Abstract
Inflammation of adipose tissue induces metabolic derangements associated with obesity. Thus, determining ways to control or inhibit inflammation in adipose tissue is of clinical interest. The present study tested the hypothesis that in mouse adipose tissue, endogenous prostaglandin E2 (PGE2) negatively regulates inflammation via activation of prostaglandin E receptor 4 (EP4). PGE2 (5–500nM) attenuated lipopolysaccharide-induced mRNA and protein expression of chemokines, including interferon-γ-inducible protein 10 and macrophage-inflammatory protein-1α in mouse adipose tissue. A selective EP4 antagonist (L161,982) reversed, and two structurally different selective EP4 agonists [CAY10580 and CAY10598] mimicked these actions of PGE2. Adipose tissue derived from EP4-deficient mice did not display this response. These findings establish the involvement of EP4 receptors in this anti-inflammatory response. Experiments performed on adipose tissue from high-fat-fed mice demonstrated EP4-dependent attenuation of chemokine production during diet-induced obesity. The anti-inflammatory actions of EP4 became more important on a high-fat diet, in that EP4 activation suppressed a greater variety of chemokines. Furthermore, adipose tissue and systemic inflammation was enhanced in high-fat-fed EP4-deficient mice compared with wild-type littermates, and in high-fat-fed untreated C57BL/6 mice compared with mice treated with EP4 agonist. These findings provide in vivo evidence that PGE2-EP4 signaling limits inflammation. In conclusion, PGE2, via activation of EP4 receptors, functions as an endogenous anti-inflammatory mediator in mouse adipose tissue, and targeting EP4 may mitigate adipose tissue inflammation.
Keywords: EP4 receptor, prostanoids, eicosanoids, chemokines, fat
The concept that chronic inflammation can accompany obesity has recently received recognition (1). Adipose tissue is not a mere depot for energy storage, but contains inflammatory cells, including macrophages and T cells that increase in number with obesity. Inflammation of adipose tissue likely contributes to insulin resistance, dyslipidemia, and hypertension associated with obesity (1, 2). Thus, determining ways to control or inhibit adipose tissue inflammation is clinically relevant. Much of the interest in this regard has focused on pro-inflammatory mediators, but the local inflammatory response likely reflects a balance between pro-inflammatory pathways and endogenous anti-inflammatory modulators. The present study tested the novel hypothesis that endogenous eicosanoids may operate in adipose tissue to modulate inflammation.
In response to various stimuli, the sequential actions of cyclooxygenase and prostaglandin E synthase produce prostaglandin E2 (PGE2) from arachidonic acid. Inflammatory sites produce PGE2 abundantly (3). This eicosanoid exerts anti-inflammatory effects by binding to prostaglandin E receptor 4 (EP4), thereby suppressing the release of cytokines and chemokines from macrophages and T cells, and inhibiting the proliferation and activation of these immune cells (4–7). EP4 agonism can attenuate atherosclerosis (8), and mice lacking EP4 in their hematopoietic cells have heightened susceptibility to experimentally produced abdominal aortic aneurysms (9). EP4 agonists effectively reduce acute cardiac rejection and prolong allograft survival by suppressing myocardial inflammation (10). They also protect the perfused myocardium against ischemic injury by reducing inflammation (11, 12). Hence, targeting EP4 may serve as therapeutic strategy for a range of inflammatory disorders (7).
Although EP4 stimulation may mitigate inflammation, no available information focuses on its role in fat inflammation. Clinical data illustrate that cyclooxgenase-2 inhibitors can increase coronary events (13, 14). Therefore, a more complete characterization of anti-inflammatory pathways stimulated by cyclooxygenase-2-dependent products is warranted. The present experiments aimed to test the hypothesis that EP4 modulates adipose tissue inflammation and thus may serve as a therapeutic target to treat the current epidemic of dysmetabolism associated with obesity.
Research Design and Methods
Mice
Male homozygous EP4 receptor-deficient (EP4−/−) mice and wild-type (EP4+/+) mice on the same genetic background were obtained by crossing mice heterozygous for the ptger4 gene mutation (EP4+/−). All genotyping was performed by PCR of DNA extracted from ear biopsies. Because EP4−/− mice do not survive on a C57BL/6 congenic strain, all EP4−/− and wild-type littermate controls used in this study were on a mixed background, composed of 129/Olac, C57BL/6, and DBA/2. The Laboratory Animal Unit of The University of Hong Kong provided the male C57BL/6N mice. On the day of harvest, the mice were anesthetized by intraperitoneal injection of either 2,3,3, tribromoethanol (2.5 mg/10 g body weight) or pentobarbital (100 mg/kg). Blood was obtained by cardiac puncture, and epididymal white adipose tissue (eWAT) was isolated for further analysis. All animal experiments were performed according to a protocol approved by the Standing Committee on Animal Welfare of The University of Hong Kong.
Diet-induced obesity
EP4+/+ and EP4−/− mice were fed a high-fat diet (RD Western Diet D2079B; Research Diets, New Brunswick, NJ; 40% kcal from fat, 1.25% cholesterol, 0% cholate) ad libitum at 10 weeks of age, and were kept on this diet for 8 or 16 weeks or as indicated. Food intake was calculated once a week, during the last 5 weeks before euthanization and expressed in kilocalories consumed per day.
Treatment with EP4 agonist
C57BL/6 (6-week-old) mice were fed a high-fat diet ad libitum and received daily subcutaneous injections of CAY10580 (a selective EP4 agonist, 200 µg/kg (15); Cayman Chemical, Ann Arbor, MI) or vehicle for 6 weeks. Food intake was calculated once a week for the whole duration of treatment and expressed in average kilocalories consumed per day.
Body composition analysis
The amount of fat and lean mass in intact, unanesthetized mice was measured using a nuclear magnetic resonance-based body composition analyzer (MiniSpec LF50; Bruker, Billerica, MA). Fat mass was normalized to lean mass (fat mass to lean mass ratio) or body weight (adiposity index) in order to calculate relative fat composition.
Adipose tissue explant culture
The eWAT (0.05 g from each mouse) was minced and incubated with 1 ml DMEM media [supplemented with 10% fetal bovine serum, 2% L-glutamine (2 mM), 1% penicillin/streptomycin (100U/ml)] or media containing the appropriate drugs. In Experiment 1, adipose tissues were pretreated with vehicle or L161,982 [a selective EP4 antagonist (16); 100 nM for 40 min, Cayman Chemical, Ann Arbor, MI] before the addition of prostaglandin E2 (PGE2; 50 nM for 1.5 h, or as stated; Cayman Chemical). In Experiment 2, adipose tissues were pretreated with CAY10580 or CAY10598 [selective EP4 agonists (15, 17), Cayman Chemical] at various concentrations for 1.5 h. In Experiment 3, adipose tissues were cultured with or without 8-bromo-cyclic AMP (8-Br-cAMP; analog of cAMP, 500 µM for 1.5 h; Sigma, St. Louis, MO), 6-monobutyryladenosine-cAMP (6-MB-cAMP; selective protein kinase A (PKA) activator; 100 µM for 30 min, BioLog Life Science Institute, Bremen, Germany), 8-(4-Chlorophenylthio)-2’-O-methyladenosine 3,5’-cyclic monophosphate monosodium [8-PCT-2’-O-ME-cAMP; selective exchange factor directly activated by cAMP (Epac) activator; 100 µM for 30 min, BioLog Life Science Institute], H89 (selective PKA inhibitor; 10 µM for 30 min, Sigma) and/or HJC0197 (selective Epac inhibitor; 10 µM for 30 min, BioLog Life Science Institute) before pretreatment with or without PGE2 (50nM for 1 h). In Experiment 4, adipose tissues were pretreated with different EP agonists [16,16-dimethyl-PGE2 (1 µM), a nonselective EP1/EP2/EP3/EP4 agonist (18); 17-phenyl-trinor-PGE2 (1 µM), a nonselective EP1/3 agonist (19); butaprost (100 nM), a selective EP2 agonist (20); 19(R)-hydroxy-PGE2 (1 µM), a selective EP2 agonist (21); sulprostone (20 nM), a selective EP3 agonist (22)] for 40 min. All EP receptor agonists were purchased from Cayman Chemical. After the different pretreatments, samples were exposed to lipopolysaccharide (LPS, from Escherichia coli O55:B5; 5 ng/ml, or as stated; Sigma) or TNF-α (50 ng/ml, human recombinant TNFα Endogen Inc., Woburn, MA) for nine h at 37°C. Adipose tissue lysates and media were collected and stored at −80°C until subsequent analysis.
