Abstract
Cerebellar development is shaped by the interplay of genetic and numerous environmental factors. Recent evidence suggests that cerebellar maturation is acutely sensitive to drugs with abuse liability including alcohol, opioids, and nicotine. Assuming substance abuse disrupts cerebellar maturation, a central question is to what are the basic mechanisms underlying potential drug-induced developmental defects. Evidence reviewed herein suggests that the maturation of granule neurons and their progeny are intrinsically affected by several classes of substances with abuse liability. Although drug abuse is also likely to target directly other cerebellar neuron and glial types, such as Purkinje cells and Bergmann glia, findings in isolated granule neurons suggest that they are often the principle target for drug actions. Developmental events that are selectively disrupted by drug abuse in granule neurons and/or their neuroblast precursors include proliferation, migration, differentiation (including neurite elaboration and synapse formation), and programmed cell death. Moreover, different classes of drugs act through distinct molecular mechanisms thereby disrupting unique aspects of development. For example, drug-induced perturbations in (i) neurotransmitter biogenesis, (ii) ligand and ion-gated receptor function and their coupling to intracellular effectors, (iii) neurotrophic factor biogenesis and signaling, and (iv) intercellular adhesion are all likely to have significant effects in shaping developmental outcome. In addition to identifying therapeutic strategies for drug abuse intervention, understanding the mechanisms by which drugs affect cellular maturation is likely to provide a better understanding of the neurochemical events that normally shape central nervous system development.
Keywords: neuroblast proliferation, cerebellar development, programmed cell death, nicotinic acetylcholinergic receptors, opioid receptors, heroin, nicotine
Introduction
A variety of classes of drugs with abuse liability affect cerebellar structure and function in adults.1 Recent evidence suggests that many of these drugs can have profound affects on cerebellar development. This includes opiates, nicotine, and alcohol, but may additionally include stimulants such as cocaine and methamphetamine, as well as the non-equilibrium N-methyl-D-aspartate (NMDA) antagonist phencyclidine (PCP),1 which are known to effect cerebellar function in adults. Neurotransmitter systems regulate many aspects of normal development. Although the mechanisms by which individual drugs of abuse affect neural maturation are not fully understood, it is assumed that many drugs act by mimicking or interfering with normal endogenous neurotransmitter-receptor interactions during maturation. The inference is that drug abuse alters neurodevelopment by disrupting the timing and sequence of developmental actions regulated by endogenous neurotransmitter systems. In this review, we focus on opioids and nicotine, and to a lesser extent alcohol (which has been more extensively reviewed elsewhere). Alcohol, opioids, and nicotine are reported to effect directly the maturation of granule cell precursors and their progeny. Stimulants such as cocaine or methamphetamine are more likely to affect neurochemical systems (e.g., norepinephrine and dopamine reuptake) that directly influence Purkinje cell maturation. Understanding how cerebellar development is perturbed will provide increasingly rationale approaches toward intervention in drug-exposed offspring, and should additionally provide insight into how neurochemical systems normally influence brain development.
The cerebellum is an important model system for understanding CNS development. The cerebellum is highly compartmentalized and the extremely ordered and stereotypic cytoarchitecture results from a tightly orchestrated production of neurons and glia during development.2,2-16 Cerebellar development proceeds with such precision that it is a useful model for identifying perturbations in CNS maturation.
A hallmark of cerebellar development is the enormous production of granule cells or neurons, which outnumber neurons in other brain regions.17 Granule neurons are relatively small in comparison to other neurons, with three-to-seven dendrites. Their axon runs vertically from the granule layer into the molecular layer, where it bifurcates as a parallel fiber and forms contacts with hundreds of Purkinje cells 13. The huge increase in the number of granule neurons during development arises from the proliferative expansion of neuroblast precursors, which occurs in several steps. Initially, neuroblast precursors originating from the rhombic lips proliferate and migrate tangentially along the surface of the incipient cerebellum to form the external granular (or germinal) layer (EGL).2,18-21 Cells within the rhombic lip express math1 and are largely committed to a granule neuron fate before they migrate to the EGL.22-24 Second, EGL neuroblasts undergo a sustained period of proliferation. Recent evidence suggests that the EGL exclusively gives rise to granule neuron progenitors, rather than other molecular layer interneurons (stellate and basket cells) as previously thought.25 Finally, postmitotic granule neurons migrate radially through the cerebellum, bypassing outwardly migrating Purkinje cells and settling in the internal granule layer (IGL).26,27 The EGL disappears when neurogenesis is complete.2,3
The period of granule neurogenesis coincides with critical periods of sensitivity to several drugs of abuse. This corresponds approximately to the first three postnatal weeks in rats and mice 4,12, and the third trimester of gestation until 1.5 years in humans.14 Superimposed on the rapid period of proliferation are significant amounts of programmed cell death.28 The intense rate of granule neurogenesis is tightly regulated and coordinated with the maturation of the entire organism. The net production of granule cells is determined by proliferation and cell death and is modified by a variety of external cues (Fig. 1). Protracted neurogenesis, combined with a high degree of sensitivity to extrinsic factors, makes developing granule cells especially vulnerable to drug abuse.
Figure 1. Summary of the principal effects of various substances on the production of granule neurons.
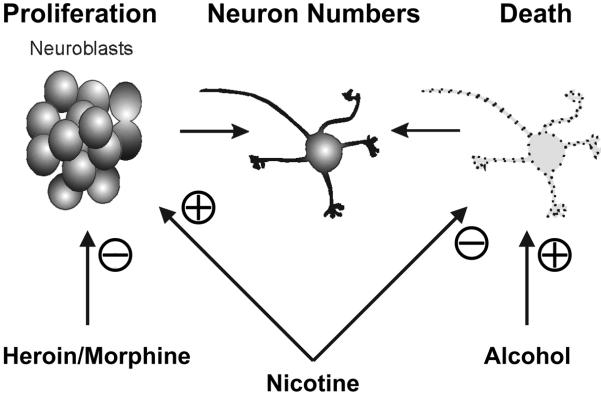
The number of granule neurons in the cerebellum is determined by two key developmental events— cell proliferation and programmed cell death. Drug abuse can independently affect each event.101 Evidence suggests that alcohol, opiate drugs (heroin and morphine), and nicotine disrupt granule neuron numbers through differing mechanisms that modulate cell proliferation and/or death.
Besides granule neurons, Purkinje neurons are a potential target for many abused substances during development (Table 1). Purkinje cells are generated before granule neurons and undergo a prolonged period of differentiation that is in part dependent on trophic support provided by granule neuron afferent synapses.13,18,29-31 The profound trophic interdependence between Purkinje and granule neurons presents challenges toward sorting causal developmental relationships.32 Despite this challenge, with recent cellular and genetic approaches, it is possible to identify key intercellular events influencing the development of each cell type. For this reason and others, we have made extensive use of in vitro methods to study how drugs intrinsically affect the maturation of granule cell precursors. However, the inherent trade-off for gaining experimental control in vitro is the loss of relevance resulting from a reductionist approach. A partial compromise has been to use organotypic culture, which retains some of the cell-to-cell interactions and three-dimensional organization inherent in the cerebellum.33,34 Nevertheless, it is important to validate in vitro findings in vivo.
Table 1.
Effects of alcohol, opiates, and nicotine on granule and Purkinje cell development in the cerebellum
| Substance | Target Cell Population | Effect (References) |
|---|---|---|
| Alcohol | Granule Neurons | Proliferation (↓ or no effect)35,204 |
| Differentiation* (Δ)40 / Migration (Δ)40,41,44 | ||
| Death (↑)38,45-50,54,66-69,73,204-206 | ||
| Purkinje Cells | Proliferation (?) | |
| Differentiation (↓)207 | ||
| Death (↑) (or reduced cell numbers)74,208,209 | ||
|
| ||
| Opioids | Granule Neurons | Proliferation (↓)90,92,155 |
| Differentiation (Δ)34,88,92 | ||
| Death (no effect)92,155 | ||
| Purkinje Cells | Proliferation (?) | |
| Differentiation (↓)33,88,106 | ||
| Death (↑)33,210 | ||
|
| ||
| Nicotine | Granule Cells | Proliferation (↑)180 |
| Differentiation (?) (Δ synaptic function)168,182,184,211,212 | ||
| Death (↓)180 | ||
| Purkinje Cells | Proliferation (?) | |
| Differentiation (?) (Δ synaptic function)185,213-217 | ||
| Death (?) | ||
Alcohol and drug effects on Purkinje cell maturation are noted because the profound interdependence of granule and Purkinje neurons during development.
Differentiation is defined specifically as an alteration in the growth or complexity of axons and/or dendrites. Key: ↑ = increased; ↓ = decreased; A = changed or disrupted; ? = uncertain
Alcohol
Alcohol has profound effects on the development of cerebellar granule neurons and their progeny. Cerebellar actions have been extensively studied as part of the global effects seen with fetal alcohol syndrome (FAS) and only briefly discussed here. One only has to attempt to “walk a straight line” following excess consumption to understand ataxia and appreciate the preferential effects of alcohol on cerebellar function as an adult. The effects of alcohol on the developing cerebellum are likely to be even more profound when alterations in neural function appear to contribute to lasting changes in the cytoarchitecture and synaptic circuitry. Alcohol perturbs all aspects of granule cell development; but is especially damaging to postmitotic cells, altering events such as neuronal migration and survival.
Studies by Li et al. demonstrate that the kinetics of the cell cycle is disrupted by exposure to ethanol. Alcohol downregulates the expression of cdk2, cyclin A and cyclin D causing a delay in the cell cycle and promoting apoptosis, which leads to an overall decrease in the cell number of granule neurons.35 Faulty migration with ectopic positioning of cerebral cortical as well as other neuronal populations seems to be a major consequence of FAS.36,37 How migration is affected is uncertain, but may relate to ethanol-induced disruptions in intracellular Ca2+ signaling,38,39 or cell adhesion,40,41 either of which might be important for granule cell migration.42,43 Alcohol has been shown to modulate the activity of cell adhesion molecules important in cell-cell and cell-matrix interactions. Physiological concentrations of ethanol impair and in some cases inhibit the function of L1, a cell adhesion molecule responsible for mediating neurite outgrowth and perhaps the migration of granule cells.40,41,44
Another important target is granule neuron survival, which is modulated by ethanol in vitro and in vivo.45-51 Alcohol exposure and withdrawal disrupts the function of NMDA38,45,52-54, AMPA/kainate55,56, and GABA 57-59 receptor-effector coupling,53,55-57,60-63 as well as voltage-dependent Ca2+ channel function.57 Ligand- and voltage-dependent channels are important regulators of neuroontogeny and survival.38,64 In addition to ion homeostasis and mitochondrial function,65 ethanol disrupts trophic factor biogenesis and neuronal responsiveness to trophic support. Pituitary adenylate cyclase-activating polypeptide66, insulin-like growth factor67,68, brain-derived neurotrophic factor 69-72, nerve growth factor and basic fibroblastic growth factor73 all attenuate ethanol-induced granule neuron death. Because ethanol seemingly influences all aspects of granule neuron maturation, suggests that the mechanisms by which alcohol acts are complex and likely affect multiple systems. The ability of ethanol to perturb ion homeostasis, neurotransmitter or trophic factor biogenesis and/or receptor-effector coupling,52,74-80, cell adhesion, as well as glial development and function81,82 (however see 83,84), are all likely to profoundly impact granule neurogenesis. As noted, excellent articles and reviews on alcohol and cerebellar development exist (Table 1).46,57,76,85,86
Opioids
The involvement of opioids in cerebellar growth regulation has been revealed by experimentally perturbing the endogenous opioid system.33,34,87-93 In this review, “opiate” refers to substances that are derived from the opium poppy such as heroin or morphine, while “opioid” refers to endogenously expressed neuropeptides and receptors.94 Heroin’s action in the CNS results in large part from its conversion to morphine. Endogenous opioid peptides and receptors are widely expressed by developing cerebellar cells.95-103 Although heroin and morphine preferentially activate μ opioid receptors, at high concentrations they can activate δ and κ receptors.104 Continuous opioid receptor blockade accelerates cerebellar growth in postnatal rats,88,93,105,106 while over-stimulating opioid receptors , as occurs with opiate drugs,107-112 retards cerebellar growth [review 88,91,93,105,106,113]. This suggests that endogenous opioids are present during cerebellar development and tonically inhibit growth.
