Abstract
Ligand-activated receptors regulate numerous genes, and mediate effects of a broad set of endogenous and exogenous chemicals in vertebrates. Understanding the roles of these transcription factors in zebrafish (Danio rerio) is important to the use of this non-mammalian model in toxicological, pharmacological, and carcinogenesis research. Response to a potential agonist for the pregnane X receptor (Pxr) [pregnenolone (PN)] was examined in developing zebrafish, to assess involvement of Pxr in regulation of selected genes, including genes in cytochrome P450 subfamilies CYP2 and CYP3. We also examined interaction of Pxr and the aryl hydrocarbon receptor (Ahr) signaling pathways. Pregnenolone caused a dose-dependent increase in mRNA levels of pxr, ahr2, CYP1A, CYP2AA1, CYP2AA12, CYP3A65, and CYP3C1, most of which peaked at 3 µM PN. The well-known Ahr agonist 3,3’,4,4’,5-pentachlorobiphenyl (PCB126) also upregulated expression of pxr, ahr2, CYP1A, CYP2AA12, CYP3A65, and CYP3C1 in a dose-dependent manner. Inhibition of pxr translation by morpholino antisense oligonucleotides (MO) suppressed PN-induced expression of pxr, ahr2, CYP3A65, and CYP3C1 genes. Levels of CYP2AA1 and CYP2AA12 mRNA were increased in the control-MO group exposed to PN; this was prevented by knocking down Pxr. Similarly, Ahr2-MO treatment blocked PCB126-induced mRNA expression of pxr, CYP1A, CYP2AA12, CYP3A65, and CYP3C1. The present study shows self-regulation of pxr by PN in developing zebrafish. Selected zebrafish CYP1, CYP2 (including several CYP2AAs) and CYP3 genes appear to be under the regulation of both Pxr and Ahr2.
Keywords: cytochrome P450, pregnane X receptor, aryl hydrocarbon receptor, zebrafish, polychlorinated biphenyl
Nuclear receptor NR1I2, the pregnane X receptor (PXR; also known as the steroid and xenobiotic receptor, SXR), and the related NR1I3, the constitutive androstane receptor (CAR) are ligand-activated transcription factors often referred to as “xenobiotic sensors” (eg, Kretschmer and Baldwin, 2005; Timsit and Negishi, 2007; Willson and Kliewer, 2002). The aryl hydrocarbon receptor (Ahr) also is a ligand-dependent transcription factor that in vertebrates is activated by xenobiotics such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and related compounds (Fernandez-Salguero et al., 1996; Safe, 1990). Together, these receptors act to protect organisms from exogenous and endogenous toxic chemicals by regulating genes involved in xenobiotic metabolism and elimination, including cytochrome P450 (CYP) genes, various transferases, and ABC transporters (Kliewer et al., 2002; Kohle and Bock, 2009; Zhang et al., 2008). The extent to which these receptors are governing responses to different chemicals is not understood.
Mammalian PXR ligands include a suite of environmental pollutants, drugs, and endogenous compounds such as bile salts and steroids (eg, Ekins et al., 2007; Kretschmer and Baldwin, 2005; Zhang et al., 2008). In cell-based assays, human PXR is activated by a large majority (234 of 320) of the EPA’s ToxCast_320 environmental substances (Martin et al., 2010). However, the ligand selectivity of PXR differs markedly among mammals. Thus, human PXR is strongly activated by rifampicin but not pregnenolone-16α-carbonitrile (PCN), whereas rodent PXR is strongly activated by PCN but not rifampicin (Jones et al., 2000; Moore et al., 2002). Similarly, clotrimazole (CLO) is an agonist for human but not mouse PXR (Moore et al., 2002). Such dramatic species differences in PXR activation have important implications for understanding effects of agonists in comparative physiology, and for the use of animal models to predict human risk.
The CAR gene diverged from PXR during the course of vertebrate evolution; however, CAR was lost from or arose after divergence of the teleost fish line (Mathas et al., 2012). Thus, teleost fish such as zebrafish do not have CAR, magnifying the potential role of PXR in xenobiotic responses in these fish. In general, narrower selectivity has been observed for fish PXR (Ekins et al., 2008; Krasowski et al., 2011; Milnes et al., 2008; Moore et al., 2002) than for human PXR, and fish PXR appears to differ from the mammalian orthologs in endogenous ligand specificity (Ekins et al., 2008; Reschly et al., 2007). These observations are derived from cell-based reporter systems, but the ligand efficacy and transcriptional landscape of Pxr in vivo in zebrafish are unknown.
Induction of rodent CYP2s and CYP3s has long been known to occur through the action of PXR and CAR (Waxman, 1999). Recently, we and others reported that chemicals identified as PXR agonists in cell-based in vitro studies were able to induce some CYP2 and CYP3A genes in fish (Bresolin et al., 2005; Kubota et al., 2013); however, the mechanism underlying chemical induction of fish CYP2 and CYP3 genes is not yet understood. Moreover, while some genes, eg, CYP3A65, are candidate targets of zebrafish Pxr, based on orthology to the PXR-regulated CYP3A genes in mammals, studies suggest that other transcription factors may be involved as well. Thus, zebrafish Ahr is responsible for dioxin and non-ortho-polychlorinated biphenyl induction of CYP1 family target genes (Handley-Goldstone et al., 2005; Jonsson et al., 2012; Kubota et al., 2011), but studies have shown that TCDD also can induce CYP3A65 in zebrafish (Chang et al., 2013; Tseng et al., 2005). Whether the Ahr is directly involved in regulating the expression of genes that also are regulated by Pxr in zebrafish is not known.