Real-time PCR
Total RNA was isolated from up to 0.05 g of adipose tissue with the RNeasy Lipid Tissue Mini Kit (Qiagen, Valencia, CA), and equal amounts were reverse-transcribed by Superscript II (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. Quantitative PCR was performed in a MyiQ Single Color Real-time PCR Detection System (Bio-Rad, Hercules, CA). The conditions for amplification were 3 min at 95°C for denaturation, 30 cycles of 40 s at 95°C, 30 s at 60°C, and 30 s at 72°C, followed by an extension for 10 min at 72°C. The mouse primer sequences used are listed in Supplementary Table 1. The mRNA levels of the various genes tested were normalized to those of β-actin, used as an internal control in all experiments.
Chemokines, cytokines, SAA, and PGE2 measurements
Interferon-γ-inducible protein 10 (IP-10), macrophage inflammatory protein (MIP)-1α, TNFα, interleukin (IL)-6, and IL-10 levels were measured using Quantikine ELISA kits (R&D Systems, Minneapolis, MN). IL-8 was measured by the IL-8 ELISA kit from Cusabio (Wuhan, Hubei, China). Plasma levels of serum amyloid A (SAA) were measured by the SAA ELISA Kit (Invitrogen). PGE2 levels were measured by the PGE2 enzymatic immunoassay kit (Cayman Chemical).
Adipocyte and stromal vascular cell fractionation
The eWAT (0.3 g) was minced in PBS containing 2% BSA and 250 U/ml of collagenase type II (Worthington, Lakewood, NJ) and incubated at 37°C for 1 h. The digested tissue was passed through a 70 µm cell strainer (BD Biosciences, Bedford, MA), and the flow through was centrifuged. The mature adipocytes from the flow through were collected for subsequent analysis. The remaining pellet was lysed with ACK lysing buffer (Gibco-BRL, Grand Island, NY) to remove red blood cells, yielding stromal vascular cells (SVC). Mature adipocytes and SVC were seeded onto culture plates with 1 ml DMEM media (supplemented with 10% fetal bovine serum, 2% L-glutamine [2 mM], and 1% penicillin/streptomycin [100 U/ml]). After 1 day of equilibration, cells were pretreated with vehicle or PGE2 at 50 nM for 90 min, and then exposed to LPS (5 ng/ml) for 9 h at 37°C.
Flow cytometry
Adipose tissue-derived SVCs were washed with DMEM [supplemented with 10% fetal bovine serum, 2% L-glutamine [2 mM], and 1% penicillin/streptomycin (100 U/ml)], counted, and labeled with conjugated antibodies or their respective isotype controls before acquisition by FACScan. Single-color staining was performed to detect F4/80-, CD11c-, CD4- and CD8-positive cells (all antibodies were purchased from Biolegend, San Diego, CA). Triple staining with PE anti-mouse F4/80 (Biolegend), FITC anti-mouse CD11c (Biolegend), and Alexa Fluor® 647 anti-mouse CD206 (Biolegend) were used to identify M1 and M2 macrophages.
Immunohistochemistry
eWAT was fixed with 4% paraformaldehyde in 0.1M phosphate buffer and embedded in paraffin. Five µm sections were stained with hematoxylin and eosin Y. Cross-sectional area of adipocytes (in three consecutive visual fields at the same magnification per sample) was calculated using ImageJ (The National Institutes of Health; Bethesda, MD).
Statistical analyses
Data are expressed as means ± SEM. All statistical analysis was performed using GraphPad Prism software 5.0 (San Diego, CA). The two-tailed Mann-Whitney t-test was used for comparisons between two experimental groups or ANOVA for multiple group comparisons. Differences were considered to be statistically significant when p was less than 0.05.
Results
Chemokine production in adipose tissue
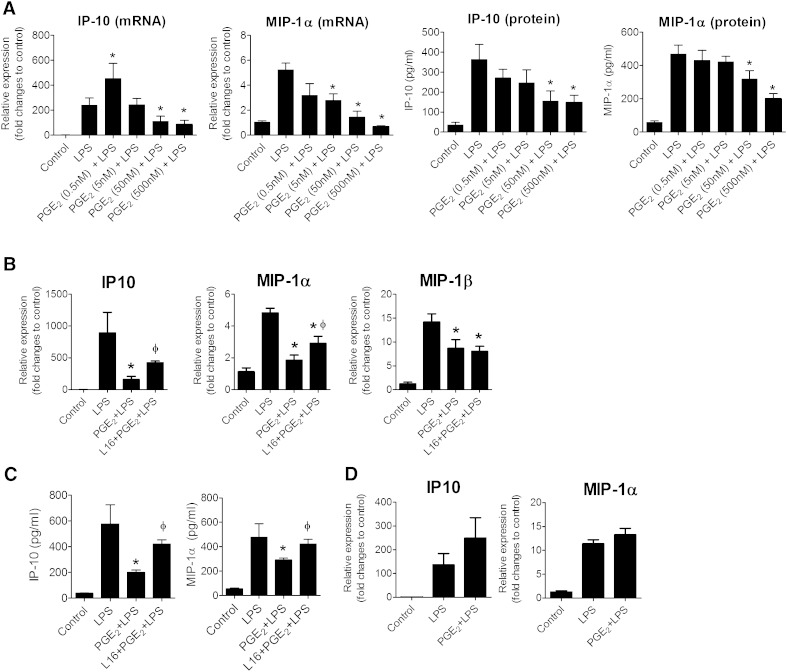
To test whether PGE2 has anti-inflammatory actions on adipose tissue, explants of mice eWAT were pretreated with different concentrations of PGE2 (for 1.5 h) before exposure to LPS (5 ng/ml, for 9 h). We focused on measuring the effect of PGE2 on IP-10, MIP-1α, MIP-1β, and monocyte chemoattractant protein (MCP)-1 expression as the inflammatory output because PGE2 attenuated the expression of these chemokines in LPS-treated human macrophages (4). LPS stimulated an increase in IP-10 and MIP-1α mRNA expression in adipose tissue explants (Fig. 1A, B). Preincubation with PGE2 significantly reduced IP-10 and MIP-1α mRNA responses to LPS in a concentration-dependent manner, with the strongest inhibition observed at 500 nM (Fig. 1A). Concentration-dependent inhibition of IP-10 and MIP-1α by PGE2 also occurred at the protein level (Fig. 1C). PGE2 decreased levels of IP-10 and MIP-1α, but did not alter mRNA levels of MCP-1 and of regulated on activation normal T cell expressed and secreted (RANTES) in LPS-stimulated adipose tissue from healthy mice (Supplementary Fig. 1). Based on these findings, PGE2 pretreatment potentially reduces the release of IP-10 and MIP-1α from LPS-stimulated adipose tissue explants of healthy mice.
Fig. 1.
PGE2-mediated suppression of chemokines in mouse adipose tissue depends on EP4 receptor. A: PGE2 reduces IP-10 and MIP-1α expression in LPS-activated adipose tissue explants. RNA was isolated from adipose tissue explants pretreated with different concentrations of PGE2 (0.5 to 500 nM for 1.5 h) before exposure to LPS (5 ng/ml) for 9 h. The mRNA expression of IP-10 and MIP-1α for different treatment groups was expressed in relative amount produced in control. IP-10 and MIP-1α protein production in the medium was measured by ELISA and expressed in absolute values. N = 6; *, P < 0.05 versus LPS. B: Adipose tissue explants of EP4+/+ mice were treated with PGE2 (50 nM) in the presence or absence of a selective EP4 antagonist (L161,982). After 1.5 h of treatment, the cells were stimulated with LPS for 9 h. The mRNA expression of IP-10, MIP-1α, and MIP-1β in different samples was quantified. Data are expressed in relative amount produced in control. N = 6; *, P < 0.05 versus LPS; ɸ, P < 0.05 versus PGE2+LPS. C: IP-10 and MIP-1β production in the medium released from adipose tissue of EP4+/+ mice, as measured by ELISA. N = 6; *, P < 0.05 versus LPS; ɸ, P < 0.05 versus PGE2+LPS. D: PGE2 did not suppress LPS-stimulated IP-10 and MIP-1α in EP4-deficient adipose tissue. RNA was isolated from eWAT derived from EP4−/− cultured under various conditions, controls, LPS (5 ng/ml for 9 h), PGE2 (50 nM, pretreated for 2 h) + LPS (5 ng/ml for 9 h), and amounts of IP-10 and MIP-1α in different treatment groups were quantified and expressed against that of controls. N = 6. All data are expressed as means ± SEM.