In the cerebellum, acute opioid exposure (≤ 72 h) typically inhibits the proliferation of cerebellar neuroblasts and astroglia,88,93,105,105,106,109,114-119 and can affect cell differentiation33,88,105 and survival.33 Opioid actions are complex and affect each cerebellar cell type differently.120 For example, unlike neuroblasts and astroglia in which morphine inhibits cell replication, morphine is mitogenic to immature oligodendrocytes.121 In another example, cell death is not seen with high concentrations of morphine (>1 μM) in cultured mouse granule cells33,92 or astrocytes (unpublished, see also 33,122,123), but cell death is evident in cultured Purkinje cells with more chronic exposure (~7 days).33 The mechanism underlying Purkinje cell death is uncertain, but may result from morphine-induced reductions in parallel fiber afferents from granule cells.33 Purkinje cell losses have been reported in chronic heroin abusers who are HIV-seronegative.124
Toxic heroin leukoencephalopathy
Recently a heroin induced spongiform leukoencephalopathy has been described that effects predominately the posterior fossa structures including the cerebellum.125,126 Ultrastructural studies show vacuolar changes in the myelin. Recent identification of this entity, most likely reflects the increased popularity of the practice of "dragon chasing”.127 In this mode of heroin abuse, powdered heroin is placed on a piece of aluminum foil and heated from below with a flame. The oil content allows the heroin to liquefy and vaporize, producing a plume that is inhaled through the mouth with a straw or aluminum foil tube. This practice is distinct from smoking or sniffing heroin. Heroin chasers tend to be younger than heroin injectors, and this route of administration seems to appeal to users trying to avoid intravenous heroin use.128 As drug users explore modes of administration that avoid the risk of HIV exposure, they may resort to heroin inhalation. This condition has never been reported in persons using heroin by other means, such as injection or snorting, which suggests that the extreme toxicity arises from the formation of one or more toxic byproducts during heroin volatilization or from the unusual pharmacodynamics of heroin exposure through this unique route of administration. The toxin in heroin-induced leukoencephalopathy is unknown, but progression in this condition might be due to coasting, or, alternatively, to persistent metabolic changes in the affected white matter such as ongoing oxidative damage initiated by a toxin. The illness is extremely grave, with no known treatment and progression to akinetic mutism and death in approximately 20% of reported cases.129
Opioid receptor and peptide expression in the cerebellum
There is considerable discrepancy between opioid peptide and receptor expression in immature and adult granule cells.92,95-98,130,131 Immature cells within the EGL display proenkephalin mRNA and/or peptide products, which are lost with maturation.96,97,130 Ontogenetic changes in proenkephalin expression within individual cells are manifest as dynamic spatiotemporal gradients in opioid neuropeptide levels throughout the entire cerebellum.96
Interestingly, high-levels of opioid receptor binding coincide with the transient appearance of the EGL.98,130,132 Immature EGL cells from postnatal mouse cerebellum display immunocytochemical and functional evidence of μ and δ, but not κ, opioid receptor expression in vitro.92 EGL cells express a putative ζ opioid receptor with high affinity for Met-enkephalin.98,131 Initial reports identifying opioid receptors in the immature cerebellum were viewed with some skepticism, because the adult cerebellum in rats and mice has been traditionally described as being largely devoid of opioid receptors. More recently, low levels of δ receptor expression have been reported in mature granule cells in rodents,133,134 while μ receptors are reported absent. In contrast, in the human cerebellum, high affinity opioid binding sites are associated with the EGL130 and μ opioid receptors are widely expressed in adult granule cells,135,136 indicating species differences in the types opioid receptors present. The transient and coordinated expression of the opioid peptides and receptors in the developing cerebellum infers that they are functionally related to growth, and suggests granule cells are important in opioid-dependent maturation in the cerebellum.
Opioids and granule cell precursor maturation.
Preliminary evidence implicating endogenous opioids in neural development came from findings that heroin, morphine (much of heroin’s actions in the CNS result from its conversion to morphine), or other preferential μ opioid receptor agonists inhibited the growth of the brain including the cerebellum.118,137-139,139 We tested whether opiates intrinsically affect the growth of granule cell precursors by studying the response of mouse precursors to morphine in vitro. Morphine (1 μM) exposure caused significant reductions in DNA synthesis at 24 h with subsequent reductions in DNA content at 48 h.92 Importantly, because morphine does not increase EGL cell death, suggests that morphine reduces granule neuron numbers by inhibiting neuroblast proliferation. Lastly, the antiproliferative effects of morphine appear to be mediated by μ opioid receptors, since granule cell precursor proliferation was unaffected by δ opioid receptor agonists and κ receptors are not expressed by these cells (Fig. 2).92
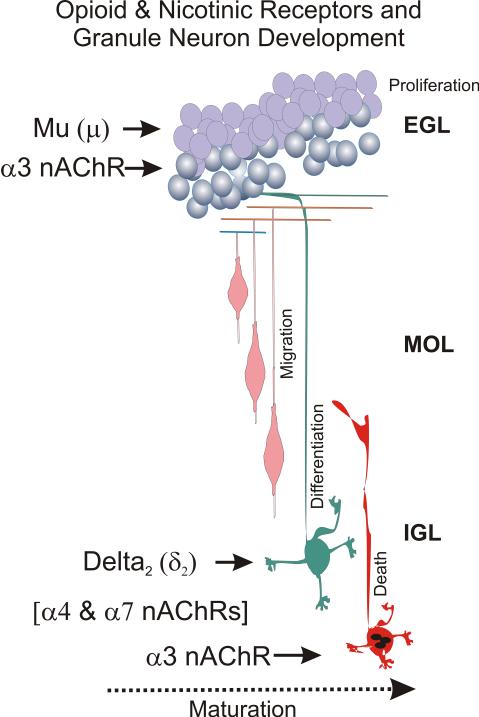
Figure 2. Summary: Opioid and nicotine actions during granule cell development.
Cerebellar granule neuron proliferation occurs in the external granular layer (EGL), a transient layer of proliferating cells that disappears in the adult. After cell division is completed, the neurons migrate past the molecular (MOL) and Purkinje cell (PC) layers to their adult positions in the internal granule layer (IGL) where they differentiate forming dendrites and synapses. Programmed cell death (apoptosis) can occur (perhaps by independent processes) in both the EGL and the IGL. Opiate drugs of abuse, such as heroin, inhibit cell replication through direct actions on μ opioid receptors, while δ2 opioid receptor agonists inhibit differentiation. Nicotine directly increases granule neuron numbers by independently increasing granule cell proliferation, while attenuating cell death. Both the mitogenic and antiapoptotic effects are likely mediated by α3 nicotinic AChR subunits. In contrast, α7 and α4 nAChR subunits (α4 & α7 nAChRs) are expressed later and potentially affect the maturation of more mature granule neurons, although there is no direct evidence for this at present.
Opioids can modulate dendritic growth and/or potentially retraction.34,88,92 In immature cerebellar granule cells, δ2 receptor agonists, but not μ receptor agonists, preferentially inhibit neurite elaboration. Proenkephalin gene-derived products have been noted in mossy fibers in a variety of species.140,141 In another model system, Met-enkephalin, acting through δ receptors, significantly increases the phosphorylation of the Src kinase substrate cortactin and vinculin at focal adhesion sites,112 suggesting one possible mechanism for opioid-induced changes in neurites. Prenatal exposure to morphine alters catecholamine levels in the cerebellum via a sexually dimorphic mechanism and might affect other neurotransmitters.142
Opioids act through multiple pathways and downstream effectors, including MAP kinase and/or focal adhesion kinase.92,112,143-148 Opioids can also affect cell growth through pathways more traditionally ascribed to opioids, such as by augmenting phosphatidylinositol (PI) turnover, or by increasing PI-3-kinase and/or Ca2+-mobilization.145,149-152 The ability to stimulate multiple signaling cascades may explain how opioids can have varied effects on cell growth.120,121,153,154
Little is known whether opiates modulate development by altering key trophic factors and/or their receptors. We have found that heparin-binding epidermal growth factor (Hb-EGF) negates the antiproliferative actions of morphine in isolated mouse EGL cells.155 Conversely, opiates can modify EGFR function through convergent signaling events.148 Similarly, different classes of opioid receptors can transactivate one another156-158 and interact directly with important non-opioid signaling pathways affecting growth.159 Interactions between opiates and other trophic regulators, such as sonic hedgehog (Shh)-patched2 and/or EGF-erbB receptor interactions between Purkinje and granule neurons have not been fully explored in the developing cerebellum. Assuming Shh-patched2 and/or EGF-erbB receptor interactions drive the near-exponential increases in granule neurogenesis, how might opioids interact with these potent trophic factors? We propose that opioids are strategically positioned to finely tune and coordinate developmental details, and are likely to function at later stages during development, after trophic factors such as EGF or hedgehog have served their main functions. In addition, as noted, opioid and growth factor signaling pathways can overlap intracellularly. Because opioid peptides and receptors are widely expressed by developing neurons, astroglia, and oligodendroglia suggest that opioids are strategically positioned to coordinate the proliferation and differentiation of neurons and glia.92,160,161 This might include the regulation of neuronal and glial numbers, or physical or functional interactions among cells.120 Irrespective of a particular mechanism, current evidence suggests that opioids affect cerebellar maturation by interfering directly with granule cell development.
