased BMP7 gene expression (Fig. 1A ) and its GFP florescence (Fig. 1B) without showing cytotoxicity. BMP7 protein secretion to the medium was also confirmed by ELISA (Fig. 1C) and western blot analysis (Fig. 1D).
Figure 1. Preparation of BMP7 conditioned medium using Ad-BMP7.
A. BMP7 mRNA expression at 72 h after Ad-BMP7-GFP infection (0–5 MOI), B. Florescent image of hASC infected with GFP-tagged Ad-BMP7BMP7, C. Secretion to the media at 72 h after Ad-BMP7 infection (0–5 MOI) by ELISA. D. Western blot analysis of 1μl of BMP7-containing condition medium (from MOI 5 infection). Values not sharing a common letter differ significantly by one-way ANOVA.
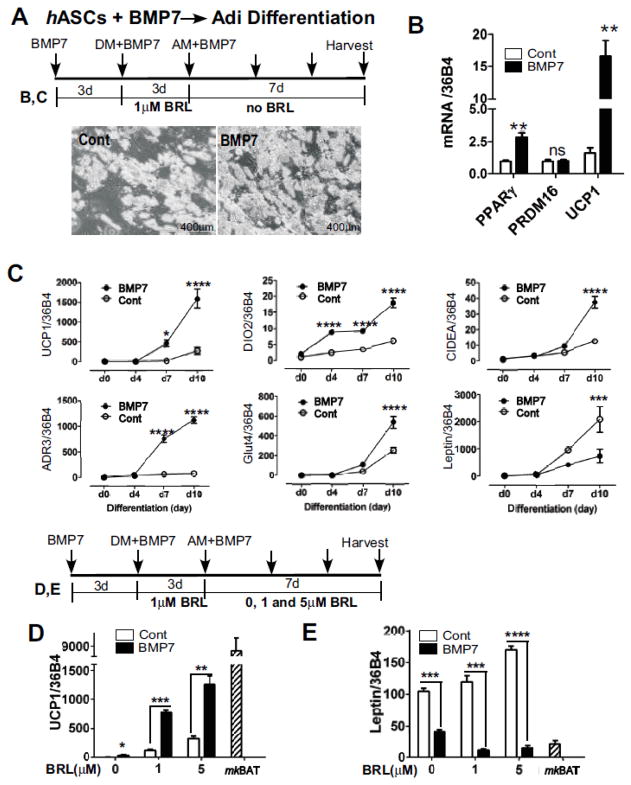
The BMP7-induced adipocyte cultures were prepared by adding ~100 ng/mL of BMP7 for three days followed by exposure to the adipogenic stimulation cocktail for three days and terminal maturation for seven more days (Fig. 2A upper). Consistent with Schulz et al., BMP7 treatment was more adipogenic than control (Fig. 2A lower) [24]. There was an increased expression in both PPARγ (2.5-fold) and uncoupling protein 1 (UCP1) (~15-fold), but no significant changes in PR domain containing 16 (PRDM16) gene expression level. (Fig. 2B). Next, we examined the changes in mRNA levels of brown adipocyte-specific genes during differentiation process. The brown marker genes including UCP1, cell-death inducing DFF45-like effector A (CIDEA), deiodinase II (DIO2), glucose transporter 4 (Glut4), and β3-adrenoceptor (ADRB3) were rapidly increased after 7 days of differentiation in BMP7 treated adipocytes compared to control adipocytes. The increase of leptin gene expression was higher in control than BMP7 treated adipocytes (Fig. 2C).
Figure 2. BMP7 promotes brown adipocyte-like gene profiles in human adipocytes.
A. hASC treated with or without BMP7 conditioned medium were differentiated into adipocyte. Phase contrast images were taken 10 d after differentiation. Arrows indicate timeline for BMP7 addition and initiation of adipogenic differentiation by adding differentiation medium (DM) and adipocyte maintenance medium (AM), B. BMP7-mediated changes in white- and brown-adipocyte gene expression measured after 10 d of differentiation by qPCR. C. Kinetics of white- and brown-adipocyte gene expression with or without BMP7 incubation to hASC during 10 d of adipogenic differentiation. Changes of UCP1 (D) and Leptin E) gene expression in response to( continuous exposure BRL (1 and 5 μmol/L) in differentiated adipocytes with or without BMP7. BRL treatment after first 3 days of differentiation has shown for experiment in D and E. Classical BAT from monkey (mkBAT) was used as a classical brown fat control for UCP1 and leptin expression. In B (n=4), D and E (n=3), *p<0.05 and **p<0.01, ***p<0.001 and ****p<0.0001 by student t-tests, ns= not significant. In C, *p<0.05, ***p<0.001, ****p<0.0001 by two-way ANOVA with multiple comparisons at each time point (n=3).
