Abstract
Opiate drug abuse, through selective actions at μ opioid receptors (MOR), exacerbates the pathogenesis of human immunodeficiency virus-1 (HIV-1) in the CNS by disrupting glial homeostasis, increasing inflammation, and decreasing the threshold for pro-apoptotic events in neurons. Neurons are affected directly and indirectly by opiate-HIV interactions. Although most opiate drugs have some affinity for κ (KOR) and/or δ (DOR) opioid receptors, their neurotoxic effects are largely mediated through MOR. Besides direct actions on the neurons themselves, opiates directly affect MOR-expressing astrocytes and microglia. Because of their broad-reaching actions in glia, opiate abuse causes widespread metabolic derangement, inflammation, and the disruption of neuron-glial relationships, which likely contribute to neuronal dysfunction, death, and HIV encephalitis. In addition to direct actions on neural cells, opioids modulate inflammation and disrupt normal intercellular interactions among immunocytes (macrophages and lymphocytes), which on balance further promote neuronal dysfunction and death. The neural pathways involved in opiate enhancement of HIV-induced inflammation and cell death, appear to involve MOR activation with downstream effects through PI3-kinase/Akt and/or MAPK signaling, which suggests possible targets for therapeutic intervention in neuroAIDS.
Keywords: AIDS, chemokines, μ-opioid receptors, neurons, astroglia, microglia, neuroimmunology, CNS inflammation
INTRODUCTION
Drug Abuse and HIV/AIDS as Interlinked Epidemics
Injection drug users are at higher risk for contracting HIV infection and for developing neurological and systemic complications. Injection drug use is a major risk factor and is the fastest growing means for spread of HIV infection in the United States (Beyrer et al., 2000; De Castro and Sabate, 2003; Francis, 2003; Vaswani and Desai, 2004). Patients who engage in high-risk sexual behavior frequently abuse drugs (Heckman et al., 1999; Miller, 2003). In addition to the drug abusing population, patients with HIV infection who develop persistent headaches or intractable pain syndromes associated with peripheral neuropathies require long-term use of opiate drugs for treatment. This group is therefore also at risk for any deleterious effects of HIV-opiate interactions (Mirsattari et al., 1999). Although methamphetamine, cocaine, and opiates (heroin, oxycodone) are detrimental in neuroAIDS, recent findings indicate that the opioid system intrinsically regulates the host CNS response to HIV. Moreover, current understanding regarding methamphetamine and cocaine in HIV progression have been recently reviewed elsewhere (Goodkin et al., 1998; Pellegrino and Bayer, 1998; Tyor and Middaugh, 1999; Nath et al., 2000; Nath et al., 2002). Recreational drug use appears to limit the immune system’s ability to fight HIV (Nair et al., 2004). Unique aspects of opiate drug abuse-HIV interactions are emphasized in this review and key considerations for understanding the interactive effects are listed below:
HIV/AIDS is a global pandemic with severe human and economic consequences.
Drug abuse and HIV are interlinked epidemics—In the United States, AIDS is now largely spread through injection drug use or through the exchange of sex for drugs.
Opiate drugs exacerbate the pathogenesis and neurological complications of HIV through direct actions in the CNS.
Opioid-HIV interactions in the CNS are complex, and mediated through direct actions in neurons, astroglia, and microglia. Although occasionally reported to be beneficial, the net consequences of opioid actions in the CNS are deleterious.
Opiate drugs act by disrupting the endogenous opioid system. Understanding how the opioid system modulates neuropathogenesis will be fundamental toward understanding neuroAIDS, and will provide insight regarding the potential role of endogenous opioids in neuroAIDS.
Opiate drug use and AIDS
Globally, 34–46 million people are currently infected with HIV (UNAIDS, 2003) and injection drug use is a major factor in the spread of the disease. HIV-1 infected opiate injection drug abusers undergo an accelerated rate of progression to AIDS and HIV dementia (Nath et al., 1999a). Not only does injection drug use increase the probability of contracting HIV (Nath et al., 1999a), but perhaps more importantly, opiate drugs intrinsically alter the pathogenesis of HIV. As noted, in addition to the drug abusing population, patients with HIV infection who develop persistent headaches or intractable pain syndromes can require long-term use of opiate drugs for treatment, thereby placing this group also at risk for any deleterious effects of HIV-opiate interactions (Mirsattari et al., 1999). In addition to experimental findings, epidemiological studies are also beginning to establish links between opiate drug use and AIDS progression (Sheng et al., 1997; Donahoe and Vlahov, 1998), although the extent to which opiates per se contribute to AIDS progression has been controversial (Everall, 2004; Donahoe, 2004; Ansari, 2004). This may be in part due to the fact that the mechanisms underlying opiate-HIV interactions are incompletely understood (Everall, 2004; Donahoe, 2004). In studies of infected humans, drug histories are frequently not reliable, and patients have not been routinely screened for drug use or systematically screened for neurologic complications (Avison et al., 2003). Varying results are obtained depending on the parameter measured, the cell type affected, and timing of the insult. Since the consequences of drug interactions in peripheral organs may differ from those in the brain (Donahoe, 2004; Ansari, 2004), findings from periphery may not be readily extrapolated to the CNS (Nath et al., 2002). In fact, the CNS appears to be preferentially vulnerable to opiate drug-HIV interactions (Nath et al., 2002; Donahoe, 2004). Recent findings indicate that chronic opiate abuse increases viral load in the serum and CSF of non-human primates (Kumar et al., 2004) and causes significantly worse CD4 cell recovery in clinical studies (Dronda et al., 2004), suggesting the progression of the disease is markedly affected by opiates. Opiates also appear to increase perivascular B-lymphocytes in presymptomatic, HIV-positive individuals who preferentially abuse opiates (Anthony et al., 2004). Lastly, opiate drug use may promote the spread and pathogenesis of other diseases that hasten HIV infection. For example, morphine increases hepatitis C virus (HCV) replication and attenuates the antiviral effects of IFN-γ in Huh.8 and FCA-1 cells (Li et al., 2003). These effects are mediated through activation of NFκB and antagonized by both naltrexone and β-FNA suggesting they are mediated through opioid receptors and μ opioid receptors (MOR) in particular (Li et al., 2003). Although HCV has been detected in monocytic cells in the CNS and in cerebrospinal fluid of infected individuals, the consequences of HCV on CNS function are not fully understood (Morsica et al., 1997; Radkowski et al., 2002). Interestingly, individuals co-infected with HCV were more likely to have abused opiates or stimulants and tended to have more neurocognitive defects than individuals infected with HIV alone (Ryan et al., 2004).
Opiates and immune modulation
In this review, the plant alkaloids derived from the opium poppy will be referred to as opiates (e.g., opium, heroin, morphine) and the broader class of related endogenous peptides and receptors as opioids. Morphine is the major metabolite of heroin in the CNS and preferentially activates MOR (Sawynok, 1986). Opiates intrinsically modify immune function and have been proposed as potential cofactors in the pathogenesis of AIDS (Donahoe and Vlahov, 1998). This is based in part on the ability of opiates to modulate HIV propagation in immune cells (Carr et al., 1996; Nyland et al., 1998; Sharp et al., 1998a; Wetzel et al., 2000; McCarthy et al., 2001) and is upheld by recent evidence that opioids and chemokine receptors undergo heterologous, bidirectional cross-desensitization (Rogers et al., 2000; Rogers and Peterson, 2003). Morphine or the endogenous peptide β-endorphin (Sundar et al., 1995), which preferentially activate MOR, potentiate the expression of HIV in acutely infected peripheral blood mononuclear cells (Peterson et al., 1990; Carr and Serou, 1995), and this may result from the enhanced production of TGF-β (Chao et al., 1992; Peterson et al., 1993a; Peterson et al., 1993b). The endogenous peptide endomorphin-1, which activates MOR, increases HIV replication in isolated human brain cells (Peterson et al., 1999). Although most opiate drugs with abuse liability activate MOR preferentially, some can activate DOR and KOR opioid receptors especially at high concentrations. Alternatively, selective MOR activation can alter the expression of DOR and KOR, as well as endogenous opioid peptides that act through all three receptor types.
Besides actions at MOR, activation of DOR and KOR can also modify HIV progression. Many of the components of the endogenous opioid system (peptides and receptors) can modulate the host response to HIV-1 infection (Adler et al., 1993; Sheng et al., 1997; Peterson et al., 1998; Rogers and Peterson, 2003). MOR, DOR and KOR can have different effects. For example, subsets of leukocytes express MOR, DOR, and KOR, as well as endogenous opioid peptides such as enkephalins, and opioids can modulate neuroimmune function through complex (direct and indirect) actions that involve both peripheral and central neural and non-neural mechanisms (Mellon and Bayer, 1998; Chang et al., 1998; Sharp et al., 1998b; Ignatowski and Bidlack, 1999; Bidlack, 2000; McCarthy et al., 2001). Although the effects of opioids on immune function have been previously reviewed (Adler et al., 1993; Sharp et al., 1998a; Bidlack, 2000; Peterson et al., 2001), an emerging concept is that the opioid system can have complex (positive and negative) effects on HIV infection and/or replication in immunocytes. For example, MOR stimulation increases HIV expression in monocytic cells (Peterson et al., 1993a; Peterson et al., 1993b; Peterson et al., 1999), while kappa receptor activation can have an opposing inhibitory effect on HIV expression in monocytic and lymphocytic cells (Chao et al., 1996; Peterson et al., 2001; Chao et al., 2001; Gekker et al., 2004). MOR and KOR have been noted to mediate opposing actions in other systems (Bohn et al., 2000). Interestingly, MOR activation can increase the expression of cytokine receptors that serve as co-receptors for HIV in susceptible cells including CCR3, CCR5, and CXCR4, while KOR stimulation can increase CCR2, while decreasing CCR5, expression (review (Rogers and Peterson, 2003)). To add to this complexity, as noted earlier, there is evidence for bi-directional heterologous interactions between opioid and chemokine receptors (Rogers et al., 2000; Rogers and Peterson, 2003; Chen et al., 2004). There is also evidence that opiate withdrawal markedly destabilizes immune function. Following opiate withdrawal, immune homeostasis is disrupted as characterized by a reduced ability to mount an immune response (Rahim et al., 2003; Rahim et al., 2004).
CNS complications related to HIV
Although AIDS is clearly a disease that affects multiple systems and cell types, it was appreciated very early that the CNS was highly vulnerable to HIV. Neurological complications of HIV infection may range from peripheral neuropathies to CNS infections and tumors (Avison et al., 2003; Estanislao et al., 2003). Of these complications, dementia is the most debilitating and is in part characterized by sub-cortical dementia accompanied by motor symptomatology including paralysis and ataxia (Berger and Nath, 1997; Factor et al., 2003). AIDS dementia complex (Navia et al., 1986b), HIV-1 associated cognitive-motor complex (Janssen et al., 1989), or HIV-1 associated dementia (HAD) (synonymous terms) are neurological syndromes that typically occur in conjunction with advanced immune suppression. Neurons, in particular, were an inevitable target of the disease despite findings that the neurons themselves are normally not infected. Pathological correlates include neuronal loss (Everall et al., 1993), myelin pallor, reactive astrogliosis, dendritic pathology, and especially an increased presence of microglia and multinucleated giant cells (Navia et al., 1986a; Glass et al., 1993; Bell, 1998). The combined pathological changes are referred to as HIV encephalitis (HIVE). Mononuclear phagocytes (macrophages and microglia) are key cellular intermediaries in the neuropathogenesis of HIV. Monocyte/macrophages in the periphery become infected and subsequently cross the blood brain barrier (Trojan horse hypothesis) preferentially invading the parenchyma at perivascular sites (Meltzer et al., 1990; Persidsky and Gendelman, 2003). Although some infection of astrocytes and endothelial cells are reported, HIV is concentrated and retained in brain microglia and infiltrating macrophages (Wiley and Achim, 1994), which constitute a major cellular reservoir of HIV-1 in the brain (Nath, 1999; Kaul et al., 2001; Garden, 2002; Persidsky and Gendelman, 2003; Rock et al., 2004). Macrophages/microglia are the major site of production of new virions and toxic viral proteins (e.g., gp120, Tat) and the source of many toxic cellular products. Toxins released by microglia include cytokines such as tumor necrosis factor alpha (TNF-α), interleukins, platelet activating factor, as well as arachidonic acid, L-cysteine, glutamate, quinolinic acid, and nitric oxide (Persidsky and Gendelman, 2003).
Immune modulators contribute significantly to the pathogenesis of HIV infection (Poli and Fauci, 1992). Monocytes/macrophages are major contributors of cytokines. Infected macrophages show increased expression of TNF-α, TNF-α receptors, interleukin-1 (IL-1), interferon-γ and nitric oxide synthase (Tyor et al., 1992; Wesselingh et al., 1993; Sippy et al., 1995). Opioids can also behave as cytokines (Peterson et al., 1998), or modulate the response of leukocytes to cytokines (Rogers and Peterson, 2003), thereby mediating the host response to infection by acting as the chemical signal between immune cells and/or cells that intercede during the host response to infection. The neurotoxic properties of gp120 and Tat are in part mediated by their ability to induce inflammation and perhaps directly via cytokine mimicry (Murphy, 2001). Hence, opioids may modulate the response to virotoxins (and perhaps vice versa) by interacting with particular cytokine/chemokine receptor signaling pathways. Interactions likely occur at multiple levels. For example, MOR have been reported to heterodimerize directly with CCR5 chemokine receptors (Suzuki et al., 2002), although the functional significance of these interactions is not fully understood (Miller and Falke, 2004; Milligan, 2004)
Opiate Drug Abuse and neuroAIDS
Neurocognitive and motor dysfunction frequently occurs with HIV infection, and several prominent pathological studies indicate that patients with HIV infection and a history of opiate drug abuse are more likely to develop HIVE (Davies et al., 1997; Bell et al., 1998; Nath et al., 2002). Both HIV and opiate drug abuse increase the proportion of macrophages/microglia in the CNS (Bell et al., 2002), which likely sets the stage for subsequent pathophysiological change. Despite this, however, opiate-HIV interactions are incompletely understood and the degree to which opiate abuse per se contributes to the progression of AIDS is controversial (Everall, 2004; Donahoe, 2004). Varying results are obtained depending on the parameter measured, the cell type affected, and timing of the insult. In studies of infected humans, drug histories are frequently not reliable, and patients are not routinely screened for drug use. As noted earlier, the consequences of drug interactions may be particularly deleterious in CNS (Donahoe, 2004; Ansari, 2004).