Expanding our knowledge of the role of Pxr in regulating the expression of genes in vivo, and of the nature of cross-regulation of genes by Ahr and Pxr, is critical to establishing a mechanistic foundation for understanding and screening for chemical effects in this premiere toxicological model. We recently reported cloning, expression, and activation of full-length Pxr in zebrafish (Bainy et al., 2013). Here we address the role of zebrafish Pxr and Ahr2 in regulation of target CYP genes in vivo in developing zebrafish. Pxr involvement in response to agonists was established using morpholino antisense oligonucleotides (MO) to knock down translation of pxr, coupled with a search for putative Pxr response elements in proximal promoters of target genes. We also examined the role of Ahr2 in regulating the expression of the same genes examined for Pxr regulation, seeking cross-talk between the Pxr and Ahr in zebrafish.
FOOTNOTES
Reference to Gene Name
Zebrafish cytochrome P450 family genes/mRNAs and proteins are referred to as CYP and CYP according to Nelson et al. (1996). For other genes/mRNAs and proteins in zebrafish, we have followed the approved guidelines for zebrafish, eg, pxr and Pxr (https://wiki.zfin.org/display/general/ZFIN+Zebrafish+Nomenclature+Guidelines). When not referring to a particular species, capitalized abbreviations are used, eg, PXR and PXR.
MATERIALS AND METHODS
Fish Husbandry
The Tupfel/long fin wild-type strain of zebrafish was used. Fertilized eggs were obtained by breeding multiple groups of 30 females and 15 males as described previously (Jonsson et al., 2007). The day after fertilization, unfertilized eggs and dead embryos were removed. Embryos and eleutheroembryos (hatched embryos which depend on yolk-derived nutrition) were held in 0.3×Danieau’s solution at 28.5°C and at a 14-h light/10-h dark diurnal cycle. The experimental procedures were approved by the Animal Care and Use Committee of the Woods Hole Oceanographic Institution.
Chemical Exposure
A candidate PXR agonist 5-pregnen-3β-diol-20-one [pregnenolone (PN), Sigma-Aldrich, St. Louis, MO] was tested. Embryos at 48 hours post fertilization (hpf) were exposed to vehicle (0.1% DMSO, v/v) alone or containing various concentrations of PN (1–10 µM). Twenty embryos per replicate were exposed in polystyrene petri dishes containing 20 mL of 0.3×Danieau’s solution. Embryos were collected at 72 hpf. Dose–response studies for PN were repeated twice. We also used a model AHR agonist, 3,3’4,4’,5-pentachlorobiphenyl (PCB126), to assess the potential for Ahr to interact with zebrafish Pxr signaling. For this purpose, embryos were exposed to graded concentrations of PCB126 (0.5–10 nM) for 24 hours beginning at 24 hpf and collected at 96 hpf (Jonsson et al., 2012). We performed repetitive dose–response studies for PCB126, and collected half of the samples at 72 hpf (n = 2) and the rest at 96 hpf (n = 2). From each treatment group, eleutheroembryos were collected, flash frozen in liquid nitrogen, and stored at −80°C until used for quantitative real-time PCR.
Morpholino Knock Down of Pxr and Ahr2
To examine the role of zebrafish Pxr in chemical effects on gene expression, we treated zebrafish embryos with MO to block translation of pxr. We also examined the role of Ahr2 in chemical effect on gene expression using a similar approach. Morpholinos targeting the translation start site of pxr (Pxr-MO; 5′-CATGTCATATAAGCGGGACATTGAC-3′), the translation start site of ahr2 (Ahr2-MO; 5′-TGTACCGATACCCGCCGACATGGTT-3′) (Dong et al., 2004) and a placebo control MO (Ctl-MO; 5′-CCTCTTACCTCAGTTACAATTTATA-3′) were synthesized by Gene Tools (Philomath, OR). They contained a fluorescein tag to enable the selection of successfully injected embryos. A Narishige IM-300 microinjector (Tokyo, Japan) was used to inject approximately 2 nL (0.36 pmoles) of morpholinos into the yolk of one- to four-cell-stage embryos. Embryos were screened at 24 hpf by fluorescence microscopy to verify incorporation and homogeneous distribution of morpholinos. Any damaged embryo or those not displaying homogeneous fluorescence were removed. In the PN exposure experiment, embryos from each morpholino group were exposed to either 3 µM PN or DMSO (0.1%). Groups of uninjected embryos were also exposed to PN or DMSO. Groups of 20 embryos per replicate were exposed at 48 hpf in polystyrene petri dishes containing 20 mL 0.3×Danieau’s solution. After 24 hours, the exposed embryos were collected. We studied effects of Ahr2 knock down and subsequent exposure to PCB126 (5 nM), following the protocol reported in Jonsson et al. (2012). cDNA samples from prior Ahr2-MO studies (Jonsson et al., 2012) were also used for quantification of gene expression.
Confirmation of Efficacy and Specificity of Pxr-MO
The efficacy and specificity of the Pxr-MO were determined by its ability to block in vitro translation of zebrafish pxr coding sequence, cloned into pGEM-T Easy, using the Promega TNT® rabbit reticulocyte T7 Quick Coupled Translation system. Transcend™ biotinylated t-Lysyl-RNA was used to label the translated protein. One to five microliters of neat or acetone-precipitated reaction mixture (per kit protocol) was resolved on 10% polyacrylamide gels and transferred to Hoefer 0.22 µm nitrocellulose membrane. LiCor blocker was then applied and membrane was incubated with LiCor Streptavidin IRDye 680™. Fluorimetric detection was operated with the Licor Odyssey™ near-IR laser using the 700-nm excitation wavelength to visualize labeled proteins and co-resolved BioRad Precision Plus™ All Blue prestained molecular weight standards.