There is evidence that chronic exposure to PPARγ agonist induces brown-like phenotypes in primary cultures of human adipocytes [29]. To determine whether BMP7 has similar effects to PPARγ agonist, adipocytes grown with or without BMP7 were treated with 1 or 5 μmol/L BRL during the whole differentiation process. BRL, a PPARγ agonist, significantly increased the UCP1 gene expression in a dose-dependent manner in both cultures with or without BMP7, but to a higher magnitude in BMP7 treated adipocytes (Fig. 2D). Intriguingly, leptin gene expression increased in response to BRL concentration in control white adipocytes while leptin gene expression was not increased in response to BRL in BMP7 treated adipocytes, suggesting that BMP7-mediated brown-like features are different from those caused by chronic treatment with PPARγ agonist on white adipocytes (Fig. 2E ).
BMP7 increases beige specific marker expression
BMP7 has been suggested to contribute to commit rodent embryonic stem cells [18]. However, less information is available for the cellular identity of BMP7 induced human brown adipocytes. As the hASC possess bidirectional potential to differentiate into either adipocyte or myocyte [30–32], it was uncertain whether BMP7 transforms hASC into classical brown adipocytes (myocyte origin) or beige adipocytes (white adipocyte origin). As it is difficult to acquire human BAT, we collected BAT depots from nonhuman primates as a positive control. BAT of nonhuman primates housed at ambient temperature was identified by its distinct brown color in the supraclavicular area. The characteristics of BAT and WAT from nonhuman primates were summarized in Supplement Fig. 1.
It has been shown that beige adipocytes are distinguishable from classical brown adipocytes by their unique expression of surface proteins such as CD137 and TMED26 [7]. As we expected, CD137 and TMEM26 expression levels were not upregulated in BAT compared to subQWAT from nonhuman primates, confirming these monkey fat depots were composed of classical brown adipocytes (Fig. 3A). In contrast, both CD137 (5.8-fold) and TMEM26 (60-fold) expression levels were remarkably increased in adipocyte cultures treated with BMP7 compared to white adipocyte counterparts (Fig. 3B). The beige selective gene expression levels were also increased in control adipocytes in the presence of cAMP analogue, but the magnitudes of the increases were less than in BMP7 treated adipocytes (Fig. 3C). We also measured LHX8 gene expression as a classical brown adipocyte selective marker [17]. LHX8 gene expression was highly prevalent (~6000-fold) in nonhuman primate BAT compared to WAT. Unexpectedly, LHX8 gene expression in adipocytes with BMP7 was slightly but significantly higher than with control. Interestingly, LHX8 expression was inversely regulated by Bt2-cAMP treatment in both cultures of adipocytes (Fig. 3D). The other classical brown adipocyte markers ZIC1 and EPSTL1 were undetectable in both cultures of adipocytes (data not shown). Collectively, these data indicate that the cellular identity of BMP7-driven adipocytes is closer to beige adipocytes rather than classical brown adipocytes.
Figure 3. Expression of beige vs. classical brown adipocyte selective markers in BMP7 treated adipocytes.