Opioid-HIV interactions in the CNS are highly complex, and although sometimes beneficial, most consequences of opiate abuse are deleterious. Although opiate use increases the progression to HIVE, the specific mechanisms underlying the accelerated neuropathogenesis remain uncertain (Nath et al., 2002). Neurons (Gurwell et al., 2001), astrocytes (Khurdayan et al., 2004), and microglia (Tomassini et al., 2004) express opioid receptors and each cell type displays unique responses to opiates and HIV alone or in combination. Experimental findings are supported by clinical studies. The presence of giant cells and/or HIV p24 immunoreactivity in the CNS was found more frequently in preferential opiate drug users (56%) than in non-drug-abusing homosexual men (17%) with AIDS (Bell et al., 1998). The findings are from a well-characterized cohort that is unique in the extent to which they preferentially abuse opiates. HIV-1 infected individuals who inject opiate drugs have been reported to undergo an accelerated rate of progression to HIVE and/or HIV dementia (Bell et al., 1998; Nath et al., 1999a; Nath et al., 2002; Arango et al., 2004). Not only does injection drug use increase the probability of contracting HIV (Nath et al., 1999a), but perhaps more importantly, opiate drugs may intrinsically alter the pathogenesis of HIV through direct effects on neurons and glia (Gurwell et al., 2001; Khurdayan et al., 2004; El-Hage et al., 2005). Although considerable evidence indicates that opiate abuse increases the HIV progression in the CNS, the mechanism by which opiates exacerbate CNS pathology and neurological complications are not understood. Relatively straightforward questions, such as are opiates per se acting principally on compromised neurons or infected glia to accelerate HIVE, remain unclear (Nath et al., 2002). Thus, there are multiple, compelling reasons to investigate the interactions between opiate abuse and HIV infection that accelerate neuropathogenesis. Efforts are only beginning to be directed toward answering these extremely important questions in the CNS, and toward understanding the cellular mechanisms underlying neuropathogenesis due to HIV infection alone and when superimposed with opiate abuse. It is important to consider past findings when exploring opiate-HIV interactions in the CNS, which include:
Opiate-HIV interactions in the CNS are complex, and although some isolated effects may be beneficial, the net consequence of opiate drug abuse is deleterious.
Although multiple opioid receptor types are expressed, the MOR is the principal site of action for opiate drugs with abuse liability. Most evidence thus far indicates that the neurotoxic effects of morphine alone or in combination with HIV are mediated by MOR.
Neurons, astrocytes, and microglia can express MOR and MOR activation affects each cell type differently. Opiates and HIV have unique effects in each neural cell type.
The collective CNS response to opiate drug abuse and HIV differs from the response of individual cell types. Defining the intercellular (glia-glia, glia-neuron) interactions and their integration is key to understanding how opiate drugs exacerbate neuropathogenesis.
Neuronal dysfunction and death is caused by the accumulated direct and indirect effects of opiates and HIV.
MOR activation and neuronal death—convergence with Tat/gp120 proapoptotic cascades?
Most opioids with abuse liability are ligands for MOR, and MOR activation can affect cell viability (Grode and Murray, 1973; Meriney et al., 1985; Meriney et al., 1991; Goswami et al., 1998). Interestingly, depending on the cell type and opioid dosage, opioids can have paradoxical—neuroprotective or neurodegenerative effects (Meriney et al., 1985; Meriney et al., 1991; Faden, 1996; Goswami et al., 1998). Morphine or Met-enkephalin can inhibit neuron death in the avian ciliary ganglion (Meriney et al., 1985; Meriney et al., 1991). In a cell line transfected with MOR, the MOR agonist DAMGO ([D-Ala2,N-Me- Phe4,Gly5-ol]-enkephalin) activates Akt-induced neuroprotection (Polakiewicz et al., 1998). Alternatively, morphine can be toxic to Purkinje cells (Hauser et al., 1994) and neuronal cell lines (Hu et al., 2002). Also, fentanyl-related compounds, which are highly selective μ-agonists can be neurotoxic (Sinz et al., 2000; Kofke et al., 2002). In other studies, μ-opioids are not overtly toxic but enhance cell losses when apoptosis is induced by other factors (Dawson et al., 1997; Goswami et al., 1998). For example, the μ-agonist morphiceptin enhances staurosporine or wortmannin-induced apoptosis in chick embryonic cerebral cortex neurons (Goswami et al., 1998). Together, the above findings suggest that opioid effects on cell death are variable and cell type specific. Importantly, we have described a pattern of synergism between morphine and Tat in causing the death of striatal neurons. Although morphine by itself is not toxic to striatal neurons in culture, it significantly potentiates Tat toxicity in uninfected striatal neurons (Gurwell et al., 2001; Turchan et al., 2002). The observation that opioid exposure can exacerbate viral protein induced neurotoxicity has important implications for the neuropathology of AIDS dementia and gives impetus to the exploration of intracellular mechanisms of opioid-virotoxin induced neurotoxicity.
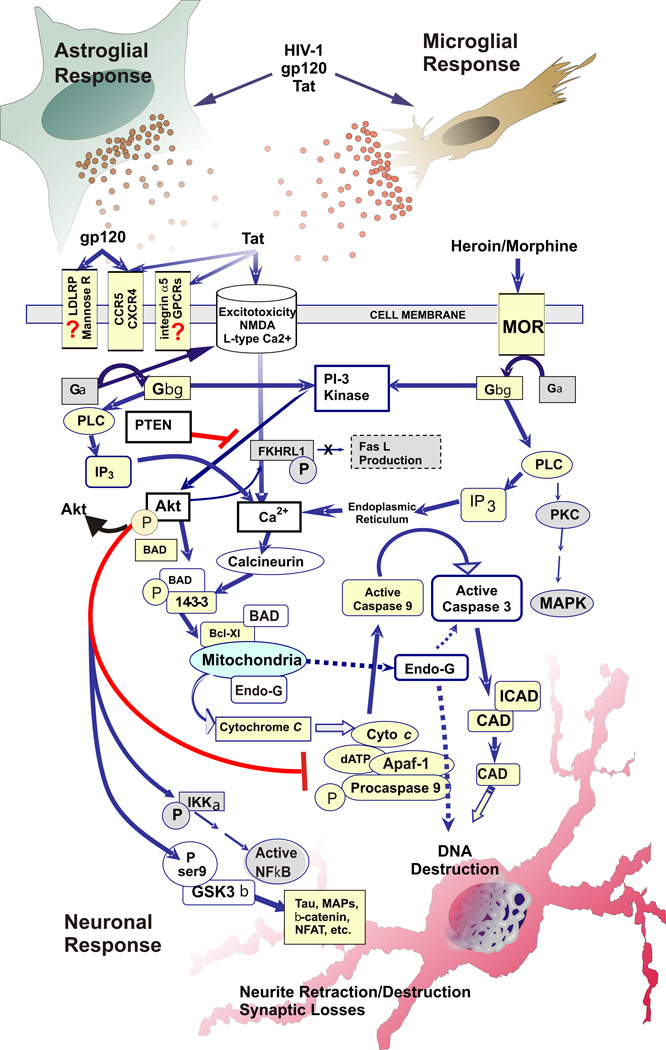
Opioids were first described as modulating cAMP, opening K+ channels, and closing Ca2+ channels. Opioids are also known to have excitatory actions in many cell types (Crain and Shen, 1990; Wang and Gintzler, 1994; Huang, 1995; Hauser and Mangoura, 1998). More recently, opioids have been tethered to intracellular signaling pathways that have direct bearing on cell survival. MORs are typical G-protein coupled receptors. They can also activate mitogen-activated protein kinases (MAPK), and some other phosphotyrosines (Georgoussi et al., 1997; Mangoura, 1997; Wilson et al., 1997; Mullaney et al., 1997; Hauser and Mangoura, 1998) through either Gβγ subunits and/or SH-2 adapter proteins (Wilson et al., 1997; Mullaney et al., 1997; Belcheva et al., 1998; Ignatova et al., 1999; Belcheva et al., 2002; Belcheva et al., 2003). Acute activation of opiate receptors activates Gαi/o and Gβγ subunits of GTP-protein coupled receptors, which inhibit adenylyl cyclase and cyclic AMP production, and activate phosphatidylinositol 3-kinase (PI3-kinase or PI3K) and PLC, respectively (Ai et al., 1999; Persson et al., 2003a). PI3K and Akt, in particular, are involved with MOR mediated modulation of apoptosis (Goswami et al., 1998; Polakiewicz et al., 1998; Goswami et al., 2000; Kim et al., 2001; Iglesias et al., 2003). Interestingly, and of importance clinically, chronic exposure of neurons to morphine, lasting from hours to days, gradually leads to molecular and cellular adaptations of most pathways that differ greatly from the acute effects (Nestler, 2001; Tan et al., 2003). Chronic morphine activates ERK, c-jun-N-terminal kinase (JNK), and p38 MAPK (p38-kinase) pathways, as well as causing super-activation of adenylyl cyclase (AC) (Ma et al., 2001). Increased AC activity is accompanied by increases in PKA and CREB in some neuronal types. Many of these same pathways in T-lymphocytes are also affected by morphine, but with somewhat different effects on cell viability compared to neurons (Singhal et al., 2001; Singhal et al., 2002; Suzuki et al., 2003; Wang et al., 2003a). And although studies in other cell types reveal important potential mechanisms of opioid action, there are aspects of opioid signaling and their functional consequences that are specific to neurons and cannot be generalized to other cell types (Hauser and Mangoura, 1998; Tan et al., 2003).
As noted, both Tat and gp120 have been shown to be directly neurotoxic. Although the literature on Tat signaling in neurons is not extensive, Tat exposure alone does appear to modulate a number of intracellular pathways that can affect cell survival. PC12 cells exposed to Tat alone display altered PI3-kinase signaling with downstream effects on Akt, cAMP and CREB (Milani et al., 1998; Zauli et al., 2001). Interestingly, the PI3-kinase effects for short term (< 24 h) and more chronic (> 24 h) exposure were different, with more chronic exposure driving cells increasingly towards apoptosis. GSK3β, a downstream target for PI3-kinase-Akt signaling, is also activated by Tat, leading to neurotoxic events in neurons (Maggirwar et al., 1999b; Tong et al., 2001). Tat is known to induce p53 expression in neurons (Silva et al., 2003), although this effect may depend on the specific Tat sequence (Ariumi et al., 2001). Crosstalk between PTEN (phosphatase and tensin homolog deleted on chromosome 10) and p53 may provide another signaling avenue for affecting cell survival (Stambolic et al., 2001; Freeman et al., 2003). Tat can also signal through MAPK and/or ERK1/2 events in neurons (Menegon et al., 1997; Peruzzi et al., 2002). Interestingly, gp120 can also interact in neurons with several of these signaling pathways and intermediaries including MAPK (Leoni et al., 1997), p42-ERK/JNK (Lannuzel et al., 1997), p53 (Yeung et al., 1998), and GSK3β (Everall et al., 2002). Tat and gp120 can also both modulate intracellular Ca2+ levels through effects on IP3-dependent stores. Taken together, the findings cited above show that HIV viral toxins and opioids both stimulate a variety of overlapping pathways that can affect neuronal survival.
Potential targets of opiate-HIV interactions to improve survival
Previous work has shown that neurons which die in individuals with AIDS dementia or HIVE show activation of post-mitochondrial events typical of apoptosis including activation of the effector caspase-3 (Kruman et al., 1998; James et al., 1999; Garden et al., 2002). Our own work has shown that opioids synergize with HIV neurotoxins in activating caspase-3 and/or promoting neuronal losses (Gurwell et al., 2001; Singh et al., 2004). Similar events are activated in other conditions involving neurodegeneration suggesting that some of these findings are broadly applicable to other neurodegenerative disorders. Numerous in vitro models have shown that caspase-3 inhibitors can stop or delay neuronal death, but interrupting caspase-mediated pathways may only delay death instead of preventing it. Interrupting apoptosis in its final stages, i.e., at the stage of caspase-3 activation, may not permit neurons to retain functional capacity. Intervention at early step(s) preceding apoptosis may maximize the opportunity to retain neurons and their function. Since neuronal loss in HIV involves multiple toxic agents, and since opiate drug exposure is a co-morbid factor, several apoptotic intermediates are likely involved. Targeting sites where multiple toxic signaling pathways converge would be advantageous.
One likely target for therapy is the serine/threonine kinase Akt, or protein kinase B (PKB). Akt1/PKBα is the predominant isoform in most mammalian tissues including CNS (Kandel and Hay, 1999). Akt activation is associated with numerous examples of receptor-mediated cell survival induced by growth factors and cytokines including PDGF, IGF-1, NGF, M-CSF and IL-3 among others (Franke et al., 1995; Yao and Cooper, 1995; Songyang et al., 1997; Dudek et al., 1997; Kelley et al., 1999; Vaillant et al., 1999). Akt is activated by PI 3-kinases, largely through the binding of the major PI 3-kinase lipid product PI(3,4,5)P3 to the plekstrin homology (PH) domain of Akt. Binding of PIP3, and to a lesser extent PI(3,4)P2, to the PH domain of Akt causes its translocation to the plasma membrane where Akt is phosphorylated on thr308 and ser473 by 3-phosphoinositide-dependent kinase 1 (PDK1) (Datta et al., 1997; Kandel and Hay, 1999; Cross et al., 2000). Once activated, it promotes survival by phosphorylating substrates involved in apoptosis (Yao and Cooper, 1995; Datta et al., 1997; Dudek et al., 1997; Cardone et al., 1998; Cross et al., 2000) (see Fig. 1). pAkt phosphorylates and inactivates BAD and caspase-9, thus limiting activation of the effector caspase-3. pAkt also inactivates members of the FOXO family of proapoptotic transcription factors (FKHR-L1, AFX, FKHR) whose activity drives apoptosis through multiple mechanisms, including activation of FasL and Bim, and repression of Bcl-6 (Birkenkamp and Coffer, 2003). pAkt also activates IKK-α, the kinase that regulates IκB, leading to activation of transcription factor NFκB which generally inhibits apoptosis via transcriptional regulation of a number of survival factors. A more recently described target for Akt is glycogen synthase kinase 3β (GSK3β). Akt inactivates GSK3β by phosphorylation at ser9. GSK3β has recently been implicated in a number of neurodegenerative pathologies including Alzheimer’s disease (Kaytor and Orr, 2002) and can promote neuronal apoptosis through interference with the activation of neuroprotective transcriptional factors such as nuclear factor of activated T cells (NFAT) and heat shock transcription factor-1 (HSF-1), and modulation of cellular levels and localization of tau, MAPs, and β-catenin. Interestingly, both Tat and gp120 appear able to activate GSK3β and inhibitors of GSK3β such as lithium and valproate are able to ameliorate virotoxin-induced neuronal death (Maggirwar et al., 1999a; Everall et al., 2002; Dou et al., 2003). There is also increasing evidence for crosstalk between signaling pathways typically activated by MOR and other G-protein coupled receptors (ras/MAPK) and the PI3K/Akt/GSK3ß pathway (Chu et al., 1996; Duckworth and Cantley, 1997; Lopez-Ilasaca et al., 1997; Polakiewicz et al., 1998; Scheid et al., 1999; Tan et al., 2003; Jones et al., 2003). Such interactions could mediate synergistic opiate-Tat-induced neurotoxicity.
Fig. 1.
Pro-apoptotic signaling events that can be activated by opiate drugs and HIV, and potential sites of convergence underlying synergistic neurotoxicity. Potential sites where opioids impact Tat/gp120-mediated neurotoxicity include PI3-kinase, PTEN, Akt, Ca2+, calcineurin, BAD, GSK3β, caspase-3 and endonuclease-G (endo-G) (arrows indicate activation; lines ending in “T” indicate inhibition; broken arrows indicate multi-step and/or incompletely understood pathways; pathways & abbreviations are noted in the text).