The effect of PGE2 on cytokine production was also investigated. PGE2 significantly reduced LPS-induced release of IL-6 and IL-8 but not TNFα and IL-10 (Supplementary Fig. 2). Hence, PGE2 selectively limits the production of some cytokines in adipose tissue from healthy mice.
Inhibition of chemokines by PGE2 depends on EP4
In eWAT from EP4+/+ mice, PGE2 reduced LPS-mediated IP-10 and MIP-1α mRNA expression (Fig. 1B). Pretreatment with L161,982 (a selective EP4 antagonist; 100 nM) reversed these effects of PGE2 (Fig. 1B). Pretreatment with PGE2 significantly reduced the presence of IP-10 and MIP-1α at the protein level, and L161,982 reversed this inhibition (Fig. 1C). PGE2 also limited LPS-induced MIP-1β mRNA expression, but L161,982 did not prevent this effect (Fig. 1B). PGE2 did not reduce IP-10 and MIP-1α in eWAT from EP4−/− mice (Fig. 1D). In eWAT from C57BL/6 mice, two structurally different selective EP4 agonists, CAY10590 and CAY10598, concentration-dependently reduced LPS-stimulated release of IP-10 (at both the mRNA and protein levels; Supplementary Fig. 3). These data collectively indicate that the inhibition of IP-10 and MIP-1α by PGE2 depends on EP4 activation.
Cell types involved in the anti-inflammatory effect
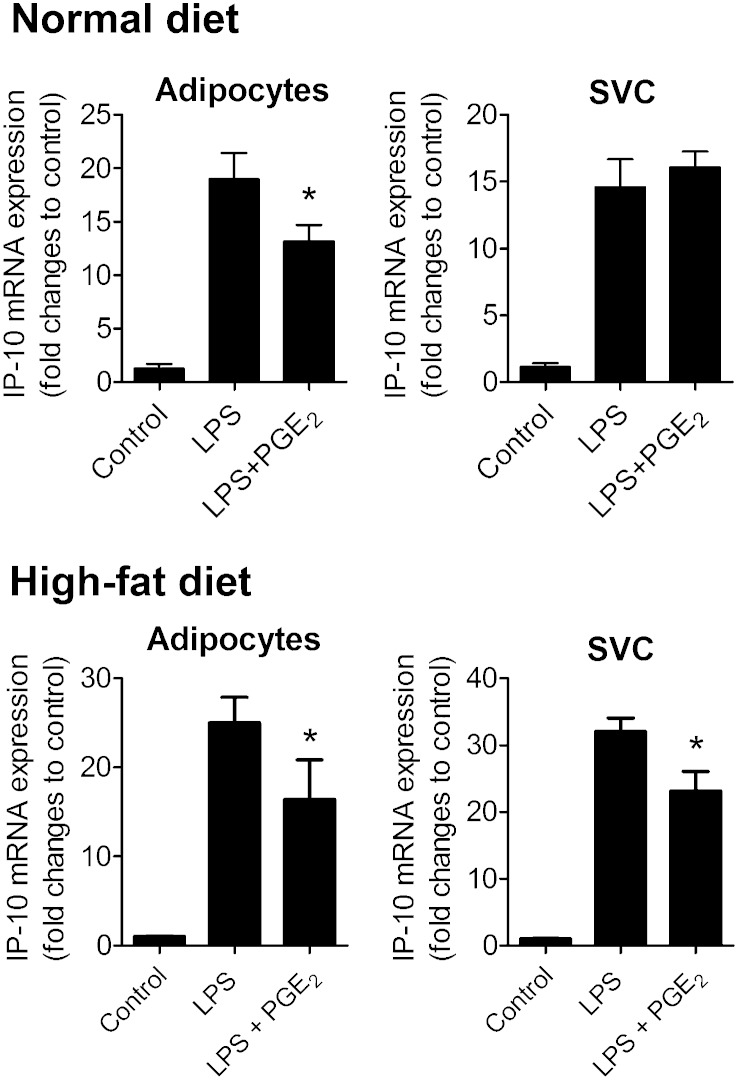
Adipose tissue consists of adipocytes and SVCs and both cell fractions can release chemokines and cytokines. In mature adipocytes, PGE2 significantly reduced LPS-induced increases in IP-10 (Fig. 2) and MIP-1α (Supplementary Fig. 4) mRNA and protein levels (Supplementary Fig. 5). SVCs had a higher capacity than adipocytes to produce IP-10 and MIP-1α protein on a per-cell basis (Supplementary Fig. 5), but PGE2 did not significantly affect the mRNA (Fig. 2 and Supplementary 4) and protein levels of those chemokines in this cell preparation (Supplementary Fig. 5). These findings suggest that PGE2 predominately exerts its anti-inflammatory effect on adipocytes. In line with this, PGE2 significantly lowered LPS-stimulated IL-6 and IL-8 protein levels in mature adipocytes but did not affect the protein levels of these cytokines in SVCs (Supplementary Fig. 2). TNFα and IL-10 production was undetectable in adipocytes and their production was unaffected by PGE2 in LPS-activated SVCs (Supplementary Fig. 2).
Fig. 2.
The effect of PGE2 on IP-10 mRNA in LPS-activated adipocytes and SVCs from normal diet or high-fat diet mice. Adipocytes and SVCs separated by collagenase digestion of EP4+/+ adipose tissue were cultured in the presence or absence of PGE2 (50 nM for 1.5 h), and then stimulated with LPS (5 ng/ml for 9 h). RNA then was isolated in each condition and IP-10 mRNA was quantified. N = 6; *, P < 0.05 versus LPS. All data are expressed as means ± SEM.
The amount of infiltrated inflammatory cells within the SVCs of relatively healthy mice is probably minimal. Therefore, SVCs from high-fat diet-fed mice (after 11 weeks of high-fat diet), which presumably contain more infiltrated inflammatory cells, were isolated and the effect of PGE2 on them was tested. PGE2 significantly reduced LPS-mediated IP-10 mRNA in both adipocytes and SVC fractions when adipose tissue from high-fat-fed mice was used (Fig. 2).
cAMP and PKA
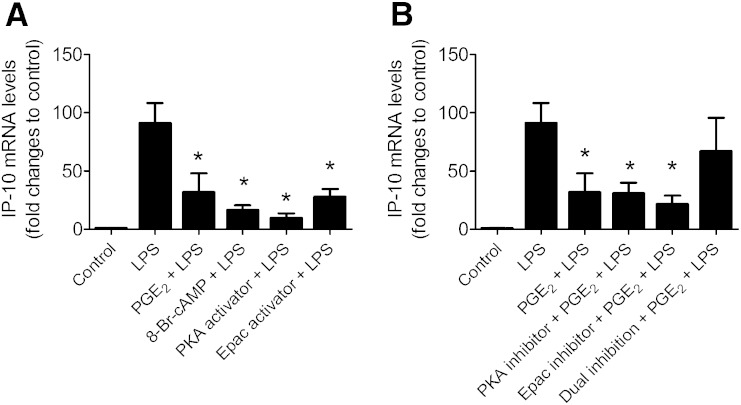
The downstream signaling pathway for EP4 receptor includes adenylyl cyclase and is suggested to involve cAMP-dependent PKA (4). However, adenylyl cyclase may also activate the Epac pathway. We examined the signaling pathway in the effect of PGE2 on adipose tissue explants by using a 8-bromo-cAMP, cell membrane-permeable analog of cAMP (8-Br-cAMP; 500 µM); 6-MB-cAMP, a selective PKA activator (100 µM) or 8-PCT-2’-O-ME-cAMP, a selective Epac activator (100 µM). Similar to PGE2, pretreatment with 8-Br-cAMP, 6-MB-cAMP, or 8-PCT-2’-O-ME-cAMP suppressed IP-10 (Fig. 2) in LPS-activated adipose tissue (Fig. 2). These observations suggest that release of cAMP and activation of PKA and Epac can mimic the anti-inflammatory effect of PGE2. To consolidate the participation of cAMP, PKA, and Epac in the response, the effect of selective PKA (H-89; 10 µM) and Epac inhibitors (HJC0197; 10 µM) on LPS-mediated IP-10 was tested. H-89 and HJC0197 alone, however, did not reverse this inhibitory effect of PGE2 (Fig. 3B). The inhibitory effect of PGE2 was significantly reversed only when PKA and Epac were simultaneously inhibited [H89 + HJC0197(dual inhibition); Fig. 3B].