Nicotine
AChR and transmitter expression in the developing cerebellum
The abundance of cholinergic synthetic enzymes and receptors in the developing cerebellum suggests that acetylcholine might potentially influence postnatal maturation in this region.162-165 The maturation of cholinergic systems164,166-168 coincides with critical periods of granule neurogenesis in rodents.4,12,169 During this time, choline acetyltransferase levels are generally higher than levels of the degradative enzyme for acetylcholine, acetylcholinesterase.163,164,170 Granule neurons receive cholinergic mossy fiber innervation from dorsal pontine brainstem nuclei late during development,168 suggesting that acetylcholine affects synaptogenesis and neuromodulation.171 Choline itself, which is plentiful during development, may activate α7 nicotinic AChRs and act as a partial agonist for α3 nicotinic AChRs.172-174
Both muscarinic and nicotinic AChR subtypes are present in perinatal rat175-177 and human brains.167,178 Nicotinic AChRs are expressed in the EGL in humans167 and in granule and/or Purkinje neurons in rodents165,175,179,180 and can precede the ingrowth of cholinergic axons,181 suggesting that nicotinic agonists could act directly on granule neuron precursors. Our immunocytochemical findings show α3, but not α4, nicotinic AChRs in cultured EGL cells prior to the formation of neurites.180 Interestingly, transcripts of multiple nicotinic AChR subtypes, including α3, α4, α5, α7, β2, and β4, have been detected in more mature cultured rat granule neurons that have formed axons and dendrites.182 Similarly, α4β2 and/or α3β4 nicotinic AChR subunits are expressed by granule neurons in cerebellar slices from 5 to 14 day-old rats,183 suggesting that nicotinic AChR subunit composition is developmentally regulated and that non-α3 subtypes might be more important for neurite outgrowth and synapse formation. Additional support for this is prompted by the finding that α7 subunits are segregated to the dendrites, but not cell bodies of granule cells, suggesting α7 subunits are preferentially involved in differentiation, synaptogenesis, and/or postsynaptic function.184 In contrast, α3 nicotinic AChR subunits are localized on the cell bodies of neuroblasts long-before neurites are formed.180 In 5-to-10 day old rats, nicotine significantly enhances synaptic activity of the Purkinje cells via presynaptic nicotinic receptors on the excitatory and inhibitory interneurons, while in older rats such an effect is barely noticeable.185
Nicotine and granule cell precursor maturation
Together, these findings suggest that the cholinergic system is important in cerebellar maturation, at least in part, by directly influencing the proliferation and survival of granule cell precursors. Nicotine administration has been reported to decrease DNA synthesis in several rat brain regions including the cerebellum,186-188 although a recent report shows increased numbers of mitotic neural cells in rat embryos exposed to nicotine in vitro.189 However, similar to opioids, nicotinic AChR activation has potent systemic effects, which include alterations in cardiovascular, respiratory, and endocrine function that are likely to influence neurogenesis. Activation of presynaptic nicotinic AChRs might modulate the release of other neurotransmitters. Recently, abnormalities in nicotinic AChR subunit levels have been reported in autism.190
To understand better the intrinsic effects of nicotine on the development of granule cell precursors, we examined the effect of nicotine on EGL cells isolated in vitro. We found that nicotine caused concentration-dependent increases in DNA content and synthesis in EGL neuroblasts implying increases in cell proliferation. Pretreatment of cultures with the nicotinic AChR antagonist dihydro-β-erythroidine (DHBE) significantly attenuated nicotine-induced increases in cell replication. To further determine whether α3 or α4 subunits are preferentially involved in neural proliferation, EGL cultures were continuously exposed for 7 days to selective α3/α4 (epibatidine) or α4 (cytisine) agonists or partial agonists, and DNA content and synthesis were examined.180 Epibatidine, but not cytisine, caused concentration-dependent increases in DNA synthesis and DNA levels in EGL cells indicating that α3 nicotinic AChR activation is mitogenic. Moreover, significant effects were seen with a 1 pM concentration of epibatidine, and were markedly attenuated by concurrent administration of DHBE suggesting the involvement of specific nicotinic AChRs. In summary, these data provide novel evidence that nicotinic AChRs directly affect the development of granule cell precursors and further suggest that the effects are mediated through α3 nicotinic AChR subtypes. It is interesting to speculate that other nicotinic AChR subtypes also regulate unique aspects of development.
Cell Death
Granule neuron death occurs during normal cerebellar development in vivo28 and in vitro.191 Nicotinic AChR activation can have paradoxical proapoptotic or neuroprotective effects depending on cell type and developmental stage, pharmacodynamics of drug exposure, and the particular nicotinic AChR subtype affected. Chronic nicotine exposure is neuroprotective in organotypic cultures of the hippocampus by upregulating calbindin expression, which buffers toxic increases in intracellular Ca2+.192 In hippocampal neurons, neurotoxicity is associated with the activation of α7 nicotinic AChR subtypes, which permit Ca2+ influx and α7 antagonists can be neuroprotective.193,194 In contrast, there are numerous examples in which nicotine is neurotoxic. Nicotine promotes death in some cell types, e.g., embryonic rat neural cells189 and in vascular cells.195 Nicotine is neurotoxic at high concentrations in whole rat embryos,189 and causes apoptosis in cultured hippocampal neurons.193
We found that EGL cell viability was enhanced following chronic nicotine treatment for 7 days in vitro (DIV), but not 4 DIV.180 Importantly, the neuroprotective effects of nicotine were completely blocked by the nicotinic AChR antagonist DHBE and mimicked by α3, but not α4, selective agonists. Chronic exposure may be neuroprotective by causing adaptive responses within cells.192 Alternatively, the background rate of cell death was greater in our 7 DIV cultures and this might better reveal nicotine neuroprotection.
Collectively, the cell proliferation and survival data suggest that nicotine has both mitogenic and neuroprotective effects in EGL cells, and these effects are mediated through α3 nicotinic AChR subunits (Fig. 2). Interestingly, Yan and coworkers196 reported that acetylcholine prevented apoptosis in cultured granule neurons via an interaction with muscarinic AChRs. It is conceivable that activation of both nicotinic AChR and muscarinic AChRs regulate the maturation of cerebellar granule neurons as has been suggested in retinal ganglion cells.197 Alternatively, nicotinic AChR stimulation might also induce acetylcholine release.
Despite findings suggesting that nicotinic AChR activation directly affects neuroblast development, the mechanisms by which this occurs are not understood. Recent reports suggest that nicotine can regulate the synthesis and/or degradation of trophic factors, including platelet derived growth factor, tumor necrosis factor-α, and transforming growth factor-β , which can enhance or impede cell growth in transformed cell lines.198,199 Alternatively, nicotinic AChRs may directly couple to mitogenic signaling events as shown in cell lines, as well as in primary retinal and hippocampal neurons.200-202 Irrespective of the mechanisms involved, it appears that nicotine per se can directly modulate cerebellar development by affecting granule cell maturation. For this reason, recent suggestions that nicotine replacement therapy be used during pregnancy as a substitute for cigarette smoking, should be judiciously approached.203 While this seems a prudent measure because the myriad products in cigarette smoke besides nicotine are likely to be far more adverse than nicotine alone, it might only a partial solution assuming nicotine itself effects neural maturation.
Conclusions
Despite findings that opiate drugs and/or nicotine can intrinsically affect the maturation and survival of isolated EGL cells in culture, caution should be used before generalizing these results to effects in the whole organism. In the absence of the complex cues normally present within the microenvironment of the developing brain, it is premature to speculate whether granule cells might respond similarly in vivo, or whether the untimely exposure to opiate drugs or nicotine during maturation might have similar influences on human cerebellar development. Moreover, pharmacodynamic differences in drug exposure make it challenging to generalize experimental findings from in vivo or in vitro animal models to human development.
An underlying assumption is that drug abuse impacts cerebellar maturation by modulating the degree and timing of ongoing developmental events. In addition to the potential for additive and synergistic interactions, pharmacodynamic differences in drug exposure (versus the neurochemical systems they mimic) potentially activate novel signaling events and genes. The response of a cell to a single drug such as heroin likely reflects the synergistic effect of heroin’s actions through multiple signaling cascades and downstream effectors. Future studies are beginning to tackle this complexity using gene microarrays, proteomics, and new means of combinatorial analysis of complex data sets. The cerebellum, in general, and granule cells, in particular, which display highly delineated spatial and temporal patterns of development, will continue to provide an excellent model system to elucidate how drug abuse disrupts the CNS maturation.
Acknowledgements
We gratefully acknowledge the support of the Kentucky Tobacco and Health Research Institute and NIH grants DA 08443 and DA 06204. The authors wish to thank Ms. Sarah E. Lutz for helpful editorial comments.
References
- 1.Manto M-U, Jacquy J. Other cerebellotoxic agents. In: Manto M-U, Pandolfo M, editors. The Cerebellum and Its Disorders. 1 Cambridge University Press; Cambridge: 2002. pp. 342–366. [Google Scholar]
- 2.Ramóny Cajal S. Studies on Vertebrate Neurogenesis. Charles C. Thomas; Springfield, Il.: 1960. [Google Scholar]