Further proof that Akt regulates cell survival can be seen in the effects of the tumor suppressor gene PTEN. PTEN is a lipid phosphatase that antagonizes PI 3-kinase by promoting dephosphorylation of PI(3,4,5)P3 to PI(4,5)P2, thus downregulating levels of pAkt. PTEN(−/−) and (+/−) cells exhibit high basal pAkt activity and are resistant to apoptotic induction to the point where transgenic animals are highly susceptible to lymphoma (Stambolic et al., 1998; Suzuki et al., 1998; Sun et al., 1999). Akt is an attractive target for therapeutic manipulation since it is an intermediary in multiple apoptotic pathways and promotes survival by effects on cytochrome-c signaling (through BAD and caspase-9), Fas ligand-death domain induction (through FKHRL1), NFκB transcription and GSK3β phosphorylation. Experimental strategies using wild-type or constitutively active forms of Akt or v-Akt have rescued neurons and other cell types after survival factor withdrawal (Kennedy et al., 1997; Philpott et al., 1997; Songyang et al., 1997; Dudek et al., 1997; Crowder and Freeman, 1998) or other apoptotic stimuli (Khwaja et al., 1997; Rohn et al., 1998; Goswami et al., 1999). Since Akt can act at a premitochondrial portion of the pathway, it may also limit the activation of caspase-independent apoptotic pathways involving components that are released from mitochondria, such as endonuclease-G. Excitotoxic increases in Ca2+ and calcineurin activation are directly opposed by Akt, which inactivates BAD. In theory, any treatment that increases Akt activity could interrupt calcineurin-mediated activation of BAD.
Opiate-HIV interactions in astroglia
Astroglia are a significant target for HIV and their function is markedly impacted by the disease (Brack-Werner, 1999; Nath, 1999; Nath et al., 1999b; Wang et al., 2004). Astroglia can express MOR, DOR, and/or KOR (Stiene-Martin and Hauser, 1990; Eriksson et al., 1990; Eriksson et al., 1991; Stiene-Martin and Hauser, 1991; Ruzicka et al., 1995; Gurwell et al., 1996; Hauser et al., 1996; Stiene-Martin et al., 1998; Thorlin et al., 1998; Stiene-Martin et al., 2001), and there are regional differences in opioid receptor expression among astrocytes in different brain regions (Hauser and Stiene-Martin, 1991; Eriksson et al., 1992; Ruzicka et al., 1994; Ruzicka et al., 1995; Gurwell et al., 1996; Stiene-Martin et al., 1998; Thorlin et al., 1999). Activation of MOR, KOR or DOR causes cellular hypertrophy (Stiene-Martin and Hauser, 1991; Gurwell et al., 1996; Hauser et al., 1996). The reactive cellular hypertrophy is mediated by increases in intracellular Ca2+ ([Ca2+]i) (Gurwell et al., 1996; Hauser et al., 1996), and is accompanied by increased GFAP immunoreactivity and may mimic reactive astrogliosis in vivo (Hauser et al., 1996; Hauser et al., 1998).
There are mixed reports regarding the extent to which astrocytosis occurs in the CNS of opiate abusers (Gosztonyi et al., 1993; Oehmichen et al., 1996; Buttner et al., 2000; Anderson et al., 2003). An assessment of astrocytosis in the frontal lobes found increases in glial fibrillary acidic protein (GFAP) immunoreactivity in of HIVE subjects, but not in drug abusers or pre-AIDS individuals (Anderson et al., 2003). This is perhaps not surprising since astroglia are highly plastic and their response to insults may be transient and reversible, and may differ among brain regions. Also, microglia increases, which occur with opiate abuse, are not apparent in the frontal lobes, but are seen in the thalamus and to a lesser extent in the hippocampus (see below) (Tomlinson et al., 1999; Arango et al., 2004). Especially, with respect to opioids, the response to morphine and the expression of MOR, DOR and KOR by astroglia differs among brain regions, is developmentally regulated, and cell cycle dependent (Hauser and Stiene-Martin, 1991; Eriksson et al., 1992; Ruzicka et al., 1995; Stiene-Martin et al., 1998; Thorlin et al., 1999). Therefore, the response of astrocytes to opiates and HIV is also likely to differ among brain regions, with age, and may change dramatically with disease progression.
Opiates and HIV-1 Tat synergistically disrupt astroglial function
Findings that both MOR activation (Hauser et al., 1996), and Tat (Haughey et al., 1999), synergistically increase Ca2+ release from IP3-dependent intracellular stores, and that the interactions can be blocked by the PI3K inhibitor LY294002, suggest that interactions occur at the level of [Ca2+]i in astroglia (El-Hage et al., 2005). Synergistic increases in [Ca2+]i were markedly suppressed by inhibiting IP3-dependent increases in [Ca2+]i and by inhibiting Ca2+–induced Ca2+ release (CICR) from internal stores (El-Hage et al., 2005). Last, the MOR antagonist, β-FNA, and the PI3K inhibitor, LY297856, also completely blocked synergistic increases in [Ca2+]i. PI3K can influence [Ca2+]i by modulating PLCγ activity or by regulating Ca2+ influx (Barker et al., 1999). Synergistic increases in reactive oxygen species are also evident following combined opiate-HIV exposure (El-Hage and Sullivan, unpublished). Our findings suggest that PI3K is acting downstream of the MOR and mediating Tat and/or Tat-morphine induced increases in [Ca2+]i. Striatal neurons and astrocytes display similar patterns of [Ca2+]i responsiveness to opiates and HIV Tat (Hauser, unpublished).
Astroglial release of cytokines
Astrocytes are strategically positioned to monitor physiological changes within the extracellular space (ECS) (e.g., transmitter levels, ion fluxes) and maintain steady state within that compartment (Vargova et al., 2001; Sykova, 2005). By disrupting normal astrocyte function, opiate drug abuse likely subverts the ability of astrocytes to maintain steady state levels of neurochemicals within the ECS. Moreover, morphine can modify cytokine production by astroglia (Mahajan et al., 2002). This is worsened by HIV infection in which the ECS is further compromised by viral (e.g., HIV virions, gp120, and Tat) and toxic cellular products (e.g., glutamate, ROS or cytokines such as TNF-α, IFN-γ, and IL-6) creating pathophysiological conditions that are detrimental for neurons (Genis et al., 1992; Bell, 1998; Nath et al., 1999b; Kaul et al., 2001; Garden, 2002; Persidsky and Gendelman, 2003).
There is considerable evidence that Tat can modulate chemokine release by astrocytes (Conant et al., 1998; Nath et al., 1999b), and more recent evidence that opioids modulate the response of astroglia to a Tat insult (Sheng et al., 2003). Morphine exposure exacerbates Tat-induced chemokine (MCP-1, RANTES, MCP-5) and cytokine (IL-6 and perhaps IL-12) production by astrocytes (El-Hage et al., 2005). Interestingly, morphine does not appear to induce novel cytokines to be released; rather, morphine furthered an existing response of astrocytes to Tat (El-Hage et al., 2005). TNF-α reportedly has a priming effect on RANTES production, which in turn triggers MCP-1, RANTES and IL-6 production (Ng et al., 1994; Oh et al., 1999; Luo et al., 2003). Interestingly, morphine-induced increases in Tat-evoked MCP-1, RANTES, and IL-6 production are not preceded by synergistic increases in TNF-α, suggesting that morphine’s effects are not mediated by increases in TNF-α levels beyond those seen with Tat alone. Thus, morphine-induced increases in MCP-1, RANTES, and IL-6 seemingly occur through actions that are downstream of TNF-α. Morphine’s effects on chemokine production appear to be mediated through MOR since β-FNA, but not nor-BNI, significantly attenuated the effects of morphine plus Tat on levels of MCP-1 and RANTES transcripts at 4 h and 12 h (El-Hage et al., 2005). Interestingly, and commensurate with the concept that MOR and KOR can sometimes have opposing effects, KOR activation inhibits Tat-induced MCP-1 expression in human astrocytes (Sheng et al., 2003). These studies revealed an important temporal requirement that KOR be activated for 6 h or 24 h before suppression of Tat-induced MCP-1 increases were noted. This suggests that KOR activation represses MCP-1 production through alterations in gene expression, which is not evident if Tat and KOR agonists are given concurrently (Sheng et al., 2003; El-Hage et al., 2005). Conversely, MOR activation increases Tat-induced MCP-1 mRNA levels and protein release within 4 h, suggesting new genes are likely to be not involved in the acute response (El-Hage et al., 2005).
Opiate HIV interactions in microglia
The role of the endogenous opioid system in pain and in mediating inflammatory events in immunocompetent cells especially in the peripheral nervous system is well established (see (Machelska and Stein, 2000; Walker, 2003) for review). Opioid receptors are widely expressed on immune cells and opiates can function similarly to cytokines, modulating immune activities (Donahoe and Falek, 1988; Rouveix, 1992; Sheng et al., 1997; Peterson et al., 1998). Indeed, immune function is markedly altered with chronic opioid abuse (Donahoe and Falek, 1988; Novick et al., 1991). More specifically, considerable evidence now suggests that opioids affect immune responses directly within the brain (Sheng et al., 1997; Nelson et al., 2000; Dimitrijevic et al., 2000). Treatment of microglia with specific opioid ligands has been shown to alter their phagocytic (Peterson et al., 1995), chemotactic (Chao et al., 1997), and free radical producing properties (Hu et al., 1998; Liu et al., 2002), as well as other functions (Eisenstein and Hilburger, 1998), including cytokine production (Wetzel et al., 2000; Peng et al., 2000). Opiates can also induce apoptosis in macrophages (Singhal et al., 1998; Singhal et al., 2002). Opioid-induced changes in the function of microglial cells may have critical implications for HIVD, in which microglial activation is thought to play a prominent role (Tyor et al., 1995; Glass et al., 1995; Gray et al., 2001). Morphine interacts with gp160 to modulate apoptosis in monocytes (Kapasi et al., 2004). Bell and coworkers find that the number of microglia tend to be increased in a cohort of HIVE-positive drug abusers who preferentially use opiates (Tomlinson et al., 1999; Arango et al., 2004). The number of microglia in HIV seropositive drug abusers compared to non-abusers tended to greater depending on the brain region studied. In particular, trends toward increased numbers of microglia in the gray matter of the thalamus and to a lesser extent temporal hippocampus were evident in drug abusers, while drug abuse did not appear to increase microglia in the frontal lobe (Arango et al., 2004). Despite wide-spread evidence for opiate-HIV interactions in macrophages/microglia, the exact intracellular mechanisms whereby opioids alter microglial activation are unclear. However, it is known that macrophage/microglia activation with HIV infection is accompanied by free radical production (including superoxide, hydrogen peroxide, hydroxyl radical, and peroxynitrite), as well as the release of other toxic substances, and may be a major factor in the neuropathology (Gendelman et al., 1997; Nath, 1999; Xiong et al., 2000; Kaul et al., 2001; Persidsky and Gendelman, 2003). Assuming opiates exacerbate these effects; this would be an important mechanism by which opiates hasten the pathogenesis of the disease. We propose that microglial free radical production, and opioid-induced increases in free radical production, are very important to immune signaling and the promulgation of brain inflammation though redox-signaling in microglia. Interestingly, a role for free radicals in the signal transduction machinery responsible for microglial activation has recently been proposed, and the term “redoxsignaling” has been coined to describe intracellular signaling that is dependent on free radical production [for review see (Forman and Torres, 2001; Droge, 2002)].
Opiate HIV interactions in glial precursors
Neural progenitors express several key chemokine receptors (Tran and Miller, 2003; Tran et al., 2004), including CXCR4 and CCR5, that are cofactors involved in HIV infection (Lazarini et al., 2000; Peng et al., 2004; Kao and Price, 2004). Multipotential neural progenitors can become infected with HIV-1 and serve as a viral reservoir (Lawrence et al., 2004). Interestingly, viral production increases if the infected neural progenitors are allowed to differentiate toward an astroglial phenotype (Lawrence et al., 2004). HIV exposure inhibits the proliferation of neural progenitors and this reportedly occurs through CCR3 and CXCR4 chemokine receptors (Krathwohl and Kaiser, 2004). In addition, opioid receptors are widely expressed by neural progenitors within the ventricular zone (Reznikov et al., 1999), the subventricular zone (Reznikov et al., 1999; Stiene-Martin et al., 2001), the cerebellar external granular layer (Hauser et al., 2003), and the subgranular layer of the dentate gyrus (Persson et al., 2003a; Persson et al., 2003b).
We have found that exposure to morphine and Tat viral protein causes the preferential death of glial precursors (Khurdayan et al., 2004). The toxic effects of morphine and Tat on glial precursors are synergistic, mediated by MOR, and accompanied by the activation of caspase-3 and an inability to exclude ethidium monoazide (Khurdayan et al., 2004). Further characterization of the affected cells indicates that a majority were dual-fated oligodendroglia-type 2 astroglia (O2A) progenitors (Raff et al., 1983; Fulton et al., 1992). Because a vast majority of the O2A progenitors in our cultures would have developed into oligodendroglia with further maturation, we speculate that cells committed to an oligodendroglial fate may be preferentially vulnerable to combined opiate-HIV-1 toxicity (Khurdayan et al., 2004). Although this was not confirmed at the limited survival times studied, oligodendrocyte numbers tended to be reduced and this might become significant with greater durations of exposure or increased time. Macroglia may possess life spans lasting years, suggesting that deficits in gliogenesis would only become evident with protracted time. Assuming glial progenitors are similarly lost in the CNS, macroglial numbers may be impacted with sustained exposure to opiates in the HIV infected CNS. Whether chronic opiate abuse contributes to the pathogenesis of neuroAIDS by reducing macroglial turnover and CNS dysfunction is uncertain; but warrants further study.
Opiate-HIV-induced changes in glia likely contribute to neurotoxicity
As noted throughout, an evolving concept is that opiate drug abuse exacerbates HIV-induced neurotoxicity through indirect actions mediated by glia in addition to the direct effects on the neurons themselves. The role of microglia in the pathogenesis of HIVE and HIVD are well documented.
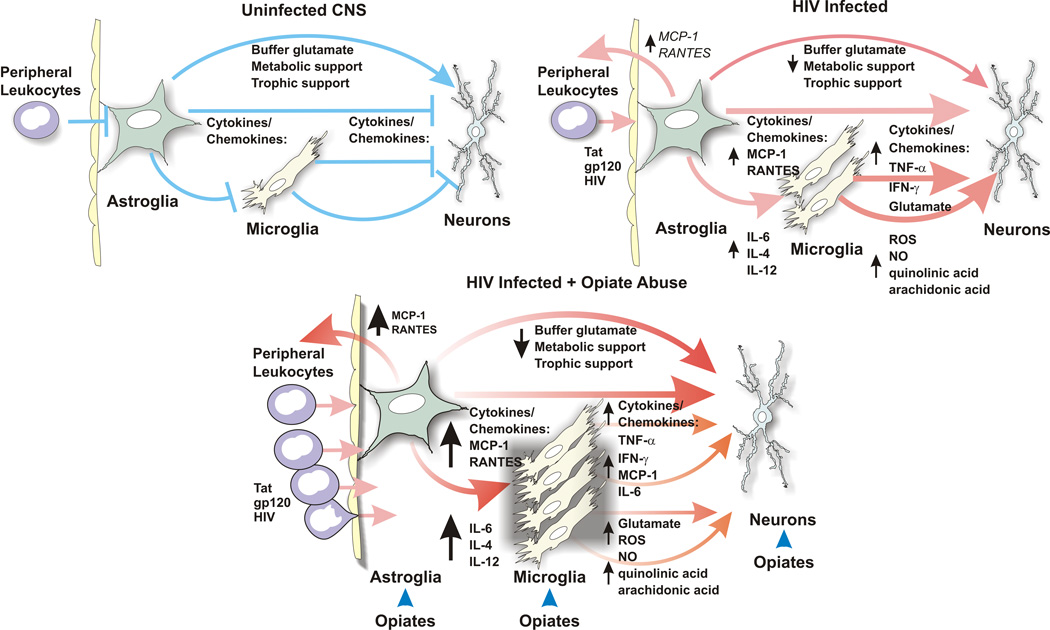
Microglia significantly modify the response of neighboring cells (neurons and glia) to HIV (Nottet et al., 1995; Tyor et al., 1995; Glass et al., 1995; Gray et al., 2001; Rogers and Peterson, 2003), Opiate-induced exacerbation of the macrophage/microglial response to HIV is likely to further contribute to neuronal dysfunction and their eventual demise. In preliminary studies, we have found that intrastriatally-injected Tat (25 μg) combined with continuous systemic morphine exposure (time-release morphine implant; 25 mg released over 5 days; NIDA) interacted significantly to increase the number of reactive microglia and astroglia at 7 days post-exposure (El-Hage, Wu and Hauser, in preparation). Although frank neuronal losses are not seen at 7 days in these studies, there are significant increases in caspase-3 activation in striatal neurons suggesting that there is impending death. Collectively, these preliminary findings in vivo suggest that opiates exaggerate HIV protein-induced reactive glial changes, which precede and likely contribute to neuronal losses.