Fig. 3.
cAMP, PKA, and Epac mediates suppression of IP-10 by PGE2 in LPS-stimulated adipose tissue. A: EP4+/+ adipose tissues were cultured with or without 8-Br-cAMP (analog of cAMP, 500 µM for 1.5 h), 6-MB-cAMP (selective PKA activator; 100µM for 30 minutes), 8-PCT-2’-O-ME-cAMP (selective Epac activator; 100µM for 30 minutes) before exposure to LPS (5ng/ml for 9 h). B: EP4+/+ adipose tissues were cultured with or without H89 (selective PKA inhibitor; 10 µM for 30 min), HJC0197 (selective Epac inhibitor; 10 µM for 30 min), or H89 + HJC0197 (dual inhibition) before pretreatment with or without PGE2 (50 nM for 1 h). Then cells were exposed to LPS (5 ng/ml) for 9 h. mRNA was quantified by PCR. N = 6; *, P < 0.05 versus LPS. Data are expressed as means ± SEM.
TNF-α-induced inflammation
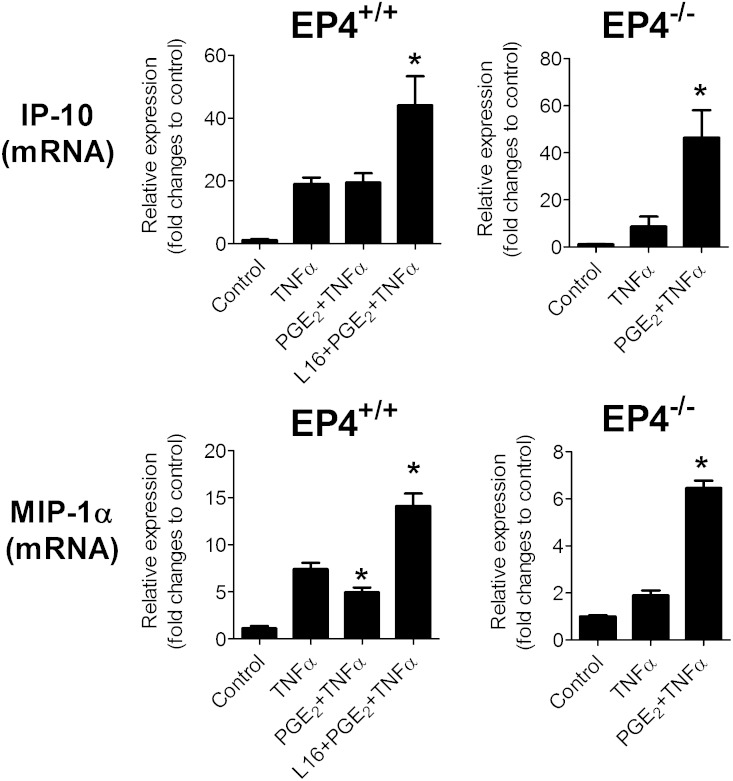
Pro-inflammatory cytokines such as TNF-α participate in adipose tissue inflammation. TNF-α directly stimulated the mRNA expression of IP-10 in mouse adipose tissue, but PGE2 did not alter this increase (Fig. 4). Yet, when the adipose tissue was treated with L161,982 before PGE2 exposure, the TNF-α–induced IP-10 mRNA production rose significantly (Fig. 4). Likewise, the addition of L161,982 before PGE2 caused a further increase in the MIP-1α expression in response to TNF-α. Thus, blockade of EP4 resulted in augmented TNFα-induced chemokine release, indicating that EP4 operates as an anti-inflammatory effector in adipose tissue. In line with this observation, in eWAT obtained from EP4−/− mice, PGE2 significantly increased TNFα-mediated IP-10 and MIP-1α mRNA expression (Fig. 4). This result indicates that pro-inflammatory responses prevail in adipose tissue lacking the anti-inflammatory receptor EP4. As PGE2 can directly reduce LPS- but not TNFα-induced inflammation, LPS and TNFα likely interact with prostaglandin signaling in different ways.
Fig. 4.
Effect of EP4 inhibition on TNFα-induced inflammation. EP4+/+ or EP4−/− adipose tissue explants were treated with PGE2 (50 nM) in the presence or absence of a selective EP4 antagonist (L161,982). After 1.5 h of treatment, the cells were stimulated with TNF-α (50 ng/ml) for 9 h. mRNA expression of IP-10 and MIP-1α for different treatment groups was expressed as fold changes from control (without TNF-α stimulation). N = 6; *, P < 0.05 versus TNF-α. Data are expressed as means ± SEM.
Role of other EP receptors
Three synthases produce PGE2, membrane prostaglandin E2 synthase (mPGES)-1, mPGES-2, and cytosolic prostaglandin E2 synthase (cPGES), and this prostanoid can engage four receptors: EP1, EP2, EP3, and EP4 (23). Thus, receptors other than EP4 may participate in regulating the inflammatory response in fat. Real-time PCR demonstrated mRNA expression of all known EP receptor subtypes and prostaglandin synthases in mouse eWAT (Supplementary Figs. 6, 7). Comparing the critical threshold values for EP1–EP4 (obtained from the same samples in the same experimental trial), the abundance of EP receptors in eWAT can be estimated as follows: EP3 (most abundant)> EP4 = EP2 > EP1. As for the synthases, their abundance in mouse eWAT can be estimated as: cPGES (most abundant) > mPGES-2 > mPGES-1 (Supplementary Fig. 6).
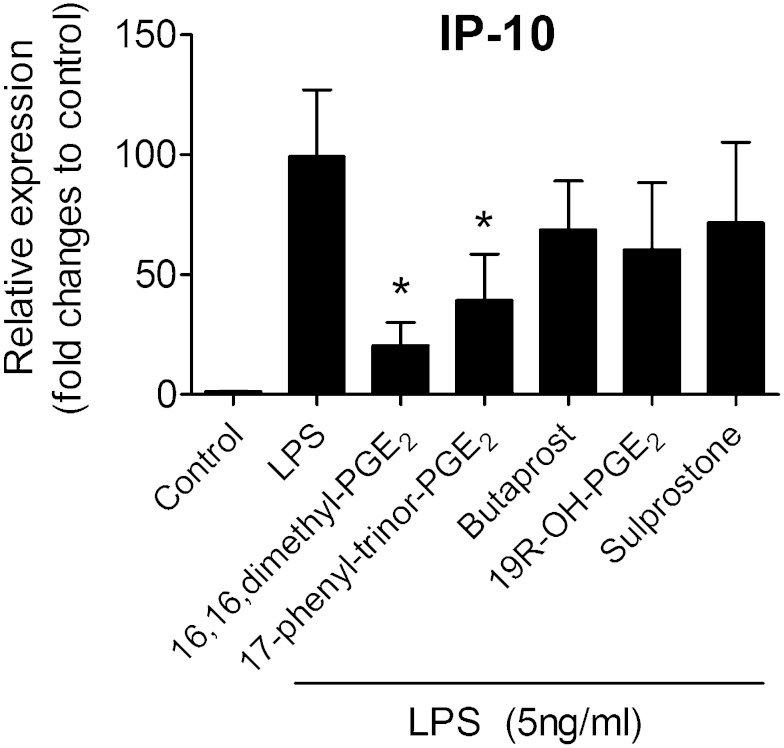
To test directly whether other EP receptor subtypes modulate chemokine production, a range of EP receptor agonists were used to test their effects on LPS-induced IP-10 mRNA expression. The nonselective EP agonist 16,16,dimethyl-PGE2 significantly reduced the LPS-induced increase in mRNA expression of IP-10, mimicking the effects produced by PGE2 (Fig. 5). Likewise, 17-phenyl-trinor-PGE2 (an agonist at both EP1 and EP3 receptors), significantly suppressed IP-10 mRNA expression elicited by LPS. The selective EP3 agonist sulprostone and the selective EP2 agonists, butaprost and 19(R)-hydroxyl-PGE2, had no significant effect on LPS-induced IP-10 mRNA (Fig. 5). The present results do not support roles for EP2 and EP3 acting as anti-inflammatory mediators in adipose tissue, but they do not exclude the involvement of EP1.
Fig. 5.
Role of other EP receptors in adipose tissue inflammation.C57BL/6 adipose tissue explants were pretreated with different EP agonists [16,16-dimethyl-PGE2 (1 µM), a nonselective EP1/EP2/EP3/EP4 agonist; 17-phenyl-trinor-PGE2 (1 µM), a nonselective EP1/3 agonist; butaprost (100 nM), a selective EP2 agonist; 19(R)-hydroxyl-PGE2 (1 µM), a selective EP2 agonist; sulprostone (20 nM), a selective EP3 agonist] for 40 min before the exposure to LPS (5 ng/ml for 9 h). mRNA expression of IP-10 for different treatment groups was expressed as fold changes to control (without LPS stimulation). N = 5; *P < 0.05 vs. LPS. All data are expressed as means ± SEM.