- 3.Rakic P. Neuron-glia relationship during granule cell migration in developing cerebellar cortex. A Golgi and electronmicroscopic study in Macacus rhesus. J Comp Neurol. 1971;141:283–312. doi: 10.1002/cne.901410303. [DOI] [PubMed] [Google Scholar]
- 4.Altman J. Postnatal development of the cerebellar cortex in the rat. I. The external germinal layer and the transitional molecular layer. J Comp Neurol. 1972;145:353–398. doi: 10.1002/cne.901450305. [DOI] [PubMed] [Google Scholar]
- 5.Herrup K, Kuemerle B. The compartmentalization of the cerebellum. Annu Rev Neurosci. 1997;20:61–90. doi: 10.1146/annurev.neuro.20.1.61. 61-90. [DOI] [PubMed] [Google Scholar]
- 6.Hawkes R, Herrup K. Aldolase C/zebrin II and the regionalization of the cerebellum. J Mol Neurosci. 1995;6:147–158. doi: 10.1007/BF02736761. [DOI] [PubMed] [Google Scholar]
- 7.Goldowitz D, Hamre KM, Przyborski SA, Ackerman SL. Granule cells and cerebellar boundaries: analysis of Unc5h3 mutant chimeras. J Neurosci. 2000;20:4129–4137. doi: 10.1523/JNEUROSCI.20-11-04129.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldowitz D, Hamre K. The cells and molecules that make a cerebellum. Trends Neurosci. 1998;21:375–382. doi: 10.1016/s0166-2236(98)01313-7. [DOI] [PubMed] [Google Scholar]
- 9.Mullen RJ, Hamre KM, Goldowitz D. Cerebellar mutant mice and chimeras revisited. Perspect Dev Neurobiol. 1997;5:43–55. [PubMed] [Google Scholar]
- 10.Larsell O. The morphogenesis and adult pattern of the lobules and fissures of the cerebellum of the white rat. J Comp Neurol. 1952;97:281–356. doi: 10.1002/cne.900970204. [DOI] [PubMed] [Google Scholar]
- 11.Mugnaini E. Ultrastructural studies on the cerebellar histogenesis. II. Maturation of nerve poplations and establishment of synaptic connections in the cerebellar cortex of the chick. In: Llinas R, editor. Neurobiology of cerebellar evolution and development. Institute for Biomedical Research, American Medical Association; Chicago: 1969. pp. 749–801. [Google Scholar]
- 12.Miale IL, Sidman RL. An autoradiographic analysis of histogenesis in the mouse cerebellum. Exp Neurol. 1961;4:277–296. doi: 10.1016/0014-4886(61)90055-3. [DOI] [PubMed] [Google Scholar]
- 13.Palay SL, Chan-Palay V. The Cerebellar Cortex, Cytology and Organization. Springer-Verlag; New York: 1974. pp. 1–348. [Google Scholar]
- 14.Bayer SA, Altman J, Russo RJ, Zhang X. Timetables of neurogenesis in the human brain based on experimentally determined patterns in the rat. Neurotoxicol. 1993;14:83–144. [PubMed] [Google Scholar]
- 15.Altman J, Bayer SA. Development of the Cerebellar System. (1) 1997;1:1–783. [Google Scholar]
- 16.Wingate RJ. The rhombic lip and early cerebellar development. Curr Opin Neurobiol. 2001;11:82–88. doi: 10.1016/s0959-4388(00)00177-x. [DOI] [PubMed] [Google Scholar]
- 17.Wechsler-Reya RJ, Scott MP. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron. 1999;22:103–114. doi: 10.1016/s0896-6273(00)80682-0. [DOI] [PubMed] [Google Scholar]
- 18.Altman J, Bayer SA. Prenatal development of the cerebellar system in the rat. I. Cytogenesis and histogenesis of the deep nuclei and the cortex of the cerebellum. J Comp Neurol. 1978;179:23–48. doi: 10.1002/cne.901790104. [DOI] [PubMed] [Google Scholar]
- 19.Maricich SM, Gilmore EC, Herrup K. The role of tangential migration in the establishment of mammalian cortex. Neuron. 2001;31:175–178. doi: 10.1016/s0896-6273(01)00370-1. [DOI] [PubMed] [Google Scholar]
- 20.Wang VY, Zoghbi HY. Genetic regulation of cerebellar development. Nat Rev Neurosci. 2001;2:484–491. doi: 10.1038/35081558. [DOI] [PubMed] [Google Scholar]
- 21.Wingate RJ. The rhombic lip and early cerebellar development. Curr Opin Neurobiol. 2001;11:82–88. doi: 10.1016/s0959-4388(00)00177-x. [DOI] [PubMed] [Google Scholar]
- 22.Helms AW, Johnson JE. Progenitors of dorsal commissural interneurons are defined by MATH1 expression. Development. 1998;125:919–928. doi: 10.1242/dev.125.5.919. [DOI] [PubMed] [Google Scholar]
- 23.Ben Arie N, Bellen HJ, Armstrong DL, McCall AE, Gordadze PR, Guo Q, et al. Math1 is essential for genesis of cerebellar granule neurons. Nature. 1997;390:169–172. doi: 10.1038/36579. [DOI] [PubMed] [Google Scholar]
- 24.Hatten ME, Alder J, Zimmerman K, Heintz N. Genes involved in cerebellar cell specification and differentiation. Curr Opin Neurobiol. 1997;7:40–47. doi: 10.1016/s0959-4388(97)80118-3. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Goldman JE. Generation of cerebellar interneurons from dividing precursors in white matter. Neuron. 1996;16:47–54. doi: 10.1016/s0896-6273(00)80022-7. [DOI] [PubMed] [Google Scholar]
- 26.Wingate RJ. The rhombic lip and early cerebellar development. Curr Opin Neurobiol. 2001;11:82–88. doi: 10.1016/s0959-4388(00)00177-x. [DOI] [PubMed] [Google Scholar]
- 27.Komuro H, Rakic P. Orchestration of neuronal migration by activity of ion channels, neurotransmitter receptors, and intracellular Ca2+ fluctuations. J Neurobiol. 1998;37:110–130. [PubMed] [Google Scholar]
- 28.Wood KA, Dipasquale B, Youle RJ. In situ labeling of granule cells for apoptosis-associated DNA fragmentation reveals different mechanisms of cell loss in developing cerebellum. Neuron. 1993;11:621–632. doi: 10.1016/0896-6273(93)90074-2. [DOI] [PubMed] [Google Scholar]
- 29.Altman J, Bayer SA. Embryonic development of the rat cerebellum. III. Regional differences in the time of origin, migration, and settling of Purkinje cells. J Comp Neurol. 1985;231:42–65. doi: 10.1002/cne.902310105. [DOI] [PubMed] [Google Scholar]
- 30.Altman J. Experimental reorganization of the cerebellar cortex. VII. Effects of late X-irradiation schedules that interfere with cell acquisition after stellate cells are formed. J Comp Neurol. 1976;165:65–76. doi: 10.1002/cne.901650106. [DOI] [PubMed] [Google Scholar]
- 31.Ito M. The cerebellum and neural control. Raven Press; New York: 1984. [Google Scholar]
- 32.Baptista CA, Hatten ME, Blazeski R, Mason CA. Cell-cell interactions influence survival and differentiation of purified Purkinje cells in vitro. Neuron. 1994;12:243–260. doi: 10.1016/0896-6273(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 33.Hauser KF, Gurwell JA, Turbek CS. Morphine inhibits Purkinje cell survival and dendritic differentiation in organotypic cultures of the mouse cerebellum. Exp Neurol. 1994;130:95–105. doi: 10.1006/exnr.1994.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hauser KF, Stiene-Martin A. Opiates and the regulation of nervous system development: Evidence from in vitro studies. In: Hammer RP Jr., editor. Neurobiology of Opiates. CRC Press; Boca Raton, Florida: 1993. pp. 23–61. [Google Scholar]
- 35.Li Z, Lin H, Zhu Y, Wang M, Luo J. Disruption of cell cycle kinetics and cyclin-dependent kinase system by ethanol in cultured cerebellar granule progenitors. Brain Res Dev Brain Res. 2001;132:47–58. doi: 10.1016/s0165-3806(01)00294-2. [DOI] [PubMed] [Google Scholar]
- 36.Miller MW. Migration of cortical neurons is altered by gestational exposure to ethanol. Alcohol Clin Exp Res. 1993;17:304–314. doi: 10.1111/j.1530-0277.1993.tb00768.x. [DOI] [PubMed] [Google Scholar]
- 37.Miller MW. Effects of alcohol on the generation and migration of cerebral cortical neurons. Science. 1986;233:1308–1311. doi: 10.1126/science.3749878. [DOI] [PubMed] [Google Scholar]
- 38.Gruol DL, Ryabinin AE, Parsons KL, Cole M, Wilson MC, Qiu Z. Neonatal alcohol exposure reduces NMDA induced Ca2+ signaling in developing cerebellar granule neurons. Brain Res. 1998;793:12–20. doi: 10.1016/s0006-8993(98)00014-6. [DOI] [PubMed] [Google Scholar]
- 39.Przewlocki R, Parsons KL, Sweeney DD, Trotter C, Netzeband JG, Siggins GR, et al. Opioid enhancement of calcium oscillations and burst events involving NMDA receptors and L-type calcium channels in cultured hippocampal neurons. J Neurosci. 1999;19:9705–9715. doi: 10.1523/JNEUROSCI.19-22-09705.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bearer CF, Swick AR, O'Riordan MA, Cheng G. Ethanol inhibits L1-mediated neurite outgrowth in postnatal rat cerebellar granule cells. J Biol Chem. 1999;274:13264–13270. doi: 10.1074/jbc.274.19.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Burden-Gulley SM, Pendergast M, Lemmon V. The role of cell adhesion molecule L1 in axonal extension, growth cone motility, and signal transduction. Cell Tissue Res. 1997;290:415–422. doi: 10.1007/s004410050948. [DOI] [PubMed] [Google Scholar]
- 42.Komuro H, Rakic P. Intracellular Ca2+ fluctuations modulate the rate of neuronal migration. Neuron. 1996;17:275–285. doi: 10.1016/s0896-6273(00)80159-2. [DOI] [PubMed] [Google Scholar]
- 43.Komuro H, Rakic P. Dynamics of granule cell migration: a confocal microscopic study in acute cerebellar slice preparations. J Neurosci. 1995;15:1110–1120. doi: 10.1523/JNEUROSCI.15-02-01110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liesi P. Ethanol-exposed central neurons fail to migrate and undergo apoptosis. J Neurosci Res. 1997;48:439–448. doi: 10.1002/(sici)1097-4547(19970601)48:5<439::aid-jnr5>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 45.Pantazis NJ, Dohrman DP, Luo J, Thomas JD, Goodlett CR, West JR. NMDA prevents alcohol-induced neuronal cell death of cerebellar granule cells in culture. Alcohol Clin Exp Res. 1995;19:846–853. doi: 10.1111/j.1530-0277.1995.tb00957.x. [DOI] [PubMed] [Google Scholar]
- 46.West JR, Chen WJ, Pantazis NJ. Fetal alcohol syndrome: the vulnerability of the developing brain and possible mechanisms of damage. Metab Brain Dis. 1994;9:291–322. doi: 10.1007/BF02098878. [DOI] [PubMed] [Google Scholar]
- 47.West JR. Acute and long-term changes in the cerebellum following developmental exposure to ethanol. Alcohol Alcohol Suppl. 1993;2:199–202. [PubMed] [Google Scholar]
- 48.Pantazis NJ, West JR, Dai D. The nitric oxide-cyclic GMP pathway plays an essential role in both promoting cell survival of cerebellar granule cells in culture and protecting the cells against ethanol neurotoxicity. J Neurochem. 1998;70:1826–1838. doi: 10.1046/j.1471-4159.1998.70051826.x. [DOI] [PubMed] [Google Scholar]
- 49.Oberdoerster J, Rabin RA. Enhanced caspase activity during ethanol-induced apoptosis in rat cerebellar granule cells. Eur J Pharmacol. 1999;385:273–282. doi: 10.1016/s0014-2999(99)00714-1. [DOI] [PubMed] [Google Scholar]