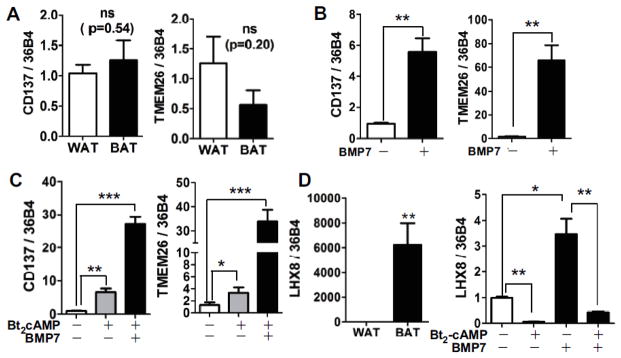
mRNA expression of beige adipocyte-selective markers CD137 and TMEM26 in supraclavicular BAT collected from nonhuman primates (A), cultured adipocytes with or without BMP7 (B), and in the presence and absence of Bt2-cAMP stimulation (C). D. Classical BAT marker LHX8 in BAT of nonhuman primates and cultured adipocytes with or without BMP7 infection. ns = not significant, *p<0.05, **p<0.01 and ***p<0.001 by student t-tests (n=4–5 for each experiments).
BMP7-mediated beige adipocytes are metabolically active than white adipocytes
Brown adipocytes are key metabolic sites to dispose glucose and fatty acids [33]. To determine whether BMP7-driven beige adipocytes are more metabolically active compared to white adipocytes, we measured glucose and fatty acid uptake, fatty acid release (basal lipolysis), and oxygen consumption rate. In agreement with an increase of Glut4 gene expression (Fig. 2C), both basal- and insulin-stimulated glucose uptake were significantly higher in BMP7 treated adipocytes compared to control assessed by 2-[3H]-deoxy-glucose (2-DOG)uptake (Fig. 4A). Similarly, fatty acid uptake was determined by adding 14C-oleate (OLA) to the medium. The BMP7 treated adipocytes exhibited a higher fatty acid uptake rate than control; 79.5 % of 14C-OLA was internalized by adipocytes with BMP7, while only 56.3 % 14C-OLA was internalized by control adipocytes after a 2 hr incubation with 14C-OLA (Fig. 4B). To determine the fatty acid release to the medium, we first incubated 14C-OLA overnight (> 95 % of 14C-OLA was loaded in both adipocytes, data not shown). The medium was replaced with fresh medium and the release of 14C-OLA to the medium was determined for 2 h. 14C-OLA release to the medium was significantly lowered in adipocytes treated with BMP7 (3.75 %) compared to control adipocytes (7.6 %) (Fig. 4C). We have measured respiration rate using the commercial oxygen-sensitive probe as a surrogate marker for oxygen concentration. This oxygen-sensing system was validated in adipocytes by measuring oxygen consumption with or without the respiration inhibitor Antimycin A (AA) (Fig. 5A). Oxygen consumption rate (OCR) was markedly enhanced in adipocytes treated with BMP7 compared control (Fig. 5B). OCR was increased by 1.76-fold in BMP7 treated adipocytes versus control adipocytes in basal level. More interestingly, an increase of OCR upon stimulation with Bt2-cAMP was significantly higher in BMP7 treated beige adipocytes compare to white adipocyte control (Fig 5C). Taken together, these data clearly demonstrated that BMP7-driven brown adipocytes have acquired both the gene signature profile and metabolic features of beige adipocytes.
Figure 4. Measurement of metabolic capacity in BMP7-derived beige adipocytes.

A. [3H]-2-deoxy glucose uptake (2-DOG) in the presence and absence of insulin (100 nmol/L) in triplicate samples (n=3), B. Fatty acid uptake using [14C]-oleic acid over 4 h, n=6 per each time point C. Lipolysis was determined by measuring [14C]-oleate release to the medium for 2 h (n=3). Data expressed as relative [14C] radioactivity to the cellular radioactivity, *p<0.05, **p<0.01, and ***p<0.001 by student t-tests.
Figure 5. Measurement of oxygen consumption rate in BMP7-derived beige adipocytes.

Oxygen consumption was measured using a fluorescent oxygen probe. A. Effectiveness of oxygen quenching was validated in the presence (+) and absence (−) of Antimycin A (AA) for 4 h. B. Oxygen consumption in adipocytes grown with or without BMP7. C. Cultured adipocytes grown with or without BMP7 then stimulated with or without of Bt2-cAMP for last 12 hr before measurement of oxygen consumption rate (OCR). Slope of OCR was obtained by linear regression for all data points (n=8–10 per group). RPU; relative phosphorescent unit, OCR; oxygen consumption rate, Values not sharing a common letter differ significantly by one-way ANOVA.