Although astroglia are normally neuroprotective, this appears to be short-circuited by opiate and HIV exposure. We propose that several critical mechanisms are involved in the altered relationship between astrocytes and neurons. First, astrocytes lose the ability to provide neurons with metabolic and trophic support. Second, astrocytes directly drive neuron toxicity through release of inflammatory signals (ROS and chemokines/cytokines). There is a rapid loss in intracellular metabolic and ion homoeostasis within astrocytes. Tat and morphine (the major product of heroin in the CNS) appear to interact and synergize, affecting critical astrocyte parameters and functions [Ca2+]i and increased ROS production. Besides disrupting astroglial function intracellularly, ROS derived from astroglia can have toxic bystander effects on neighboring neurons. A likely consequence of loss of ion homeostasis is an increasing inability to buffer extracellular potassium and glutamate, both of which would lower the threshold for excitotoxicity in neurons. Other intracellular responses which may be part of an internal astroglial defense mechanism, but which also have the potential to disrupt neuronal function, include alterations in transcription and expression of key regulatory molecules. This may include changes in expression of the glutamate transporters GLAST (EAAT1) and GLT-1 (EAAT2), which are affected by morphine (Ozawa et al., 2001; Mao et al., 2002) or HIV (Wang et al., 2003b), respectively. In response to combined morphine and Tat, astrocytes also release proinflammatory cytokines and chemokines, many of which can be directly neurotoxic (e.g., TNF-α, IL-6), while others recruit monocytes/macrophages (e.g., MCP-1, MCP-5, and RANTES) and activate microglia, which in turn likely contribute to neurotoxicity. Thus, astroglia potentially mediate a substantial portion of the enhanced neuropathology seen in HIV infected heroin abusers through two different yet related mechanisms.
Opioid antagonists and agonists have been proposed as a treatment for HIV dementia
Based on its ability to inhibit HIV-1 expression in CD4+ lymphocytes, Peterson and coworkers have proposed that naltrexone be used as an adjunctive therapy to HAART in the treatment of HIV (Peterson et al., 2001). Our findings showing that MOR agonists increase the release of inflammatory cytokines by astroglia (El-Hage et al., 2005), while augmenting the toxicity of HIV proteins in neurons (Gurwell et al., 2001), glial precursors (Khurdayan et al., 2004), and to a lesser extent astrocytes (Khurdayan et al., 2004), support the notion that opioid antagonists would be neuroprotective in neuroAIDS. MOR antagonists in particular should be beneficial; since we find that opiate synergism with HIV toxicity is almost exclusively mediated through MOR. In addition, based on findings that KOR agonists can suppress viral replication and MCP-1 release by astrocytes, KOR agonists have also been proposed as potentially beneficial for neuroAIDS (Sheng et al., 2003). Based on the above experimental findings, opiate drugs with mixed agonist and antagonist properties would be predicted to modify HIV neuropathogenesis through complex actions at multiple opioid receptor types. For example, treatment with buprenorphine, which is a partial MOR agonist and KOR antagonist, and is currently used to treat heroin addiction, might actually negatively impact disease progression. Alternatively, mixed KOR agonists and MOR agonists/antagonists have been proposed as beneficial in the treatment of addiction and pain (Bidlack et al., 2000; Neumeyer et al., 2001), and may also be beneficial in treating neuroAIDS. Lastly, although the precise role of endogenous MOR peptides in neuroAIDS is not been firmly established, the possible use of therapies involving opioid receptor antagonists to limit endogenous opioid peptide-opioid receptor interactions in non-opiate abusing patients with neuroAIDS might eventually need to be considered.
The therapeutic value of these novel strategies for treating neuroAIDS needs to be further explored. There seems to be little doubt that chronic opiate abuse significantly modulates the progression of neuroAIDS and it is likely that opiates worsen disease outcome and contribute to the development of HIV dementia. Work during the past few years has led to significant advances in our understanding of the cellular mechanisms that underlie this synergy between HIV/HIV viral proteins and opiates. Most importantly, we are now in a position to propose novel therapeutic strategies for treating neuroAIDS which are being explored in multiple experimental settings.
Fig. 2.
Summary of key intercellular events by which opiates may exacerbate the pathogenesis of HIV in the CNS. Opiates exaggerate the neuropathogenesis of HIV through direct actions on opioid receptor-expressing neurons, astrocytes, and microglia (blue arrowheads in “HIV-Infected + Opiate Abuse”). Note that opiates amplify the toxic actions of HIV, but do not result in additional insults. Opiates affect each cell type differently, which collectively results in disruptions in neuronal dysfunction and death. Leukocytes, within CNS blood vessels (Peripheral Leukocytes), including monocytes/macrophages and T-lymphocytes, can express opioid receptors and respond directly to opiates (arrows indicate intercellular signaling events; lines ending in a “T” indicate quiescent signaling; pathways & abbreviations are noted in the text).
Acknowledgments
This work was supported by NIH grants DA13559, DA13728, T32 DA16176-01, and P20RR015592.
ABBREVIATIONS
- AC
adenylyl cyclase
- CXCR
alpha chemokine receptor
- Apaf-1
apoptosis protease activating factor-1
- CCL
beta chemokine ligand
- CCR
beta chemokine receptor
- DOR
δ-opioid receptor
- β-FNA
β-funaltrexamine
- CAD
caspase activated DNAse
- JNK
c-jun-N-terminal kinase
- CREB
cAMP response element-binding protein
- EAAT2
excitatory amino acid transporter-2
- ERK
extracellular-regulated kinase
- GPCR
G-protein coupled receptor
- GSK3β
glycogen synthase kinase 3β
- FKHRL or FOXO
forkhead transcription factor
- HCV
hepatitis C virus
- HAD
HIV associated dementia
- HIV
human immunodeficiency virus
- HIVE
human immunodeficiency virus encephalitis
- IKK
IκB kinase
- IP3
inositol trisphosphate
- IFN
interferon
- IL
interleukin
- [Ca2+]i
intracellular Ca2+
- KOR
κ-opioid receptor
- LDLRP
low-density lipoprotein receptor-related protein
- mannose receptor
mannose R
- MAPs
microtubule associated proteins
- MAPK
mitogen-activated protein kinase
- MCP-1 or CCL2
monocyte chemoattractant protein-1
- NFκB
nuclear factor-κB
- MOR
μ-opioid receptor
- NO
nitric oxide
- nor-BNI
nor-binaltorphimine
- NFκB
nuclear factor κB
- O2A
oligodendroctye-type-2 astrocyte progenitor cell
- PI3-kinase or PI3K
phosphatidylinositol 3-kinase
- PDK1
phosphoinositide-dependent kinase 1
- PLCγ
phospholipase C-γ
- PTEN
phosphatase and tensin homolog deleted on chromosome 10
- PH
pleckstrin homology
- PKA
protein kinase A
- PKB, also known as Akt
protein kinase B
- PKC
protein kinase C
- RANTES
regulated on activation, normal T cell expressed and secreted
- ROS
reactive oxygen species
- Tat
transactivator of transcription
- TNF-α
tumor necrosis factor-α
References
- Adler MW, Geller EB, Rogers TJ, Henderson EE, Eisenstein TK. Opioids, receptors, and immunity. Adv Exp Med Biol. 1993;335:13–20. doi: 10.1007/978-1-4615-2980-4_3. 13–20. [DOI] [PubMed] [Google Scholar]
- Ai W, Gong J, Yu L. MAP kinase activation by mu opioid receptor involves phosphatidylinositol 3-kinase but not the cAMP/PKA pathway. FEBS Lett. 1999;456:196–200. doi: 10.1016/s0014-5793(99)00949-7. [DOI] [PubMed] [Google Scholar]
- Anderson CE, Tomlinson GS, Pauly B, Brannan FW, Chiswick A, Brack-Werner R, Simmonds P, Bell JE. Relationship of Nef-positive and GFAP-reactive astrocytes to drug use in early and late HIV infection. Neuropathol Appl Neurobiol. 2003;29:378–388. doi: 10.1046/j.1365-2990.2003.00475.x. [DOI] [PubMed] [Google Scholar]
- Ansari AA. Drugs of abuse and HIV--a perspective. J Neuroimmunol. 2004;147:9–12. doi: 10.1016/j.jneuroim.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Anthony IC, Crawford DH, Bell JE. Effects of human immunodeficiency virus encephalitis and drug abuse on the B lymphocyte population of the brain. J Neurovirol. 2004;10:181–188. doi: 10.1080/13550280490444100. [DOI] [PubMed] [Google Scholar]
- Arango JC, Simmonds P, Brettle RP, Bell JE. Does drug abuse influence the microglial response in AIDS and HIV encephalitis? AIDS. 2004;18(Suppl 1):S69–S74. S69–S74. [PubMed] [Google Scholar]
- Ariumi Y, Kaida A, Hatanaka M, Shimotohno K. Functional cross-talk of HIV-1 Tat with p53 through its C-terminal domain. Biochem Biophys Res Commun. 2001;287:556–561. doi: 10.1006/bbrc.2001.5626. [DOI] [PubMed] [Google Scholar]
- Avison M, Berger JR, McArthur JC, Nath A. HIV meningitis and dementia. In: Nath A, Berger JR, editors. Clinical Neurovirology. New York: Marcel Dekker, Inc.; 2003. pp. 251–276. [Google Scholar]
- Barker SA, Lujan D, Wilson BS. Multiple roles for PI 3-kinase in the regulation of PLCgamma activity and Ca2+ mobilization in antigen-stimulated mast cells. J Leukoc Biol. 1999;65:321–329. doi: 10.1002/jlb.65.3.321. [DOI] [PubMed] [Google Scholar]
- Belcheva MM, Haas PD, Tan Y, Heaton VM, Coscia CJ. The fibroblast growth factor receptor is at the site of convergence between mu-opioid receptor and growth factor signaling pathways in rat C6 glioma cells. J Pharmacol Exp Ther. 2002;303:909–918. doi: 10.1124/jpet.102.038554. [DOI] [PubMed] [Google Scholar]
- Belcheva MM, Tan Y, Heaton VM, Clark AL, Coscia CJ. {micro} Opioid Transactivation and Down-Regulation of the Epidermal Growth Factor Receptor in Astrocytes: Implications for Mitogen-Activated Protein Kinase Signaling. Mol Pharmacol. 2003;64:1391–1401. doi: 10.1124/mol.64.6.1391. [DOI] [PubMed] [Google Scholar]
- Belcheva MM, Vogel Z, Ignatova E, Avidor-Reiss T, Zippel R, Levy R, Young EC, Barg J, Coscia CJ. Opioid modulation of extracellular signal-regulated protein kinase activity is ras-dependent and involves Gβγ subunits. J Neurochem. 1998;70:635–645. doi: 10.1046/j.1471-4159.1998.70020635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell JE. The neuropathology of adult HIV infection. Rev Neurol (Paris) 1998;154:816–829. [PubMed] [Google Scholar]
- Bell JE, Arango JC, Robertson R, Brettle RP, Leen C, Simmonds P. HIV and Drug Misuse in the Edinburgh Cohort. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S35–S42. doi: 10.1097/00126334-200210012-00003. S35–S42. [DOI] [PubMed] [Google Scholar]
- Bell JE, Brettle RP, Chiswick A, Simmonds P. HIV encephalitis, proviral load and dementia in drug users and homosexuals with AIDS. Effect of neocortical involvement. Brain. 1998;121:2043–2052. doi: 10.1093/brain/121.11.2043. [DOI] [PubMed] [Google Scholar]
- Berger JR, Nath A. HIV dementia and the basal ganglia. Intervirology. 1997;40:122–131. doi: 10.1159/000150539. [DOI] [PubMed] [Google Scholar]
- Beyrer C, Razak MH, Lisam K, Chen J, Lui W, Yu XF. Overland heroin trafficking routes and HIV-1 spread in south and south-east Asia. AIDS. 2000;14:75–83. doi: 10.1097/00002030-200001070-00009. [DOI] [PubMed] [Google Scholar]
- Bidlack JM. Detection and function of opioid receptors on cells from the immune system. Clin Diagn Lab Immunol. 2000;7:719–723. doi: 10.1128/cdli.7.5.719-723.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidlack JM, McLaughlin JP, Wentland MP. Partial opioids. Medications for the treatment of pain and drug abuse. Ann N Y Acad Sci. 2000;909:1–11. doi: 10.1111/j.1749-6632.2000.tb06672.x. 1–11. [DOI] [PubMed] [Google Scholar]
- Birkenkamp KU, Coffer PJ. Regulation of cell survival and proliferation by the FOXO (Forkhead box, class O) subfamily of Forkhead transcription factors. Biochem Soc Trans. 2003;31:292–297. doi: 10.1042/bst0310292. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Belcheva MM, Coscia CJ. Mu-opioid agonist inhibition of kappa-opioid receptor-stimulated extracellular signal-regulated kinase phosphorylation is dynamin-dependent in C6 glioma cells. J Neurochem. 2000;74:574–581. doi: 10.1046/j.1471-4159.2000.740574.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis [editorial] [In Process Citation] AIDS. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- Buttner A, Mall G, Penning R, Weis S. The neuropathology of heroin abuse. Forensic Sci Int. 2000;113:435–442. doi: 10.1016/s0379-0738(00)00204-8. [DOI] [PubMed] [Google Scholar]
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, Stanbridge E, Frisch S, Reed JC. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282:1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- Carr DJ, Rogers TJ, Weber RJ. The relevance of opioids and opioid receptors on immunocompetence and immune homeostasis. Proc Soc Exp Biol Med. 1996;213:248–257. doi: 10.3181/00379727-213-44056. [DOI] [PubMed] [Google Scholar]
- Carr DJ, Serou M. Exogenous and endogenous opioids as biological response modifiers. Immunopharmacology. 1995;31:59–71. doi: 10.1016/0162-3109(95)00033-6. [DOI] [PubMed] [Google Scholar]
- Chang SL, Wu GD, Patel NA, Vidal EL, Fiala M. The effects of interaction between morphine and interleukin-1 on the immune response. Adv Exp Med Biol. 1998;437:67–72. doi: 10.1007/978-1-4615-5347-2_8. [DOI] [PubMed] [Google Scholar]