- 50.Bhave SV, Snell LD, Tabakoff B, Hoffman PL. Chronic ethanol exposure attenuates the anti-apoptotic effect of NMDA in cerebellar granule neurons. J Neurochem. 2000;75:1035–1044. doi: 10.1046/j.1471-4159.2000.0751035.x. [DOI] [PubMed] [Google Scholar]
- 51.Borges S, Lewis PD. Effects of ethanol on postnatal cell acquisition in the rat cerebellum. Brain Res. 1983;271:388–391. doi: 10.1016/0006-8993(83)90308-6. [DOI] [PubMed] [Google Scholar]
- 52.Zhang FX, Rubin R, Rooney TA. N-Methyl-D-aspartate inhibits apoptosis through activation of phosphatidylinositol 3-kinase in cerebellar granule neurons. A role for insulin receptor substrate-1 in the neurotrophic action of n-methyl-D-aspartate and its inhibition by ethanol. J Biol Chem. 1998;273:26596–26602. doi: 10.1074/jbc.273.41.26596. [DOI] [PubMed] [Google Scholar]
- 53.Snell LD, Bhave SV, Tabakoff B, Hoffman PL. Chronic ethanol exposure delays the 'developmental switch' of the NMDA receptor 2A and 2B subunits in cultured cerebellar granule neurons. J Neurochem. 2001;78:396–405. doi: 10.1046/j.1471-4159.2001.00424.x. [DOI] [PubMed] [Google Scholar]
- 54.Hoffman PL, Iorio KR, Snell LD, Tabakoff B. Attenuation of glutamate-induced neurotoxicity in chronically ethanol-exposed cerebellar granule cells by NMDA receptor antagonists and ganglioside GM1. Alcohol Clin Exp Res. 1995;19:721–726. doi: 10.1111/j.1530-0277.1995.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 55.Akinshola BE, Stewart RR, Karvonen LL, Taylor RE, Liesi P. Involvement of non-NMDA receptors in the rescue of weaver cerebellar granule neurons and sensitivity to ethanol of cerebellar AMPA receptors in oocytes. Brain Res Mol Brain Res. 2001;93:8–17. doi: 10.1016/s0169-328x(01)00152-8. [DOI] [PubMed] [Google Scholar]
- 56.Valenzuela CF, Bhave S, Hoffman P, Harris RA. Acute effects of ethanol on pharmacologically isolated kainate receptors in cerebellar granule neurons: comparison with NMDA and AMPA receptors. J Neurochem. 1998;71:1777–1780. doi: 10.1046/j.1471-4159.1998.71041777.x. [DOI] [PubMed] [Google Scholar]
- 57.Littleton J, Little H. Current concepts of ethanol dependence. Addiction. 1994;89:1397–1412. doi: 10.1111/j.1360-0443.1994.tb03736.x. [DOI] [PubMed] [Google Scholar]
- 58.Hsiao SH, West JR, Mahoney JC, Frye GD. Postnatal ethanol exposure blunts upregulation of GABAA receptor currents in Purkinje neurons. Brain Res. 1999;832:124–135. doi: 10.1016/s0006-8993(99)01480-8. [DOI] [PubMed] [Google Scholar]
- 59.Hsiao S, Parrish A, Nahm S, Abbott L, McCool B, Frye G. Effects of early postnatal ethanol intubation on GABAergic synaptic proteins. Brain Res Dev Brain Res. 2002:138–177. doi: 10.1016/s0165-3806(02)00470-4. [DOI] [PubMed] [Google Scholar]
- 60.Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, et al. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome [see comments] Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- 61.Littleton JM, Lovinger D, Liljequist S, Ticku R, Matsumoto I, Barron S. Role of Polyamines and NMDA Receptors in Ethanol Dependence and Withdrawal. Alcohol Clin Exp Res. 2001;25:132S–136S. doi: 10.1097/00000374-200105051-00023. [DOI] [PubMed] [Google Scholar]
- 62.Cebers G, Hou YN, Cebere A, Terenius L, Liljequist S. Chronic ethanol enhances NMDA-induced AP-1 activity in cultured rat cerebellar granule cells. Neuroreport. 1996;8:217–220. doi: 10.1097/00001756-199612200-00044. [DOI] [PubMed] [Google Scholar]
- 63.Cebers G, Cebere A, Zharkovsky A, Liljequist S. Glycine does not reverse the inhibitory actions of ethanol on NMDA receptor functions in cerebellar granule cells. Naunyn Schmiedebergs Arch Pharmacol. 1996;354:736–745. doi: 10.1007/BF00166900. [DOI] [PubMed] [Google Scholar]
- 64.Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated 'menage a trois'. Trends Neurosci. 1997;20:523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- 65.de la Monte SM, Neely TR, Cannon J, Wands JR. Ethanol impairs insulin-stimulated mitochondrial function in cerebellar granule neurons. Cell Mol Life Sci. 2001;58:1950–1960. doi: 10.1007/PL00000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vaudry D, Rousselle C, Basille M, Falluel-Morel A, Pamantung TF, Fontaine M, et al. Pituitary adenylate cyclase-activating polypeptide protects rat cerebellar granule neurons against ethanol-induced apoptotic cell death. Proc Natl Acad Sci U S A. 2002;99:6398–6403. doi: 10.1073/pnas.082112699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hallak H, Seiler AE, Green JS, Henderson A, Ross BN, Rubin R. Inhibition of insulin-like growth factor-I signaling by ethanol in neuronal cells. Alcohol Clin Exp Res. 2001;25:1058–1064. [PubMed] [Google Scholar]
- 68.Zhang FX, Rubin R, Rooney TA. Ethanol induces apoptosis in cerebellar granule neurons by inhibiting insulin-like growth factor 1 signaling. J Neurochem. 1998;71:196–204. doi: 10.1046/j.1471-4159.1998.71010196.x. [DOI] [PubMed] [Google Scholar]
- 69.Bhave SV, Ghoda L, Hoffman PL. Brain-derived neurotrophic factor mediates the anti-apoptotic effect of NMDA in cerebellar granule neurons: signal transduction cascades and site of ethanol action. J Neurosci. 1999;19:3277–3286. doi: 10.1523/JNEUROSCI.19-09-03277.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Light KE, Brown DP, Newton BW, Belcher SM, Kane CJ. Ethanol-induced alterations of neurotrophin receptor expression on Purkinje cells in the neonatal rat cerebellum. Brain Res. 2002;924:71–81. doi: 10.1016/s0006-8993(01)03224-3. [DOI] [PubMed] [Google Scholar]
- 71.Light KE, Ge Y, Belcher SM. Early postnatal ethanol exposure selectively decreases BDNF and truncated TrkB-T2 receptor mRNA expression in the rat cerebellum. Brain Res Mol Brain Res. 2001;93:46–55. doi: 10.1016/s0169-328x(01)00182-6. [DOI] [PubMed] [Google Scholar]
- 72.Heaton MB, Madorsky I, Paiva M, Mayer J. Influence of ethanol on neonatal cerebellum of BDNF gene-deleted animals: analyses of effects on Purkinje cells, apoptosis-related proteins, and endogenous antioxidants. J Neurobiol. 2002;51:160–176. doi: 10.1002/neu.10051. [DOI] [PubMed] [Google Scholar]
- 73.Luo J, West JR, Pantazis NJ. Nerve growth factor and basic fibroblast growth factor protect rat cerebellar granule cells in culture against ethanol-induced cell death. Alcohol Clin Exp Res. 1997;21:1108–1120. [PubMed] [Google Scholar]
- 74.Chen WJ, Parnell SE, West JR. Neonatal alcohol and nicotine exposure limits brain growth and depletes cerebellar Purkinje cells. Alcohol. 1998;15:33–41. doi: 10.1016/s0741-8329(97)00084-0. [DOI] [PubMed] [Google Scholar]
- 75.McAlhany RE, Jr, West JR, Miranda RC. Glial-derived neurotrophic factor rescues calbindin-D28k-immunoreactive neurons in alcohol-treated cerebellar explant cultures. J Neurobiol. 1997;33:835–847. [PubMed] [Google Scholar]
- 76.Luo J, Miller MW. Growth factor-mediated neural proliferation: target of ethanol toxicity. Brain Res Brain Res Rev. 1998;27:157–167. doi: 10.1016/s0165-0173(98)00009-5. [DOI] [PubMed] [Google Scholar]
- 77.Di Chiara G, Acquas E, Tanda G. Ethanol as a neurochemical surrogate of conventional reinforcers: the dopamine-opioid link. Alcohol. 1996;13:13–17. doi: 10.1016/0741-8329(95)02034-9. [DOI] [PubMed] [Google Scholar]
- 78.Fitzgerald LW, Nestler EJ. Molecular and cellular adaptations in signal transduction pathways following ethanol exposure. Clin Neurosci. 1995;3:165–173. [PubMed] [Google Scholar]
- 79.Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, et al. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- 80.Maier SE, Chen WJ, West JR. Prenatal binge-like alcohol exposure alters neurochemical profiles in fetal rat brain. Pharmacol Biochem Behav. 1996;55:521–529. doi: 10.1016/s0091-3057(96)00282-1. [DOI] [PubMed] [Google Scholar]
- 81.Guerri C, Renau-Piqueras J. Alcohol, astroglia, and brain development. Mol Neurobiol. 1997;15:65–81. doi: 10.1007/BF02740616. [DOI] [PubMed] [Google Scholar]
- 82.Aschner M, Allen JW. Astrocytes in methylmercury, ammonia, methionine sulfoximine and alcohol-induced neurotoxicity. Neurotoxicology. 2000;21:573–579. [PubMed] [Google Scholar]
- 83.Rintala J, Jaatinen P, Kiianmaa K, Riikonen J, Kemppainen O, Sarviharju M, et al. Dose-dependent decrease in glial fibrillary acidic protein-immunoreactivity in rat cerebellum after lifelong ethanol consumption. Alcohol. 2001;23:1–8. doi: 10.1016/s0741-8329(00)00116-6. [DOI] [PubMed] [Google Scholar]
- 84.Dlugos CA, Pentney RJ. Quantitative immunocytochemistry of glia in the cerebellar cortex of old ethanol-fed rats. Alcohol. 2001;23:63–69. doi: 10.1016/s0741-8329(00)00143-9. [DOI] [PubMed] [Google Scholar]
- 85.Fadda F, Rossetti ZL. Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol. 1998;56:385–431. doi: 10.1016/s0301-0082(98)00032-x. [DOI] [PubMed] [Google Scholar]
- 86.Guerri C. Neuroanatomical and neurophysiological mechanisms involved in central nervous system dysfunctions induced by prenatal alcohol exposure. Alcohol Clin Exp Res. 1998;22:304–312. doi: 10.1111/j.1530-0277.1998.tb03653.x. [DOI] [PubMed] [Google Scholar]
- 87.Crofford M, Smith AA. Growth retardation in young mice treated with dl-methadone. Science. 1973;181:947–949. doi: 10.1126/science.181.4103.947. [DOI] [PubMed] [Google Scholar]
- 88.Hauser KF, McLaughlin PJ, Zagon IS. Endogenous opioid systems and the regulation of dendritic growth and spine formation. J Comp Neurol. 1989;281:13–22. doi: 10.1002/cne.902810103. [DOI] [PubMed] [Google Scholar]
- 89.Hauser KF, Stiene-Martin A. Characterization of opioid-dependent glial development in dissociated and organotypic cultures of mouse central nervous system: Critical periods and target specificity. Dev Brain Res. 1991;62:245–255. doi: 10.1016/0165-3806(91)90172-f. [DOI] [PubMed] [Google Scholar]
- 90.Hauser KF. Morphine regulates DNA synthesis in cerebellar neuroblasts in vitro. Dev Brain Res. 1992;70:291–297. doi: 10.1016/0165-3806(92)90210-n. [DOI] [PubMed] [Google Scholar]
- 91.Hammer RP, Jr., Hauser KF. Consequences of early exposure to opioids on cell proliferation and neuronal morphogenesis. In: Miller M, editor. Development of the Central Nervous System: Effects of Alcohol and Opiates. Wiley-Liss; New York: 1992. pp. 319–339. [Google Scholar]