High-fat diet
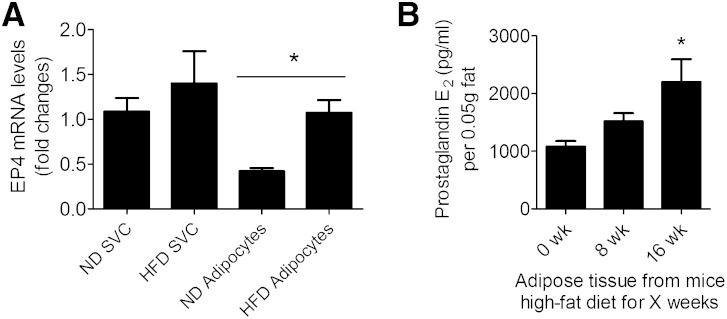
Quantitative RT-PCR measurements were performed to examine whether the expression of EP receptors changes in eWAT during consumption of a high-fat diet. The mRNA level of EP1, mPGES-1, mPGES-2, and cPGES-2 did not change significantly in either EP4+/+ and EP4−/− mice consuming a high-fat diet (Supplementary Fig. 7), but the mRNA levels of EP2, EP3, and EP4 increased with the duration of the high-fat diet in EP4+/+ mice (Supplementary Fig. 7). Similar observations were made for the mRNA levels of EP2 and EP3 in eWAT from EP4−/− mice (Supplementary Fig. 7). To identify the cell origin of EP4 within adipose tissues, RT-PCR was performed on adipocytes and SVCs from normal or high-fat-fed mice (Fig. 6A). The data indicate that high-fat feeding enhanced the expression of EP4 on adipocytes, but not on SVCs. We also measured PGE2 in the supernatant of minced eWAT to show that adipose tissue directly produces this prostanoid. The amount of PGE2 released by adipose tissue increased with the duration of the high-fat diet (Fig. 6B).
Fig. 6.
Expression of EP4 receptors and production of PGE2 during consumption of a high-fat diet. A: mRNA expression of EP4 in adipocytes and SVCs from normal (ND, for 16 weeks) or high-fat diet-fed (HFD, for 16 weeks) EP4 wild-type mice. N = 5. *, P < 0.05 ND adipocytes versus HFD adipocytes. B: Amount of PGE2 produced per 0.05 g of adipose tissue in 1 ml media. N = 6; *, P < 0.05 versus 0 weeks. All data are expressed as means ± SEM.
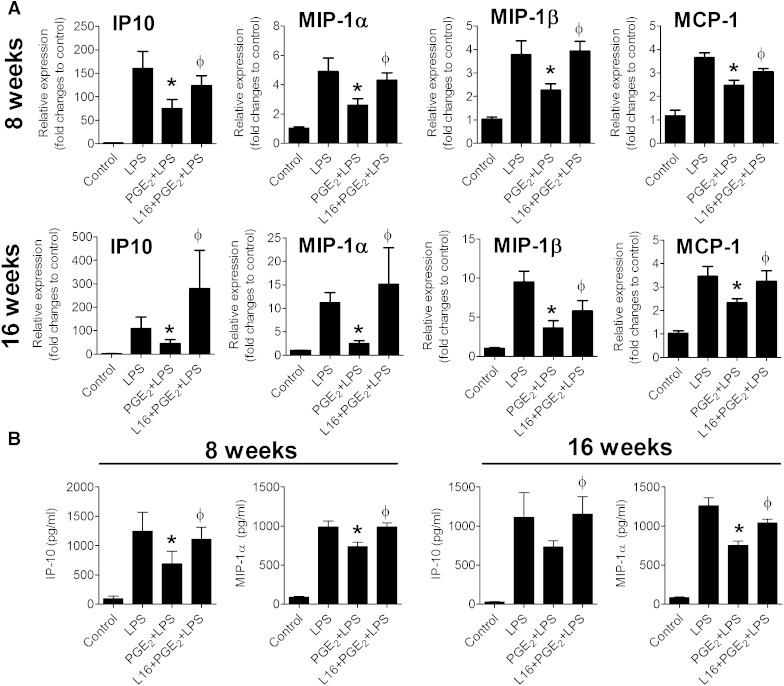
We then examined whether PGE2-EP4 dependent anti-inflammatory responses persist during consumption of a high-fat diet. Explants of adipose tissue derived from EP4+/+ mice fed a high-fat diet for 8 or 16 weeks were exposed to PGE2 (50 nM) with or without preexposure to L161,982 (100 nM). PGE2 suppressed LPS-stimulated IP-10 and MIP-1α mRNA expression in adipose tissue from high-fat-fed mice and L161,982 prevented this suppression (Fig. 7A). Altered IP-10 and MIP-1α protein levels paralleled the mRNA changes (Fig. 7B). In addition, PGE2 suppressed MIP-1β and MCP-1 expression in adipose tissue explants from high-fat-fed mice (8 or 16 weeks of high-fat diet) and L161,982 reversed this suppression (Fig. 7A). PGE2 did not affect RANTES expression in adipose tissue from mice fed a high-fat diet for 8 or 16 weeks (Supplementary Fig. 8). These results not only indicate that EP4-dependent anti-inflammatory responses persisted during high-fat diet, but also that EP4 could suppress a broader range of chemokines during high-fat diet challenge.
Fig. 7.
Adipose tissues of high-fat-fed mice display EP4-dependent suppression of chemokine expression. Adipose tissue explants from EP4+/+ mice fed a high-fat diet for 8 or 16 weeks were treated with PGE2 (50 nM) in the presence or absence of a selective EP4 antagonist (L161,982). After 1.5 h of treatment, the cells were stimulated with LPS for 9 h. A: mRNA expression of IP-10, MIP-1α, MIP-1β, and MCP-1 in different samples were quantified. Data are expressed in relative amount produced in control. N = 6; *, P < 0.05 versus LPS; ɸ, P < 0.05 versus PGE2+LPS. B: IP-10 and MIP-1α protein production in the medium as measured by ELISA. N = 6; *P < 0.05 versus LPS; ɸ, P < 0.05 versus PGE2+LPS. All data are expressed as means ± SEM.
EP4 deficiency and inflammation in vivo
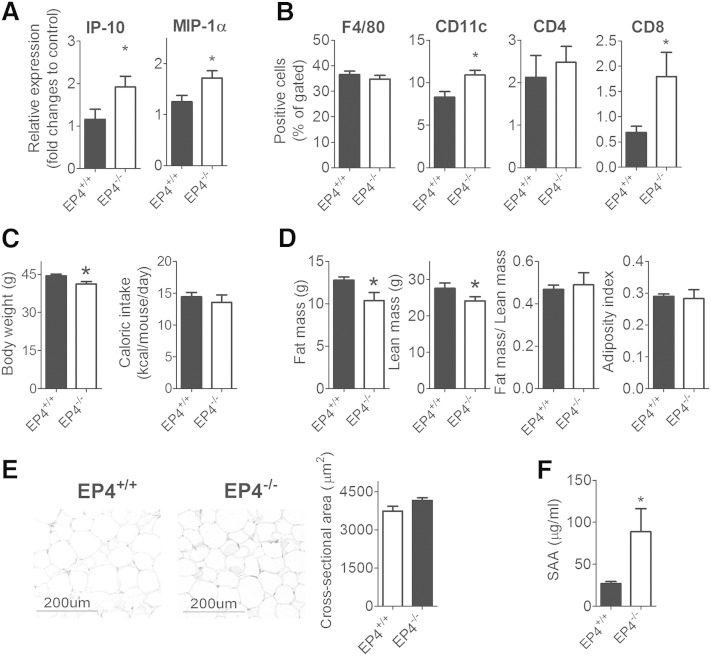
To demonstrate that EP4 deficiency in high-fat-fed mice aggravates adipose tissue and systemic inflammation, adipose tissue from EP4+/+ and EP4−/− mice that consumed a high-fat diet for 16 weeks was used for analysis. The eWAT from EP4−/− mice had significantly higher levels of IP-10 and MIP-1α mRNA than that of EP4+/+ mice (Fig. 8A). Flow cytometry on SVCs isolated from adipose tissue quantified inflammatory cells. Deficiency of EP4 in mice did not alter the amount of F4/80 (pan-macrophage marker)- and CD4-positive cells within eWAT (Fig. 8B). However, eWAT from EP4−/− mice contained more CD11c (a marker of M1 macrophages)- and CD8-positive cells than that of EP4+/+ mice (Fig. 8B).
Fig. 8.