Real-Time RT-PCR
Total RNA was isolated and treated with DNase using Aurum kits (Bio-Rad, Hercules, CA) following the manufacturer’s instruction. The concentration and integrity of RNA were determined spectrophotometrically (NanoDrop ND-1000; NanoDrop Technologies, Wilmington, DE). Total RNA (1 µg per sample) was reverse-transcribed using the iScript cDNA Synthesis kit (Bio-Rad). Gene-specific primers for real-time PCR were synthesized by Eurofins MWG Operon (Huntsville, AL). Primer sequences for pxr, ahr2, CYP1A, CYP2AA1, CYP2AA2, CYP2AA12, CYP3A65, CYP3C1, arnt2, and ef1α are listed in Table 1. Real-time PCR was performed using the iQ SYBR Green Supermix (Bio-Rad) in a MyiQ Single-Color Real-Time PCR Detection System (Bio-Rad), according to the manufacturer’s instruction. In each sample, the genes were analyzed in duplicate with the following protocol: 95°C for 3 min and 95°C for 15 s/62°C for 1 min (45 cycles). A melt curve analysis was performed at the end of each PCR run to ensure that a single product was amplified. Relative mRNA expression of each target gene was normalized to that of arnt2 or ef1α (E−ΔCt; where ΔCt = [Ct(target genes) −Ct(arnt2 or ef1α)]). In dose-response studies, relative changes due to treatment (PN or PCB126) were determined by E−ΔΔCt (E−ΔCt[sample] / mean E−ΔCt[control]). In morpholino knock down studies, relative changes were determined by E−ΔΔCt (E−ΔCt[sample] / mean E−ΔCt[Ctl-MO]). Polymerase chain reaction efficiencies (E) for within-experiment amplicon groups were determined as described previously (Kubota et al., 2013). Selection of a reference gene depends on chemicals; arnt2 was used for the PN exposure study, whereas ef1α was used for the PCB126 exposure study (Jonsson et al., 2012).
TABLE 1.
Primer sequences used for quantification of transcript levels of pxr, ahr2, and CYP genes by quantitative real-time PCR
| Gene | Primer sense | Primer antisense | References |
|---|---|---|---|
| pxr | 5′-GCATTCGCGTCCATATCACAGAG | 5′-CTAACTAGGGCTCCACTTCCTGG | Bainy et al. (2013) |
| ahr2 | 5′-CTACTTGGGCTTCCATCAGTCG | 5′-GTCACTTGAGGGATTGAGAGCG | |
| CYP1A | 5′-GCATTACGATACGTTCGATAAGGAC | 5′-GCTCCGAATAGGTCATTGACGAT | Jonsson et al. (2007) |
| CYP2AA1 | 5′-TTCCATTTTCACTGGGACCG | 5′-CGAACAAGACCCATGATGCC | Kubota et al. (2013) |
| CYP2AA2 | 5′-GCCTTTTGTGGGAAACTTAC | 5′-AGCCAGTTGGATTGTATTGATGC | Kubota et al. (2013) |
| CYP2AA12 | 5′-CCAGGTCATAAAGGAAGCCATAG | 5′-CAGTGATCCAGGTTAAAATCGG | |
| CYP3A65 | 5′-ATGGTGCCGACCTACGCCCTC | 5′-GGGCCCAGACCGAACGGCAT | Bainy et al. (2013) |
| CYP3C1 | 5′-TGGTGAGCATTAGTGTACATGAGC | 5′-GAGGGTTATGACCAGAACCACC | |
| arnt2 | 5′-CACCTTTGGATCACATCTCATTG | 5′-TCACCCTCCTTAGACGGACC | Goldstone et al. (2010) |
| ef1α | 5′-CAACCCCAAGGCTCTCAAATC-3′ | 5′-AGCGACCAAGAGGAGGGTAGGT-3′ | Goldstone et al. (2010) |
Promoter Analysis
The zebrafish pxr, ahr2, CYP1A, CYP2AA1, CYP2AA2, CYP2AA12, CYP3A65, and CYP3C1 genes are localized in Zv9 in Ensembl (Flicek et al., 2013), and the regions 0–10-kb upstream of the translational start site of these genes were downloaded. Some putative PXR response elements (PXREs), including direct repeat 3 and 4 (DR3, DR4), everted repeat 6 and 8 (ER6, ER8), and inverted repeat 0 (IR0) (Goodwin et al., 2002; Kast et al., 2002; Sonoda et al., 2002; Sueyoshi and Negishi, 2001) were identified using NHR scan (Sandelin and Wasserman, 2005). Putative xenobiotic response elements [XREs; also known as dioxin response elements (DREs)] also were searched using the XRE consensus sequence identified by Fujisawa-Sehara et al. (1987) and studied in zebrafish by Zeruth and Pollenz (2007).
Statistics
Data are presented as means ± SD. Significance of difference between control and treatment groups was determined by one-way ANOVA followed by Dunnett’s multiple comparisons test. Outlier data were excluded based on the Grubbs test. The significance level was set at 0.05. All statistical analyses were performed with GraphPad Prism 5.0 (GraphPad Software, San Diego, CA).
RESULTS
Putative Pxr and Ahr Response Elements in Proximal Promoters
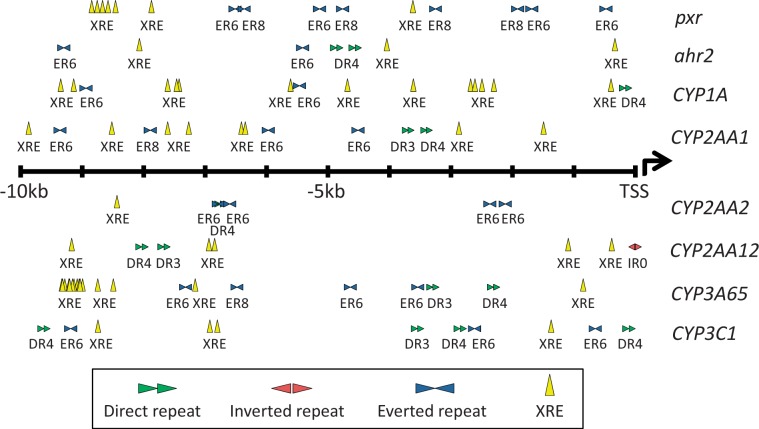
First, we screened proximal promoters of candidate Pxr and Ahr target genes for direct repeat 3 and 4 (DR3, DR4), everted repeat 6 and 8 (ER6, ER8), and inverted repeat 0 (IR0) sequences, which are putative PXREs in mammalian models (Goodwin et al., 2002; Kast et al., 2002; Sonoda et al., 2002; Sueyoshi and Negishi, 2001). We also searched for known AHR response elements (XRE or DRE) (Fujisawa-Sehara et al., 1987). The numbers of putative PXREs identified in 10-kb upstream of the genes examined were 8 for pxr, 4 for ahr2, 3 for CYP1A, 6 for CYP2AA1, 5 for CYP2AA2, 3 for CYP2AA12, 6 for CYP3A65, and 7 for CYP3C1 (Figure 1). There also were multiple XREs in the 10-kb upstream of all of these genes except CYP2AA2, which has a single XRE (Figure 1). Interestingly, both pxr and CYP3A65 had clusters of XREs in their 10-kb region upstream of the translation start sites.