- 92.Hauser KF, Houdi AA, Turbek CS, Elde RP, Maxson W., III Opioids intrinsically inhibit the genesis of mouse cerebellar granule cell precursors in vitro: Differential impact of μ and δ receptor activation on proliferation and neurite elongation. Eur J Neurosci. 2000;12:1281–1293. doi: 10.1046/j.1460-9568.2000.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zagon IS, McLaughlin PJ. Increased brain size and cellular content in infant rats treated with an opioid antagonist. Science. 1983;221:1179–1180. doi: 10.1126/science.6612331. [DOI] [PubMed] [Google Scholar]
- 94.Akil H, Watson SJ, Young E, Lewis ME, Khachaturian H, Walker JM. Endogenous Opioids: Biology and Function. Ann Rev Neurosci. 1984;7:223–255. doi: 10.1146/annurev.ne.07.030184.001255. [DOI] [PubMed] [Google Scholar]
- 95.Leslie FM, Chen Y, Winzer-Serhan UH. Opioid receptor and peptide mRNA expression in proliferative zones of fetal rat central nervous system. Can J Physiol Pharmacol. 1998;76:284–293. [PubMed] [Google Scholar]
- 96.Osborne JG, Kindy MS, Spruce BA, Hauser KF. Ontogeny of proenkephalin mRNA and enkephalin peptide expression in the cerebellar cortex of the rat: Spatial and temporal patterns of expression follow maturational gradients in the external granular layer and in Purkinje cells. Dev Brain Res. 1993;76:1–12. doi: 10.1016/0165-3806(93)90117-s. [DOI] [PubMed] [Google Scholar]
- 97.Zagon IS, Rhodes RE, McLaughlin PJ. Distribution of enkephalin immunoreactivity in germinative cells of developing rat cerebellum. Science. 1985;227:1049–1051. doi: 10.1126/science.3883485. [DOI] [PubMed] [Google Scholar]
- 98.Zagon IS, Gibo DM, McLaughlin PJ. Zeta (ζ), a growth-related opioid receptor in developing rat cerebellum: Identification and characterization. Brain Res. 1991;551:28–35. doi: 10.1016/0006-8993(91)90909-f. [DOI] [PubMed] [Google Scholar]
- 99.Hauser KF, Osborne JG, Stiene-Martin A, Melner MH. Cellular localization of proenkephalin mRNA and enkephalin peptide products in cultured astrocytes. Brain Res. 1990;522:347–353. doi: 10.1016/0006-8993(90)91482-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spruce BA, Curtis R, Wilkin GP, Glover DM. A neuropeptide precursor in cerebellum: Proenkephalin exists in subpopulations of both neurons and astrocytes. EMBO J. 1990;9:1787–1795. doi: 10.1002/j.1460-2075.1990.tb08303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shinoda H, Marini AM, Cosi C, Schwartz JP. Brain region and gene specificity of neuropeptide gene expression in cultured astrocytes. Science. 1989;245:415–417. doi: 10.1126/science.2569236. [DOI] [PubMed] [Google Scholar]
- 102.Stiene-Martin A, Osborne JG, Hauser KF. Co-localization of proenkephalin mRNA using cRNA probes and a cell-type-specific marker for intact astrocytes in vitro. J Neurosci Methods. 1991;36:119–126. doi: 10.1016/0165-0270(91)90037-z. [DOI] [PubMed] [Google Scholar]
- 103.Zhu Y, Hsu MS, Pintar JE. Developmental expression of the mu, kappa, and delta opioid receptor mRNAs in mouse. J Neurosci. 1998;18:2538–2549. doi: 10.1523/JNEUROSCI.18-07-02538.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Goldstein A. Binding selectivity profiles for ligands of multiple receptor types: focus on opioid receptors. Trends Pharmacol Sci. 1987;8:456–459. [Google Scholar]
- 105.Zagon IS, McLaughlin PJ. Opioid antagonist (naltrexone) modulation of cerebellar development: histological and morphometric studies. J Neurosci. 1986;6:1424–1432. doi: 10.1523/JNEUROSCI.06-05-01424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hauser KF, McLaughlin PJ, Zagon IS. Endogenous opioids regulate dendritic growth and spine formation in developing rat brain. Brain Res. 1987;416:157–161. doi: 10.1016/0006-8993(87)91509-5. [DOI] [PubMed] [Google Scholar]
- 107.Vernadakis A, Estin C, Gibson DA, Amott S. Effects of methadone on ornithine decarboxylase and cyclic nucleotide phosphohydrolase in neuronal and glial cell cultures. J Neurosci Res. 1982;7:111–117. doi: 10.1002/jnr.490070203. [DOI] [PubMed] [Google Scholar]
- 108.Sakellaridis N, Vernadakis A. An unconventional response of adenylate cyclase to morphine and naloxone in the chicken during early development. Proc Natl Acad Sci (USA) 1986;83:2738–2742. doi: 10.1073/pnas.83.8.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kornblum HI, Loughlin SE, Leslie FM. Effects of morphine on DNA synthesis in neonatal rat brain. Dev Brain Res. 1987;31:45–52. doi: 10.1016/0165-3806(87)90081-2. [DOI] [PubMed] [Google Scholar]
- 110.Hammer RP., Jr. Effects of opioids on the developing brain. In: Hammer RP Jr., editor. The Neurobiology of Opiates. CRC Press; Boca Raton, Florida: 1993. pp. 1–21. [Google Scholar]
- 111.Bartolome JV, Alicke B, Bartolome MB. Central administration of morphine inhibits brain and liver ornithine decarboxylase activity in neonatal rats: involvement of transcription- and non-transcription-dependent mechanisms. Eur J Pharmacol. 1997;331:145–153. doi: 10.1016/s0014-2999(97)01045-5. [DOI] [PubMed] [Google Scholar]
- 112.Mangoura D. mu-Opioids activate tyrosine kinase focal adhesion kinase and regulate cortical cytoskeleton proteins cortactin and vinculin in chick embryonic neurons. J Neurosci Res. 1997;50:391–401. doi: 10.1002/(SICI)1097-4547(19971101)50:3<391::AID-JNR5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 113.Slotkin T. Perinatal exposure to methadone: how do early biochemical alterations cause neurofunctional disturbances? Prog Brain Res. 1988;73:265–279. doi: 10.1016/S0079-6123(08)60509-9. [DOI] [PubMed] [Google Scholar]
- 114.Schmahl W, Funk R, Miaskowski U, Plendl J. Long-lasting effects of naltrexone, an opioid receptor antagonist, on cell proliferation in developing rat forebrain. Brain Res. 1989;486:297–300. doi: 10.1016/0006-8993(89)90515-5. [DOI] [PubMed] [Google Scholar]
- 115.Steele WJ, Johannesson T. Effects of Prenatally-administered Morphine on Brain Development and Resultant Tolerance to the Analgesic Effect of Morphine in Offspring of Morphine Treated Rats. Acta Pharmacol Toxicol. 1975;36:243–256. doi: 10.1111/j.1600-0773.1975.tb00792.x. [DOI] [PubMed] [Google Scholar]
- 116.Hammer RP, Jr., Ricalde AA, Seatriz JV. Effects of opiates on brain development. Neurotoxicol. 1989;10:475–484. [PubMed] [Google Scholar]
- 117.Seatriz JV, Hammer RP., Jr. Effects of opiates on neuronal development in the rat cerebral cortex. Brain Res Bull. 1993;30:523–527. doi: 10.1016/0361-9230(93)90078-p. [DOI] [PubMed] [Google Scholar]
- 118.Lorber BA, Freitag SK, Bartolome JV. Effects of beta-endorphin on DNA synthesis in brain regions of preweanling rats. Brain Res. 1990;531:329–332. doi: 10.1016/0006-8993(90)90795-d. [DOI] [PubMed] [Google Scholar]
- 119.Zagon IS, McLaughlin PJ. Endogenous opioid systems regulate cell proliferation in the developing rat brain. Brain Res. 1987;412:68–72. doi: 10.1016/0006-8993(87)91440-5. [DOI] [PubMed] [Google Scholar]
- 120.Hauser KF, Mangoura D. Diversity of the endogenous opioid system in development: novel signal transduction translates multiple extracellular signals into neural cell growth and differentiation. Perspect Dev Neurobiol. 1998;5:437–449. [PubMed] [Google Scholar]
- 121.Knapp PE, Maderspach K, Hauser KF. Endogenous opioid system in developing normal and jimpy oligodendrocytes: μ and κ opioid receptors mediate differential mitogenic and growth responses. Glia. 1998;22:189–201. doi: 10.1002/(sici)1098-1136(199802)22:2<189::aid-glia10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 122.Gurwell JA, Hauser KF. Morphine does not affect astrocyte survival in developing primary mixed-glial cultures. Dev Brain Res. 1993;76:293–298. doi: 10.1016/0165-3806(93)90222-v. [DOI] [PubMed] [Google Scholar]
- 123.Stiene-Martin A, Zhou R, Hauser KF. Regional, developmental, and cell cycle-dependent differences in μ, δ, and κ–opioid receptor expression among cultured mouse astrocytes. Glia. 1998;22:249–259. [PMC free article] [PubMed] [Google Scholar]
- 124.Oehmichen M, Meissner C, Reiter A, Birkholz M. Neuropathology in non-human immunodeficiency virus-infected drug addicts: hypoxic brain damage after chronic intravenous drug abuse. Acta Neuropathol (Berl) 1996;91:642–646. doi: 10.1007/s004010050478. [DOI] [PubMed] [Google Scholar]
- 125.Kriegstein AR, Armitage BA, Kim PY. Heroin inhalation and progressive spongiform leukoencephalopathy. N Engl J Med. 1997;336:589–590. doi: 10.1056/NEJM199702203360818. [DOI] [PubMed] [Google Scholar]
- 126.Kriegstein AR, Shungu DC, Millar WS, Armitage BA, Brust JC, Chillrud S, et al. Leukoencephalopathy and raised brain lactate from heroin vapor inhalation ("chasing the dragon") Neurology. 1999;53:1765–1773. doi: 10.1212/wnl.53.8.1765. [DOI] [PubMed] [Google Scholar]
- 127.Strang J, Griffiths P, Gossop M. Heroin smoking by 'chasing the dragon': origins and history. Addiction. 1997;92:673–683. doi: 10.1046/j.1360-0443.1997.9266734.x. [DOI] [PubMed] [Google Scholar]
- 128.Gossop M, Griffiths P, Strang J. Chasing the dragon: characteristics of heroin chasers. Br J Addict. 1988;83:1159–1162. doi: 10.1111/j.1360-0443.1988.tb03022.x. [DOI] [PubMed] [Google Scholar]
- 129.Wolters EC, van Wijngaarden GK, Stam FC, Rengelink H, Lousberg RJ, Schipper ME, et al. Leucoencephalopathy after inhaling "heroin" pyrolysate. Lancet. 1982;2:1233–1237. doi: 10.1016/s0140-6736(82)90101-5. [DOI] [PubMed] [Google Scholar]
- 130.Kinney HC, White WF. Opioid receptors localize to the external granular cell layer of the developing human cerebellum. Neuroscience. 1991;45:13–21. doi: 10.1016/0306-4522(91)90099-a. [DOI] [PubMed] [Google Scholar]
- 131.Zagon IS, Verderame MF, McLaughlin PJ. The biology of the opioid growth factor receptor (OGFr) Brain Res Brain Res Rev. 2002;38:351–376. doi: 10.1016/s0165-0173(01)00160-6. [DOI] [PubMed] [Google Scholar]
- 132.Coyle JT, Pert CB. Ontogenetic development of [3H]naloxone binding in rat brain. Neuropharmacol. 1976;15:555–560. doi: 10.1016/0028-3908(76)90107-6. [DOI] [PubMed] [Google Scholar]
- 133.Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, et al. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: An in situ hybridization study. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- 134.Abeyta A, Dettmer TS, Barnes A, Vega D, Carta M, Gallegos N, et al. Delta opioid receptor localization in the rat cerebellum. Brain Res. 2002;931:100–105. doi: 10.1016/s0006-8993(02)02248-5. [DOI] [PubMed] [Google Scholar]
- 135.Platzer S, Winkler A, Schadrack J, Dworzak D, Tolle TR, Zieglgansberger W, et al. Autoradiographic distribution of mu-, delta- and kappa 1-opioid stimulated [35S]guanylyl-5'-O-(gamma-thio)-triphosphate binding in human frontal cortex and cerebellum. Neurosci Lett. 2000;283:213–216. doi: 10.1016/s0304-3940(00)00943-5. [DOI] [PubMed] [Google Scholar]
