Summary
Axonal death disrupts functional connectivity of neural circuits and is a critical feature of many neurodegenerative disorders. Pathological axon degeneration often occurs independently of known programmed death pathways, but the underlying molecular mechanisms remain largely unknown. Using traumatic injury as a model, we systematically investigate mitogen-activated protein kinase (MAPK) families, and delineate a MAPK cascade that represents the early degenerative response to axonal injury. The adaptor protein Sarm1 is required for activation of this MAPK cascade, and this Sarm1-MAPK pathway disrupts axonal energy homeostasis, leading to ATP depletion before physical breakdown of damaged axons. The protective cytoNmnat1/Wlds protein inhibits activation of this MAPK cascade. Further, MKK4, a key component in the Sarm1-MAPK pathway, is antagonized by AKT signaling, which modulates the degenerative response by limiting activation of downstream JNK signaling. Our results reveal a regulatory mechanism that integrates distinct signals to instruct pathological axon degeneration.
Introduction
Axons connect neurons with their innervating targets, forming the intricate neural circuits underlying perception, motility, cognition and memory. However, the enormous distance axons can span (e.g., over a meter for certain axons in humans) creates a challenge for maintaining their structural and functional integrity. In fact, axonal death has been observed as a key pathological feature in many neurodegenerative disorders including traumatic injury, Alzheimer's disease, Parkinson's disease, glaucoma, multiple sclerosis, and amyotrophic lateral sclerosis (Conforti et al., 2014; Wang et al., 2012).
A common form of pathological axonal death is observed in segments distal to a site of traumatic injury, a process known as Wallerian degeneration. The discovery of the Wallerian Degeneration Slow mouse (Wlds) and characterization of the Wlds protein, whose gain-of-function can dramatically prolong axonal survival after injury, has suggested that death of injured axons is not simply due to loss of trophic support from neuronal cell bodies, but is instead triggered by an intrinsic signal(s) in axons. The Wlds protein can suppress axon degeneration in many neurodegeneration models besides traumatic injury (Conforti et al., 2014; Wang et al., 2012), suggesting that a common mechanism might regulate axonal death in different neurodegenerative conditions.
Despite morphological similarities to cells undergoing apoptosis, including membrane blebbing and cytoskeletal fragmentation, axon degeneration under pathological conditions often does not involve the classic apoptotic pathway. For instance, genetic deletion of apoptotic regulators like BAX and caspases has no effect on Wallerian degeneration (Simon et al., 2012; Whitmore et al., 2003). In addition, at least during traumatic injury, axon degeneration also appears independent of the necroptotic pathway, since treatment with necroptosis inhibitors or lentiviral-shRNA knockdown of signaling components of necroptosis pathway like RIP3 and MLKL do not protect axons (data not shown). Therefore, axonal death in traumatic injury, as well as in neurodegeneration more broadly, likely represents a yet-to-be-characterized death program critical for human diseases. In-depth understanding of this degeneration program should illuminate neurodegenerative diseases and reveal novel therapeutic targets to battle them.
Our colleagues and we recently showed that loss-of-function of the adaptor protein Sarm1 significantly delays degeneration of injured axons (Gerdts et al., 2013; Osterloh et al., 2012). Despite this breakthrough, signaling components in the Sarm1-mediated degenerative response are largely unknown. Previous studies have described roles for Sarm1 or its C. elegans ortholog tir-1 in neural development (Chen et al., 2011; Chuang and Bargmann, 2005), stress responses (Couillault et al., 2004; Kurz et al., 2007), and non-apoptotic cell death (Blum et al., 2012). While the identity of signaling components varies in different settings, a MAPK cascade downstream of Sarm1 / tir-1 has been observed in each case, suggesting potential conservation of the Sarm1-MAPK signaling axis. In addition, genetic deletion of the mouse MAPK kinase kinase (MAPKKK) member DLK transiently delayed, though to a significantly lesser extent than in Wlds mice, the degeneration of injured axons in sciatic nerves in vivo and in cultured sensory neurons in vitro (Miller et al., 2009; Shin et al., 2012), protection in vitro was also seen with a pharmacological inhibitor of MAPKs of the JNK family (Chen et al., 2012; Miller et al., 2009). Weak protection of injured axons was also reported with deletion of the DLK homolog Wallenda in Drosophila in some neurons (Miller et al., 2009), though not others (Osterloh et al., 2012; Xiong and Collins, 2012).
These observations prompted us to systematically investigate MAPK family members, and we delineate a MAPK cascade that is central in axon degeneration under pathological conditions. This cascade represents the early degenerative response, and Sarm1 is required for its activation after injury. Activation of the Sarm1-MAPK pathway in turn disrupts local energy homeostasis in injured axons, leading to ATP depletion before activation of calpains and breakdown of axonal structures. We find that a cytosolic version of Nmnat1 (nicotinamide mononucleotide adenylyltransferase 1, an enzyme in the NAD+ synthesis pathway), which mimics the functional moiety of the Wlds protein (Sasaki et al., 2009), inhibits activation of this MAPK pathway. Furthermore, MKK4, a central component in the pathway, is antagonized by AKT signaling, which modulates the early degenerative response by limiting activation of downstream JNKs. Our results reveal that distinct signals can converge onto a central MAPK pathway that triggers local energy deficit to promote pathological axon degeneration – a regulatory mechanism with potentially broad implications in neurodegenerative disorders.
Results
Fluorescence-labeling approach to examine axons undergoing degeneration
To facilitate in vivo analysis of injury-induced axon degeneration, we established an approach to examine the axons of retinal ganglion cells (RGCs) undergoing degeneration in mouse optic nerves. RGCs were sparsely labeled with the TdTomato fluorescent protein via intravitreal delivery of adeno-associated virus 2 (AAV2). Degeneration of TdTomato-positive RGC axons was visualized at different time points following traumatic injury (optic nerve crush). Across experiments, 70% to 80% of TdTomato-positive axons showed large swellings and/or fragmentation and were scored as degenerated 3 days after injury, and all axons had fragmented by 6 days (Fig. S1A and S1B). This approach gave equivalent results to electron microscopic analysis (Fig. S1C and S1D), i.e., about 80% of all RGC axons underwent demyelination and/or cytoskeletal destruction at 3 days, with complete breakdown of axonal structures by 6 days.
To validate this approach further, we examined the cytosolic version of Nmnat1 (cytoNmnat1). As expected, in a transgenic mouse line expressing cytoNmnat1 driven by the prion protein promoter (PrP) (Sasaki et al., 2009), most TdTomato-positive axons remained intact 6 days after injury (Fig. S1A and S1B). Transduction of wildtype axons with a virus expressing TdTomato-P2A-cytoNmnat1 (TdTomato and cytoNmnat1 proteins linked by a porcine teschovirus-derived self-cleaving 2A peptide) similarly resulted in robust protection, with over 60% of TdTomato-positive axons being intact at 6 days (Fig. S1A and S1B). Of note, the protection level observed with TdTomato-P2A-cytoNmnat1 was modestly weaker than that in cytoNmnat1-transgenic mice, even though the level of cytoNmnat1 protein expressed by AAV2 was higher than by the transgene (Fig. S1E). We attribute this to the fact that, in TdTomato-P2A-cytoNmnat1 optic nerves, unlabeled RGC axons (>99% of all axons) still degenerated normally as shown by electron microscopy (Fig. S1C and S1D). There was profound activation of microglia and astrocytes in TdTomato-P2A-cytoNmnat1 nerves, but not in cytoNmnat1-transgenic nerves (Fig. S1F), and it seems possible that this inflammatory response might secondarily influence axonal survival.
Further confirming the effectiveness of our approach, we found that RGC axons of wildtype mice transduced with TdTomato/Sarm1-shRNA (TdTomato together with a specific shRNA targeting Sarm1) virus were highly resistant to degeneration after nerve injury, in contrast to those transduced with control TdTomato/scrambled-shRNA virus (Fig. S2D and S2E), consistent with the fact that Sarm1 mRNA is expressed in RGCs (Fig. S2A) and degeneration of injured RGC axons was largely blocked in Sarm1−/− mice (Fig. S2B and S2C).
Finally, degeneration of traumatically-injured axons depends on calpain activation as it can be delayed by expression of the endogenous calpain inhibitor calpastatin (Yang et al., 2013). Confirming this, RGC axons of CAPNS1flox/flox mice transduced with a TdTomato-P2A-Cre virus, which results in disruption of both calpain-1 and calpain-2 activity through deletion of their common small subunit (Tan et al., 2006), showed significantly delayed degeneration compared to those of wildtype mice (Fig. S2F and S2G). Together, our results show that this labeling approach can provide effective cell-autonomous manipulation of signaling components involved in axon degeneration.