- Chao CC, Gekker G, Hu S, Sheng WS, Shark KB, Bu DF, Archer S, Bidlack JM, Peterson PK. kappa opioid receptors in human microglia downregulate human immunodeficiency virus 1 expression. Proc Natl Acad Sci U S A. 1996;93:8051–8056. doi: 10.1073/pnas.93.15.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, Gekker G, Sheng WS, Hu S, Peterson PK. U50488 inhibits HIV-1 expression in acutely infected monocyte-derived macrophages. Drug Alcohol Depend. 2001;62:149–154. doi: 10.1016/s0376-8716(00)00185-x. [DOI] [PubMed] [Google Scholar]
- Chao CC, Hu S, Molitor TW, Zhou Y, Murtaugh MP, Tsang M, Peterson PK. Morphine potentiates transforming growth factor-β release from human peripheral blood mononuclear cell cultures. J Pharmacol Exp Ther. 1992;262:19–24. [PubMed] [Google Scholar]
- Chao CC, Hu S, Shark KB, Sheng WS, Gekker G, Peterson PK. Activation of mu opioid receptors inhibits microglial cell chemotaxis. J Pharmacol Exp Ther. 1997;281:998–1004. [PubMed] [Google Scholar]
- Chen C, Li J, Bot G, Szabo I, Rogers TJ, Liu-Chen LY. Heterodimerization and cross-desensitization between the mu-opioid receptor and the chemokine CCR5 receptor. Eur J Pharmacol. 2004;483:175–186. doi: 10.1016/j.ejphar.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Chu B, Soncin F, Price BD, Stevenson MA, Calderwood SK. Sequential phosphorylation by mitogen-activated protein kinase and glycogen synthase kinase 3 represses transcriptional activation by heat shock factor-1. J Biol Chem. 1996;271:30847–30857. doi: 10.1074/jbc.271.48.30847. [DOI] [PubMed] [Google Scholar]
- Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci U S A. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain SM, Shen KF. Opioids can evoke direct receptor-mediated excitatory effects on sensory neurons. Trends Pharmacol Sci. 1990;11:77–81. doi: 10.1016/0165-6147(90)90322-y. [DOI] [PubMed] [Google Scholar]
- Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M, Lord JM. Serine/threonine protein kinases and apoptosis. Exp Cell Res. 2000;256:34–41. doi: 10.1006/excr.2000.4836. [DOI] [PubMed] [Google Scholar]
- Crowder RJ, Freeman RS. Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J Neurosci. 1998;18:2933–2943. doi: 10.1523/JNEUROSCI.18-08-02933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91:231–241. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- Davies J, Everall IP, Weich S, McLaughlin J, Scaravilli F, Lantos PL. HIV-associated brain pathology in the United Kingdom: an epidemiological study. AIDS. 1997;11:1145–50. doi: 10.1097/00002030-199709000-00010. [DOI] [PubMed] [Google Scholar]
- Dawson G, Dawson SA, Goswami R. Chronic exposure to kappa-opioids enhances the susceptibility of immortalized neurons (F-11kappa 7) to apoptosis-inducing drugs by a mechanism that may involve ceramide. J Neurochem. 1997;68:2363–2370. doi: 10.1046/j.1471-4159.1997.68062363.x. [DOI] [PubMed] [Google Scholar]
- De Castro S, Sabate E. Adherence to heroin dependence therapies and human immunodeficiency virus/acquired immunodeficiency syndrome infection rates among drug abusers. Clin Infect Dis. 2003;37(Suppl 5):S464–S467. doi: 10.1086/377561. S464–S467. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic M, Stanojevic S, Kovacevic-Jovanovic V, Miletic T, Vujic-Redzic V, Radulovic J. Modulation of humoral immune responses in the rat by centrally applied Met-Enk and opioid receptor antagonists: functional interactions of brain OP1, OP2 and OP3 receptors. Immunopharmacology. 2000;49:255–262. doi: 10.1016/s0162-3109(00)00213-7. [DOI] [PubMed] [Google Scholar]
- Donahoe RM. Multiple ways that drug abuse might influence AIDS progression: clues from a monkey model. J Neuroimmunol. 2004;147:28–32. doi: 10.1016/j.jneuroim.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Donahoe RM, Falek A. Neuroimmunomodulation by opiates and other drugs of abuse: relationship to HIV infection and AIDS. Adv Biochem Psychopharmacol. 1988;44:145–158. [PubMed] [Google Scholar]
- Donahoe RM, Vlahov D. Opiates as potential cofactors in progression of HIV-1 infections to AIDS. J Neuroimmunol. 1998;83:77–87. doi: 10.1016/s0165-5728(97)00224-5. [DOI] [PubMed] [Google Scholar]
- Dou H, Birusingh K, Faraci J, Gorantla S, Poluektova LY, Maggirwar SB, Dewhurst S, Gelbard HA, Gendelman HE. Neuroprotective activities of sodium valproate in a murine model of human immunodeficiency virus-1 encephalitis. J Neurosci. 2003;23:9162–9170. doi: 10.1523/JNEUROSCI.23-27-09162.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Dronda F, Zamora J, Moreno S, Moreno A, Casado JL, Muriel A, Perez-Elias MJ, Antela A, Moreno L, Quereda C. CD4 cell recovery during successful antiretroviral therapy in naive HIV-infected patients: the role of intravenous drug use. AIDS. 2004;18:2210–2212. doi: 10.1097/00002030-200411050-00018. [DOI] [PubMed] [Google Scholar]
- Duckworth BC, Cantley LC. Conditional inhibition of the mitogen-activated protein kinase cascade by wortmannin. Dependence on signal strength. J Biol Chem. 1997;272:27665–27670. doi: 10.1074/jbc.272.44.27665. [DOI] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Eisenstein TK, Hilburger ME. Opioid modulation of immune responses: effects on phagocyte and lymphoid cell populations. J Neuroimmunol. 1998;83:36–44. doi: 10.1016/s0165-5728(97)00219-1. [DOI] [PubMed] [Google Scholar]
- El-Hage N, Gurwell JA, Singh IN, Knapp PE, Nath A, Hauser KF. Synergistic increases in intracellular Ca2+, and the release of MCP-1, RANTES, and IL-6 by astrocytes treated with opiates and HIV-1 Tat. Glia. 2005;50:91–106. doi: 10.1002/glia.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson PS, Hansson E, Rönnbäck L. δ and kappa opiate receptors in primary astroglial cultures from rat cerebral cortex. Neurochem Res. 1990;15:1123–1126. doi: 10.1007/BF01101714. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Hansson E, Rönnbäck L. Mu and delta opiate receptors in neuronal and astroglial primary cultures from various regions of the brain-coupling with adenylate cyclase, localisation on the same neurones and association with dopamine (D1) receptor adenylate cyclase. Neuropharmacology. 1991;30:1233–1239. doi: 10.1016/0028-3908(91)90170-g. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Hansson E, Rönnbäck L. δ and kappa opiate receptors in primary astroglial cultures. Part II: Receptor sets in cultures from various brain regions and interactions with β-receptor activated cyclic AMP. Neurochem Res. 1992;17:545–551. doi: 10.1007/BF00968781. [DOI] [PubMed] [Google Scholar]
- Estanislao L, Geraci A, Simpson DM, Di Rocco A. HIV myelopathy, peripheral neuropathy, and myopathy. In: Nath A, Berger JR, editors. Clinical Neurovirology. New York: Marcel Dekker, Inc.; 2003. pp. 277–296. [Google Scholar]
- Everall I, Luthert P, Lantos P. A review of neuronal damage in human immunodeficiency virus infection: its assessment, possible mechanism and relationship to dementia. J Neuropathol Exp Neurol. 1993;52:561–566. doi: 10.1097/00005072-199311000-00002. [DOI] [PubMed] [Google Scholar]
- Everall IP. Interaction between HIV and intravenous heroin abuse? J Neuroimmunol. 2004;147:13–15. doi: 10.1016/j.jneuroim.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Everall IP, Bell C, Mallory M, Langford D, Adame A, Rockestein E, Masliah E. Lithium ameliorates HIV-gp120-mediated neurotoxicity. Mol Cell Neurosci. 2002;21:493–501. doi: 10.1006/mcne.2002.1196. [DOI] [PubMed] [Google Scholar]
- Factor SA, Troche-Panetto M, Weaver SA. Dystonia in AIDS: report of four cases. Mov Disord. 2003;18:1492–1498. doi: 10.1002/mds.10602. [DOI] [PubMed] [Google Scholar]
- Faden AI. Neurotoxic versus neuroprotective actions of endogenous opioid peptides: implications for treatment of CNS injury. NIDA Res Monogr. 1996;163:318–330. [PubMed] [Google Scholar]
- Forman HJ, Torres M. Redox signaling in macrophages. Mol Aspects Med. 2001;22:189–216. doi: 10.1016/s0098-2997(01)00010-3. [DOI] [PubMed] [Google Scholar]
- Francis H. Substance abuse and HIV infection. Top HIV Med. 2003;11:20–24. [PubMed] [Google Scholar]
- Franke TF, Yang SI, Chan TO, Datta K, Kazlauskas A, Morrison DK, Kaplan DR, Tsichlis PN. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- Freeman DJ, Li AG, Wei G, Li HH, Kertesz N, Lesche R, Whale AD, Martinez-Diaz H, Rozengurt N, Cardiff RD, Liu X, Wu H. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell. 2003;3:117–130. doi: 10.1016/s1535-6108(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Fulton BP, Burne JF, Raff MC. Visualization of O-2A progenitor cells in developing and adult rat optic nerve by quisqualate-stimulated cobalt uptake. J Neurosci. 1992;12:4816–4833. doi: 10.1523/JNEUROSCI.12-12-04816.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA. Microglia in human immunodeficiency virus-associated neurodegeneration. Glia. 2002;40:240–251. doi: 10.1002/glia.10155. [DOI] [PubMed] [Google Scholar]
- Garden GA, Budd SL, Tsai E, Hanson L, Kaul M, D'Emilia DM, Friedlander RM, Yuan J, Masliah E, Lipton SA. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci. 2002;22:4015–4024. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekker G, Hu S, Wentland MP, Bidlack JM, Lokensgard JR, Peterson PK. Kappa-opioid receptor ligands inhibit cocaine-induced HIV-1 expression in microglial cells. J Pharmacol Exp Ther. 2004;309:600–606. doi: 10.1124/jpet.103.060160. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Persidsky Y, Ghorpade A, Limoges J, Stins M, Fiala M, Morrisett R. The neuropathogenesis of the AIDS dementia complex. AIDS. 1997;11:35–45. [PubMed] [Google Scholar]
- Genis P, Jett M, Bernton EW, Boyle T, Gelbard HA, Dzenko K, Keane RW, Resnick L, Mizrachi Y, Volsky DJ, Epstein LG, Gendelman HE. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: Implications for the neuropathogenesis of HIV disease. J Exp Med. 1992;176:1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgoussi Z, Merkouris M, Mullaney I, Megaritis G, Carr C, Zioudrou C, Milligan G. Selective interactions of mu-opioid receptors with pertussis toxin-sensitive G proteins: involvement of the third intracellular loop and the c-terminal tail in coupling. Biochim Biophys Acta. 1997;1359:263–274. doi: 10.1016/s0167-4889(97)00097-9. [DOI] [PubMed] [Google Scholar]
- Glass JD, Fedor H, Wesselingh SL, McArthur JC. Immunocytochemical quantitation of human immunodeficiency virus in the brain: correlations with dementia. Ann Neurol. 1995;38:755–762. doi: 10.1002/ana.410380510. [DOI] [PubMed] [Google Scholar]
- Glass JD, Wesselingh SL, Selnes OA, McArthur JC. Clinical-neuropathologic correlation in HIV-associated dementia. Neurology. 1993;43:2230–2237. doi: 10.1212/wnl.43.11.2230. [DOI] [PubMed] [Google Scholar]
- Goodkin K, Shapshak P, Metsch LR, McCoy CB, Crandall KA, Kumar M, Fujimura RK, McCoy V, Zhang BT, Reyblat S, Xin KQ, Kumar AM. Cocaine abuse and HIV-1 infection: epidemiology and neuropathogenesis. J Neuroimmunol. 1998;83:88–101. doi: 10.1016/s0165-5728(97)00225-7. [DOI] [PubMed] [Google Scholar]
- Goswami R, Dawson SA, Dawson G. Cyclic AMP protects against staurosporine and wortmannin-induced apoptosis and opioid-enhanced apoptosis in both embryonic and immortalized (F-11kappa7) neurons. J Neurochem. 1998;70:1376–1382. doi: 10.1046/j.1471-4159.1998.70041376.x. [DOI] [PubMed] [Google Scholar]
- Goswami R, Dawson SA, Dawson G. Multiple polyphosphoinositide pathways regulate apoptotic signalling in a dorsal root ganglion derived cell line. J Neurosci Res. 2000;59:136–144. [PubMed] [Google Scholar]
- Goswami R, Kilkus J, Dawson SA, Dawson G. Overexpression of Akt (protein kinase B) confers protection against apoptosis and prevents formation of ceramide in response to pro-apoptotic stimuli. J Neurosci Res. 1999;57:884–893. [PubMed] [Google Scholar]
- Gosztonyi G, Schmidt V, Nickel R, Rothschild MA, Camacho S, Siegel G, Zill E, Pauli G, Schneider V. Neuropathologic analysis of postmortal brain samples of HIV-seropositive and - seronegative i.v. drug addicts. Forensic Sci Int. 1993;62:101–105. doi: 10.1016/0379-0738(93)90052-c. [DOI] [PubMed] [Google Scholar]
- Gray F, dle-Biassette H, Chretien F, Lorin de la GG, Force G, Keohane C. Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments. Clin Neuropathol. 2001;20:146–155. [PubMed] [Google Scholar]
- Grode ML, Murray MR. Effects of methadone-HCl on dorsal root ganglia in organotypic culture. Exp Neurol. 1973;40:68–81. doi: 10.1016/0014-4886(73)90124-6. [DOI] [PubMed] [Google Scholar]