FIG. 1.
Putative PXR response elements (DR3, 4, ER6, 8, IR0) and xenobiotic response elements (XREs) in 10-kb upstream of the translation start site (TSS) of pxr, ahr2, CYP1A, CYP2AA, CYP3A, and CYP3C genes.
Responses to a Potential Pxr Agonist (PN) in Developing Zebrafish
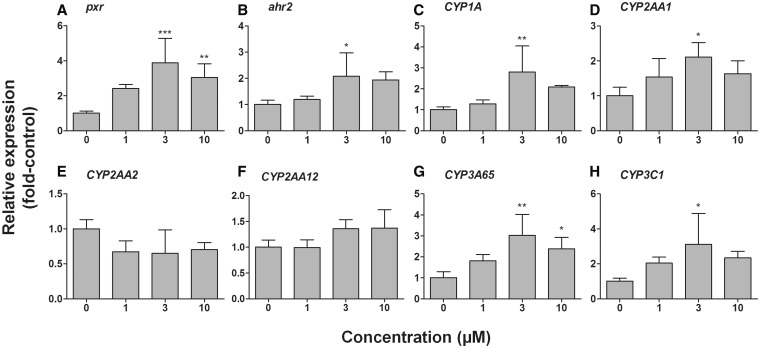
Pregnenolone, previously identified as an agonist for zebrafish Pxr in transactivation assays (Moore et al., 2002), was examined for effects on expression of selected genes in zebrafish eleutheroembryos. Our preliminary study to optimize the exposure protocol for PN revealed stronger effects on the mRNA expression of CYPs and receptors when zebrafish embryos were exposed for 24 h continuously from 48 to 72 hpf and were collected at 72 hpf for the analysis, as compared to those embryos that were exposed for 48 h continuously from 48 to 96 hpf and were collected at 96 hpf (data not shown). Thus, we chose 72 hpf as a time point for PN. Pregnenolone significantly induced mRNA expression of pxr, ahr2, CYP1A, CYP2AA1, CYP3A65, and CYP3C1 in a dose-dependent manner (Figure 2). Expression of most of these genes peaked at 3 µM PN and then declined at the highest dose (10 µM). CYP2AA2 was not induced by PN exposure compared with vehicle control. The dose-response studies with PN were repeated and the results were essentially the same each time, with the exception of CYP2AA12, which gave inconsistent induction results in the two experiments (data not shown).
FIG. 2.
Dose-response relationships for effects of pregnenolone on the mRNA expression of pxr (A), ahr2 (B), CYP1A (C), CYP2AA1 (D), CYP2AA2 (E), CYP2AA12 (F), CYP3A65 (G), and CYP3C1 (H) in developing zebrafish (determined at 72 hpf). Embryos were exposed to carrier (0.1% DMSO) or differing concentrations of pregnenolone (PN; 1, 3 or 10 µM) for 24 h starting at 48 hpf. At 72 hpf, eleutheroembryos were sampled for quantitative real-time PCR analysis. Relative expression (fold-control) was calculated by E−ΔΔCt using arnt2 as a reference gene. Statistical differences between control and treatment group were determined by one-way ANOVA followed by Dunnett’s multiple comparison test and are shown by asterisks (*p < .05 and **p < .01, ***p < .001, n = 4).
Effect of Pxr Knock Down on Basal and PN-Induced Expression of Pxr, Ahr2 and CYPs
One approach to determining the role of Pxr in regulation of CYP genes is to block pxr translation with morpholino oligonucleotides. We first tested the efficacy and specificity of a Pxr-MO by assessing the inhibitory effect on in vitro translation of the pxr transcript (Supplementary Figure S1). A plasmid containing a pxr insert produced a specific protein band of the expected molecular weight. Pxr-MO inhibited the translation of pxr by more than 95%, whereas the placebocontrol morpholino (Ctl-MO) did not inhibit the translation of pxr.
We next investigated whether the basal expression of pxr, ahr2, and CYP genes was affected by Pxr knock down in 72 hpf zebrafish eleutheroembryos (Supplementary Table S1). The basal level of pxr expression was repressed by 47 ± 8% (mean ± SD of four independent experiments, with each determination at least in duplicates). A weaker but consistent repression was seen also for ahr2 (25 ± 11%), CYP1A (23 ± 14%), CYP2AA1 (24 ± 31%), and CYP3A65 (31 ± 18%).