A central MAPK pathway in axon degeneration after traumatic injury
Sarm1 was recently shown to regulate axon degeneration after traumatic injury (Gerdts et al., 2013; Osterloh et al., 2012). As mentioned, in other biological processes Sarm1 and its ortholog tir-1 were linked to MAPK signaling, and prior genetic analyses implicated the MAPKKK member DLK as a weak regulator in degeneration of injured axons (Miller et al., 2009; Osterloh et al., 2012; Shin et al., 2012), prompting us to investigate the function of MAPK families more systematically.
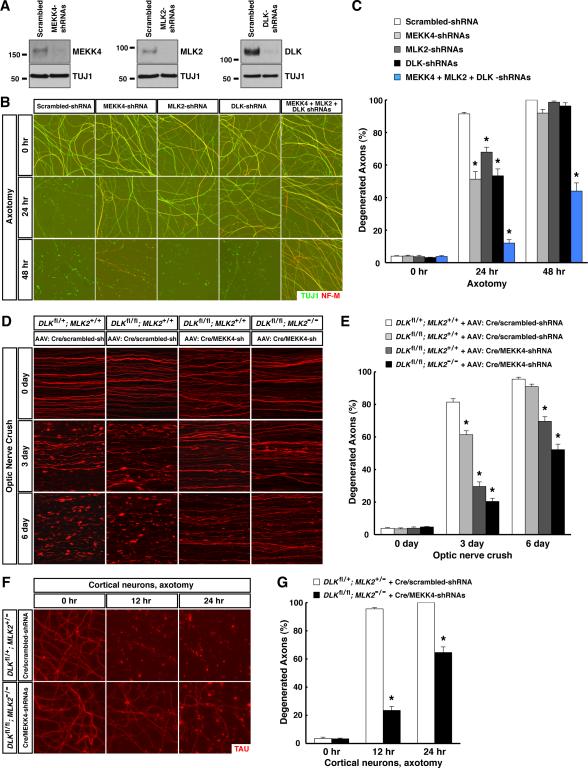
There are 23 members of the MAPKKK family in mouse. Using cultured sensory neurons, we found that the highly specific inhibitor PLX-4720 for three MAPKKKs, A-raf, B-raf and C-raf, did not offer protection after axotomy (data not shown). We therefore performed lentiviral-shRNA knockdown screening of the remaining 20 MAPKKK members (Table S1), and observed that knockdown of each of three MAPKKK members, MEKK4, MLK2, and DLK, in sensory axons partially protected (Fig. 1A to 1C). Moreover, MEKK4 / MLK2 / DLK function additively, as knockdown of any two produced a stronger phenotype (data not shown), and knockdown of all three resulted in the strongest protection, with about 60% of axons intact 48 hr after axotomy (Fig. 1A to 1C). In parallel, we examined expression of mRNAs for the 23 MAPKKK members in adult mouse retina (Fig. S3A). Many show no or very low expression (e.g., MAP3K15), but a few are strongly expressed (e.g., B-raf). Importantly, MEKK4, MLK2, and DLK mRNAs are all expressed in RGCs. In functional analyses, we found that RGC axons in DLKflox/flox mice transduced by viral delivery of Cre together with a control scrambled-shRNA (Cre/scrambled-shRNA) showed a modest delay of degeneration compared to DLKflox/+ mice 3 days, but not 6 days, after injury (Fig. 1D and 1E), in line with the transient protection of injured axons in mouse sciatic nerve after DLK deletion (Shin et al., 2012). Greater protection was seen after further knockdown of MEKK4 in RGC axons of DLKflox/flox mice with a Cre/MEKK4-shRNA virus, and even more with MLK2 deletion through delivery of that virus into DLKflox/flox; MLK2−/− mice, with 50% of TdTomato-positive axons remaining intact 6 days after injury (Fig. 1D and 1E). Although we cannot rule out that other MAPKKKs might exert a minor role, our results identify MEKK4 / MLK2 / DLK as the predominant MAPKKKs regulating this degeneration process.
Figure 1. MEKK4 / MLK2 / DLK function as the predominant MAPKKKs in axon degeneration following traumatic injury. See also Figure S3 and Table S1.
(A to C) MEKK4 / MLK2 / DLK regulate degeneration of injured sensory axons. Cultures of embryonic DRG neurons were subjected to lentiviral-shRNA knockdown of MEKK4, MLK2 and DLK, individually or in combination. The efficiency of lentiviral-shRNAs was examined by immunoblot analysis of axonal proteins harvested from each condition (A). Axon degeneration at indicated time points following axotomy was visualized by immunostaining (B), and degeneration was quantified (C), n=3 for each condition. (D and E) MEKK4 / MLK2 / DLK regulate degeneration of injured RGC axons in vivo. RGCs of the mice with indicated genotypes were transduced with TdTomato virus together with either Cre/scrambled-shRNA or Cre/MEKK4-shRNA. Degeneration of TdTomato-positive axons was visualized at indicated time points after optic nerve crush (D), and degeneration was quantified (E), n=4 for each condition. (F and G) MEKK4 / MLK2 / DLK regulate degeneration of injured cortical axons. Cultures of embryonic cortical neurons with indicated genotypes were transduced with lentivirus expressing Cre together with scrambled-shRNA or MEKK4-shRNAs. Axon degeneration at indicated time points following axotomy was visualized by immunostaining (F), and degeneration was quantified (G), n=3 for each condition. Values are presented as mean ± SEM; *, p < 0.01.
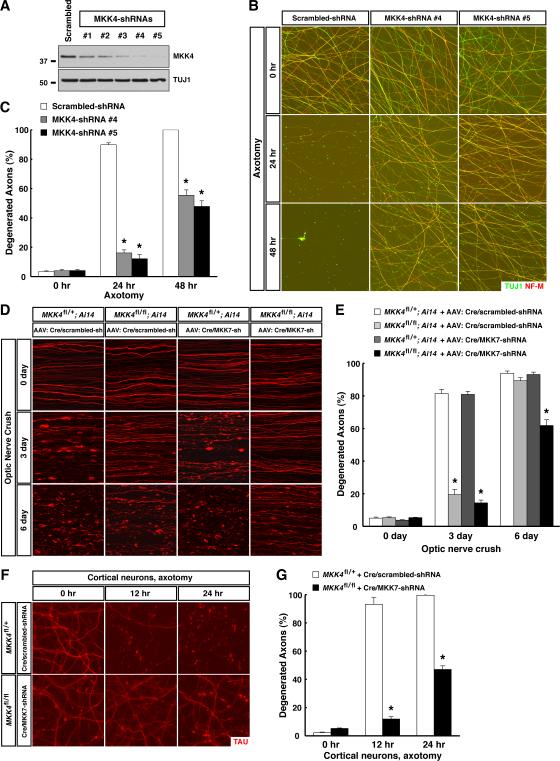
We next explored the 7 members of MAPK kinase (MAPKK) family. Inhibition of MEK1 / MEK2 with the highly specific inhibitor PD-0325901 failed to protect sensory axons after injury in vitro (data not shown). We therefore screened lentiviral-shRNAs against the other five MAPKK members (Table S1). Knockdown of MKK4 (also known as SEK1) led to strong protection of injured sensory axons, i.e., with either of the two most efficient shRNAs, axon degeneration was almost completely suppressed at 24 hr, and about 50% of injured axons persisted even at 48 hr (Fig. 2A to 2C). In parallel, we examined expression of six MAPKK members in adult mouse retina, and found that mRNAs for all six, including MKK4, are expressed in RGCs (Fig. S3B). To determine the function of MKK4 in vivo, RGC axons of MKK4flox/flox mice were transduced with a Cre/scrambled-shRNA virus. TdTomato-positive axons were dramatically protected against degeneration compared to those in MKK4flox/+ mice 3 days after injury (Fig. 2D and 2E). However, RGC axons in MKK4flox/flox mice still exhibited significant degeneration at 6 days, raising the possibility that other MAPKKs might contribute. Indeed, further knockdown of MKK7 in RGC axons of MKK4flox/flox mice using a Cre/MKK7-shRNA virus led to significant enhancement of protection, i.e., over 40% of TdTomato-positive axons were intact at 6 days (Fig. 2D and 2E). Of note, transduction of RGC axons of MKK4flox/+ mice with the Cre/MKK7-shRNA virus did not offer protection (Fig. 2D and 2E), and shRNA knockdown of MKK3 or MKK6 failed to enhance protection in MKK4flox/flox mice (data not shown). These results identify MKK4 as the predominant MAPKK in axon degeneration after injury, with MKK7 exerting a moderate redundant function.