- Gurwell JA, Duncan MJ, Maderspach K, Stiene-Martin A, Elde RP, Hauser KF. κ-Opioid receptor expression defines a phenotypically distinct subpopulation of astroglia: relationship to Ca2+ mobilization, development, and the antiproliferative effect of opioids. Brain Res. 1996;737:175–187. doi: 10.1016/0006-8993(96)00728-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurwell JA, Nath A, Sun Q, Zhang J, Martin KM, Chen Y, Hauser KF. Synergistic neurotoxicity of opioids and human immunodeficiency virus-1 Tat protein in striatal neurons in vitro. Neuroscience. 2001;102:555–563. doi: 10.1016/s0306-4522(00)00461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haughey NJ, Holden CP, Nath A, Geiger JD. Involvement of inositol 1,4,5-trisphosphate-regulated stores of intracellular calcium in calcium dysregulation and neuron cell death caused by HIV-1 Protein Tat. J Neurochem. 1999;73:1363–1374. doi: 10.1046/j.1471-4159.1999.0731363.x. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Gurwell JA, Turbek CS. Morphine inhibits Purkinje cell survival and dendritic differentiation in organotypic cultures of the mouse cerebellum. Exp Neurol. 1994;130:95–105. doi: 10.1006/exnr.1994.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Harris-White ME, Jackson JA, Opanashuk LA, Carney JM. Opioids disrupt Ca2+ homeostasis and induce carbonyl oxyradical production in mouse astrocytes in vitro: transient increases and adaptation to sustained exposure. Exp Neurol. 1998;151:70–76. doi: 10.1006/exnr.1998.6788. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Khurdayan VK, Goody RJ, Nath A, Pauly JR, Saria A. Selective vulnerability of cerebellar granule neuroblasts and their progeny to drugs with abuse liability. The Cerebellum. 2003;2:184–195. doi: 10.1080/14734220310016132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser KF, Mangoura D. Diversity of the endogenous opioid system in development: novel signal transduction translates multiple extracellular signals into neural cell growth and differentiation. Perspect Dev Neurobiol. 1998;5:437–449. [PubMed] [Google Scholar]
- Hauser KF, Stiene-Martin A. Characterization of opioid-dependent glial development in dissociated and organotypic cultures of mouse central nervous system: Critical periods and target specificity. Dev Brain Res. 1991;62:245–255. doi: 10.1016/0165-3806(91)90172-f. [DOI] [PubMed] [Google Scholar]
- Hauser KF, Stiene-Martin A, Mattson MP, Elde RP, Ryan SE, Godleske CC. μ-Opioid receptor-induced Ca2+ mobilization and astroglial development: Morphine inhibits DNA synthesis and stimulates cellular hypertrophy through a Ca2+-dependent mechanism. Brain Res. 1996;720:191–203. doi: 10.1016/0006-8993(96)00103-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckman TG, Kelly JA, Bogart LM, Kalichman SC, Rompa DJ. HIV risk differences between African-American and white men who have sex with men. J Natl Med Assoc. 1999;91:92–100. [PMC free article] [PubMed] [Google Scholar]
- Hu S, Peterson PK, Chao CC. Kappa-opioid modulation of human microglial cell superoxide anion generation. Biochem Pharmacol. 1998;56:285–288. doi: 10.1016/s0006-2952(98)00162-2. [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng WS, Lokensgard JR, Peterson PK. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42:829–836. doi: 10.1016/s0028-3908(02)00030-8. [DOI] [PubMed] [Google Scholar]
- Huang L-YM. Cellular mechanisms of excitatory and inhibitory actions of opioids. In: Tseng LF, editor. The Pharmacology of Opioid Peptides. Harwood Academic Publishers; 1995. pp. 131–149. [Google Scholar]
- Iglesias M, Segura MF, Comella JX, Olmos G. Mu-opioid receptor activation prevents apoptosis following serum withdrawal in differentiated SH-SY5Y cells and cortical neurons via phosphatidylinositol 3-kinase. Neuropharmacology. 2003;44:482–492. doi: 10.1016/s0028-3908(03)00024-8. [DOI] [PubMed] [Google Scholar]
- Ignatova EG, Belcheva MM, Bohn LM, Neuman MC, Coscia CJ. Requirement of receptor internalization for opioid stimulation of mitogen-activated protein kinase: biochemical and immunofluorescence confocal microscopic evidence. J Neurosci. 1999;19:56–63. doi: 10.1523/JNEUROSCI.19-01-00056.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatowski TA, Bidlack JM. Differential kappa-opioid receptor expression on mouse lymphocytes at varying stages of maturation and on mouse macrophages after selective elicitation. J Pharmacol Exp Ther. 1999;290:863–870. [PubMed] [Google Scholar]
- James HJ, Sharer LR, Zhang Q, Wang HG, Epstein LG, Reed JC, Gelbard HA. Expression of caspase-3 in brains from paediatric patients with HIV-1 encephalitis. Neuropathol Appl Neurobiol. 1999;25:380–386. doi: 10.1046/j.1365-2990.1999.00195.x. [DOI] [PubMed] [Google Scholar]
- Janssen RS, Saykin AJ, Cannon L, Campbell J, Pinsky PF, Hessol NA, O'Malley PM, Lifson AR, Doll LS, Rutherford GW. Neurological and neuropsychological manifestations of HIV-1 infection: association with AIDS-related complex but not asymptomatic HIV-1 infection. Ann Neurol. 1989;26:592–600. doi: 10.1002/ana.410260503. [DOI] [PubMed] [Google Scholar]
- Jones DM, Tucker BA, Rahimtula M, Mearow KM. The synergistic effects of NGF and IGF-1 on neurite growth in adult sensory neurons: convergence on the PI 3-kinase signaling pathway. J Neurochem. 2003;86:1116–1128. doi: 10.1046/j.1471-4159.2003.01925.x. [DOI] [PubMed] [Google Scholar]
- Kandel ES, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res. 1999;253:210–229. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- Kao AW, Price RW. Chemokine receptors, neural progenitor cells, and the AIDS dementia complex. J Infect Dis. 2004;190:211–215. doi: 10.1086/422012. [DOI] [PubMed] [Google Scholar]
- Kapasi AA, Coscia SA, Pandya MP, Singhal PC. Morphine modulates HIV-1 gp160-induced murine macrophage and human monocyte apoptosis by disparate ways. J Neuroimmunol. 2004;148:86–96. doi: 10.1016/j.jneuroim.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kaytor MD, Orr HT. The GSK3 beta signaling cascade and neurodegenerative disease. Curr Opin Neurobiol. 2002;12:275–278. doi: 10.1016/s0959-4388(02)00320-3. [DOI] [PubMed] [Google Scholar]
- Kelley TW, Graham MM, Doseff AI, Pomerantz RW, Lau SM, Ostrowski MC, Franke TF, Marsh CB. Macrophage colony-stimulating factor promotes cell survival through Akt/protein kinase B. J Biol Chem. 1999;274:26393–26398. doi: 10.1074/jbc.274.37.26393. [DOI] [PubMed] [Google Scholar]
- Kennedy SG, Wagner AJ, Conzen SD, Jordan J, Bellacosa A, Tsichlis PN, Hay N. The PI 3-kinase/Akt signaling pathway delivers an anti-apoptotic signal. Genes Dev. 1997;11:701–713. doi: 10.1101/gad.11.6.701. [DOI] [PubMed] [Google Scholar]
- Khurdayan VK, Buch S, El-Hage N, Lutz SE, Goebel SM, Singh IN, Knapp PE, Turchan-Cholewo J, Nath A, Hauser KF. Preferential vulnerability of astroglia and glial precursors to combined opioid and HIV-1 Tat exposure in vitro. Eur J Neurosci. 2004;19:3171–3182. doi: 10.1111/j.0953-816X.2004.03461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Cheong YP, So HS, Lee KM, Kim TY, Oh J, Chung YT, Son Y, Kim BR, Park R. Protective effects of morphine in peroxynitrite-induced apoptosis of primary rat neonatal astrocytes: potential involvement of G protein and phosphatidylinositol 3-kinase (PI3 kinase) Biochem Pharmacol. 2001;61:779–786. doi: 10.1016/s0006-2952(01)00541-x. [DOI] [PubMed] [Google Scholar]
- Kofke WA, Attaallah AF, Kuwabara H, Garman RH, Sinz EH, Barbaccia J, Gupta N, Hogg JP. The neuropathologic effects in rats and neurometabolic effects in humans of large-dose remifentanil. Anesth Analg. 2002;94:1229–36, table. doi: 10.1097/00000539-200205000-00033. [DOI] [PubMed] [Google Scholar]
- Krathwohl MD, Kaiser JL. HIV–1 promotes quiescence in human neural progenitor cells. J Infect Dis. 2004;190:216–226. doi: 10.1086/422008. [DOI] [PubMed] [Google Scholar]
- Kruman II, Nath A, Mattson MP. HIV-1 protein Tat induces apoptosis of hippocampal neurons by a mechanism involving caspase activation, calcium overload, and oxidative stress. Exp Neurol. 1998;154:276–288. doi: 10.1006/exnr.1998.6958. [DOI] [PubMed] [Google Scholar]
- Kumar R, Torres C, Yamamura Y, Rodriguez I, Martinez M, Staprans S, Donahoe RM, Kraiselburd E, Stephens EB, Kumar A. Modulation by morphine of viral set point in rhesus macaques infected with simian immunodeficiency virus and simian-human immunodeficiency virus. J Virol. 2004;78:11425–11428. doi: 10.1128/JVI.78.20.11425-11428.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannuzel A, Barnier JV, Hery C, Huynh VT, Guibert B, Gray F, Vincent JD, Tardieu M. Human immunodeficiency virus type 1 and its coat protein gp120 induce apoptosis and activate JNK and ERK mitogen-activated protein kinases in human neurons. Ann Neurol. 1997;42:847–856. doi: 10.1002/ana.410420605. [DOI] [PubMed] [Google Scholar]
- Lawrence DM, Durham LC, Schwartz L, Seth P, Maric D, Major EO. Human immunodeficiency virus type 1 infection of human brain-derived progenitor cells. J Virol. 2004;78:7319–7328. doi: 10.1128/JVI.78.14.7319-7328.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarini F, Casanova P, Tham TN, De Clercq E, Arenzana-Seisdedos F, Baleux F, Dubois-Dalcq M. Differential signalling of the chemokine receptor CXCR4 by stromal cell-derived factor 1 and the HIV glycoprotein in rat neurons and astrocytes. Eur J Neurosci. 2000;12:117–125. doi: 10.1046/j.1460-9568.2000.00894.x. [DOI] [PubMed] [Google Scholar]
- Leoni C, Menegon A, Benfenati F, Valtorta F. Downregulation of MAP kinase activity signalled by HIV-1-gp120 coat protein in granular neurons and glial cells from rat cerebellum. Biochem Biophys Res Commun. 1997;240:683–686. doi: 10.1006/bbrc.1997.7710. [DOI] [PubMed] [Google Scholar]
- Li Y, Zhang T, Douglas SD, Lai JP, Xiao WD, Pleasure DE, Ho WZ. Morphine enhances hepatitis C virus (HCV) replicon expression. Am J Pathol. 2003;163:1167–1175. doi: 10.1016/S0002-9440(10)63476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Qin L, Wilson BC, An L, Hong JS, Liu B. Inhibition by naloxone stereoisomers of beta-amyloid peptide (1-42)-induced superoxide production in microglia and degeneration of cortical and mesencephalic neurons. J Pharmacol Exp Ther. 2002;302:1212–1219. doi: 10.1124/jpet.102.035956. [DOI] [PubMed] [Google Scholar]
- Lopez-Ilasaca M, Crespo P, Pellici PG, Gutkind JS, Wetzker R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through PI 3-kinase gamma. Science. 1997;275:394–397. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- Luo Y, Berman MA, Abromson-Leeman SR, Dorf ME. Tumor necrosis factor is required for RANTES-induced astrocyte monocyte chemoattractant protein-1 production. Glia. 2003;43:119–127. doi: 10.1002/glia.10231. [DOI] [PubMed] [Google Scholar]
- Ma W, Zheng WH, Powell K, Jhamandas K, Quirion R. Chronic morphine exposure increases the phosphorylation of MAP kinases and the transcription factor CREB in dorsal root ganglion neurons: an in vitro and in vivo study. Eur J Neurosci. 2001;14:1091–1104. doi: 10.1046/j.0953-816x.2001.01731.x. [DOI] [PubMed] [Google Scholar]
- Machelska H, Stein C. Pain control by immune-derived opioids. Clin Exp Pharmacol Physiol. 2000;27:533–536. doi: 10.1046/j.1440-1681.2000.03287.x. [DOI] [PubMed] [Google Scholar]
- Maggirwar SB, Tong N, Ramirez S, Gelbard HA, Dewhurst S. HIV-1 Tat-mediated activation of glycogen synthase kinase-3beta contributes to Tat-mediated neurotoxicity. J Neurochem. 1999a;73:578–586. doi: 10.1046/j.1471-4159.1999.0730578.x. [DOI] [PubMed] [Google Scholar]
- Maggirwar SB, Tong N, Ramirez S, Gelbard HA, Dewhurst S. HIV-1 Tat-mediated activation of glycogen synthase kinase-3beta contributes to Tat-mediated neurotoxicity. J Neurochem. 1999b;73:578–586. doi: 10.1046/j.1471-4159.1999.0730578.x. [DOI] [PubMed] [Google Scholar]
- Mahajan SD, Schwartz SA, Shanahan TC, Chawda RP, Nair MP. Morphine regulates gene expression of alpha- and beta-chemokines and their receptors on astroglial cells via the opioid mu receptor. J Immunol. 2002;169:3589–3599. doi: 10.4049/jimmunol.169.7.3589. [DOI] [PubMed] [Google Scholar]
- Mangoura D. mu-Opioids activate tyrosine kinase focal adhesion kinase and regulate cortical cytoskeleton proteins cortactin and vinculin in chick embryonic neurons. J Neurosci Res. 1997;50:391–401. doi: 10.1002/(SICI)1097-4547(19971101)50:3<391::AID-JNR5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22:8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy L, Wetzel M, Sliker JK, Eisenstein TK, Rogers TJ. Opioids, opioid receptors, and the immune response. Drug Alcohol Depend. 2001;62:111–123. doi: 10.1016/s0376-8716(00)00181-2. [DOI] [PubMed] [Google Scholar]
- Mellon RD, Bayer BM. Evidence for central opioid receptors in the immunomodulatory effects of morphine: review of potential mechanism(s) of action. J Neuroimmunol. 1998;83:19–28. doi: 10.1016/s0165-5728(97)00217-8. [DOI] [PubMed] [Google Scholar]
- Meltzer MS, Skillman DR, Gomatos PJ, Kalter DC, Gendelman HE. Role of mononuclear phagocytes in the pathogenesis of human immunodeficiency virus infection. Annu Rev Immunol. 1990;8:169–194. doi: 10.1146/annurev.iy.08.040190.001125. 169–194. [DOI] [PubMed] [Google Scholar]
- Menegon A, Leoni C, Benfenati F, Valtorta F. Tat protein from HIV-1 activates MAP kinase in granular neurons and glial cells from rat cerebellum. Biochem Biophys Res Commun. 1997;238:800–5. doi: 10.1006/bbrc.1997.7393. [DOI] [PubMed] [Google Scholar]
- Meriney SD, Ford MJ, Oliva D, Pilar G. Endogenous opioids modulate neuronal survival in the developing avian ciliary ganglion. J Neurosci. 1991;11:3705–3717. doi: 10.1523/JNEUROSCI.11-12-03705.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meriney SD, Gray DB, Pilar G. Morphine-induced delay of normal cell death in the avian ciliary ganglion. Science. 1985;228:1451–1453. doi: 10.1126/science.2990029. [DOI] [PubMed] [Google Scholar]
- Milani D, Mazzoni M, Borgatti P, Zauli G, Cantely L, Capitani S. Extracellular human immunodeficiency virus type-1 Tat protein activates phosphatidylinositol 3-kinase in PC-12 neuronal cells. J Biol Chem. 1998;271:22961–22964. doi: 10.1074/jbc.271.38.22961. [DOI] [PubMed] [Google Scholar]