EP4 deficiency in high-fat-fed mice aggravates adipose tissue and systemic inflammation but did not alter adiposity in vivo. A: IP-10 and MIP-1α mRNA levels in eWAT of EP4+/+ and EP4−/− mice; B: SVCs isolated from adipose tissues of EP4+/+ and EP4−/− mice were stained with F4/80-, CD11c-, CD4-, and CD8-specific antibodies; C: Body weight and caloric intake of EP4+/+ and EP4−/− mice. D: Fat and lean mass composition of EP4+/+ and EP4−/− mice; E: Histology and adipocyte size of eWAT from EP4+/+ and EP4−/− mice; F: The amount of SAA in plasma of EP4+/+ and EP4−/− mice. N = 6; *, P < 0.05 versus EP4+/+. All data are expressed as means ± SEM.
After 16 weeks of high-fat diet, the body weight of EP4−/− was significantly lower than that of EP4+/+ mice (Fig. 8C). EP4 deficiency did not alter calorie intake (Fig. 8C). EP4−/− mice had an 18.73% reduction in fat mass and 12.65% reduction in lean mass compared with EP4+/+ mice. However, the fat to lean mass ratio or the adiposity index did not differ between EP4+/+ and EP4−/− mice (Fig. 8D). Dissection of adipose tissues revealed that EP4−/− mice had significantly less subcutaneous white adipose tissue and brown adipose tissue than wild-type mice (Supplementary Fig. 9). EP4−/− mice also appeared to have less epididymal and peri-renal white adipose tissue but this difference did not reach statistical significant (Supplementary Fig. 9). EP4 deficiency did not alter the size of adipocytes within eWAT (Fig. 8E). EP4−/− mice had greater plasma levels of SAA, indicating increased systemic inflammation (Fig. 8F).
EP4 agonist and inflammation in vivo
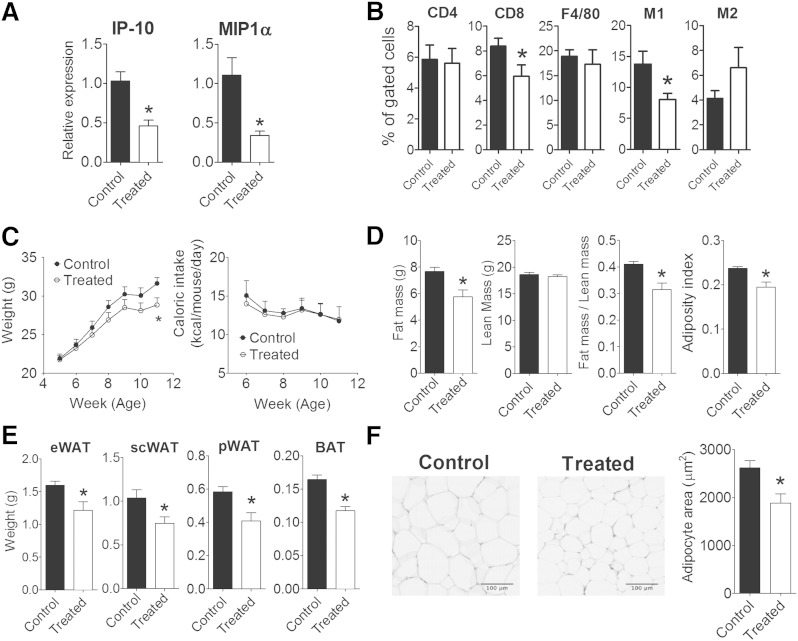
To further confirm the relevance of EP4 in the inflammatory network in vivo, whether or not pharmacological activation of EP4 is effective against the development of diet-induced obesity was examined. High-fat-fed C57BL/6 mice administrated with the EP4 agonist [CAY10580; 200 µg/kg (15)] or solvent for 6 weeks provided adipose tissue for analysis. EP4 agonist-treated mice had significantly lower IP-10 and MIP-1α mRNA levels in their eWAT than control mice (Fig. 9A). The results from the high-fat-fed EP4+/+ and EP4−/− mouse study suggest that subclasses of macrophages (e.g., CD11c-bearing cells) may vary in quantity between EP4+/+ and EP4−/− mice, despite a lack of difference in total macrophage numbers (as quantified by F4/80-bearing cells). To test this interpretation, SVCs from adipose tissue underwent triple staining to delineate a profile attributed to M1 macrophages [F4/80+CD11c+CD206-(24)] or one marking M2 macrophages [F4/80+CD11c-CD206+(24)] in mice, along with CD4+, and CD8+ staining to quantify the involvement of different T lymphocyte subclasses during chronic EP4 activation. EP4 agonist-treated and control mice fed on high-fat diet had comparable amounts of F4/80-positive cells, as determined by flow cytometry (Fig. 9B). The triple staining revealed that EP4 agonist-treated mice have a significantly lower amount of M1 pro-inflammatory macrophages (F4/80+CD11c+CD206−) but a higher amount of M2 anti-inflammatory macrophages (F4/80+CD11c-CD206+) in their adipose tissue than control mice (Fig. 9B). The amounts of CD4+-positive cells did not differ significantly, but adipose tissue of EP4 agonist-treated mice contained significantly fewer CD8+-positive cells than that of control mice (Fig. 9B).
Fig. 9.
Administration of EP4 agonist limits adipose tissue inflammation and decreased adiposity in vivo. A: IP-10 and MIP-1α mRNA levels in eWAT of C57BL/6 mice without (control) or with (treated) EP4 agonist (CAY10580; 200 µg/kg) treatment; B: SVCs isolated from adipose tissue of control or treated C57BL/6 mice were singly-stained with CD4- or CD8- specific antibodies, or triple-stained with PE anti-mouse F4/80, FITC anti-mouse CD11c, and Alexa Fluor® 647 anti-mouse CD206 to identify M1 macrophages (F4/80+CD11c+CD206−) and M2 macrophages (F4/80+CD11c-CD206+). C: The body weight and caloric intake of control and treated C57BL/6 mice. D: Fat and lean mass composition of control and treated C57BL/6 mice. E: Weight of epididymal (eWAT), subcutaneous (scWAT), peri-renal (pWAT) white adipose tissue and brown adipose tissue (BAT) of control and treated C57BL/6 mice. F: Histology and adipocyte size of eWAT from control and treated C57BL/6 mice. N = 6; *, P < 0.05 versus control. All data are expressed as means ± SEM.
After 6 weeks of EP4 agonist treatment, the body weight of mice was significantly decreased (by 8.95%) when compared with vehicle-treated mice (Fig. 9C). Administration of the EP4 agonist did not alter calorie intake (Fig. 9C). The reduced weight gain was attributed to reduce fat depositions (by 24.7% compared with vehicle-treated mice) without alteration in lean mass. The fat to lean mass ratio and the adiposity index of EP4 agonist-treated mice were reduced by 23.2% and 20.8%, respectively, when compared with vehicle-treated mice (Fig. 9D). Treatment with the EP4 agonist reduced the mass of adipose tissue depots (including epididymal, subcutaneous, peri-renal white adipose depots and brown adipose tissue; Fig. 9E) and the size of adipocytes within the adipose tissue (Fig. 9F).
Discussion
PGE2 suppresses inflammation via activation of EP4 receptors in mouse adipose tissue. This conclusion emerged from the observations that PGE2 attenuated LPS-induced mRNA and protein expression of chemokines, including IP-10 and MIP-1α (and also cytokines IL-6 and IL-8) in mouse adipose tissue. An EP4 antagonist (L161,982) reversed this anti-inflammatory response. Furthermore, adipose tissue derived from EP4-deficient mice did not exhibit this effect, confirming its dependency on EP4. The ability of EP4 agonists (CAY10580 and CAY10598) to suppress chemokines from stimulated adipose tissue independently supported the involvement of EP4 in the response. These experiments identify a novel counterregulatory property of an endogenously produced prostanoid, and suggest that activators of EP4 receptor represent a novel strategy to combat inflammation in fat.
To further evaluate the relevance of EP4 in the inflammatory network in vivo, whether or not pharmacological activation of EP4 is effective against the development of diet-induced obesity was examined. Indeed, administration of an EP4 agonist to high-fat-fed C57BL/6 mice decreased the expression of the pro-inflammatory chemokines IP-10 and MIP1α in adipose tissue. The reduced accumulation of CD8-positive cells (a marker of cytotoxic lymphocytes) and the shifting of the macrophage polarization from the pro-inflammatory (M1) to the anti-inflammatory (M2) profile in the adipose tissue affirmed the functional in vivo consequences of decreased mRNA levels of these pro-inflammatory chemokines following EP4 activation. The EP4 agonist reduced weight gain in mice and reduced fat depositions (in terms of mass and size of adipocytes) without altering food intake. Therefore, activation of EP4 by CAY10580 limited adipose tissue inflammation in vivo and provided protection against diet induced-obesity.