Figure 2. MKK4 functions as the predominant MAPKK assisted by MKK7 in axon degeneration following traumatic injury. See also Figure S3 and Table S1.
(A to C) MKK4 regulates degeneration of injured sensory axons. Cultures of embryonic DRG neurons were subjected to lentiviral-shRNA knockdown of MKK4. Efficiency of lentiviral-shRNAs was examined by immunoblot analysis of axonal proteins harvested from each condition (A). Degeneration of axons transduced with the two most efficient lentiviral-shRNAs was visualized by immunostaining at indicated time points after axotomy (B), and degeneration was quantified (C), n=3 for each condition. (D and E) MKK4 functions with MKK7 to regulate degeneration of injured RGC axons in vivo. RGCs of the mice with indicated genotypes were transduced with Cre/scrambled-shRNA or Cre/MKK7-shRNA virus. Ai14 represents the Rosa26-CAG-LSL-TdTomato reporter. Degeneration of TdTomato-positive axons was visualized at indicated time points after optic nerve crush (D), and degeneration was quantified (E), n=4 for each condition. (F and G) MKK4 / MKK7 regulate degeneration of injured cortical axons. Cultures of embryonic cortical neurons with indicated genotypes were transduced with lentivirus expressing Cre together with scrambled-shRNA or MKK7-shRNA. Axon degeneration at indicated time points following axotomy was visualized by immunostaining (F), and degeneration was quantified (G), n=3 for each condition. Values are presented as mean ± SEM; *, p < 0.01.
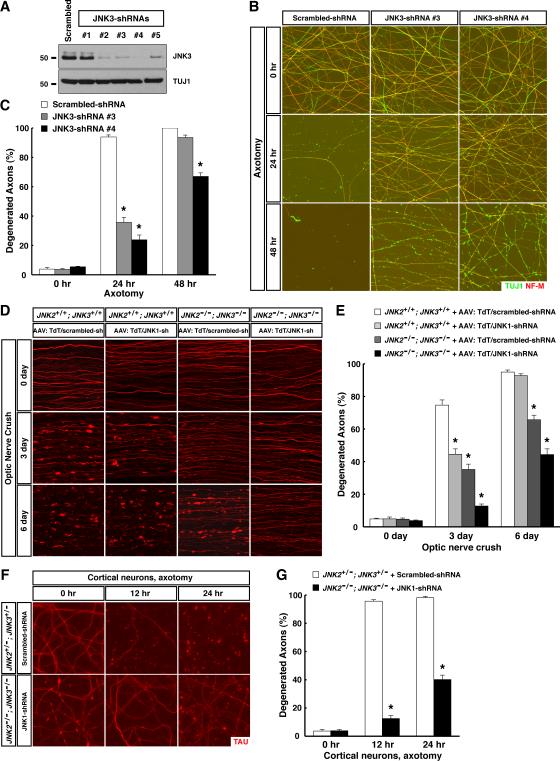
MKK4 activates MAPK members of the JNK and p38 families, whereas MKK7 only activates JNK family members (Weston and Davis, 2007). mRNAs of all three JNKs and two p38s (p38alpha and p38delta) are expressed in RGCs in adult mouse retina (Fig. S4A). In vitro treatment of injured RGC axons with a JNK inhibitor but not a p38 inhibitor delayed degeneration (Fig. S4C and S4D), consistent with previous reports (Chen et al., 2012; Miller et al., 2009). As well, in the lentiviral-shRNA screening of 13 MAPK family members (Table S1), knockdown of JNK3 significantly protected sensory axons in vitro, i.e., degeneration was strongly suppressed 24 hr after axotomy with either of the two most efficient shRNAs, and a sizable fraction of injured axons still remained intact at 48 hr with the most efficient shRNA (Fig. 3A to 3C). Consistent with the in vitro results, degeneration of RGC axons was significantly delayed 6 days after injury in JNK3−/- mice, and the protection level was greater in JNK2−/−; JNK3−/− mice, while there was no effect in JNK2−/− mice (Fig. S4E and S4F). Further, shRNA-knockdown of JNK1 in wildtype RGC axons inhibited degeneration after injury (Fig. 3D and 3E). More importantly, survival of RGC axons in JNK2−/−; JNK3−/− mice was enhanced by shRNA-knockdown of JNK1, with about 60% of TdTomato-positive axons being intact at 6 days (Fig. 3D and 3E). Therefore, JNK1 / JNK2 / JNK3 function to regulate the degeneration of injured axons, with JNK1 and JNK3 playing predominant roles.
Figure 3. JNK1 and JNK3 function as the predominant MAPKs assisted by JNK2 in axon degeneration after traumatic injury. See also Figure S4 and Table S1.
(A to C) JNK3 regulates degeneration of injured sensory axons. Cultures of embryonic DRG neurons were subjected to lentiviral-shRNA knockdown of JNK3. Efficiency of lentiviral-shRNAs was examined by immunoblot analysis of axonal proteins harvested from each condition (A). Degeneration of axons transduced with the two most efficient lentiviral-shRNAs was visualized by immunostaining at indicated time points after axotomy (B), and degeneration was quantified (C), n=3 for each condition. (D and E) JNK1 and JNK3 function as the predominant MAPKs in regulating degeneration of injured RGC axons in vivo. RGCs of the mice with indicated genotypes were transduced with TdTomato/scrambled-shRNA or TdTomato/JNK1-shRNA virus. Degeneration of TdTomato-positive axons was visualized at indicated time points after optic nerve crush (D), and degeneration was quantified (E), n=4 for each condition. (F and G) JNK1 / JNK2 / JNK3 regulate degeneration of injured cortical axons. Cultures of embryonic cortical neurons with indicated genotypes were transduced with lentivirus expressing scrambled-shRNA or JNK1-shRNA. Axon degeneration at indicated time points following axotomy was visualized by immunostaining (F), and degeneration was quantified (G), n=3 for each condition. Values are presented as mean ± SEM; *, p < 0.01.
MAPK signaling can be modulated by various scaffold proteins, including the JIPs (JNK-interacting proteins) (Morrison and Davis, 2003). mRNAs of three JIPs are expressed in adult mouse RGCs (Fig. S4B). Knockdown of JIP3 in wildtype RGCs with a TdTomato/JIP3-shRNA virus protected their axons at 3 days, though not 6 days, after injury (Fig. S4G and S4H, data not shown), whereas shRNA-knockdown of JIP1 or JIP2 did not (data not shown), showing specific involvement of JIP3 in this process.
Thus, using sensory neurons and RGCs as models, we delineate a MAPK cascade comprising three MAPKKKs (MEKK4 / MLK2 / DLK), two MAPKKs (MKK4 / MKK7), three MAPKs (JNK1 / JNK2 / JNK3), and the scaffold protein JIP3, as a central pathway in axon degeneration after traumatic injury. This MAPK cascade is also critical for the degeneration of injured cortical axons, as combinatorial depletion of MEKK4 / MLK2 / DLK (Fig. 1F and 1G), MKK4 / MKK7 (Fig. 2F and 2G), or JNK1 / JNK2 / JNK3 (Fig. 3F and 3G) in cultured cortical neurons all strongly protected against injury, with the protective level comparable to that in Sarm1−/− cortical neurons (Osterloh et al., 2012).