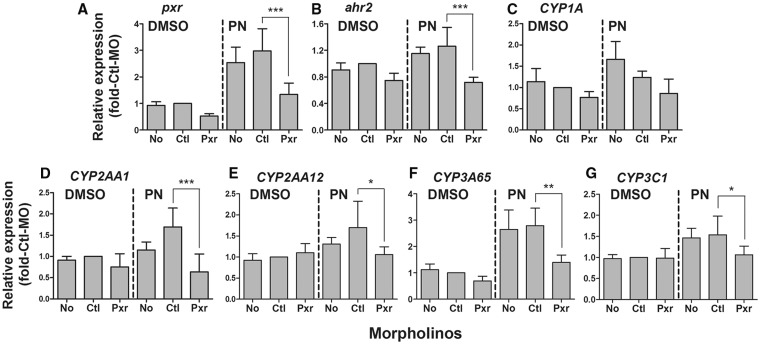
To assess the involvement of Pxr in PN-induced gene expression, we used 3 µM PN, the dose that gave the maximum induction of most of the genes examined in the dose-response studies detailed above. There were significant decreases in the PN induction of pxr, ahr2, CYP3A65, and CYP3C1 expression at 72 hpf in the Pxr-MO group relative to the Ctl-MO group (Figure 3; Supplementary Table S1). The induction of CYP2AA1 and CYP2AA12 by PN tended to be enhanced in the Ctl-MO-treated embryos, for unknown reasons. However, these increases were suppressed significantly by knocking down Pxr. Pxr morphants also exhibited a trend to a reduced CYP1A expression in the PN-exposed group. Notably, there was no statistical difference in transcript levels of any of the genes examined when comparing the vehicle control group and the Pxr-MO + PN group.
FIG. 3.
Effect of Pxr-morpholino (Pxr-MO) treatment on the mRNA expression of pxr (A), ahr2 (B), CYP1A (C), CYP2AA1 (D), CYP2AA12 (E), CYP3A65 (F), and CYP3C1 (G) in 72 hpf eleutheroembryos exposed to pregnenolone (PN; 3 µM) or vehicle (0.1% DMSO). Data are normalized to the expression levels in the control morpholino (Ctl-MO) + DMSO group and are shown as mean + SD for four independent experiments with each determination made at least in duplicates. Significant decreases in the PN induction of expression of these genes were observed in the Pxr-MO group relative to the Ctl-MO group (*p < .05, **p < .01, ***p < .001).
Effect of Ahr2 Knock Down on PN-Induced Expression of Pxr, Ahr2, and CYP3A65
We then examined the effect of Ahr2 knock down on the PN-induced expression of pxr and ahr2, as well as one of the most typical target genes for zebrafish Pxr, CYP3A65 (as shown above), in 72 hpf eleutheroembryos. Knock down of Ahr2 did not suppress the PN-induced expression of pxr or CYP3A65 (Supplementary Figure S2). Expression of ahr2 also was not inhibited in the Ahr2 morphants exposed to PN (data not shown).
Responses to an Ahr Agonist (PCB126) in Developing Zebrafish
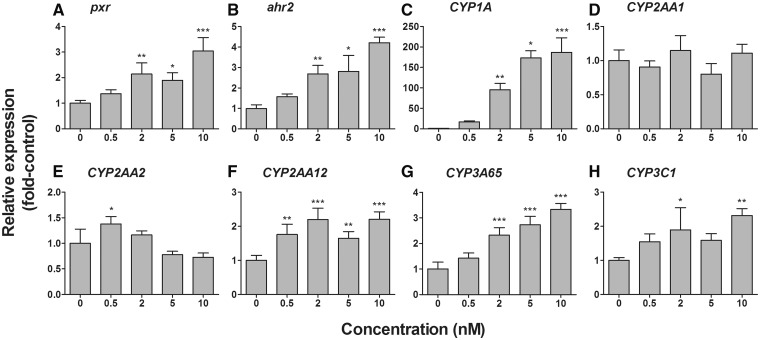
An AHR agonist, PCB126, was also tested for its potency to affect expression of pxr, ahr2, CYP2AA, and CYP3 genes, as well as the known target CYP1A in developing zebrafish. Eleutheroembryos harvested at 96 hpf (Jonsson et al., 2012) were first used for mRNA quantification due to availability of samples. PCB126 caused dose-dependent increases in pxr, ahr2, CYP1A, CYP2AA12, CYP3A65, and CYP3C1 mRNA expression in these samples (Figure 4). Expression of CYP2AA2 was slightly increased only at the lowest dose examined. No induction of CYP2AA1 by PCB126 was observed at the doses used in these studies. A repeated experiment with PCB126 confirmed the pattern of the dose-response relationships for selected genes (ie, pxr, CYP2AA12, and CYP3A65) (data not shown). We also examined mRNA expression of pxr, CYP2AA12, and CYP3A65 with eleutheroembryos collected at 72 hpf, and found increased transcript levels, however with relatively smaller fold changes compared to 96 hpf (except CYP3A65 that showed an equivalent level of induction) (Supplementary Figure S3). Thus, the following studies involving Ahr2-MO injection and subsequent exposure to PCB126 were performed with eleutheroembryos collected at 96 hpf.
FIG. 4.
Dose-response relationships for effects of PCB126 on the mRNA expression of pxr (A), ahr2 (B), CYP1A (C), CYP2AA1 (D), CYP2AA2 (E), CYP2AA12 (F), CYP3A65 (G), and CYP3C1 (H) in developing zebrafish (determined at 96 hpf). Embryos were exposed to carrier (0.02% DMSO) or differing concentrations of PCB126 (0.5, 2, 5, or 10 nM) for 24 h starting at 24 hpf. At 96 hpf, eleutheroembryos were sampled for quantitative real-time PCR analysis. Relative expression (fold-control) was calculated by E−ΔΔCt using ef1α as a reference gene (Jonsson et al., 2007, 2012). Other conditions are the same as given in the legend of Figure 2.