- Miller AF, Falke JJ. Chemotaxis receptors and signaling. Adv Protein Chem. 2004;68:393–444. doi: 10.1016/S0065-3233(04)68011-9. 393–444. [DOI] [PubMed] [Google Scholar]
- Miller M. The dynamics of substance use and sex networks in HIV transmission. J Urban Health. 2003;80:iii88–iii96. doi: 10.1093/jurban/jtg086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G. G protein-coupled receptor dimerization: function and ligand pharmacology. Mol Pharmacol. 2004;66:1–7. doi: 10.1124/mol.104.000497.. [DOI] [PubMed] [Google Scholar]
- Mirsattari SM, Power C, Nath A. Primary headaches with HIV infection. Headache. 1999;39:3–10. doi: 10.1046/j.1526-4610.1999.3901003.x. [DOI] [PubMed] [Google Scholar]
- Morsica G, Bernardi MT, Novati R, Uberti FC, Castagna A, Lazzarin A. Detection of hepatitis C virus genomic sequences in the cerebrospinal fluid of HIV-infected patients. J Med Virol. 1997;53:252–254. doi: 10.1002/(sici)1096-9071(199711)53:3<252::aid-jmv12>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Mullaney I, Carr IC, Burt AR, Wilson M, Anderson NG, Milligan G. Agonist-mediated tyrosine phosphorylation of isoforms of the shc adapter protein by the delta opioid receptor. Cell Signal. 1997;9:423–429. doi: 10.1016/s0898-6568(96)00188-x. [DOI] [PubMed] [Google Scholar]
- Murphy PM. Viral exploitation and subversion of the immune system through chemokine mimicry. Nat Immunol. 2001;2:116–122. doi: 10.1038/84214. [DOI] [PubMed] [Google Scholar]
- Nair MP, Mahajan S, Hewitt R, Whitney ZR, Schwartz SA. Association of drug abuse with inhibition of HIV-1 immune responses: studies with long-term of HIV-1 non-progressors. J Neuroimmunol. 2004;147:21–25. doi: 10.1016/j.jneuroim.2003.10.038. [DOI] [PubMed] [Google Scholar]
- Nath A. Pathobiology of human immunodeficiency virus dementia. Semin Neurol. 1999;19:113–127. doi: 10.1055/s-2008-1040830. [DOI] [PubMed] [Google Scholar]
- Nath A, Booze RM, Hauser KF, Mactutus CF, Bell JE, Maragos WF, Berger JR. Critical questions for neuroscientists in interactions of drugs of abuse and HIV infection. NeuroAIDS. 1999a;2:1–12. [Google Scholar]
- Nath A, Conant K, Chen P, Scott C, Major EO. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes : A hit and run phenomenon. J Biol Chem. 1999b;274:17098–17102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, Cass W, Turchan JT. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S62–S69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Nath A, Jones M, Maragos W, Booze R, Mactutus C, Bell J, Hauser KF, Mattson M. Neurotoxicity and dysfunction of dopamine systems associated with AIDS dementia. Psychopharmacol. 2000;14:222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II Neuropathology. Ann Neurol. 1986a;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986b;19:517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- Nelson CJ, Schneider GM, Lysle DT. Involvement of central mu- but not delta- or kappa-opioid receptors in immunomodulation. Brain Behav Immun. 2000;14:170–184. doi: 10.1006/brbi.1999.0575. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of addiction. Am J Addict. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- Neumeyer JL, Gu XH, van Vliet LA, DeNunzio NJ, Rusovici DE, Cohen DJ, Negus SS, Mello NK, Bidlack JM. Mixed kappa agonists and mu agonists/antagonists as potential pharmacotherapeutics for cocaine abuse: synthesis and opioid receptor binding affinity of N-substituted derivatives of morphinan. Bioorg Med Chem Lett. 2001;11:2735–2740. doi: 10.1016/s0960-894x(01)00543-1. [DOI] [PubMed] [Google Scholar]
- Ng SB, Tan YH, Guy GR. Differential induction of the interleukin-6 gene by tumor necrosis factor and interleukin-1. J Biol Chem. 1994;269:19021–19027. [PubMed] [Google Scholar]
- Nottet HS, Jett M, Flanagan CR, Zhai QH, Persidsky Y, Rizzino A, Bernton EW, Genis P, Baldwin T, Schwartz J. A regulatory role for astrocytes in HIV-1 encephalitis. An overexpression of eicosanoids, platelet-activating factor, and tumor necrosis factor-alpha by activated HIV-1-infected monocytes is attenuated by primary human astrocytes. J Immunol. 1995;154:3567–3581. [PubMed] [Google Scholar]
- Novick DM, Ochshorn M, Kreek MJ. In vivo and in vitro studies of opiates and cellular immunity in narcotic addicts. Adv Exp Med Biol. 1991;288:159–70. doi: 10.1007/978-1-4684-5925-8_18. 159–170. [DOI] [PubMed] [Google Scholar]
- Nyland SB, Specter S, Im-Sin J, Ugen KE. Opiate effects on in vitro human retroviral infection. Adv Exp Med Biol. 1998;437:91–100. doi: 10.1007/978-1-4615-5347-2_11. 91–100. [DOI] [PubMed] [Google Scholar]
- Oehmichen M, Meissner C, Reiter A, Birkholz M. Neuropathology in non-human immunodeficiency virus-infected drug addicts: hypoxic brain damage after chronic intravenous drug abuse. Acta Neuropathol (Berl) 1996;91:642–646. doi: 10.1007/s004010050478. [DOI] [PubMed] [Google Scholar]
- Oh JW, Schwiebert LM, Benveniste EN. Cytokine regulation of CC and CXC chemokine expression by human astrocytes. J Neurovirol. 1999;5:82–94. doi: 10.3109/13550289909029749. [DOI] [PubMed] [Google Scholar]
- Ozawa T, Nakagawa T, Shige K, Minami M, Satoh M. Changes in the expression of glial glutamate transporters in the rat brain accompanied with morphine dependence and naloxone-precipitated withdrawal. Brain Res. 2001;905:254–258. doi: 10.1016/s0006-8993(01)02536-7. [DOI] [PubMed] [Google Scholar]
- Pellegrino T, Bayer BM. In vivo effects of cocaine on immune cell function. J Neuroimmunol. 1998;83:139–147. doi: 10.1016/s0165-5728(97)00230-0. [DOI] [PubMed] [Google Scholar]
- Peng H, Huang Y, Rose J, Erichsen D, Herek S, Fujii N, Tamamura H, Zheng J. Stromal cell-derived factor 1-mediated CXCR4 signaling in rat and human cortical neural progenitor cells. J Neurosci Res. 2004;76:35–50. doi: 10.1002/jnr.20045. [DOI] [PubMed] [Google Scholar]
- Peng X, Mosser DM, Adler MW, Rogers TJ, Meissler JJ, Eisenstein TK. Morphine enhances interleukin-12 and the production of other pro-inflammatory cytokines in mouse peritoneal macrophages. J Leukoc Biol. 2000;68:723–728. [PubMed] [Google Scholar]
- Persidsky Y, Gendelman HE. Mononuclear phagocyte immunity and the neuropathogenesis of HIV-1 infection. J Leukoc Biol. 2003;74:691–701. doi: 10.1189/jlb.0503205. [DOI] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Bull C, Eriksson PS. Opioid-induced proliferation through the MAPK pathway in cultures of adult hippocampal progenitors. Mol Cell Neurosci. 2003a;23:360–372. doi: 10.1016/s1044-7431(03)00061-7. [DOI] [PubMed] [Google Scholar]
- Persson AI, Thorlin T, Bull C, Zarnegar P, Ekman R, Terenius L, Eriksson PS. Mu- and delta-opioid receptor antagonists decrease proliferation and increase neurogenesis in cultures of rat adult hippocampal progenitors. Eur J Neurosci. 2003b;17:1159–1172. doi: 10.1046/j.1460-9568.2003.02538.x. [DOI] [PubMed] [Google Scholar]
- Peruzzi F, Gordon J, Darbinian N, Amini S. Tat-induced deregulation of neuronal differentiation and survival by nerve growth factor pathway. J Neurovirol 8 Suppl. 2002;2:91–96. doi: 10.1080/13550280290167885. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Lokensgard J, Portoghese PS, Chao CC. Endomorphin-1 potentiates HIV-1 expression in human brain cell cultures: implication of an atypical mu-opioid receptor. Neuropharmacology. 1999;38:273–278. doi: 10.1016/s0028-3908(98)00167-1. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Hu S, Sheng WS, Molitor TW, Chao CC. Morphine stimulates phagocytosis of Mycobacterium tuberculosis by human microglial cells: involvement of a G protein-coupled opiate receptor. Adv Neuroimmunol. 1995;5:299–309. doi: 10.1016/0960-5428(95)00020-3. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Lokensgard JR, Bidlack JM, Chang A, Fang X, Portoghese PS. kappa-Opioid receptor agonist suppression of HIV-1 expression in CD4(+) lymphocytes. Biochem Pharmacol. 2001;61:1145–1151. doi: 10.1016/s0006-2952(01)00574-3. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Gekker G, Schut R, Hu S, Balfour HH, Jr, Chao CC. Enhancement of HIV-1 replication by opiates and cocaine: The cytokine connection. Adv Exp Med Biol. 1993a;335:181–188. doi: 10.1007/978-1-4615-2980-4_26. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Molitor TW, Chao CC. Mechanisms of morphine-induced immunomodulation. Biochem Pharmacol. 1993b;46:343–348. doi: 10.1016/0006-2952(93)90508-t. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Molitor TW, Chao CC. The opioid-cytokine connection. J Neuroimmunol. 1998;83:63–69. doi: 10.1016/s0165-5728(97)00222-1. [DOI] [PubMed] [Google Scholar]
- Peterson PK, Sharp BM, Gekker G, Portoghese PS, Sannerud K, Balfour HH., Jr Morphine promotes the growth of HIV-1 in human peripheral blood mononuclear cell cocultures. AIDS. 1990;4:869–873. doi: 10.1097/00002030-199009000-00006. [DOI] [PubMed] [Google Scholar]
- Philpott KL, McCarthy MJ, Klippel A, Rubin LL. Activated phosphatidylinositol 3-kinase and Akt kinase promote survival of superior cervical neurons. J Cell Biol. 1997;139:809–815. doi: 10.1083/jcb.139.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakiewicz RD, Schieferl SM, Gingras AC, Sonenberg N, Comb MJ. mu-Opioid receptor activates signaling pathways implicated in cell survival and translational control. J Biol Chem. 1998;273:23534–23541. doi: 10.1074/jbc.273.36.23534. [DOI] [PubMed] [Google Scholar]
- Poli G, Fauci AS. The role of monocyte/macrophages and cytokines in the pathogenesis of HIV infection. Pathobiology. 1992;60:246–251. doi: 10.1159/000163729. [DOI] [PubMed] [Google Scholar]
- Radkowski M, Wilkinson J, Nowicki M, Adair D, Vargas H, Ingui C, Rakela J, Laskus T. Search for hepatitis C virus negative-strand RNA sequences and analysis of viral sequences in the central nervous system: evidence of replication. J Virol. 2002;76:600–608. doi: 10.1128/JVI.76.2.600-608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff MC, Miller RH, Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on the culture. Nature. 1983;303:390–396. doi: 10.1038/303390a0. [DOI] [PubMed] [Google Scholar]
- Rahim RT, Feng P, Meissler JJ, Rogers TJ, Zhang L, Adler MW, Eisenstein TK. Paradoxes of immunosuppression in mouse models of withdrawal. J Neuroimmunol. 2004;147:114–120. doi: 10.1016/j.jneuroim.2003.10.024. [DOI] [PubMed] [Google Scholar]
- Rahim RT, Meissler JJ, Zhang L, Adler MW, Rogers TJ, Eisenstein TK. Withdrawal from morphine in mice suppresses splenic macrophage function, cytokine production, and costimulatory molecules. J Neuroimmunol. 2003;144:16–27. doi: 10.1016/s0165-5728(03)00273-x. [DOI] [PubMed] [Google Scholar]
- Reznikov K, Hauser KF, Nazarevskaja G, Trunova Y, Derjabin V, Bakalkin G. Opioids modulate cell division in the germinal zone of the late embryonic neocortex. Eur J Neurosci. 1999;11:2711–2719. doi: 10.1046/j.1460-9568.1999.00680.x. [DOI] [PubMed] [Google Scholar]
- Rock RB, Gekker G, Hu S, Sheng WS, Cheeran M, Lokensgard JR, Peterson PK. Role of microglia in central nervous system infections. Clin Microbiol Rev. 2004;17:942–964. doi: 10.1128/CMR.17.4.942-964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers TJ, Peterson PK. Opioid G protein-coupled receptors: signals at the crossroads of inflammation. Trends Immunol. 2003;24:116–121. doi: 10.1016/s1471-4906(03)00003-6. [DOI] [PubMed] [Google Scholar]
- Rogers TJ, Steele AD, Howard OM, Oppenheim JJ. Bidirectional heterologous desensitization of opioid and chemokine receptors. Ann N Y Acad Sci. 2000;917:19–28. doi: 10.1111/j.1749-6632.2000.tb05369.x. [DOI] [PubMed] [Google Scholar]
- Rohn JL, Hueber AO, McCarthy NJ, Lyon D, Navarro P, Burgering BM, Evan GI. The opposing roles of the Akt and c-Myc signalling pathways in survival from CD95-mediated apoptosis. Oncogene. 1998;17:2811–2818. doi: 10.1038/sj.onc.1202393. [DOI] [PubMed] [Google Scholar]
- Rouveix B. Opiates and immune function. Consequences on infectious diseases with special reference to AIDS. Therapie. 1992;47:503–512. [PubMed] [Google Scholar]
- Ruzicka BB, Fox CA, Thompson RC, Akil H, Watson SJ. Opioid receptor mRNA expression in primary cultures of glial cells derived from different rat brain regions. Regul Pept. 1994;54:251–252. [Google Scholar]
- Ruzicka BB, Fox CA, Thompson RC, Meng F, Watson SJ, Akil H. Primary astroglial cultures derived from several rat brain regions differentially expressμ, δ and kappa opioid receptor mRNA. Mol Brain Res. 1995;34:209–220. doi: 10.1016/0169-328x(95)00165-o. [DOI] [PubMed] [Google Scholar]
- Ryan EL, Morgello S, Isaacs K, Naseer M, Gerits P. Neuropsychiatric impact of hepatitis C on advanced HIV. Neurology. 2004;62:957–962. doi: 10.1212/01.wnl.0000115177.74976.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawynok J. The therapeutic use of heroin: a review of the pharmacological literature. Can J Physiol Pharmacol. 1986;64:1–6. doi: 10.1139/y86-001. [DOI] [PubMed] [Google Scholar]
- Scheid MP, Schubert KM, Duronio V. Regulation of bad phosphorylation and association with Bcl-x(L) by the MAPK/Erk kinase. J Biol Chem. 1999;274:31108–31113. doi: 10.1074/jbc.274.43.31108. [DOI] [PubMed] [Google Scholar]