The MAPK cascade functions downstream of Sarm1 as the early degenerative response to axonal injury
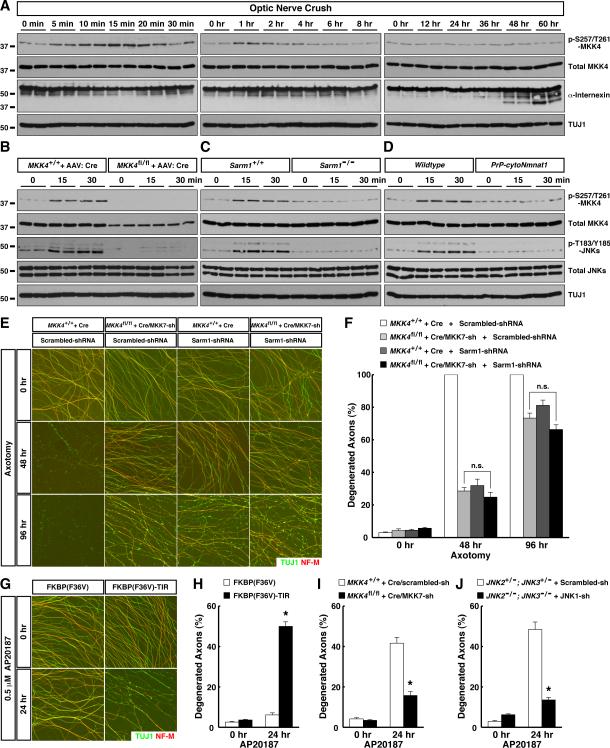
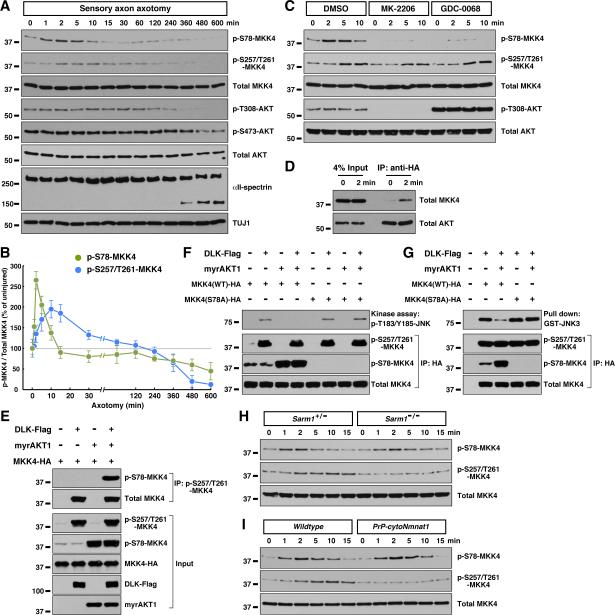
We next examined activation of this MAPK pathway after axonal injury. Ser257 / Thr261 phosphorylation of MKK4, which reflects activity of upstream MAPKKKs, was evident as little as 5 min after optic nerve injury. MKK4 activation peaked between 15 to 30 min, then returned to baseline between 2 to 4 hr (Fig. 4A and Fig. S5A). This signaling represents the early degenerative response, occurring days before physical breakdown of axons, as assessed by proteolysis of cytoskeletal components starting around 48 hr (e.g., α-internexin, Fig. 4A).
Figure 4. Sarm1 is required for activation of the MAPK cascade as an early response to axonal injury. See also Figure S5.
(A to D) Nerve samples were harvested at indicated time points after optic nerve crush for immunoblot analysis (2 mice per time point). (A) Activation of the MAPK cascade represents an early response to axonal injury. (B) MKK4 functions as the predominant MAPKK for activation of downstream JNKs. RGCs of wildtype or MKK4flox/flox mice were subjected to saturated transduction of Cre virus for 6 weeks prior to optic nerve crush. (C) Sarm1 is required for activation of the MAPK cascade. (D) Activation of the MAPK cascade is suppressed by the cytoNmnat1 transgene. (E and F) Sarm1 and MKK4 / MKK7 function in the same genetic pathway. Cultures of sensory neurons with indicated genotypes were transduced with lentivirus expressing scrambled-shRNA or Sarm1-shRNA. Axon degeneration at indicated time points following axotomy was visualized by immunostaining (E), and degeneration was quantified (F), n=3 for each condition. (G and H) Induced dimerization of the Sarm1 TIR-domain causes axon degeneration. Cultures of sensory neurons were transduced with lentivirus expressing FKBP(F36V) or FKBP(F36V)-TIR. Axon degeneration at 24 hr after treatment with 0.5 μM AP20187 was visualized by immunostaining (G), and degeneration was quantified (H), n=3 for each condition. (I and J) Axon degeneration induced by the Sarm1 TIR-domain requires the MAPK cascade. Cultures of sensory neurons with indicated genotypes were transduced with lentivirus expressing FKBP(F36V)-TIR. Axon degeneration at 24 hr after treatment with 0.5 μM AP20187 was quantified, n=3 for each condition. Values are presented as mean ± SEM; *, p < 0.01; n.s., not significant.
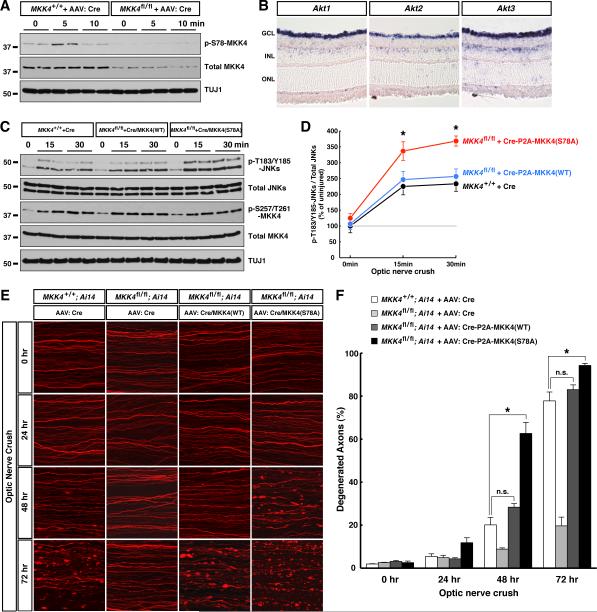
MKK4 activation in injured optic nerves was specific to RGC axons. When we transduced RGCs of MKK4flox/flox mice with a saturating amount of Cre virus, which depleted MKK4 protein in over 90% of RGCs (Fig. S5C) and decreased total MKK4 protein in optic nerves, this in turn eliminated MKK4 phosphorylation on Ser257 / Thr261 in injured nerves (Fig. 4B and Fig. S5B). Deletion of MKK4 in RGC axons also largely diminished activation of downstream JNKs (Fig. 4B). To determine the physiological role of MKK4 phosphorylation on Ser257 / Thr261 in degeneration, we depleted endogenous MKK4 protein in RGCs of MKK4flox/flox mice with Cre, and concurrently delivered into these cells wildtype MKK4 or the MKK4(S257A/T261A) mutant. After nerve injury, RGC axons of MKK4flox/flox mice transduced with the Cre-P2A-MKK4(WT) virus degenerated normally (Fig. 7E and 7F). In contrast, RGC axons transduced with the Cre-P2A-MKK4(S257A/T261A) virus exhibited strong reduction of degeneration at 3 days (Fig. S5D and S5E) similar to that seen in MKK4flox/flox mice transduced with the Cre virus (Fig. 2D and 2E), showing that MKK4(S257A/T261A) failed to rescue the function of endogenous MKK4. Moreover, MKK4(S257A/T261A) had a dominant-negative effect, since RGC axons of wildtype mice transduced with the Cre-P2A-MKK4(S257A/T261A) virus showed delayed degeneration 3 days after injury (Fig. S5D and S5E).
Figure 7. Antagonism of pro-degenerative function of MKK4 by AKT modulates the early response to axonal injury. See also Figure S6.
(A) MKK4 phosphorylation on Ser78 in the early response to axonal injury in vivo. RGCs of wildtype or MKK4flox/flox mice were subjected to saturated transduction of Cre virus. Nerve samples were harvested at indicated time points after optic nerve crush for immunoblot analysis. (B) mRNA expression of the 3 members of the AKT family in adult mouse retina was determined by in situ hybridization. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer. (C and D) MKK4 phosphorylation on Ser78 limits activation of downstream JNKs. RGCs of wildtype or MKK4flox/flox mice were subjected to saturated transduction of indicated virus. Nerve samples were harvested at indicated time points after optic nerve crush for immunoblot analysis (C), and JNK phosphorylation on Thr183/Tyr185 was quantified (D, n=3 for each condition). Values are presented as mean ± SD. (E and F) MKK4 phosphorylation on Ser78 modulates degeneration of injured RGC axons in vivo. RGCs of mice with indicated genotypes were transduced with indicated viruses. Degeneration of TdTomato-positive axons was visualized at indicated time points after optic nerve crush (E), and degeneration was quantified (F), n=3 for each condition. Ai14 is the Rosa26-CAG-LSL-TdTomato reporter. Values are presented as mean ± SEM. *, p < 0.01; n.s., not significant.
We also studied MKK4 activation in the in vitro degeneration model, which allows us to obtain preparations of axonal proteins excluding neuronal cell bodies and non-neuronal cells, and therefore to focus on axon-specific events (Yang et al., 2013). Similar to our in vivo data, MKK4 phosphorylation on Ser257 / Thr261 occurred during the early degenerative response in vitro, peaking around 10 to 15 min after axotomy (Fig. 6A and 6B), but hours before proteolysis of cytoskeletal proteins (e.g., αII-spectrin). In addition, treatment of RGC axons in vitro with the JNK inhibitor during the first 1 hr after injury was sufficient to suppress degeneration (data not shown), similar to a previous observation implying an early role of JNK signaling in the degeneration of injured sensory axons (Miller et al., 2009).
Figure 6. Sarm1-independent regulation of MKK4 by AKT signaling in the early response to axonal injury. See also Figure S6.