Effect of Ahr2 Knock Down on PCB126-Induced Expression of Pxr and CYPs
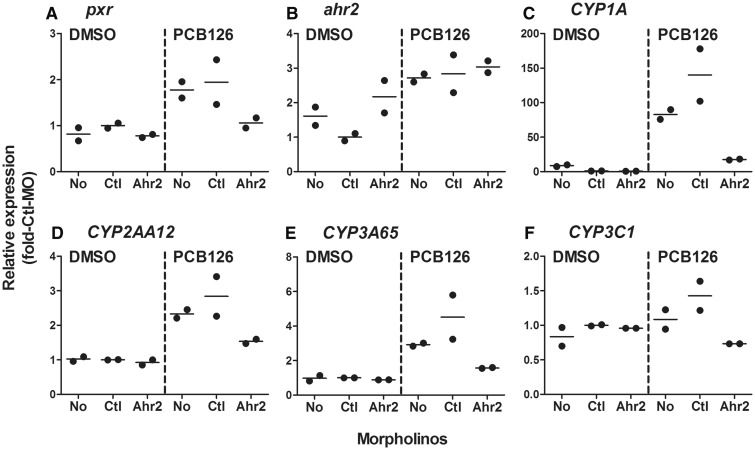
We next sought to determine whether Ahr2 is involved in PCB126-induced expression of pxr and CYP genes, which also are under the regulation of Pxr (Figure 3; Supplementary Table S1). It has been well documented that treatment with Ahr2-MO blocks PCB126-induced expression of CYP1s in developing zebrafish (Jonsson et al., 2007). Here, knocking down Ahr2 was found to suppress the PCB126-induced expression of pxr, CYP2AA12, and CYP3A65, but not ahr2 (Figure 5; Supplementary Table S2).
FIG. 5.
Effect of Ahr2 morpholino (Ahr2-MO) treatment on the mRNA expression of pxr (A), ahr2 (B), CYP1A (C), CYP2AA12 (D), CYP3A65 (E), and CYP3C1 (F) in 96 hpf eleutheroembryos exposed to PCB126 (5 nM) or vehicle (0.02% DMSO). Data are normalized to the expression levels in the control morpholino (Ctl-MO) + DMSO group and individual data are plotted to show the difference in the expression between two replicates of a single experiment (average values are shown with lines).
We repeated the Ahr2-MO studies and found an essentially similar pattern of the morpholino effect (data not shown); Ahr2 knock down blocked PCB126-induced expression of CYP2AA12 and CYP3A65 gene transcripts, although in this repeated experiment there was not a significant induction of pxr by PCB126, precluding detection of an Ahr2-MO effect on pxr expression. As expected, the induction of CYP1A by PCB126 was significantly reduced in the Ahr2 morphants. Supplementary Figure S4 shows effects of Ahr2 knock down on the PCB126-induced expression of CYP2AA12 and CYP3A65 after combining data from the two independent experiments. Induction of CYP2AA12 and CYP3A65 by PCB126 was knocked down to the levels of the Ctl-MO + DMSO group, with a significant reduction as compared to the levels of the Ctl-MO + PCB126 group.
Effect of Pxr Knock Down on the PCB126-Induced Expression of Pxr and CYPs
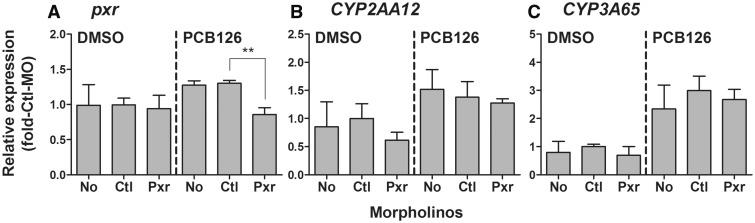
To examine the role of Pxr in regulation of PCB126-induced expression of pxr target genes, we knocked down Pxr and then exposed embryos to PCB126. For this purpose, we measured transcript levels of CYP2AA12 and CYP3A65 in eleutheroembryos (96 hpf), which together with pxr had shown induction by PCB126. Injection of Pxr-MO did not suppress the PCB126-induced expression of either CYP2AA12 or CYP3A65, although the Pxr-MO blocked the slight induction of pxr transcript by PCB126 (Figure 6).
FIG. 6.
Effect of Pxr morpholino (Pxr-MO) treatment on the mRNA expression of pxr (A), CYP2AA12 (B), and CYP3A65 (C) in 96 hpf eleutheroembryos exposed to PCB126 (5 nM) or vehicle (0.02% DMSO). Data are normalized to the expression levels in the control morpholino (Ctl-MO) + DMSO group and are shown as mean + SD of four replicates of a single experiment. No significant suppression of PCB126 induction of CYP2AA12 or CYP3A65 was observed in the Pxr-MO group relative to the Ctl-MO group (p > .05), when statistical analysis was conducted by one-way ANOVA followed by Dunnett’s multiple comparison test (p > .05), whereas a significant decrease in the PCB126 induction of pxr expression was observed in the same comparison (**p < .01).
DISCUSSION
Self-Regulation of Pxr
The results here show that PN, an agonist for zebrafish Pxr as shown in cell-based reporter gene assay (Ekins et al., 2008), increased expression levels of pxr transcripts in a dose-dependent manner in zebrafish eleutheroembryos. Furthermore, we show that blocking of pxr translation by Pxr-MO suppressed in part the basal levels of pxr transcripts, indicating that Pxr is involved in the constitutive expression of pxr in certain early stage of zebrafish development. The Pxr-MO study further demonstrated that Pxr participates in the PN-induced expression of pxr. This highlights that Pxr can self-regulate pxr expression in vivo in developing zebrafish.
The basal level of pxr expression in 96 hpf eleutheroembryos was not blocked by knocking down Pxr translation (see Figure 6), which is in contrast to what was observed in 72 hpf eleutheroembryos injected with Pxr-MO (see Figure 3). It is possible that some unidentified mechanism(s) involved in regulating the basal expression of pxr begins to be active after 72 hpf, and compensates at 96 hpf for the Pxr signaling that is knocked down. This remains to be examined.