- Sharp BM, Gekker G, Li MD, Chao CC, Peterson PK. Delta-opioid suppression of human immunodeficiency virus-1 expression in T cells (Jurkat) Biochem Pharmacol. 1998a;56:289–292. doi: 10.1016/s0006-2952(98)00155-5. [DOI] [PubMed] [Google Scholar]
- Sharp BM, Roy S, Bidlack JM. Evidence for opioid receptors on cells involved in host defense and the immune system. J Neuroimmunol. 1998b;83:45–56. [PubMed] [Google Scholar]
- Sheng WS, Hu S, Gekker G, Zhu S, Peterson PK, Chao CC. Immunomodulatory role of opioids in the central nervous system. Arch Immunol Ther Exp (Warsz ) 1997;45:359–366. [PubMed] [Google Scholar]
- Sheng WS, Hu S, Lokensgard JR, Peterson PK. U50,488 inhibits HIV-1 Tat-induced monocyte chemoattractant protein-1 (CCL2) production by human astrocytes. Biochem Pharmacol. 2003;65:9–14. doi: 10.1016/s0006-2952(02)01480-6. [DOI] [PubMed] [Google Scholar]
- Silva C, Zhang K, Tsutsui S, Holden JK, Gill MJ, Power C. Growth hormone prevents human immunodeficiency virus-induced neuronal p53 expression. Ann Neurol. 2003;54:605–614. doi: 10.1002/ana.10729. [DOI] [PubMed] [Google Scholar]
- Singh IN, Goody RJ, Dean C, Ahmad NM, Lutz SE, Knapp PE, Nath A, Hauser KF. Apoptotic death of striatal neurons induced by HIV-1 Tat and gp120: differential involvement of caspase-3 and endonuclease G. J Neurovirol. 2004;10:141–151. doi: 10.1080/13550280490441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal P, Kapasi A, Reddy K, Franki N. Opiates promote T cell apoptosis through JNK and caspase pathway. Adv Exp Med Biol. 2001;493:127–135. doi: 10.1007/0-306-47611-8_15. [DOI] [PubMed] [Google Scholar]
- Singhal PC, Bhaskaran M, Patel J, Patel K, Kasinath BS, Duraisamy S, Franki N, Reddy K, Kapasi AA. Role of p38 mitogen-activated protein kinase phosphorylation and Fas-Fas ligand interaction in morphine-induced macrophage apoptosis. J Immunol. 2002;168:4025–4033. doi: 10.4049/jimmunol.168.8.4025. [DOI] [PubMed] [Google Scholar]
- Singhal PC, Sharma P, Kapasi AA, Reddy K, Franki N, Gibbons N. Morphine enhances macrophage apoptosis. J Immunol. 1998;160:1886–1893. [PubMed] [Google Scholar]
- Sinz EH, Kofke WA, Garman RH. Phenytoin, midazolam, and naloxone protect against fentanyl-induced brain damage in rats. Anesth Analg. 2000;91:1443–1449. doi: 10.1097/00000539-200012000-00027. [DOI] [PubMed] [Google Scholar]
- Sippy BD, Hofman FM, Wallach D, Hinton DR. Increased expression of tumor necrosis factor-alpha receptors in the brains of patients with AIDS. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;10:511–21. [PubMed] [Google Scholar]
- Songyang Z, Baltimore D, Cantley LC, Kaplan DR, Franke TF. Interleukin 3-dependent survival by the Akt protein kinase. Proc Natl Acad Sci U S A. 1997;94:11345–11350. doi: 10.1073/pnas.94.21.11345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V, MacPherson D, Sas D, Lin Y, Snow B, Jang Y, Benchimol S, Mak TW. Regulation of PTEN transcription by p53. Mol Cell. 2001;8:317–325. doi: 10.1016/s1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- Stambolic V, Suzuki A, de la Pompa JL, Brothers GM, Mirtsos C, Sasaki T, Ruland J, Penninger JM, Siderovski DP, Mak TW. Negative regulation of PKB/Akt-dependent cell survival by the tumor suppressor PTEN. Cell. 1998;95:29–39. doi: 10.1016/s0092-8674(00)81780-8. [DOI] [PubMed] [Google Scholar]
- Stiene-Martin A, Hauser KF. Opioid-dependent growth of glial cultures: Suppression of astrocyte DNA synthesis by Met-enkephalin. Life Sci. 1990;46:91–98. doi: 10.1016/0024-3205(90)90041-o. [DOI] [PubMed] [Google Scholar]
- Stiene-Martin A, Hauser KF. Glial growth is regulated by agonists selective for multiple opioid receptor types in vitro. J Neurosci Res. 1991;29:538–548. doi: 10.1002/jnr.490290415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiene-Martin A, Knapp PE, Martin KM, Gurwell JA, Ryan S, Thornton SR, Smith FL, Hauser KF. Opioid system diversity in developing neurons, astroglia, and oligodendroglia in the subventricular zone and striatum: impact on gliogenesis in vivo. Glia. 2001;36:78–88. [PMC free article] [PubMed] [Google Scholar]
- Stiene-Martin A, Zhou R, Hauser KF. Regional, developmental, and cell cycle-dependent differences in μ, δ, and κ-opioid receptor expression among cultured mouse astrocytes. Glia. 1998;22:249–259. [PMC free article] [PubMed] [Google Scholar]
- Sun H, Lesche R, Li DM, Liliental J, Zhang H, Gao J, Gavrilova N, Mueller B, Liu X, Wu H. PTEN modulates cell cycle progression and cell survival by regulating phosphatidylinositol 3,4,5,-trisphosphate and Akt/protein kinase B signaling pathway. Proc Natl Acad Sci U S A. 1999;96:6199–6204. doi: 10.1073/pnas.96.11.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundar KS, Kamaraju LS, Dingfelder J, McMahon J, Gollapudi S, Wilson WH, Kong LY, Hong JS, Weiss JM, Lee JE. Beta-endorphin enhances the replication of neurotropic human immunodeficiency virus in fetal perivascular microglia. J Neuroimmunol. 1995;61:97–104. doi: 10.1016/0165-5728(95)00089-k. [DOI] [PubMed] [Google Scholar]
- Suzuki A, de la Pompa JL, Stambolic V, Elia AJ, Sasaki T, del BB I, Ho A, Wakeham A, Itie A, Khoo W, Fukumoto M, Mak TW. High cancer susceptibility and embryonic lethality associated with mutation of the PTEN tumor suppressor gene in mice. Curr Biol. 1998;8:1169–1178. doi: 10.1016/s0960-9822(07)00488-5. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Chuang LF, Doi RH, Chuang RY. Morphine suppresses lymphocyte apoptosis by blocking p53-mediated death signaling. Biochem Biophys Res Commun. 2003;308:802–808. doi: 10.1016/s0006-291x(03)01472-4. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Chuang LF, Yau P, Doi RH, Chuang RY. Interactions of opioid and chemokine receptors: oligomerization of mu, kappa, and delta with CCR5 on immune cells. Exp Cell Res. 2002;280:192–200. doi: 10.1006/excr.2002.5638. [DOI] [PubMed] [Google Scholar]
- Sykova E. Glia and volume transmission during physiological and pathological states. J Neural Transm. 2005;112:137–147. doi: 10.1007/s00702-004-0120-4. [DOI] [PubMed] [Google Scholar]
- Tan M, Groszer M, Tan AM, Pandya A, Liu X, Xie CW. Phosphoinositide 3-kinase cascade facilitates mu-opioid desensitization in sensory neurons by altering G-protein-effector interactions. J Neurosci. 2003;23:10292–10301. doi: 10.1523/JNEUROSCI.23-32-10292.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorlin T, Eriksson PS, Persson PA, Aberg ND, Hansson E, Ronnback L. Delta-opioid receptors on astroglial cells in primary culture: mobilization of intracellular free calcium via a pertussis sensitive G protein. Neuropharmacology. 1998;37:299–311. doi: 10.1016/s0028-3908(98)00026-4. [DOI] [PubMed] [Google Scholar]
- Thorlin T, Persson PA, Eriksson PS, Hansson E, Ronnback L. Delta-opioid receptor immunoreactivity on astrocytes is upregulated during mitosis. Glia. 1999;25:370–378. doi: 10.1002/(sici)1098-1136(19990215)25:4<370::aid-glia6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Tomassini N, Renaud F, Roy S, Loh HH. Morphine inhibits Fc-mediated phagocytosis through mu and delta opioid receptors. J Neuroimmunol. 2004;147:131–133. doi: 10.1016/j.jneuroim.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Tomlinson GS, Simmonds P, Busuttil A, Chiswick A, Bell JE. Upregulation of microglia in drug users with and without pre-symptomatic HIV infection. Neuropathol Appl Neurobiol. 1999;25:369–379. doi: 10.1046/j.1365-2990.1999.00197.x. [DOI] [PubMed] [Google Scholar]
- Tong N, Sanchez JF, Maggirwar SB, Ramirez SH, Guo H, Dewhurst S, Gelbard HA. Activation of glycogen synthase kinase 3 beta (GSK-3beta) by platelet activating factor mediates migration and cell death in cerebellar granule neurons. Eur J Neurosci. 2001;13:1913–1922. doi: 10.1046/j.0953-816x.2001.01572.x. [DOI] [PubMed] [Google Scholar]
- Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nat Rev Neurosci. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- Tran PB, Ren D, Veldhouse TJ, Miller RJ. Chemokine receptors are expressed widely by embryonic and adult neural progenitor cells. J Neurosci Res. 2004;76:20–34. doi: 10.1002/jnr.20001. [DOI] [PubMed] [Google Scholar]
- Turchan J, Chauchan A, Liu Y, Gartner S, Conant K, Hauser KF, Nath A. Increased vulnerability of ApoE positive neurons to chronic morphine and Tat exposure: protection by diosgenin and selegiline. J.Neurovirol. 2002;8(1):20. Ref Type: Abstract. [Google Scholar]
- Tyor WR, Glass JD, Becker S, Griffin J, Bezman L, McArthur J, Griffin DE. Cytokine expression in the brain during AIDS. Ann Neurol. 1992;31:349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- Tyor WR, Middaugh LD. Do alcohol and cocaine abuse alter the course of HIV-associated dementia complex? J Leukoc Biol. 1999;65:475–481. doi: 10.1002/jlb.65.4.475. [DOI] [PubMed] [Google Scholar]
- Tyor WR, Wesselingh SL, Griffin JW, McArthur JC, Griffin DE. Unifying hypothesis for the pathogenesis of HIV-associated dementia complex, vacuolar myelopathy, and sensory neuropathy. Journal of AIDS and Human Retroviruses. 1995;9:379–388. [PubMed] [Google Scholar]
- UNAIDS. Global summary of the HIV/AIDS epidemic. The Joint United Nations Programme on HIV/AIDS, UNAIDS; 2003. Dec, 12-1-2003. Ref Type: Electronic Citation. [Google Scholar]
- Vaillant AR, Mazzoni I, Tudan C, Boudreau M, Kaplan DR, Miller FD. Depolarization and neurotrophins converge on the phosphatidylinositol 3-kinase-Akt pathway to synergistically regulate neuronal survival. J Cell Biol. 1999;146:955–966. doi: 10.1083/jcb.146.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargova L, Jendelova P, Chvatal A, Sykova E. Glutamate, NMDA, and AMPA induced changes in extracellular space volume and tortuosity in the rat spinal cord. J Cereb Blood Flow Metab. 2001;21:1077–1089. doi: 10.1097/00004647-200109000-00005. [DOI] [PubMed] [Google Scholar]
- Vaswani M, Desai NG. HIV infection and high-risk behaviors in opioid dependent patients: the Indian context. Addict Behav. 2004;29:1699–1705. doi: 10.1016/j.addbeh.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Walker JS. Anti-inflammatory effects of opioids. Adv Exp Med Biol. 2003;521:148–160. [PubMed] [Google Scholar]
- Wang J, Barke RA, Charboneau R, Loh HH, Roy S. Morphine negatively regulates interferon-gamma promoter activity in activated murine T cells through two distinct cyclic AMP-dependent pathways. J Biol Chem. 2003a;278:37622–37631. doi: 10.1074/jbc.M301224200. [DOI] [PubMed] [Google Scholar]
- Wang L, Gintzler AR. Bimodal opioid regulation of cyclic AMP formation: Implications for positive and negative coupling of opiate receptors to adenylyl cyclase. J Neurochem. 1994;63:1726–1730. doi: 10.1046/j.1471-4159.1994.63051726.x. [DOI] [PubMed] [Google Scholar]
- Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, Rothstein JD, Volsky DJ. Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology. 2003b;312:60–73. doi: 10.1016/s0042-6822(03)00181-8. [DOI] [PubMed] [Google Scholar]
- Wang Z, Trillo-Pazos G, Kim SY, Canki M, Morgello S, Sharer LR, Gelbard HA, Su ZZ, Kang DC, Brooks AI, Fisher PB, Volsky DJ. Effects of human immunodeficiency virus type 1 on astrocyte gene expression and function: potential role in neuropathogenesis. J Neurovirol. 2004;10(Supp 1):25–32. doi: 10.1080/753312749. 25–32. [DOI] [PubMed] [Google Scholar]
- Wesselingh SL, Power C, Glass JD, Tyor WR, McArthur JC, Farber JM, Griffin JW, Griffin DE. Intracerebral cytokine messenger RNA expression in acquired immunodeficiency syndrome dementia. Ann Neurol. 1993;33:576–582. doi: 10.1002/ana.410330604. [DOI] [PubMed] [Google Scholar]
- Wetzel MA, Steele AD, Eisenstein TK, Adler MW, Henderson EE, Rogers TJ. Mu-opioid induction of monocyte chemoattractant protein-1, RANTES, and IFN-gamma-inducible protein-10 expression in human peripheral blood mononuclear cells. J Immunol. 2000;165:6519–6524. doi: 10.4049/jimmunol.165.11.6519. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Achim C. Human immunodeficiency virus encephalitis is the pathological correlate of dementia in acquired immunodeficiency syndrome. Ann Neurol. 1994;36:673–676. doi: 10.1002/ana.410360422. [DOI] [PubMed] [Google Scholar]
- Wilson MA, Burt AR, Milligan G, Anderson NG. Mitogenic signalling by delta opioid receptors expressed in rat-1 fibroblasts involves activation of the p70s6k/p85s6k S6 kinase. Biochem J. 1997;325:217–222. doi: 10.1042/bj3250217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong H, Zeng YC, Lewis T, Zheng J, Persidsky Y, Gendelman HE. HIV-1 infected mononuclear phagocyte secretory products affect neuronal physiology leading to cellular demise: relevance for HIV-1-associated dementia. J Neurovirol. 2000;6(Suppl 1):S14–S23. S14–S23. [PubMed] [Google Scholar]
- Yao R, Cooper GM. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- Yeung MC, Geertsma F, Liu J, Lau AS. Inhibition of HIV-1 gp120-induced apoptosis in neuroblastoma SK-N-SH cells by an antisense oligodeoxynucleotide against p53. AIDS. 1998;12:349–354. doi: 10.1097/00002030-199804000-00002. [DOI] [PubMed] [Google Scholar]
- Zauli G, Milani D, Mirandola P, Mazzoni M, Secchiero P, Miscia S, Capitani S. HIV-1 Tat protein down-regulates CREB transcription factor expression in PC12 neuronal cells through a phosphatidylinositol 3-kinase/AKT/cyclic nucleoside phosphodiesterase pathway. Faseb J. 2001;15:483–491. doi: 10.1096/fj.00-0354com. [DOI] [PubMed] [Google Scholar]