(A and B) Two temporally distinct MKK4 phosphorylation events in response to axonal injury. Axonal proteins of cultured sensory neurons were harvested at indicated time points after axotomy for immunoblot analysis (A), and MKK4 phosphorylation on Ser78 or Ser257/Thr261 was quantified (B). Values are presented as mean ± SD. (C) Inhibition of AKT signaling abolishes MKK4 phosphorylation on Ser78 in injured axons. Cultures of sensory neurons were treated with 5 μM MK-2206 or 5 μM GDC-0068 for 1 hr prior to axotomy. Axonal proteins harvested at indicated time points after axotomy were subjected to immunoblot analysis. (D) Axonal injury promotes interaction between AKT and MKK4. Cultures of sensory neurons were transduced with lentivirus expressing AKT1 with a C-terminal HA-tag, and axonal proteins were subjected to anti-HA immunoprecipitation. (E) Ser257/Thr261 and Ser78 phosphorylation can occur simultaneously on MKK4. HEK293 cells were transfected with plasmids expressing MKK4, together with DLK or myrAKT1. Ser257/Thr261-phosphorylated MKK4 was immunoprecipitated by p-S257/T261-MKK4 specific antibody. (F and G) Ser78 phosphorylation inhibits MKK4 kinase activity towards JNK. HEK293 cells were transfected with plasmids expressing MKK4 or MKK4(S78A) mutant with C-terminal HA-tag, together with DLK or myrAKT1. MKK4 proteins were immunoaffinity-purified, then subjected to kinase assay (F) or pull-down assay (G) with recombinant GST-JNK3(K55R). (H and I) MKK4 phosphorylation on Ser78 is independent of Sarm1 (H) or cytoNmnat1 (I). Axonal proteins of cultured sensory neurons with indicated genotypes were harvested at indicated time points after axotomy for immunoblot analysis.
Supporting the hypothesis of an evolutionarily conserved Sarm1-MAPK signaling axis, early activation of MKK4 and downstream JNKs in injured optic nerves was abolished in Sarm1−/− mice (Fig. 4C and Fig. S5B). Similarly, MKK4 phosphorylation on Ser257 / Thr261 was absent in Sarm1−/− sensory axons in the in vitro model (Fig. 6H). To further determine the genetic interaction between Sarm1 and the MAPK cascade, we examined additivity of the protective effects afforded by depletion of Sarm1 or MKK4 / MKK7. Combinatorial depletion of MKK4 / MKK7 in sensory axons resulted in strong protection until 48 hr after axotomy, but most injured axons eventually degenerated at 96 hr (Fig. 4E and 4F). While shRNA knockdown of Sarm1 gave rise to a similar protection, Sarm1-shRNA did not further potentiate protection by MKK4 / MKK7 depletion (Fig. 4E and 4F). Similarly, though Sarm1−/− sensory axons were highly resistant to degeneration, the majority degenerated 96 hr after axotomy (Fig. S5F and S5G). shRNA knockdown of MKK4 / MKK7 also strongly protected, but failed to further extend survival of Sarm1−/− axons. The non-additivity of protective effects implies that Sarm1 and the MAPK cascade reside in the same genetic pathway.
Domain analyses of Sarm1 or its ortholog tir-1 have indicated that the SAM domains mainly function in protein dimerization, which enables the C-terminal TIR domain to activate downstream signaling. The N-terminal ARM domain exerts an auto-inhibitory role, because Sarm1 or TIR-1 without the ARM domain appears constitutively active (Chuang and Bargmann, 2005; Gerdts et al., 2013). Consistent with this, expression of a Sarm1 mutant lacking the N-terminal ARM domain led to axon degeneration about 4 to 6 days after viral transduction (data not shown). To control the pro-degenerative Sarm1 signal in a temporally-defined manner, we fused the Sarm1 TIR-domain to FKBP(F36V), which could be dimerized by the cell-permeable chemical AP20187. Treatment of sensory neurons expressing FKBP(F36V)-TIR with AP20187 induced degeneration of distal axons (Fig. 4G and 4H), which appears independent of the apoptotic pathway since it was not affected by genetic deletion of caspase-9 or overexpression of Bcl-xl (data not shown). Importantly, axons of neurons expressing FKBP(F36V)-TIR showed rapid MKK4 phosphorylation on Ser257 / Thr261 in response to AP20187 (Fig. S5H and S5I), and their degeneration was largely blocked by combinatorial depletion of MKK4 / MKK7 or JNK1 / JNK2 / JNK3 (Fig. 4I and 4J). Although care must be taken in interpreting the effect of Sarm1 gain-of-function, especially as FKBP(F36V)-TIR dimerization also led to atrophy and death of neuronal cell bodies similar to that observed with constitutively-active Sarm1 (Gerdts et al., 2013; Summers et al., 2014), the results nonetheless further support the model that Sarm1 functions upstream of the MAPK cascade.
The axonal protective function of the Wlds protein has been known for decades, but its underlying mechanism remains elusive. To determine its relationship to MAPK signaling, we examined activation of the pathway in cytoNmnat1-transgenic mice. Early activation of MKK4 and downstream JNKs in injured optic nerves in vivo was suppressed in these mice (Fig. 4D and Fig. S5B), and MKK4 phosphorylation on Ser257 / Thr261 was absent from their sensory axons after axonal injury in vitro (Fig. 6I), suggesting that cytoNmnat1/Wlds might protect by inhibiting the MAPK pathway.
The Sarm1-MAPK pathway triggers local energy deficit leading to morphological degeneration
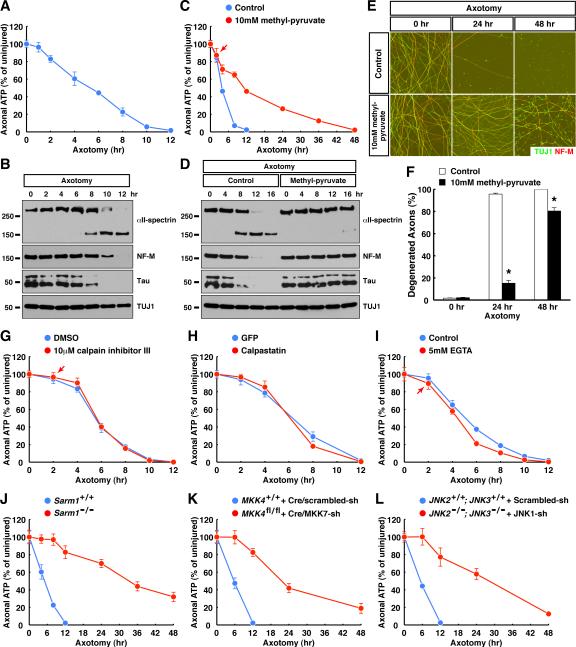
Activation of the Sarm1-MAPK pathway occurs minutes after injury, but hours or even days before breakdown of axonal structures, prompting us to explore the connection between these processes. A previous study reported a decrease of ATP level in injured axons that could be inhibited by the Wlds protein (Wang et al., 2005), but the relevance of ATP depletion was questioned by other studies (Press and Milbrandt, 2008; Summers et al., 2014). Of note, the original measurement of ATP levels was carried out with axonal samples (Wang et al., 2005), but in later studies was done with whole neuronal cultures including cell bodies, and we reasoned that this difference might explain the discrepancy. Taking advantage of our in vitro axonal enrichment method and also a newly-devised luciferase-based ATP measurement method (instead of classic HPLC), we followed ATP levels exclusively in axons after injury. Consistent with the original study (Wang et al., 2005), there was continuous depletion of axonal ATP in injured sensory axons, which preceded proteolysis of cytoskeletal proteins and morphological degeneration (Fig. 5A and 5B, and data not shown). That study also showed that ATP levels in axons could be rescued by metabolic fuel such as methyl-pyruvate (a cell-permeable form of pyruvate) provided prior to injury (Wang et al., 2005). We extended that finding by treating injured axons with methyl-pyruvate 2 hr post-axotomy, i.e., after the early activation of Sarm1-MAPK pathway in this in vitro model (Fig. 6A and 6B), and observed that it could also significantly delay the ATP depletion (Fig. 5C). Because calpain activation is responsible for proteolysis of cytoskeletal components and physical breakdown of axonal structures (Fig. S2F and S2G) (Yang et al., 2013), we examined the relationship between this local energy deficit and calpain activation. Methyl-pyruvate treatment, which delayed ATP depletion, strongly suppressed calpain-mediated proteolysis of cytoskeletal proteins (Fig. 5D) as well as morphological degeneration of injured axons (Fig. 5E and Fig. 5F). To further confirm that calpains function downstream of ATP depletion, we treated injured axons 2 hr post-axotomy with calpain inhibitor III, which is relatively selective to calpains (Yang et al., 2013), or transduced the neurons with lentivirus expressing the endogenous specific calpain inhibitor calpastatin. Both methods strongly inhibited calpain activation and morphological degeneration (Yang et al., 2013), but neither ameliorated the energy deficit in injured axons (Fig. 5G and 5H). Similarly, treatment of injured axons 2 hr post-axotomy with EGTA to chelate Ca2+ that is essential for calpain activation also failed to rescue axonal ATP levels (Fig. 5I). The results together argue that the local energy deficit in axons precedes, and is causative to, calpain activation and morphological degeneration after injury.