Although we used a single morpholino for pxr, there is a recent study by Chang et al. (2013) who succeeded in blocking constitutive CYP3A65 expression by a Pxr-MO that has a slightly different sequence from ours (ours: 5′-CATGTCATATAAGCGGGACATTGAC-3′ vs Chang et al.’s; 5′-TCATATAAGCGGGACATTGACGTAC-3′, with italicized characters that are overlapped sequences). Both of the two Pxr-MOs target blocking translation. Thus, it is unlikely that the ability of these two Pxr-MOs to suppress target gene expression is due to off-target effects that rely on the morpholino sequences.
Chemicals that are PXR agonists, including PCN, dexamethasone, and phenobarbital (PB) have been shown to induce PXR expression in vivo in rats (Ejiri et al., 2005; Zhang et al., 1999) and mice (Maglich et al., 2002). A similar increase in pxr expression was observed in adult zebrafish treated with PCN (Bresolin et al., 2005), PB and TCPOBOP (3,3’,5,5’-tetrachloro-1,4-bis(pyridyloxy)benzene) (Bainy et al., 2013). Studies with pxr-null mice showed that PXR is necessary for the PCN-induced expression of PXR (Maglich et al., 2002). Thus, PXR self-regulation in vivo appears to be conserved between zebrafish and rodents.
CYP2 and CYP3 Genes as Targets for Pxr
The current data showed that knocking down Pxr slightly suppressed basal levels of some of CYP2 and CYP3 genes in developing zebrafish. This indicates that Pxr could be involved, at least partly, in regulation of constitutive expression of these CYP genes. It is possible that the residual level of Pxr in the Pxr morphants could be enough to maintain basal expression of some target genes. As well, receptors other than Pxr could participate in regulation of the Pxr signaling, including Ahr (see below).
The Pxr-MO studies with zebrafish embryos exposed to PN show that some CYP2 and CYP3 genes are targets for Pxr. It has been well documented that a number of CYP2 and CYP3 genes are under regulation by PXR and CAR in mammals (Waxman, 1999), which mediate responses to inducers such as PB, TCPOBOP, and PCN. Teleost fish show little or no induction of microsomal enzyme activity or CYP protein by PB (eg, Elskus and Stegeman, 1989; Goksoyr et al., 1987). Otherwise, there is limited information available on induction or regulation of expression of CYP2 and CYP3 genes in fish by these or other chemicals. Induction of CYP3A65 by dexamethasone has been shown in developing zebrafish (Tseng et al., 2005). Rat hepatocytes treated with dexamethasone also showed enhanced CYP3A expression, but this appears to be the result of upregulation of PXR, and the PXR upregulation was mediated by the glucocorticoid receptor (Shi et al., 2010). Thus, the mechanism behind upregulation of CYP3A65 by dexamethasone could be different from that of the upregulation of pxr and other target genes, including induction of CYP3A65 by PN in the current study.
The general lack of evidence for in vivo responses to PXR or CAR agonists could be due to lack of information on relevant endpoints in fish. We recently described a novel CYP2 family in zebrafish, CYP2AA, with 10 genes identified in a cluster in the genome, and the expression of two of these genes, CYP2AA1 and CYP2AA2, was increased by TCPOBOP and PCN (Kubota et al., 2013). A question raised in our recent paper (Kubota et al., 2013) involves mechanisms underlying induction of CYP2AA genes in adult zebrafish treated with TCPOBOP and PCN. The current results showing involvement of Pxr in the PN-induced expression of several CYP2AAs in developing fish, together with the fact that zebrafish do not possess a CAR gene, suggest that a Pxr-mediated signaling could be involved in the CYP2AA induction found in adult zebrafish treated with PB or TCPOBOP. The nature of regulation of the suite of CYP2AA genes remains to be examined.
CYP2 and CYP3 Genes as Targets for Ahr2
Previous studies with developing zebrafish reported that CYP3A65 could be induced by TCDD via Ahr2 (Chang et al., 2013; Tseng et al., 2005). Tseng et al. (2005) also showed that basal expression of CYP3A65 was low at 72 hpf and dramatically increased in foregut at 96 hpf. Such increase was abolished by knocking down Ahr2. Recent studies revealed that knocking down either Ahr2 or Pxr blocked basal expression of CYP3A65 (Chang et al., 2013), which is consistent with the data we present (see Supplementary Table S1 and Supplementary Figure S2), suggesting that more than one receptor contributes to maintaining constitutive expression. We found that expression of pxr, CYP2AA12, CYP3A65, and CYP3C1, as well as that of the cardinal Ahr battery gene CYP1A, was induced by PCB126 in developing zebrafish and that this was dependent on Ahr2. This suggestion that Ahr2 can upregulate pxr expression and expression of Pxr target genes is supported by the presence of multiple XREs in the 10-kb upstream of the translation start sites of these genes. Notably, pxr and CYP3A65 both have clusters of XREs, similar to those found upstream of CYP1A (Zeruth and Pollenz, 2007). It could be that these XREs are involved in the induction of pxr, CYP2AA12, and CYP3C1 by PCB126, in a common mechanism with CYP3A65 involving binding of Ahr-ligand complex to XREs (Chang et al., 2013). This remains to be tested.
An intriguing finding is that induction of pxr by PCB126 was suppressed by knocking down either Ahr2 or Pxr, whereas knocking down Ahr2, but not Pxr, inhibited PCB126-induced expression of CYP2AA12 and CYP3A65. Suppression of pxr expression observed in the Pxr morphants exposed to PCB126 is likely due to self-regulation of pxr. It will be interesting to determine whether upregulation of pxr is involved in the PCB126-induced expression of the CYP2 and CYP3 genes.