Discussion
Recent evidence has revealed that the prevalence of BAT is negatively associated with obesity and diabetes [7,17,34], suggesting that activation of adaptive thermogenesis is a promising therapeutic target. Despite the rapid advances in the field of brown adipocyte biology, development of therapeutic strategies to increase “browning of human WAT” is lagging, partly due to ethical concerns surrounding human research and the lack of adequate cell models. There are clear needs to verify relevant cell models for screening novel agents expected to stimulate human beige adipogenesis. In this study, we have confirmed that continuous exposure of BMP7 directs to differentiate uncommitted hASC into metabolically active beige adipogenesis rather than conventional white adipocytes (Fig. 4, 5).
It has been shown that brown and white adipocytes originate from progenitors with different cellular lineages [16]. One way to obtain primary cultures of classical brown adipocytes is to induce adipogenic differentiation from the brown precursor cells prepared from iBAT of rodents. In addition, much effort has been made to identify human brown adipose-derived stem cells [35], or manipulate human pluripotent stem cells (embryonic stem cells) into brown-specific progenitor cells [36,37]. These classical brown adipocyte models have significantly contributed to the identification of the transcriptional regulatory pathways of brown adipocytes and possess great therapeutic potential for the treatment of obesity [38]. However, classical brown adipocyte cultures are not an attractive model for studying human adaptive thermogenesis due to the paucity of classical BAT in humans; even in infants, beige adipocytes seem to be more prevalent than classical brown fat [7,17].
Seminal research regarding the role of BMP7 on browning has been conducted by Tseng’s group [18]. BMP7 seems to direct uncommitted mesenchymal stem cells into brown precursor cells through cross-talk between SMAD, insulin [39], and mTOR signaling pathways [40]. Schulz et al. have shown that subpopulation of Sca-1+ progenitor cells from subQ fat and muscle, but not omental or mesenteric fat, are prone to acquire brown-like phenotype upon BMP7 exposure in rodents and humans [24]. Notably, Schulz et al. have also reported brown conversion of human progenitor cells (in terms of increase of signature gene UCP1 expression) requires continuous exposure of BMP7 throughout the whole differentiation process, while mouse precursor cells require BMP7 exposure only before differentiation [24]. These findings implicate that there are species differences between human and rodent in terms of when their progenitor cells will be programmed into white vs. brown adipocytes. However, the cellular identity of BMP7-derived brown adipocytes has not been fully defined in this study. Regarding the metabolic function of BMP7-derived brown adipocytes, Townsend et al. have recently demonstrated that BMP7-treated mouse embryonic stem cells (C3H/10T1/2) increased fatty acid uptake by increasing expression of scavenger receptor CD36, and fatty acid oxidation by increasing mitochondrial activity through elevated expression of carnitine palmitate transferase 1 (CTP1), but not by increasing mitochondrial number [41]. These data agree with our results that BMP7-inducible human beige adipocytes have increased glucose and fatty acid uptake (Fig4. A, B) and oxygen consumption (Fig. 5C) but no change in the number of mitochondria (data not shown). More recently, Elsen et al. have also demonstrated that BMP4 as well as BMP7 promotes hASC into brown adipocytes [25]. However, Elsen et al. did not detect an increase oxygen consumption conflicting to our data (Fig 5C) and Townsend et al [41]. Although molecular regulation by BMP7 of brown adipogenesis has been established by Tseng’s group, our study is the first to demonstrate that unsorted hASC from subQ fat is a useful source to generate metabolically functional beige adipocytes upon BMP7 exposure in a reproducible manner.
Other than BMP7, some hormones and cytokines such as BDNF, FGF21, and FNDC5 (precursor of irisin) have been shown to increase brown adipogenesis and protect mice from obesity and diabetes [42]. Several studies have attempted to increase human beige adipogenesis by applying these secretory factors to human progenitor cells in vitro. Unlike BMP7, exposure of FGF21 or FNDC5 to subQ-derived hASC failed to activate brown adipogenesis program [43]. Instead, FGF21 and irisin were effective in activating human beige adipogenic programming only when progenitor cells were isolated from human neck fat [44]. These studies indicate that neck fat is composed of preadipocytes committed to beige and classical brown adipocytes. It also implies that BMP7 may work upstream of the beige adipogenic programming driven by FNDC5 or FGF21; we speculate that 1) BMP7 may serve as a hormone to prime uncommitted hASC to beige-specific progenitors but not to classical brown progenitors, and 2) FNDC5 and FGF21 might be stimulants only working to progenitor cells that have already committed to beige adipocytes. As the routine biopsy of human neck fat presents significant technical and ethical challenges, establishing a beige adipocyte cell model using subQ-derived hASC treated with BMP7 could be reasonable and practical alternative.