Figure 5. The Sarm1-MAPK pathway triggers an axonal energy deficit following traumatic injury. See also Figure S6.
(A and B) Energy deficit precedes calpain activation in injured axons. Axonal ATP levels of cultured sensory neurons were measured at indicated time points after axotomy (A, n=6 for each condition), and in parallel, axonal proteins were harvested for immunoblot analysis (B). (C to F) Energy deficit is causative to calpain activation and morphological degeneration. Cultures of sensory neurons were provided with 10 mM methyl-pyruvate as metabolic fuel 2 hr post-axotomy. At indicated time points, axonal ATP levels were measured (C, n=6 for each condition), axonal proteins were harvested for immunoblot analysis (D), axon degeneration was visualized by immunostaining (E), and the degeneration was quantified (F, n=4 for each condition). (G to I) Calpains function downstream of axonal energy deficit. Cultures of sensory neurons were treated with 10 μM calpain inhibitor III (G, n=6 for each condition) or 5 mM EGTA (I, n=6 for each condition) 2 hr post-axotomy, or were transduced with lentivirus expressing calpastatin (H, n=4 for each condition). Axonal ATP levels were measured at indicated time points. (J to L) The Sarm1-MAPK pathway triggers local energy deficit following axonal injury. Axonal ATP levels of cultured sensory neurons with indicated genotypes were measured at indicated time points after axotomy, n=4 for each condition. Values are presented as mean ± SEM; *, p < 0.01.
Importantly, the first detectable change of axonal ATP levels occurred around 2 hr post-axotomy (e.g., Fig. 1A), after the early activation of the Sarm1-MAPK pathway (Fig. 6A and 6B), and depletion of Sarm1, MKK4 / MKK7, or JNK1 / JNK2 / JNK3, which suppressed morphological degeneration as reported above, all dramatically preserved ATP levels in injured axons (Fig. 5J to 5L), even better than methyl-pyruvate treatment. The same was also true in sympathetic (i.e., superior cervical ganglion) axons: injury triggered ATP depletion in these axons that preceded proteolysis of cytoskeletal proteins and morphological degeneration (data not shown); this energy deficit was not affected by calpain inhibition (Fig. S6A), but was significantly delayed by deletion of Sarm1 (Fig. S6B) or inhibition of JNKs (Fig. S6C). Together, our results support the model that the Sarm1-MAPK pathway triggers a local energy deficit in injured axons, which leads to calpain activation and morphological degeneration.
Pro-degenerative function of MKK4 is antagonized by AKT
While studying the phosphorylation status of Sarm1-MAPK components in injured axons, we unexpectedly found that MKK4 was also phosphorylated on Ser78. This phosphorylation increased significantly around 1 to 5 min after injury, even preceding phosphorylation on Ser257 / Thr261 (Fig. 6A and 6B). The sequence surrounding Ser78 residue of mouse MKK4 is an authentic target for AKT, and phosphorylation of Ser78 can inhibit MKK4 activity (Park et al., 2002). Two selective AKT inhibitors, i.e., the allosteric inhibitor MK-2206 and the ATP-competitive inhibitor GDC-0068, both significantly reduced MKK4 phosphorylation on Ser78 in axons after injury (Fig. 6C), though as expected from their distinct mechanisms, MK-2206 decreased while GDC-0068 increased AKT phosphorylation on Thr308 and Ser473, residues critical for AKT activation (Fig. 6C, and data not shown). We noted that phosphorylation on Thr308 and Ser473 appeared constant in injured axons and only started to decrease when degeneration began (Fig. 6A and 6C). However, there was enhanced interaction between AKT and MKK4 in axons immediately after injury (Fig. 6D), temporally correlating with increased MKK4 phosphorylation on Ser78 (Fig. 6A and 6B).
We explored how these two distinct phosphorylation events regulate MKK4 activity towards downstream JNKs. MKK4 was expressed in cultured cells with DLK (whose overexpression is self-activating, and thus mimics activation of the MAPK cascade) or myrAKT1 (a constitutively-active form of AKT1 that mimics AKT signaling). As expected, DLK significantly increased MKK4 phosphorylation on Ser257 / Thr261, and myrAKT1 enhanced MKK4 phosphorylation on Ser78 (Fig. 6E). Notably, AKT-mediated Ser78 phosphorylation did not affect the level of Ser257 / Thr261 phosphorylation induced by DLK, and vice versa. Moreover, when phospho-S257/T261-MKK4 was specifically enriched using the p-S257/T261-MKK4 antibody, Ser78 phosphorylation was retained in the immunoprecipitated sample (Fig. 6E). The results suggest that the two phosphorylation events are biochemically independent. Next, MKK4 expressed with DLK or myrAKT1 was immunoaffinity purified and subjected to a kinase assay using recombinant JNK3(K55R) as the substrate. While MKK4 activated by DLK had strong activity in phosphorylating JNK3, MKK4 expressed with DLK and AKT1 together exhibited little activity, even though the two samples had similar levels of Ser257 / Thr261 phosphorylation (Fig. 6F). This Ser78 phosphorylation-mediated inhibition of MKK4 activity correlated with a decrease of direct interaction between MKK4 and the JNK substrate in a protein pull-down assay (Fig. 6G), in line with the fact that a major JNK-binding site of MKK4 is adjacent to the Ser78 residue (Ho et al., 2003). The AKT-mediated inhibitory effects were abolished with the MKK4(S78A) mutant protein (Fig. 6F and 6G). These results show that while Ser257 / Thr261 phosphorylation of MKK4 activates its kinase activity towards JNK, Ser78 phosphorylation of MKK4 can block its interaction with the JNK substrate.
Interestingly, unlike Ser257 / Thr261 phosphorylation, MKK4 phosphorylation on Ser78 was not affected in injured sensory axons from Sarm1−/− (Fig. 6H and S6D) or cytoNmnat1-transgenic (Fig. 6I and S6D) mice. Moreover, AKT inhibition did not have a significant effect on MKK4 phosphorylation on Ser257 / Thr261 after injury (Fig. 6C). The results suggest that AKT signaling targets MKK4 in a Sarm1-independent manner.
The finding that AKT-mediated Ser78 phosphorylation can suppress Ser257/Thr261-phosphorylated MKK4 activity towards JNKs raised the possibility that it might promote axonal survival. Indeed, transient inhibition of AKT in the first 1 hr after injury significantly accelerated degeneration of RGC axons in vitro (Fig. S6G and S7H). In vivo, MKK4 phosphorylation on Ser78 was present in optic nerves and transiently increased after injury (Fig. 7A), and was dramatically reduced following deletion of MKK4 in RGCs of MKK4flox/flox mice with the Cre virus (Fig. 7A). Supporting involvement of AKT signaling, mRNAs of all three AKT members are expressed in RGCs (Fig. 7B). To determine its physiological relevance, we depleted endogenous MKK4 protein in RGCs of MKK4flox/flox mice with Cre, and concurrently delivered either wildtype MKK4 or the MKK4(S78A) mutant, which were expressed at levels comparable to endogenous MKK4 both in cultured cells (Fig. S6E) and in optic nerves (Fig. 7C). After nerve injury, MKK4flox/flox mice transduced with the Cre-P2A-MKK4(WT) virus showed a similar level of JNK activation as in wildtype mice, whereas those transduced with the Cre-P2A-MKK4(S78A) virus exhibited significantly stronger JNK activation (Fig. 7C and 7D). In the latter case, RGC axons underwent accelerated degeneration, i.e., while the vast majority of TdTomato-positive axons were intact in wildtype mice or in the Cre-P2A-MKK4(WT) condition 48 hr after injury, over 60% of axons in the Cre-P2A-MKK4(S78A) condition had already degenerated, and their degeneration was completed by 72 hr (Fig. 7E and 7F). Consistent with Ser257 / Thr261 and Ser78 phosphorylation being independent events, MKK4flox/flox mice transduced with the Cre-P2A-MKK4(WT) or Cre-P2A-MKK4(S78A) viruses exhibited similar levels of Ser257 / Thr261 phosphorylation after injury (Fig. 7C and Fig. S6F). Our results demonstrate that MKK4 is regulated by two distinct phosphorylation events in the early response to axonal injury, and Ser78 phosphorylation mediated by a Sarm1-independent AKT signal modulates the degeneration process by limiting activation of downstream JNKs.