- 136.Schadrack J, Willoch F, Platzer S, Bartenstein P, Mahal B, Dworzak D, et al. Opioid receptors in the human cerebellum: evidence from [11C]diprenorphine PET, mRNA expression and autoradiography. Neuroreport. 1999;10:619–624. doi: 10.1097/00001756-199902250-00032. [DOI] [PubMed] [Google Scholar]
- 137.Zagon IS, McLaughlin PJ. Morphine and brain growth retardation in the rat. Pharmacol. 1977;15:276–282. doi: 10.1159/000136699. [DOI] [PubMed] [Google Scholar]
- 138.Zagon IS, McLaughlin PJ. Methadone and brain development. Experientia. 1977;33:1486. doi: 10.1007/BF01918824. [DOI] [PubMed] [Google Scholar]
- 139.Willson NJ, Schneider JF, Roizin L, Fleiss JF, Rivers W, Demartini JE. Effects of methadone HCl on the growth of organotypic cerebellar cultures prepared from methadone tolerant and control rats. J Pharmacol Exp Ther. 1976;199:368–374. [PubMed] [Google Scholar]
- 140.King JS, Ho RH, Bishop GA. Anatomical evidence for enkephalin immunoreactive climbing fibres in the cerebellar cortex of the opossum. J Neurocytol. 1986;15:545–559. doi: 10.1007/BF01611856. [DOI] [PubMed] [Google Scholar]
- 141.Schulman JA, Finger TE, Brecha NC, Karten HJ. Enkephalin Immunoreactivity in Golgi Cells and Mossy Fibres of Mammalian, Avian, Amphibian and Teleost Cerebellum. Neurosci. 1981;6:2407–2416. doi: 10.1016/0306-4522(81)90026-9. [DOI] [PubMed] [Google Scholar]
- 142.Vathy I, Rimanoczy A, Eaton RC, Katay L. Sex dimorphic alterations in postnatal brain catecholamines after gestational morphine. Brain Res Bull. 1995;36:185–193. doi: 10.1016/0361-9230(94)00192-4. [DOI] [PubMed] [Google Scholar]
- 143.Barg J, Belcheva MM, Zimlichman R, Levy R, Saya D, Mchale RJ, et al. Opioids inhibit endothelin-mediated DNA synthesis, phosphoinositide turnover, and Ca2+ mobilization in rat C6 glioma cells. J Neurosci. 1994;14:5858–5864. doi: 10.1523/JNEUROSCI.14-10-05858.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Knapp PE, Hauser KF. μ-Opioid receptor activation enhances DNA synthesis in immature oligodendrocytes. Brain Res. 1996;743:341–345. doi: 10.1016/s0006-8993(96)01097-9. [DOI] [PubMed] [Google Scholar]
- 145.Hauser KF, Stiene-Martin A, Mattson MP, Elde RP, Ryan SE, Godleske CC. μ-Opioid receptor-induced Ca2+ mobilization and astroglial development: Morphine inhibits DNA synthesis and stimulates cellular hypertrophy through a Ca2+-dependent mechanism. Brain Res. 1996;720:191–203. doi: 10.1016/0006-8993(96)00103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mangoura D, Sogos V, Dawson G. Protein kinase C-epsilon is a developmentally regulated, neuronal isoform in the chick embryo central nervous system. J Neurosci Res. 1993;35:488–498. doi: 10.1002/jnr.490350505. [DOI] [PubMed] [Google Scholar]
- 147.Bohn LM, Belcheva MM, Coscia CJ. Mitogenic signaling via endogenous kappa-opioid receptors in C6 glioma cells: evidence for the involvement of protein kinase C and the mitogen-activated protein kinase signaling cascade. J Neurochem. 2000;74:564–573. doi: 10.1046/j.1471-4159.2000.740564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Belcheva MM, Szucs M, Wang D, Sadee W, Coscia CJ. mu-Opioid receptor-mediated ERK activation involves calmodulin-dependent epidermal growth factor receptor transactivation. J Biol Chem. 2001;276:33847–33853. doi: 10.1074/jbc.M101535200. [DOI] [PubMed] [Google Scholar]
- 149.Barg J, Belcheva MM, Coscia CJ. Evidence for the implication of phosphoinositol signal transduction in μ-opioid inhibition of DNA synthesis. J Neurochem. 1992;59:1145–1152. doi: 10.1111/j.1471-4159.1992.tb08357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Huang L-YM. Cellular mechanisms of excitatory and inhibitory actions of opioids. In: Tseng LF, editor. The Pharmacology of Opioid Peptides. 1 Harwood Academic Publishers; 1995. pp. 131–149. [Google Scholar]
- 151.Barg J, Belcheva M, McHale R, Levy R, Vogel Z, Coscia CJ. Beta-endorphin is a potent inhibitor of thymidine incorporation into DNA via mu- and kappa-opioid receptors in fetal rat brain cell aggregates in culture. J Neurochem. 1993;60:765–767. doi: 10.1111/j.1471-4159.1993.tb03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Polakiewicz RD, Schieferl SM, Gingras AC, Sonenberg N, Comb MJ. mu-Opioid receptor activates signaling pathways implicated in cell survival and translational control. J Biol Chem. 1998;273:23534–23541. doi: 10.1074/jbc.273.36.23534. [DOI] [PubMed] [Google Scholar]
- 153.Law PY, Bergsbaken C. Properties of delta opioid receptor in neuroblastoma NS20Y: receptor activation and neuroblastoma proliferation. J Pharmacol Exp Ther. 1995;272:322–332. [PubMed] [Google Scholar]
- 154.Lee YS, Wurster RD. Differential effects of methionine enkephalin on the growth of brain tumor cells. J Neurooncol. 1994;19:11–15. doi: 10.1007/BF01051044. [DOI] [PubMed] [Google Scholar]
- 155.Opanashuk LA, Hauser KF. Opposing actions of the EGF family and opioids: Heparin binding-epidermal growth factor (HB-EGF) protects mouse cerebellar neuroblasts against the antiproliferative effect of morphine. Brain Res. 1998;804:87–94. doi: 10.1016/s0006-8993(98)00647-7. [DOI] [PubMed] [Google Scholar]
- 156.Bohn LM, Belcheva MM, Coscia CJ. Mu-opioid agonist inhibition of kappa-opioid receptor-stimulated extracellular signal-regulated kinase phosphorylation is dynamin-dependent in C6 glioma cells. J Neurochem. 2000;74:574–581. doi: 10.1046/j.1471-4159.2000.740574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jordan B, Devi LA. Molecular mechanisms of opioid receptor signal transduction. Br J Anaesth. 1998;81:12–19. doi: 10.1093/bja/81.1.12. [DOI] [PubMed] [Google Scholar]
- 158.Gomes I, Jordan BA, Gupta A, Trapaidze N, Nagy V, Devi LA. Heterodimerization of mu and delta opioid receptors: A role in opiate synergy. J Neurosci. 2000;20:RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jordan BA, Trapaidze N, Gomes I, Nivarthi R, Devi LA. Oligomerization of opioid receptors with beta 2-adrenergic receptors: a role in trafficking and mitogen-activated protein kinase activation. Proc Natl Acad Sci U S A. 2001;98:343–348. doi: 10.1073/pnas.011384898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Knapp PE, Itkis OS, Zhang L, Spruce BA, Hauser KF. Endogenous opioids and oligodendroglial function: possible autocrine/paracrine effects on cell survival and development. Glia. 2001;35:156–165. doi: 10.1002/glia.1080. [DOI] [PubMed] [Google Scholar]
- 161.Stiene-Martin A, Knapp PE, Martin KM, Gurwell JA, Ryan S, Thornton SR, et al. Opioid system diversity in developing neurons, astroglia, and oligodendroglia in the subventricular zone and striatum: impact on gliogenesis in vivo. Glia. 2001;36:78–88. [PMC free article] [PubMed] [Google Scholar]
- 162.Gould E, Butcher LL. Transient expression of choline acetyltransferase-like immunoreactivity in Purkinje cells of the developing rat cerebellum. Brain Res. 1987;431:303–306. doi: 10.1016/0165-3806(87)90218-5. [DOI] [PubMed] [Google Scholar]
- 163.Clos J, Ghandour S, Eberhart R, Vincendon G, Gombos G. The cholinergic system in developing cerebellum: comparative study of normal, hypothyroid and underfed rats. Dev Neurosci. 1989;11:188–204. doi: 10.1159/000111898. [DOI] [PubMed] [Google Scholar]
- 164.Perry EK, Court JA, Johnson M, Smith CJ, James V, Cheng AV, et al. Autoradiographic comparison of cholinergic and other transmitter receptors in the normal human hippocampus. Hippocampus. 1993;3:307–315. doi: 10.1002/hipo.450030306. [DOI] [PubMed] [Google Scholar]
- 165.Court JA, Perry EK, Spurden D, Griffiths M, Kerwin JM, Morris CM, et al. The role of the cholinergic system in the development of the human cerebellum. Brain Res Dev Brain Res. 1995;90:159–167. doi: 10.1016/0165-3806(96)83496-1. [DOI] [PubMed] [Google Scholar]
- 166.Brooksbank BW, Walker D, Balazs R, Jorgensen OS. Neuronal maturation in the foetal brain in Down's syndrome. Early Hum Dev. 1989;18:237–246. doi: 10.1016/0378-3782(89)90019-4. [DOI] [PubMed] [Google Scholar]
- 167.Court JA, Perry EK, Spurden D, Griffiths M, Kerwin JM, Morris CM, et al. The role of the cholinergic system in the development of the human cerebellum. Brain Res Dev Brain Res. 1995;90:159–167. doi: 10.1016/0165-3806(96)83496-1. [DOI] [PubMed] [Google Scholar]
- 168.Jaarsma D, Ruigrok TJ, Caffe R, Cozzari C, Levey AI, Mugnaini E, et al. Cholinergic innervation and receptors in the cerebellum. Prog Brain Res. 1997;114:67–96. doi: 10.1016/s0079-6123(08)63359-2. [DOI] [PubMed] [Google Scholar]
- 169.Altman J. Postnatal development of the cerebellar cortex in the rat. III. Maturation of the components of the granular layer. J Comp Neurol. 1972;145:465–514. doi: 10.1002/cne.901450403. [DOI] [PubMed] [Google Scholar]
- 170.Boegman RJ, Parent A, Hawkes R. Zonation in the rat cerebellar cortex: patches of high acetylcholinesterase activity in the granular layer are congruent with Purkinje cell compartments. Brain Res. 1988;448:237–251. doi: 10.1016/0006-8993(88)91261-9. [DOI] [PubMed] [Google Scholar]
- 171.Role LW, Berg DK. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- 172.Alkondon M, Pereira EF, Cortes WS, Maelicke A, Albuquerque EX. Choline is a selective agonist of alpha7 nicotinic acetylcholine receptors in the rat brain neurons. Eur J Neurosci. 1997;9:2734–2742. doi: 10.1111/j.1460-9568.1997.tb01702.x. [DOI] [PubMed] [Google Scholar]
- 173.Albuquerque EX, Alkondon M, Pereira EF, Castro NG, Schrattenholz A, Barbosa CT, et al. Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther. 1997;280:1117–1136. [PubMed] [Google Scholar]
- 174.Albuquerque EX, Pereira EF, Alkondon M, Schrattenholz A, Maelicke A. Nicotinic acetylcholine receptors on hippocampal neurons: distribution on the neuronal surface and modulation of receptor activity. J Recept Signal Transduct Res. 1997;17:243–266. doi: 10.3109/10799899709036607. [DOI] [PubMed] [Google Scholar]
- 175.Zoli M, Le NN, Hill JAJ, Changeux JP. Developmental regulation of nicotinic ACh receptor subunit mRNAs in the rat central and peripheral nervous systems. J Neurosci. 1995;15:1912–1939. doi: 10.1523/JNEUROSCI.15-03-01912.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tice MA, Hashemi T, Taylor LA, McQuade RD. Distribution of muscarinic receptor subtypes in rat brain from postnatal to old age. Brain Res Dev Brain Res. 1996;92:70–76. doi: 10.1016/0165-3806(95)01515-9. [DOI] [PubMed] [Google Scholar]