As it is hard to obtain human beige adipocytes as a positive control, we utilized WAT and BAT that were collected from nonhuman primates. Similar to humans, African green monkeys have classical brown fat at the supraclavicular region, which served as a distinctive marker (Supplement Fig 1). In comparison to the monkey BAT control, we have definitely showed that human subQ-derived hASC are converted into beige adipocytes by BMP7 treatment (Fig. 3). There is ongoing controversy whether beige adipocytes are recruited from the progenitor cells [45], or trans-differentiated from the pre-existing adipocytes [15,16]. The BMP7-driven beige culture could be an adaptable system to investigate beige adipogenesis from hASC and manipulation of thermogenic potential (beigeness). Although there is some in vivo evidence that capsinoids [46] and some fatty acid [47] are associated with the activation of thermogenesis, screening individual dietary factors or pharmaceutical compounds in vivo would be expensive and inefficient, thus necessitating the creation of a new cell model. This BMP7-driven human beige adipocyte model could facilitate the discovery of regulators of adaptive thermogenesis.
Over the past five years, significant research effort has been focused on determining the physiological significance of BAT and identifying the regulatory circuits controlling white versus brown adipogenesis. It is anticipated that in the next decade much effort will be directed at searching for pharmaceutical and dietary compounds that manipulate beige energetics in humans. Despite some unanswered questions, we have validated the use of subQ fat-derived hASC (unsorted) for in vitro culture of human beige adipocytes and characterized its metabolic function via BMP7 treatment. This beige adipocyte system could be employed to create novel preventive and/or therapeutic strategies to combat obesity and metabolic complications by maximizing WAT beigeing and adaptive thermogenesis.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the NIH-NCRR to SC (1UL1RR029890), the NIH-1P20GM104320 (Project 5) to SC, and a NIH-NHLBI grant to RET (R00HL088528).
Abbreviation
- AA
Antimycin A
- ADRB3
β3-adrenoceptor
- ASC
Adipogenic stem cells
- BAT
Brown adipose tissue
- BDNF
Brain derived neurotrophic factor
- BRL
Rosiglitazone
- BMP7
Bone morphogenetic protein 7
- Bt2-cAMP
8-bromo cyclic AMP
- CD137
Cluster of differentiation 137
- CIDEA
Cell-death inducing DFF45-like effector A
- FGF21
Fibroblast growth factor 21
- FNDC5
Fibronectin type III domain containing 5 (precursor of Irisin)
- GLUT4
Glucose transporter 4
- OLA
Oleic acid
- PPARγ
Peroxisome proliferator-activated receptor gamma
- PRDM16
PR domain containing 16
- SubQ
Subcutaneous fat
- SV
Stromal vascular
- TAG
Triacylglycerol
- TMEM26
Transmembrane protein 26
- UCP1
Uncoupling protein 1
- WAT
White adipose tissue
Footnotes
Conflict of Interest
None
References
- 1.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 2.Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 4.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 5.Matsushita M, Yoneshiro T, Aita S, Kameya T, Sugie H, Saito M. Impact of brown adipose tissue on body fatness and glucose metabolism in healthy humans. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2013.206. [DOI] [PubMed] [Google Scholar]
- 6.Stanford KI, Middelbeek RJ, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng YH, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lidell ME, Betz MJ, Dahlqvist LO, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, Virtanen KA, Beuschlein F, Persson A, Borga M, Enerback S. Evidence for two types of brown adipose tissue in humans. Nat Med. 2013;19:631–634. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- 9.Cypess AM, White AP, Vernochet C, Schulz TJ, Xue R, Sass CA, Huang TL, Roberts-Toler C, Weiner LS, Sze C, Chacko AT, Deschamps LN, Herder LM, Truchan N, Glasgow AL, Holman AR, Gavrila A, Hasselgren PO, Mori MA, Molla M, Tseng YH. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat Med. 2013;19:635–639. doi: 10.1038/nm.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR, Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, Tavernier G, Langin D, Spiegelman BM. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 13.Ye L, Wu J, Cohen P, Kazak L, Khandekar MJ, Jedrychowski MP, Zeng X, Gygi SP, Spiegelman BM. Fat cells directly sense temperature to activate thermogenesis. Proc Natl Acad Sci U S A. 2013;110:12480–12485. doi: 10.1073/pnas.1310261110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 15.Rosenwald M, Perdikari A, Rulicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15:659–667. doi: 10.1038/ncb2740. [DOI] [PubMed] [Google Scholar]
- 16.Rosenwald M, Wolfrum C. The origin and definition of brite versus white and classical brown adipocytes. Adipocyte. 2014;3:4–9. doi: 10.4161/adip.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharp LZ, Shinoda K, Ohno H, Scheel DW, Tomoda E, Ruiz L, Hu H, Wang L, Pavlova Z, Gilsanz V, Kajimura S. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One. 2012;7:e49452. doi: 10.1371/journal.pone.0049452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tseng YH, Kokkotou E, Schulz TJ, Huang TL, Winnay JN, Taniguchi CM, Tran TT, Suzuki R, Espinoza DO, Yamamoto Y, Ahrens MJ, Dudley AT, Norris AW, Kulkarni RN, Kahn CR. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, During MJ. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14:324–338. doi: 10.1016/j.cmet.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, Kebebew E, Pacak K, Chen KY, Celi FS. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–309. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–1260. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bourin P, Bunnell BA, Casteilla L, Dominici M, Katz AJ, March KL, Redl H, Rubin JP, Yoshimura K, Gimble JM. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: a joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT) Cytotherapy. 2013;15:641–648. doi: 10.1016/j.jcyt.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, Cerletti M, McDougall LE, Giorgadze N, Tchkonia T, Schrier D, Falb D, Kirkland JL, Wagers AJ, Tseng YH. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A. 2011;108:143–148. doi: 10.1073/pnas.1010929108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elsen M, Raschke S, Tennagels N, Schwahn U, Jelenik T, Roden M, Romacho T, Eckel J. BMP4 and BMP7 induce the white-to-brown transition of primary human adipose stem cells. Am J Physiol Cell Physiol. 2014;306:C431–C440. doi: 10.1152/ajpcell.00290.2013. [DOI] [PubMed] [Google Scholar]
- 26.Zhao L, Ha JH, Okla M, Chung S. Activation of autophagy and AMPK by gamma-tocotrienol suppresses the adipogenesis in human adipose derived stem cells. Mol Nutr Food Res. 2014;58:569–579. doi: 10.1002/mnfr.201300157. [DOI] [PubMed] [Google Scholar]
- 27.Chung S, LaPoint K, Martinez K, Kennedy A, Boysen SM, McIntosh MK. Preadipocytes mediate lipopolysaccharide-induced inflammation and insulin resistance in primary cultures of newly differentiated human adipocytes. Endocrinology. 2006;147:5340–5351. doi: 10.1210/en.2006-0536. [DOI] [PubMed] [Google Scholar]
- 28.Chung S, Gebre AK, Seo J, Shelness GS, Parks JS. A novel role for ABCA1-generated large pre-beta migrating nascent HDL in the regulation of hepatic VLDL triglyceride secretion. J Lipid Res. 2010;51:729–742. doi: 10.1194/jlr.M900083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci U S A. 2006;103:12167–12172. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Planat-Benard V, Menard C, Andre M, Puceat M, Perez A, Garcia-Verdugo JM, Penicaud L, Casteilla L. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94:223–229. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 32.Rangappa S, Fen C, Lee EH, Bongso A, Sim EK. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg. 2003;75:775–779. doi: 10.1016/s0003-4975(02)04568-x. [DOI] [PubMed] [Google Scholar]