Involvement of the Sarm1-MAPK pathway in other axonal insults
So far our study has focused on axon degeneration in models of traumatic injury. To determine whether the Sarm1-MAPK pathway participates in other types of axonal insult, we examined toxic effects of vincristine, a common chemotherapy drug, and rotenone, a mitochondrial poison. Consistent with a previous report (Gerdts et al., 2013), Sarm1−/− sensory axons were resistant to vincristine toxicity. In addition, we observed that combinatorial depletion of components at each level of the MAPK cascade resulted in significant protection (Fig. S7A). Moreover, vincristine-damaged axons also exhibited local energy deficit before morphological degeneration in a Sarm1-dependent manner (Fig. S7B). Similarly, depletion of Sarm1 or components at each level of the MAPK cascade all protected rotenone-damaged sensory axons against degeneration (Fig. S7C), and rotenone-induced ATP depletion was partially blocked in Sarm1−/− axons (Fig. S7D).
Discussion
Although axonal death has been increasingly recognized as a key pathological feature of neurodegenerative disorders, the molecular mechanisms underlying the degeneration program have been poorly characterized. In this study, we delineate a MAPK cascade regulating pathological axon degeneration. Our genetic analyses show that this MAPK cascade operates downstream of Sarm1, and likely represents the major downstream signal mediated by Sarm1. Its pivotal role appears not to have been fully appreciated because of the significant redundancy at each level of the cascade. Interestingly, Sarm1-MAPK signaling triggers a local energy deficit in injured axons, which serves as the key functional link between the early degenerative response of the Sarm1-MAPK pathway and the late phase of morphological degeneration. Because the low level of cytosolic Ca2+ in axons is maintained by Ca2+-ATPases and Na+/Ca2+-exchangers, both of which directly or indirectly depend on a constant energy input, it appears plausible that energy deficit in injured axons could result in gradual accumulation of cytosolic Ca2+ which eventually activates calpains. Given the essential role of ATP in cellular processes, this energy deficit might also disrupt other vital functions in axons, which together with calpain activation would lead to final breakdown of injured axons.
Various studies have supported the possibility that axon degeneration in different pathological conditions could be under a common regulatory mechanism. Therefore, the Sarm1-MAPK pathway could play a critical role following axonal insults besides traumatic injury. Indeed, our results with vincristine and rotenone support that the signaling pathway from Sarm1 to the MAPK cascade to axonal ATP depletion and to calpain-mediated breakdown of axonal structures could be a common mechanism in different types of pathological axon degeneration. In this context, it is interesting to note that JNK3 has been implicated in the degeneration process in models of ischemic stroke (Kuan et al., 2003), Huntington's disease (Morfini et al., 2009), and Alzheimer's disease (Yoon et al., 2012), and a role for DLK has also been reported in excitotoxicity-induced degeneration (Pozniak et al., 2013). However, given the diversity of MAPK family members, especially the MAPKKKs, it will be important to determine whether additional MAPK member(s) are involved in other types of axonal insults.
Future studies will also help elucidate the biochemical mechanism underlying Sarm1-mediated activation of MAPK cascade. In our initial studies we were unable to detect a direct physical interaction between Sarm1, or its individual domains, with components in the MAPK cascade (data not shown). In addition, it will be critical to understand how the Sarm1-MAPK pathway triggers local energy deficit in response to axonal insults. Two pieces of evidence suggest that Sarm1-MAPK signaling may disrupt a step(s) in glycolysis in axons: ATP depletion in wildtype injured axons can be ameliorated by the glycolysis end-product pyruvate (Fig. 5C), and Sarm1−/− axons can maintain a fraction of their original ATP level even with ATP production from mitochondria completely blocked by rotenone (Fig. S7D). Of note, our findings on ATP depletion are consistent with a previous report that examined axonal ATP (Wang et al., 2005), but contrast with some others that examined ATP levels in whole neuronal cultures, which included both axons and cell bodies (Press and Milbrandt, 2008; Summers et al., 2014). Because ATP measurements in this study were all performed with pure axonal samples in absence of neuronal cell bodies, our results provide a more direct insight into axon-specific signaling events.
The protective cytoNmnat1/Wlds protein can inhibit activation of the MAPK cascade upon injury. The mechanism underlying cytoNmnat1/Wlds protective effect has been under debate, and our finding opens an avenue to understanding how it intersects with MAPK activation, either by altering nucleotide metabolism or by additional mechanism(s). Since cytoNmnat1/Wlds inhibits axon degeneration in many models of neurodegeneration, it will also be exciting to determine whether its protective effects in other degenerative paradigms rely on inhibition of the MAPK pathway identified here.
It remains possible that AKT signaling also functions through other targets to modulate axon degeneration. For instance, a previous study reported that AKT could protect injured axons by inhibiting GSK3β (Wakatsuki et al., 2011), although we found that pharmacological inhibition of GSK3 in injured RGC axons failed to significantly protect at least in vitro (Fig. S6I and S6J). With our finding that MKK4 is subject to the two temporally-distinct phosphorylation events, it is tempting to speculate that activation of these two antagonizing signals could enable pro-survival AKT to act immediately following injury to suppress the MAPK degenerative response, allowing axons to repair themselves if possible. Only when damage is irreversible would the Sarm1-MAPK pathway instruct the destruction of injured axons. Such a balance could represent an important checkpoint to prevent inappropriate initiation of axon degeneration. It will be of particular interest to explore whether reduction of AKT signaling in axons, resulting in loss of this antagonistic mechanism, contributes to axon degeneration in any disease condition.
In summary, our study demonstrates that distinct upstream signals can converge onto a central MAPK pathway that triggers local energy deficit to promote pathological axon degeneration (Fig. S7E), a regulatory mechanism with potentially broad implications in neurodegenerative disease.
Experimental Procedures
Intravitreal injection and optic nerve injury
All surgical procedures in mice were performed in compliance with the protocol approved by the IACUC of the Rockefeller University. RGCs were transduced by AAV2 (2 x 103 transduction units for sparse labeling; 1 × 107 transduction units for saturated labeling) delivered via intravitreal injection. After 3 to 6 weeks, optic nerve crush was performed about 1-mm distal from the eyeball. For visualization of TdTomato-labeled axons, nerve segments about 2-mm proximal to the optic chiasm were scanned by confocal microscopy. For biochemical analysis, nerve segments starting immediately distal to the injury site up to the optic chiasm were dissected and homogenized in 150 μl Urea / SDS buffer [50 mM Tris-Cl (pH 6.8), 8 M urea, 10 % SDS, 10 mM sodium EDTA, and 50 mM DTT]. 10 μg of total protein for each nerve sample was subjected to immunoblot analysis.
Neuronal cultures and analyses on axonal preparations
For RGC explant cultures, mouse retinas were dissected at postnatal day 1, cut into 200 to 300 μm square explants, and cultured in growth factor-free medium [Neurobasal medium supplemented with 2 % B-27, 2 mM glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.5 % methyl-cellulose, and 0.75 % glucose] with the RGC layer facing towards the culture surface coated with poly-D-lysine / N-cadherin / fibronectin. Cultures of mouse embryonic DRG neurons, embryonic cortical neurons, and postnatal SCG neurons were as described (Osterloh et al., 2012). For biochemical analysis of axonal samples, neuronal cell bodies and proximal axons were removed as described (Yang et al., 2013). Axons were immediately quenched in 100 % methanol pre-cooled to −20 °C, then dissolved in Urea / SDS buffer. 1 μg of total protein for each condition pooled from 3 to 6 wells of cultures was subjected to immunoblot analysis. For measurement of axonal ATP levels, neuronal cell bodies and proximal axons were removed, and axons were lysed and subjected to ATP measurement with the ATPlite Luminescence Assay System (PerkinElmer). 4 to 6 wells of cultures were included for each condition, and results are presented as mean ± SEM.
Quantification of axon degeneration
TdTomato-labeled axons in optic nerves or immunostained axons in cultures were scored as degenerated with any sign of swelling and/or fragmentation. Axons observed by electron microscopy in optic nerves were scored as degenerated with any sign of demyelination and/or destruction of axoplasm. Degeneration was quantified as the percentage of all axons scored as degenerated. Results are presented as mean ± SEM, and p-value was calculated using a two-way ANOVA post hoc test.
In situ hybridization
Retinas from 8-week old mice were dissected for 12-μm sagittal cryosections, and sections were hybridized with digoxigenin-labeled antisense or sense RNA probes. Hybridized probes were detected by alkaline phosphatase-conjugated anti-digoxigenin antibody (Roche), and revealed by chromogenic substrate NBT/BCIP (Roche).