- 177.Winzer-Serhan UH, Leslie FM. Codistribution of nicotinic acetylcholine receptor subunit alpha3 and beta4 mRNAs during rat brain development. J Comp Neurol. 1997;386:540–554. doi: 10.1002/(sici)1096-9861(19971006)386:4<540::aid-cne2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 178.Kinney HC, O'Donnell TJ, Kriger P, White WF. Early developmental changes in [3H]nicotine binding in the human brainstem. Neuroscience. 1993;55:1127–1138. doi: 10.1016/0306-4522(93)90326-b. [DOI] [PubMed] [Google Scholar]
- 179.Morley BJ. The embryonic and post-natal expression of the nicotinic receptor alpha 3-subunit in rat lower brainstem [In Process Citation] Brain Res Mol Brain Res. 1997;48:407–412. doi: 10.1016/s0169-328x(97)00159-9. [DOI] [PubMed] [Google Scholar]
- 180.Opanashuk LA, Pauly JR, Hauser KF. Effect of nicotine on cerebellar granule neuron development. Eur J Neurosci. 2001;13:48–56. [PMC free article] [PubMed] [Google Scholar]
- 181.Dominguez dT, Juiz JM, Smillie FI, Lindstrom J, Criado M. Expression of alpha 7 neuronal nicotinic receptors during postnatal development of the rate cerebellum. Brain Res Dev Brain Res. 1997;98:125–133. doi: 10.1016/s0165-3806(96)00185-x. [DOI] [PubMed] [Google Scholar]
- 182.Didier M, Berman SA, Lindstrom J, Bursztajn S. Characterization of nicotinic acetylcholine receptors expressed in primary cultures of cerebellar granule cells. Brain Res Mol Brain Res. 1995;30:17–28. doi: 10.1016/0169-328x(94)00266-h. [DOI] [PubMed] [Google Scholar]
- 183.De Filippi G, Baldwinson T, Sher E. Evidence for nicotinic acetylcholine receptor activation in rat cerebellar slices. Pharmacol Biochem Behav. 2001;70:447–455. doi: 10.1016/s0091-3057(01)00653-0. [DOI] [PubMed] [Google Scholar]
- 184.Caruncho HJ, Guidotti A, Lindstrom J, Costa E, Pesold C. Subcellular localization of the alpha 7 nicotinic receptor in rat cerebellar granule cell layer. Neuroreport. 1997;8:1431–1433. doi: 10.1097/00001756-199704140-00021. [DOI] [PubMed] [Google Scholar]
- 185.Kawa K. Acute synaptic modulation by nicotinic agonists in developing cerebellar Purkinje cells of the rat. J Physiol. 2002;538:87–102. doi: 10.1113/jphysiol.2001.012885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Navarro HA, Seidler FJ, Eylers JP, Baker FE, Dobbins SS, Lappi SE, et al. Effects of prenatal nicotine exposure on development of central and peripheral cholinergic neurotransmitter systems. Evidence for cholinergic trophic influences in developing brain. J Pharmacol Exp Ther. 1989;251:894–900. [PubMed] [Google Scholar]
- 187.McFarland BJ, Seidler FJ, Slotkin TA. Inhibition of DNA synthesis in neonatal rat brain regions caused by acute nicotine administration. Brain Res Dev Brain Res. 1991;58:223–229. doi: 10.1016/0165-3806(91)90008-7. [DOI] [PubMed] [Google Scholar]
- 188.Slotkin TA, Lappi SE, Seidler FJ. Impact of fetal nicotine exposure on development of rat brain regions: critical sensitive periods or effects of withdrawal? Brain Res Bull. 1993;31:319–328. doi: 10.1016/0361-9230(93)90224-y. [DOI] [PubMed] [Google Scholar]
- 189.Roy TS, Andrews JE, Seidler FJ, Slotkin TA. Nicotine evokes cell death in embryonic rat brain during neurulation. J Pharmacol Exp Ther. 1998;287:1136–1144. [PubMed] [Google Scholar]
- 190.Lee M, Martin-Ruiz C, Graham A, Court J, Jaros E, Perry R, et al. Nicotinic receptor abnormalities in the cerebellar cortex in autism. Brain. 2002;125:1483–1495. doi: 10.1093/brain/awf160. [DOI] [PubMed] [Google Scholar]
- 191.Gao W-Q, Heintz N, Hatten ME. Cerebellar granule cell neurogenesis is regulated by cell-cell interactions in vitro. Neuron. 1991;6:705–715. doi: 10.1016/0896-6273(91)90168-y. [DOI] [PubMed] [Google Scholar]
- 192.Prendergast MA, Harris BR, Mayer S, Holley RC, Hauser KF, Littleton JM. Chronic nicotine exposure reduces N-methyl-D-aspartate receptor-mediated damage in the hippocampus without altering calcium accumulation or extrusion: evidence for calbindin-D28K overexpression. Neuroscience. 2001;102:75–85. doi: 10.1016/s0306-4522(00)00450-4. [DOI] [PubMed] [Google Scholar]
- 193.Berger F, Gage FH, Vijayaraghavan S. Nicotinic receptor-induced apoptotic cell death of hippocampal progenitor cells. J Neurosci. 1998;18:6871–6881. doi: 10.1523/JNEUROSCI.18-17-06871.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Delbono O, Gopalakrishnan M, Renganathan M, Monteggia LM, Messi ML, Sullivan JP. Activation of the recombinant human alpha 7 nicotinic acetylcholine receptor significantly raises intracellular free calcium. J Pharmacol Exp Ther. 1997;280:428–438. [PubMed] [Google Scholar]
- 195.Villablanca AC. Nicotine stimulates DNA synthesis and proliferation in vascular endothelial cells in vitro. J Appl Physiol. 1998;84:2089–2098. doi: 10.1152/jappl.1998.84.6.2089. [DOI] [PubMed] [Google Scholar]
- 196.Yan GM, Lin SZ, Irwin RP, Paul SM. Activation of muscarinic cholinergic receptors blocks apoptosis of cultured cerebellar granule neurons. Mol Pharmacol. 1995;47:248–257. [PubMed] [Google Scholar]
- 197.Wong RO. Cholinergic regulation of [Ca2+]i during cell division and differentiation in the mammalian retina. J Neurosci. 1995;15:2696–2706. doi: 10.1523/JNEUROSCI.15-04-02696.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rakowicz-Szulczynska EM, McIntosh DG, Smith M. Growth factor-mediated mechanisms of nicotine-dependent carcinogenesis. Carcinogenesis. 1994;15:1839–1846. doi: 10.1093/carcin/15.9.1839. [DOI] [PubMed] [Google Scholar]
- 199.Rakowicz-Szulczynska EM, McIntosh DG, Perry M, Smith ML. PDGF AA as mediator in nicotine-dependent carcinogenesis. Carcinogenesis. 1996;17:1813–1818. doi: 10.1093/carcin/17.9.1813. [DOI] [PubMed] [Google Scholar]
- 200.Wong RO. Cholinergic regulation of [Ca2+]i during cell division and differentiation in the mammalian retina. J Neurosci. 1995;15:2696–2706. doi: 10.1523/JNEUROSCI.15-04-02696.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Role LW, Berg DK. Nicotinic receptors in the development and modulation of CNS synapses. Neuron. 1996;16:1077–1085. doi: 10.1016/s0896-6273(00)80134-8. [DOI] [PubMed] [Google Scholar]
- 202.Berger F, Gage FH, Vijayaraghavan S. Nicotinic receptor-induced apoptotic cell death of hippocampal progenitor cells. J Neurosci. 1998;18:6871–6881. doi: 10.1523/JNEUROSCI.18-17-06871.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dempsey DA, Benowitz NL. Risks and benefits of nicotine to aid smoking cessation in pregnancy. Drug Saf. 2001;24:277–322. doi: 10.2165/00002018-200124040-00005. [DOI] [PubMed] [Google Scholar]
- 204.Pantazis NJ, Dohrman DP, Goodlett CR, Cook RT, West JR. Vulnerability of cerebellar granule cells to alcohol-induced cell death diminishes with time in culture. Alcohol Clin Exp Res. 1993;17:1014–1021. doi: 10.1111/j.1530-0277.1993.tb05657.x. [DOI] [PubMed] [Google Scholar]
- 205.Bonthius D, Tzouras G, Karacay B, Mahoney J, Hutton A, McKim R, et al. Deficiency of neuronal nitric oxide synthase (nNOS) worsens alcohol-induced microencephaly and neuronal loss in developing mice. Brain Res Dev Brain Res. 2002;138:45. doi: 10.1016/s0165-3806(02)00458-3. [DOI] [PubMed] [Google Scholar]
- 206.Srivastava N, Vernadakis A. Maturation of cerebellar granule cells is delayed in cultures derived from ethanol-treated chick embryos: survival and proliferation studies. Int J Dev Neurosci. 1995;13:529–537. doi: 10.1016/0736-5748(95)00031-b. [DOI] [PubMed] [Google Scholar]
- 207.Gruol DL, Parsons KL. Chronic alcohol reduces calcium signaling elicited by glutamate receptor stimulation in developing cerebellar neurons. Brain Res. 1996;728:166–174. doi: 10.1016/0006-8993(96)00185-0. [DOI] [PubMed] [Google Scholar]
- 208.West JR, Parnell SE, Chen WJ, Cudd TA. Alcohol-mediated Purkinje cell loss in the absence of hypoxemia during the third trimester in an ovine model system. Alcohol Clin Exp Res. 2001;25:1051–1057. [PubMed] [Google Scholar]
- 209.Bauer-Moffett C, Altman J. The effect of ethanol chronically administered to preweanling rats on cerebellar development: a morphological study. Brain Res. 1977;119:249–268. doi: 10.1016/0006-8993(77)90310-9. [DOI] [PubMed] [Google Scholar]
- 210.Thomas JD, Goodlett CR, West JR. Alcohol-induced Purkinje cell loss depends on developmental timing of alcohol exposure and correlates with motor performance. Brain Res Dev Brain Res. 1998;105:159–166. doi: 10.1016/s0165-3806(97)00164-8. [DOI] [PubMed] [Google Scholar]
- 211.Lim DK, Park SH, Choi WJ. Subacute nicotine exposure in cultured cerebellar cells increased the release and uptake of glutamate. Arch Pharm Res. 2000;23:488–494. doi: 10.1007/BF02976578. [DOI] [PubMed] [Google Scholar]
- 212.Jaarsma D, Dino MR, Cozzari C, Mugnaini E. Cerebellar choline acetyltransferase positive mossy fibres and their granule and unipolar brush cell targets: a model for central cholinergic nicotinic neurotransmission. J Neurocytol. 1996;25:829–842. doi: 10.1007/BF02284845. [DOI] [PubMed] [Google Scholar]
- 213.Freund RK, Palmer MR. Ethanol depression of cerebellar Purkinje neuron firing involves nicotinic acetylcholine receptors. Exp Neurol. 1997;143:319–322. doi: 10.1006/exnr.1996.6371. [DOI] [PubMed] [Google Scholar]
- 214.de lG, Freedman R, Hoffer J. Nicotine-induced inhibition of cerebellar Purkinje neurons: specific actions of nicotine and selective blockade by mecamylamine. Neuropharmacology. 1989;28:495–501. doi: 10.1016/0028-3908(89)90085-3. [DOI] [PubMed] [Google Scholar]
- 215.de lG, McGuire TJ, Freedman R, Hoffer BJ. The electrophysiological effects of nicotine in the rat cerebellum: evidence for direct postsynaptic actions. Neurosci Lett. 1987;80:303–308. doi: 10.1016/0304-3940(87)90472-1. [DOI] [PubMed] [Google Scholar]
- 216.Agulhon C, Charnay Y, Vallet P, Abitbol M, Kobetz A, Bertrand D, et al. Distribution of mRNA for the alpha4 subunit of the nicotinic acetylcholine receptor in the human fetal brain. Brain Res Mol Brain Res. 1998;58:123–131. doi: 10.1016/s0169-328x(98)00113-2. [DOI] [PubMed] [Google Scholar]
- 217.Yang X, Criswell HE, Breese GR. Action of ethanol on responses to nicotine from cerebellar Purkinje neurons: relationship to methyllycaconitine (MLA) inhibition of nicotine responses. Neurochem Int. 1999;35:185–194. doi: 10.1016/s0197-0186(99)00060-1. [DOI] [PubMed] [Google Scholar]