The in vivo impact of genetic deficiency in EP4 on diet-induced adipose tissue inflammation and obesity in mice was also studied. EP4−/− mice exhibited an enhanced inflammatory status at the adipose tissue level. This is illustrated by the upregulation of the pro-inflammatory chemokines IP-10 and MIP-1α resulting in enhanced accumulation of CD8-positive and CD11c-positive (a marker of M1 macrophages) cells in the adipose tissue. Furthermore, EP4 deficiency augmented the plasma level of SAA, a prominent marker of systemic inflammation. Surprisingly, EP4−/− mice did not exhibit exacerbated diet-induced obesity. Instead, their body weight was lower than that of wild-type mice exposed to the same diet, suggesting that EP4 selectively limits the inflammatory features of diet-induced obesity rather than its metabolic phenotype. The reduced body weight in EP4−/− mice was not due to changes in food intake or reduction in adiposity. Although EP4−/− mice had less fat mass, there was no overall change in fat mass to lean mass ratio or in adiposity index because lean mass was correspondingly decreased in these mice. Sizes of adipocyte within the eWAT did not differ between EP4+/+ and EP4−/− mice, further supporting the conclusion that deficiency in EP4 did not impact on adiposity per se.
Adipose tissue consists of adipocytes and SVCs, and both cell fractions can release chemokines. Earlier studies described PGE2-EP4 anti-inflammatory signaling in macrophages, but not in vascular endothelial or smooth muscle cells (5, 25). These observations led to the expectation that PGE2’s anti-inflammatory effects in adipose tissue may predominate in SVCs, the cell fraction containing macrophages. Yet, the present findings demonstrate that, adipocytes, not SVCs, account for the anti-inflammatory effect of PGE2 in fat. The adipose tissues derived from relatively healthy mice in this study contained few macrophages or inflammatory cells within the SVC fraction, possibly accounting for this result. High-fat-fed mice, with adipose tissue that contains more inflammatory cells (24), indeed show prominent anti-inflammatory effect of PGE2 in both adipocytes and SVCs.
Adipocytes contribute to the overall inflammation status of adipose tissue in obese mice (26). Although adipocytes yield less IP-10 and MIP-1α than SVCs do on a per-cell basis, in any given fat pads, the absolute number of adipocytes usually exceeds that of SVCs. Thus, the cumulative amount of chemokines produced by adipocytes likely surpasses that produced by SVCs. Also of note, exogenous PGE2 attenuated IP-10 and MIP-1α expression to a greater extent in LPS-stimulated adipose tissue explants compared with collagenase-isolated adipocytes. The coexistence of SVCs and adipocytes in adipose tissue explants may exert synergistic effects on PGE2-EP4 dependent anti-inflammatory actions.
To investigate the signaling pathway for EP4-mediated anti-inflammatory actions, the effect of 8-Br-cAMP (the cell membrane-permeable analog of cAMP), 6-MP-cAMP, (a PKA activator) and 8-PCT-2’-O-ME-cAMP (an Epac activator) on LPS-mediated IP-10 levels was tested. Either activation of PKA or Epac mimicked PGE2-mediated suppression of IP-10 expression. Hence, the results suggest that the anti-inflammatory action of EP4 in adipose tissue involves both PKA and Epac. To further establish the role of PKA or Epac in the response, PKA or Epac inhibitors were used to test whether these agents could reverse the PGE2-mediated reduction in IP-10 levels. Only simultaneous inhibition of both PKA and Epac effectively reverses the response. Blocking one effector was not sufficient to prevent the effect; presumably the blockade of Epac pathway led to the shunting of cAMP to PKA pathway and vice versa.Only simultaneous blockage of both pathways could reverse the anti-inflammatory effects demonstrated by PGE2.
Pro-inflammatory cytokines such as TNFα participate in adipose tissue inflammation. TNF-α significantly stimulated the mRNA expression of IP-10 in mouse adipose tissue, but PGE2 did not alter this increase. Yet, when the adipose tissue was treated with L161,982 before PGE2 exposure, TNF-α-induced IP-10 mRNA production rose significantly. These results indicate that stimulation by PGE2, in the absence of EP4, promotes further production of IP-10. Although PGE2 alone exerts no overall effect on TNF-α-mediated responses, both pro-inflammatory and anti-inflammatory effects may have occurred simultaneously in TNF-α-stimulated adipose tissue such that the opposing effects counterbalance each other. Conversely, blocking of EP4 receptors with L161,982 inhibited the anti-inflammatory component and permitted the pro-inflammatory response evoked by the activation of other EP receptors to predominate. Similarly, the addition of L161,982 before that of PGE2 caused a further increase in MIP-1α expression in response to TNF-α. In line with these observations, PGE2 significantly increased IP-10 and MIP-1α mRNA expression in TNF-α-activated adipose tissue from EP4−/− mice. These results therefore demonstrate that, at least in vitro, EP4 operates as an anti-inflammatory effector in adipose tissues stimulated by TNF-α.
Activation of EP4 receptors mediates the PGE2 anti-inflammatory response in macrophages, as a selective EP4 receptor antagonist inhibits this action, and because macrophages derived from EP4−/− mice lack this response (4, 6). Human macrophages do not express EP1 and EP3, and have low levels of EP2 (4). The present study demonstrates that adipose tissue, in contrast to macrophages, contains mRNA that encodes all known EP receptors. Thus, receptors other than EP4 may modulate the inflammatory response in fat. The present results do not support roles for EP2 and EP3 acting as anti-inflammatory mediators in adipose tissue, based on the lack of effect of butaprost, 19(R)-hydroxyl-PGE2, and sulprostone on LPS-stimulated IP-10 release. However, 17-phenyl-trinor PGE2 (an agonist at both EP1 and EP3 receptors) reduced LPS-stimulated IP-10 release. As the selective EP3 agonist sulprostone did not affect the response, the effect of 17-phenyl-trinor-PGE2 presumably results from the action on EP1 rather than EP3. Taken together, the present findings imply that the activation of EP2 or of EP3 does not contribute to anti-inflammation in adipose tissue, but they do not exclude the involvement of EP1.
PGE2-EP4-dependent anti-inflammatory responses persist in adipose tissue from high-fat-fed mice. Indeed, the present findings suggest that the anti-inflammatory actions of EP4 become more important on a high-fat diet, in that EP4 activation suppresses a greater variety of chemokines. L161,982 did not reverse PGE2-mediated suppression of MIP-1β in adipose tissue explants of mice fed a normal diet, but did restore PGE2-mediated suppression of MIP-1β in such explants from high-fat-fed mice. Likewise, PGE2 did not suppress MCP-1 expression in adipose tissue of mice fed a normal diet, but it reduced MCP-1 levels in those from high-fat-fed mice. This reduction of MCP-1 by PGE2 in adipose tissue of high-fat-fed mice depends on EP4 activation, as shown by its blockade by L161,982. Perhaps EP4 more effectively suppresses the aggravated inflammation provoked by the high-fat diet. The increased expression of EP4 and the augmented production of PGE2 with the high-fat diet favor this hypothesis, and may serve as a counter-regulatory mechanism toward homeostasis in the organism stressed by obesity.
Cyclooxygenase-2 deficient mice have attenuated adipose tissue inflammation (27). Aspirin, which blocks both cyclooxygenase-1 and -2, is well-known to exert protection against coronary events (13, 14), which also involves an inflammatory component. These observations run counter to the assertion of the present study that cyclooxygenase products are anti-inflammatory. Cyclooxygenase is an upstream enzyme that produces a variety of prostaglandins that exhibit differential effects. Other prostaglandins via its respective receptor may exert pro-inflammatory effects, while PGE2 acting on EP4 regulates the anti-inflammatory arm. The production of different prostaglandins in different quantities is likely to regulate the overall inflammatory environment.
The present experiments collectively establish a primary role for EP4 receptors in the anti-inflammatory action of PGE2 in mouse adipose tissue, and illustrate that targeting these receptors could comprise a novel strategy to mitigate obesity-induced inflammation.