Experimental procedures including lentivirus production and transduction, adeno-associated virus production, and electron microscopy have been previously described (Yang et al., 2013). The lentiviral-shRNA constructs from The RNAi Consortium used in the study are listed in Table S1.
Information including antibodies and chemicals, and detailed procedures for animal surgeries, neuronal cultures, immunohistochemistry, in situ hybridization, kinase assay, and quantification methods are in Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
We thank the members of Tessier-Lavigne lab for discussion and suggestions. This work was supported by NIH grant 1R01NS083813-01A1 to M.T.-L.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
References
- Blum ES, Abraham MC, Yoshimura S, Lu Y, Shaham S. Control of nonapoptotic developmental cell death in Caenorhabditis elegans by a polyglutamine-repeat protein. Science. 2012;335:970–973. doi: 10.1126/science.1215156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Lin CW, Chang CY, Jiang ST, Hsueh YP. Sarm1, a negative regulator of innate immunity, interacts with syndecan-2 and regulates neuronal morphology. J Cell Biol. 2011;193:769–784. doi: 10.1083/jcb.201008050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Maloney JA, Kallop DY, Atwal JK, Tam SJ, Baer K, Kissel H, Kaminker JS, Lewcock JW, Weimer RM, et al. Spatially coordinated kinase signaling regulates local axon degeneration. J Neurosci. 2012;32:13439–13453. doi: 10.1523/JNEUROSCI.2039-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang CF, Bargmann CI. A Toll-interleukin 1 repeat protein at the synapse specifies asymmetric odorant receptor expression via ASK1 MAPKKK signaling. Genes Dev. 2005;19:270–281. doi: 10.1101/gad.1276505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforti L, Gilley J, Coleman MP. Wallerian degeneration: an emerging axon death pathway linking injury and disease. Nat Rev Neurosci. 2014;15:394–409. doi: 10.1038/nrn3680. [DOI] [PubMed] [Google Scholar]
- Couillault C, Pujol N, Reboul J, Sabatier L, Guichou JF, Kohara Y, Ewbank JJ. TLR-independent control of innate immunity in Caenorhabditis elegans by the TIR domain adaptor protein TIR-1, an ortholog of human SARM. Nat Immunol. 2004;5:488–494. doi: 10.1038/ni1060. [DOI] [PubMed] [Google Scholar]
- Gerdts J, Summers DW, Sasaki Y, DiAntonio A, Milbrandt J. Sarm1-mediated axon degeneration requires both SAM and TIR interactions. J Neurosci. 2013;33:13569–13580. doi: 10.1523/JNEUROSCI.1197-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho DT, Bardwell AJ, Abdollahi M, Bardwell L. A docking site in MKK4 mediates high affinity binding to JNK MAPKs and competes with similar docking sites in JNK substrates. J Biol Chem. 2003;278:32662–32672. doi: 10.1074/jbc.M304229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan CY, Whitmarsh AJ, Yang DD, Liao G, Schloemer AJ, Dong C, Bao J, Banasiak KJ, Haddad GG, Flavell RA, et al. A critical role of neural-specific JNK3 for ischemic apoptosis. Proc Natl Acad Sci U S A. 2003;100:15184–15189. doi: 10.1073/pnas.2336254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz CL, Shapira M, Chen K, Baillie DL, Tan MW. Caenorhabditis elegans pgp-5 is involved in resistance to bacterial infection and heavy metal and its regulation requires TIR-1 and a p38 map kinase cascade. Biochem Biophys Res Commun. 2007;363:438–443. doi: 10.1016/j.bbrc.2007.08.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BR, Press C, Daniels RW, Sasaki Y, Milbrandt J, DiAntonio A. A dual leucine kinase-dependent axon self-destruction program promotes Wallerian degeneration. Nat Neurosci. 2009;12:387–389. doi: 10.1038/nn.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfini GA, You YM, Pollema SL, Kaminska A, Liu K, Yoshioka K, Bjorkblom B, Coffey ET, Bagnato C, Han D, et al. Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nat Neurosci. 2009;12:864–871. doi: 10.1038/nn.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison DK, Davis RJ. Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol. 2003;19:91–118. doi: 10.1146/annurev.cellbio.19.111401.091942. [DOI] [PubMed] [Google Scholar]
- Osterloh JM, Yang J, Rooney TM, Fox AN, Adalbert R, Powell EH, Sheehan AE, Avery MA, Hackett R, Logan MA, et al. dSarm/Sarm1 is required for activation of an injury-induced axon death pathway. Science. 2012;337:481–484. doi: 10.1126/science.1223899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Kim MS, Huh SH, Park J, Chung J, Kang SS, Choi EJ. Akt (protein kinase B) negatively regulates SEK1 by means of protein phosphorylation. J Biol Chem. 2002;277:2573–2578. doi: 10.1074/jbc.M110299200. [DOI] [PubMed] [Google Scholar]
- Pozniak CD, Sengupta Ghosh A, Gogineni A, Hanson JE, Lee SH, Larson JL, Solanoy H, Bustos D, Li H, Ngu H, et al. Dual leucine zipper kinase is required for excitotoxicity-induced neuronal degeneration. J Exp Med. 2013;210:2553–2567. doi: 10.1084/jem.20122832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press C, Milbrandt J. Nmnat delays axonal degeneration caused by mitochondrial and oxidative stress. J Neurosci. 2008;28:4861–4871. doi: 10.1523/JNEUROSCI.0525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y, Vohra BP, Baloh RH, Milbrandt J. Transgenic mice expressing the Nmnat1 protein manifest robust delay in axonal degeneration in vivo. J Neurosci. 2009;29:6526–6534. doi: 10.1523/JNEUROSCI.1429-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JE, Cho Y, Beirowski B, Milbrandt J, Cavalli V, DiAntonio A. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron. 2012;74:1015–1022. doi: 10.1016/j.neuron.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DJ, Weimer RM, McLaughlin T, Kallop D, Stanger K, Yang J, O'Leary DD, Hannoush RN, Tessier-Lavigne M. A caspase cascade regulating developmental axon degeneration. J Neurosci. 2012;32:17540–17553. doi: 10.1523/JNEUROSCI.3012-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers DW, DiAntonio A, Milbrandt J. Mitochondrial dysfunction induces Sarm1-dependent cell death in sensory neurons. J Neurosci. 2014;34:9338–9350. doi: 10.1523/JNEUROSCI.0877-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Dourdin N, Wu C, De Veyra T, Elce JS, Greer PA. Conditional disruption of ubiquitous calpains in the mouse. Genesis. 2006;44:297–303. doi: 10.1002/dvg.20216. [DOI] [PubMed] [Google Scholar]
- Wakatsuki S, Saitoh F, Araki T. ZNRF1 promotes Wallerian degeneration by degrading AKT to induce GSK3B-dependent CRMP2 phosphorylation. Nat Cell Biol. 2011;13:1415–1423. doi: 10.1038/ncb2373. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhai Q, Chen Y, Lin E, Gu W, McBurney MW, He Z. A local mechanism mediates NAD-dependent protection of axon degeneration. J Cell Biol. 2005;170:349–355. doi: 10.1083/jcb.200504028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JT, Medress ZA, Barres BA. Axon degeneration: Molecular mechanisms of a self-destruction pathway. J Cell Biol. 2012;196:7–18. doi: 10.1083/jcb.201108111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19:142–149. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Whitmore AV, Lindsten T, Raff MC, Thompson CB. The proapoptotic proteins Bax and Bak are not involved in Wallerian degeneration. Cell Death Differ. 2003;10:260–261. doi: 10.1038/sj.cdd.4401147. [DOI] [PubMed] [Google Scholar]
- Xiong X, Collins CA. A conditioning lesion protects axons from degeneration via the Wallenda/DLK MAP kinase signaling cascade. J Neurosci. 2012;32:610–615. doi: 10.1523/JNEUROSCI.3586-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Weimer RM, Kallop D, Olsen O, Wu Z, Renier N, Uryu K, Tessier-Lavigne M. Regulation of axon degeneration after injury and in development by the endogenous calpain inhibitor calpastatin. Neuron. 2013;80:1175–1189. doi: 10.1016/j.neuron.2013.08.034. [DOI] [PubMed] [Google Scholar]
- Yoon SO, Park DJ, Ryu JC, Ozer HG, Tep C, Shin YJ, Lim TH, Pastorino L, Kunwar AJ, Walton JC, et al. JNK3 perpetuates metabolic stress induced by Abeta peptides. Neuron. 2012;75:824–837. doi: 10.1016/j.neuron.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.