- 33.Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmuller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 34.Ouellet V, Labbe SM, Blondin DP, Phoenix S, Guerin B, Haman F, Turcotte EE, Richard D, Carpentier AC. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122:545–552. doi: 10.1172/JCI60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silva FJ, Holt DJ, Vargas V, Yockman J, Boudina S, Atkinson D, Grainger DW, Revelo MP, Sherman W, Bull DA, Patel AN. Metabolically active human brown adipose tissue derived stem cells. Stem Cells. 2014;32:572–581. doi: 10.1002/stem.1595. [DOI] [PubMed] [Google Scholar]
- 36.Nishio M, Saeki K. Differentiation of human pluripotent stem cells into highly functional classical brown adipocytes. Methods Enzymol. 2014;537:177–197. doi: 10.1016/B978-0-12-411619-1.00010-0. [DOI] [PubMed] [Google Scholar]
- 37.Ahfeldt T, Schinzel RT, Lee YK, Hendrickson D, Kaplan A, Lum DH, Camahort R, Xia F, Shay J, Rhee EP, Clish CB, Deo RC, Shen T, Lau FH, Cowley A, Mowrer G, Al-Siddiqi H, Nahrendorf M, Musunuru K, Gerszten RE, Rinn JL, Cowan CA. Programming human pluripotent stem cells into white and brown adipocytes. Nat Cell Biol. 2012;14:209–219. doi: 10.1038/ncb2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gunawardana SC. Therapeutic value of brown adipose tissue: Correcting metabolic disease through generating healthy fat. Adipocyte. 2012;1:250–255. doi: 10.4161/adip.21042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang H, Schulz TJ, Espinoza DO, Huang TL, Emanuelli B, Kristiansen K, Tseng YH. Cross talk between insulin and bone morphogenetic protein signaling systems in brown adipogenesis. Mol Cell Biol. 2010;30:4224–4233. doi: 10.1128/MCB.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Townsend KL, Suzuki R, Huang TL, Jing E, Schulz TJ, Lee K, Taniguchi CM, Espinoza DO, McDougall LE, Zhang H, He TC, Kokkotou E, Tseng YH. Bone morphogenetic protein 7 (BMP7) reverses obesity and regulates appetite through a central mTOR pathway. FASEB J. 2012;26:2187–2196. doi: 10.1096/fj.11-199067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Townsend KL, An D, Lynes MD, Huang TL, Zhang H, Goodyear LJ, Tseng YH. Increased mitochondrial activity in BMP7-treated brown adipocytes, due to increased. Antioxid Redox Signal. 2013;19:243–257. doi: 10.1089/ars.2012.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saito M. Human brown adipose tissue: regulation and anti-obesity potential [Review] Endocr J. 2014 doi: 10.1507/endocrj.ej13-0527. [DOI] [PubMed] [Google Scholar]
- 43.Raschke S, Elsen M, Gassenhuber H, Sommerfeld M, Schwahn U, Brockmann B, Jung R, Wisloff U, Tjonna AE, Raastad T, Hallen J, Norheim F, Drevon CA, Romacho T, Eckardt K, Eckel J. Evidence against a beneficial effect of irisin in humans. PLoS One. 2013;8:e73680. doi: 10.1371/journal.pone.0073680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee P, Swarbrick MM, Zhao JT, Ho KK. Inducible brown adipogenesis of supraclavicular fat in adult humans. Endocrinology. 2011;152:3597–3602. doi: 10.1210/en.2011-1349. [DOI] [PubMed] [Google Scholar]
- 45.Yoneshiro T, Aita S, Matsushita M, Kayahara T, Kameya T, Kawai Y, Iwanaga T, Saito M. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123:3404–3408. doi: 10.1172/JCI67803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saito M, Yoneshiro T. Capsinoids and related food ingredients activating brown fat thermogenesis and reducing body fat in humans. Curr Opin Lipidol. 2013;24:71–77. doi: 10.1097/MOL.0b013e32835a4f40. [DOI] [PubMed] [Google Scholar]
- 47.Shen W, Chuang CC, Martinez K, Reid T, Brown JM, Xi L, Hixson L, Hopkins R, Starnes J, McIntosh M. Conjugated linoleic acid reduces adiposity and increases markers of browning and inflammation in white adipose tissue of mice. J Lipid Res. 2013;54:909–922. doi: 10.1194/jlr.M030924. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.