Supplementary Material
Footnotes
Abbreviations:
- 8-Br-cAMP
- 8-bromo-cyclic AMP
- 6-MB-cAMP
- N6- monobutyryladenosine-3’,5’-cyclic monophosphate
- 8-PCT-2’-O-ME-cAMP
- 8-(4-Chlorophenylthio)-2’-O-methyladenosine 3’,5’-cyclic monophosphate monosodium
- cPGES
- cytosolic prostaglandin E2 synthase
- Epac
- exchange factor directly activated by cAMP
- EP4
- prostaglandin E receptor 4
- eWAT
- epididymal white adipose tissue
- IL
- interleukin
- IP
- interferon-γ-inducible protein
- LPS
- lipopolysaccharide
- MIP
- macrophage inflammatory protein
- mPGES
- membrane prostaglandin E2 synthase
- PGE2
- prostaglandin E2
- PKA
- protein kinase A
- SAA
- serum amyloid A
- SVC
- stromal vascular cell
This work was supported in part by grants from the National Heart, Lung, and Blood Institute (RO1 HL080472), the D.W. Reynolds Foundation, the Fondation Leducq (to P.L.), The University of Hong Kong Research Centre of Heart Brain Hormone and Healthy Aging (to E.H.C.T.), The University of Hong Kong Seed Funding Programme for Basic Research (to E.H.C.T.), and RGC General Research Fund (HKU778012M; to E.H.C.T.).
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of nine figures and one table.
REFERENCES
- 1.Rocha V. Z., Libby P. 2008. The multiple facets of the fat tissue. Thyroid. 18: 175–183. [DOI] [PubMed] [Google Scholar]
- 2.Tso A. W., Xu A., Chow W. S., Lam K. S. 2008. Adipose tissue and the metabolic syndrome: focusing on adiponectin and several novel adipokines. Biomark Med. 2: 239–252. [DOI] [PubMed] [Google Scholar]
- 3.Amer M., Bead V. R., Bathon J., Blumenthal R. S., Edwards D. N. 2010. Use of nonsteroidal anti-inflammatory drugs in patients with cardiovascular disease—a cautionary tale. Cardiol. Rev. 18: 204–212. [DOI] [PubMed] [Google Scholar]
- 4.Takayama K., Garcia-Cardena G., Sukhova G. K., Comander J., Gimbrone M. A., Jr, Libby P. 2002. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J. Biol. Chem. 277: 44147–44154. [DOI] [PubMed] [Google Scholar]
- 5.Takayama K., Sukhova G. K., Chin M. T., Libby P. 2006. A novel prostaglandin E receptor 4-associated protein participates in anti-inflammatory signaling. Circ. Res. 98: 499–504. [DOI] [PubMed] [Google Scholar]
- 6.Minami M., Shimizu K., Okamoto Y., Folco E., Ilasaca M. L., Feinberg M. W., Aikawa M., Libby P. 2008. Prostaglandin E2 receptor type 4-associated protein interacts directly with NKκB1 and attenuates macrophage activation. J. Biol. Chem. 283: 9692–9703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang E. H., Libby P., Vanhoutte P. M., Xu A. 2012. Anti-inflammation therapy by activation of prostaglandin EP4 receptor in cardiovascular and other inflammatory diseases. J. Cardiovasc. Pharmacol. 59: 116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang E. H., Shimizu K., Christen T., Rocha V. Z., Shvartz E., Tesmenitsky Y., Sukhova G., Shi G. P., Libby P. 2011. Lack of EP4 receptors on bone marrow-derived cells enhances inflammation in atherosclerotic lesions. Cardiovasc. Res. 89: 234–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang E. H., Shvartz E., Shimizu K., Rocha V. Z., Zheng C., Fukuda D., Shi G. P., Sukhova G., Libby P. 2011. Deletion of EP4 on bone marrow-derived cells enhances inflammation and angiotensin II-induced abdominal aortic aneurysm formation. Arterioscler. Thromb. Vasc. Biol. 31: 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ogawa M., Suzuki J., Kosuge H., Takayama K., Nagai R., Isobe M. 2009. The mechanism of anti-inflammatory effects of prostaglandin E2 receptor 4 activation in murine cardiac transplantation. Transplantation. 87: 1645–1653. [DOI] [PubMed] [Google Scholar]
- 11.Xiao C. Y., Yuhki K., Hara A., Fujino T., Kuriyama S., Yamada T., Takayama K., Takahata O., Karibe H., Taniguchi T., et al. 2004. Prostaglandin E2 protects the heart from ischemia-reperfusion injury via its receptor subtype EP4. Circulation. 109: 2462–2468. [DOI] [PubMed] [Google Scholar]
- 12.Hishikari K., Suzuki J., Ogawa M., Isobe K., Takahashi T., Onishi M., Takayama K., Isobe M. 2009. Pharmacological activation of the prostaglandin E2 receptor EP4 improves cardiac function after myocardial ischaemia/reperfusion injury. Cardiovasc. Res. 81: 123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mukherjee D., Nissen S. E., Topol E. J. 2001. Risk of cardiovascular events associated with selective COX-2 inhibitors. JAMA. 286: 954–959. [DOI] [PubMed] [Google Scholar]
- 14.Grosser T., Fries S., FitzGerald G. A. 2006. Biological basis for the cardiovascular consequences of COX-2 inhibition: therapeutic challenges and opportunities. J. Clin. Invest. 116: 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Billot X., Chateauneuf A., Chauret N., Denis D., Greig G., Mathieu M. C., Metters K. M., Slipetz D. M., Young R. N. 2003. Discovery of a potent and selective agonist of the prostaglandin EP4 receptor. Bioorg. Med. Chem. Lett. 13: 1129–1132. [DOI] [PubMed] [Google Scholar]
- 16.Machwate M., Harada S., Leu C. T., Seedor G., Labelle M., Gallant M., Hutchins S., Lachance N., Sawyer N., Slipetz D., et al. 2001. Prostaglandin receptor EP4 mediates the bone anabolic effects of PGE2. Mol. Pharmacol. 60: 36–41. [DOI] [PubMed] [Google Scholar]
- 17.Li J. H., Chou C., Li B., Gavrilova O., Eisner C., Schnermann J., Anderson S. A., Deng C. X., Knepper M. A., Wess J. 2009. A selective EP4 PGE2 receptor agonist alleviates disease in a new mouse model of X-linked nephrogenic diabetes insipidus. J. Clin. Invest. 119: 3115–3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robert A., Schultz J. R., Nezamis J. E., Lancaster C. 1976. Gastric antisecretory and antiulcer properties of PGE2, 15-methyl PGE2, and 16,16-dimethyl PGE2. Intravenous, oral and intrajejunal administration. Gastroenterology. 70: 359–370. [PubMed] [Google Scholar]
- 19.Miller W. L., Weeks J. R., Lauderdale J. W., Kirton K. T. 1975. Biological activities of 17-phenyl-18,19,20-trinorprostaglandins. Prostaglandins. 9: 9–18. [DOI] [PubMed] [Google Scholar]
- 20.Kiriyama M., Ushikubi F., Kobayashi T., Hirata M., Sugimoto Y., Narumiya S. 1997. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 122: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woodward D. F., Protzman C. E., Krauss A. H., Williams L. S. 1993. Identification of 19(R)-OH prostaglandin E2 as a selective prostanoid EP2-receptor agonist. Prostaglandins. 46: 371–383. [DOI] [PubMed] [Google Scholar]
- 22.Negishi M., Harazono A., Sugimoto Y., Hazato A., Kurozumi S., Ichikawa A. 1994. TEI-3356, a highly selective agonist for the prostaglandin EP3 receptor. Prostaglandins. 48: 275–283. [DOI] [PubMed] [Google Scholar]
- 23.Sugimoto Y., Narumiya S. 2007. Prostaglandin E receptors. J. Biol. Chem. 282: 11613–11617. [DOI] [PubMed] [Google Scholar]
- 24.Fujisaka S., Usui I., Bukhari A., Ikutani M., Oya T., Kanatani Y., Tsuneyama K., Nagai Y., Takatsu K., Urakaze M., et al. 2009. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 58: 2574–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacKenzie K. F., Clark K., Naqvi S., McGuire V. A., Nöehren G., Kristariyanto Y., van den Bosch M., Mudaliar M., McCarthy P. C., Pattison M. J., et al. 2013. PGE(2) induces macrophage IL-10 production and a regulatory-like phenotype via a protein kinase A-SIK-CRTC3 pathway. J. Immunol. 190: 565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lumeng C. N., Liu J., Geletka L., Delaney C., Delproposto J., Desai A., Oatmen K., Martinez-Santibanez G., Julius A., Garg S., et al. 2011. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J. Immunol. 187: 6208–6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghoshal S., Trivedi D. B., Graf G. A., Loftin C. D. 2011. Cyclooxygenase-2 deficiency attenuates adipose tissue differentiation and inflammation in mice. J. Biol. Chem. 286: 889–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.