Response of Ahr2 to PCB126 in Ahr2 Morphants
The results showed that PCB126 induced transcript levels of ahr2. Induction of ahr2 by TCDD also was observed in developing zebrafish (Karchner et al., 2005; Tanguay et al., 1999). Many Ahr2 target genes including the CYP1s and AHR repressors are regulated by Ahr2 (Jenny et al., 2009; Jonsson et al., 2007), but at present the role of Ahr2 in regulation of the ahr2 gene remains unclear. Herein, we failed to see suppression of PCB126-induced expression of ahr2 in the Ahr2 morphants, whereas induction of CYP1A as well as some other CYPs (eg, CYP2AA12, CYP3A65) by PCB126 was markedly suppressed by knocking down Ahr2. This persistent induction of ahr2, but not Ahr2 target genes, in the Ahr2 morphants could indicate involvement of receptors other than Ahr2 in regulation of PCB126-induced expression of ahr2. Zebrafish have three genes of ahr (ie, ahr2, ahr1a, and ahr1b) (Karchner et al., 2005). Like Ahr2, Ahr1b also is functional in terms of ligand binding and transactivation (Karchner et al., 2005). Thus, induction of ahr2 transcripts in the Ahr2 morphants could be regulated by Ahr1b. The possibility that Ahr1b participates in regulation of ahr2 warrants further investigation. Studies to identify target genes for Ahr1b also are necessary. Alternatively, some residual Ahr2 protein in the Ahr2 morphants could be sufficient to maintain induction in a gene-specific manner, possibly reflecting differing sensitivity (ie, differing EC50) of gene induction by PCB126.
Cross-Talk between Ahr2 and Pxr Signaling Pathways
Data from current studies show cross-talk between Ahr2 and Pxr signaling pathways. This cross-talk is considered reciprocal rather than asymmetric, as Ahr2 activation caused upregulation of pxr, CYP2, and CYP3 genes, and Pxr activation caused upregulation of ahr2 and CYP1A. A cross-talk between PXR and AHR has been suggested in mammals. In primary cultures of human hepatocytes, the PXR agonist rifampicin induced AHR moderately and CYP1A1 markedly (Maglich et al., 2002). Such a cross-talk between PXR and AHR was not evident in mouse liver treated with PCN (Aleksunes and Klaassen, 2012; Maglich et al., 2002), suggesting species differences in the regulation of AHR signaling by PXR.
In Vivo Implications
Human and zebrafish PXRs are 74% identical in the DNA-binding domains, whereas the LBDs are only 56% identical (Bainy et al., 2013). The latter sequence difference is likely related to differences in ligand binding and reporter efficacy between the zebrafish and human PXR-LBD constructs with steroids, drugs, and xenobiotics (Ekins et al., 2008; Krasowski et al., 2005; Reschly et al., 2007). As most in vitro activation studies have been performed with LBD constructs rather than full-length proteins, they may not adequately represent the species differences or similarities between human and zebrafish PXR activation. Our recent studies have revealed different receptor binding specificity and lower efficacy in cells transiently transfected with full-length zebrafish Pxr compared with a zebrafish Pxr-LBD alone (Bainy et al., 2013). Thus, the ligand specificity of the isolated LBD determined in vitro may not accurately reflect the in vivo activity.
The current results establish a foundation for PXR studies with developing zebrafish to understand roles of PXR in developmental toxicology and pharmacology. The development of humanized PXR mice has been extremely important for extending the pharmacological usefulness of the murine model (eg, Igarashi et al., 2012; Ma et al., 2007; Scheer et al., 2008; Xie et al., 2000). “Humanized” mouse models have partially overcome differences in ligand activation, and provide an experimental approach to quantitatively predict xenobiotic and drug-drug interactions in humans (Hasegawa et al., 2011). The establishment of a humanized zebrafish PXR line would provide an alternate model for species-specific differences in developmental chemical toxicity.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institute of Health (R01ES015912 to J.J.S., R21HD073805 to J.V.G.); the NIEHS Superfund Research Program (5P42ES007381 to J.J.S., J.V.G., B.W.); Grant-in-Aid for Japan Society for the Promotion of Science (JSPS) Fellows (No. 194313 to A.K.); Grant-in-Aid for Research Activity Start-up (No. 26881001 to A.K.); JSPS Research Fellowships for Young Scientists (A.K.); JSPS Postdoctoral Fellowships for Research Abroad (A.K.), a Belgian American Educational Foundation fellowship (B.L.); a WHOI Summer Student Fellowship (M.T.). The sponsors had no involvement in performing or in the decision to publish this study.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Dr. Afonso Bainy for producing the pcDNA/PXR plasmid used for confirmation of efficacy and specificity of Pxr-MO by in vitro protein synthesis. JSPS Research Fellowships for Young Scientists to A.K. (No. 194313) and JSPS Postdoctoral Fellowships for Research Abroad to A.K. (No. 24820) are acknowledged. The U.S. and Japanese Governments are authorized to produce and distribute reprints for governmental purposes notwithstanding any copyright notation that may appear hereon.
REFERENCES
- Aleksunes L. M., Klaassen C. D. (2012). Coordinated regulation of hepatic phase I and II drug-metabolizing genes and transporters using AhR-, CAR-, PXR-, PPARalpha-, and Nrf2-null mice. Drug Metab. Dispos. 40, 1366–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainy A. C. D., Kubota A., Goldstone J. V., Lille-Langoy R., Karchner S. I., Celander M. C., Hahn M. E., Goksoyr A., Stegeman J. J. (2013). Functional characterization of a full length pregnane X receptor, expression in vivo, and identification of PXR alleles, in Zebrafish (Danio rerio). Aquat. Toxicol. 142–143, 447–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresolin T., de Freitas Rebelo M., Bainy A. C. D. (2005). Expression of PXR, CYP3A and MDR1 genes in liver of zebrafish. Comp. Biochem. Physiol. C 140, 403–407. [DOI] [PubMed] [Google Scholar]
- Chang C. T., Chung H. Y., Su H. T., Tseng H. P., Tzou W. S., Hu C. H. (2013). Regulation of zebrafish CYP3A65 transcription by AHR2. Toxicol. Appl. Pharmacol. 270, 174–184. [DOI] [PubMed] [Google Scholar]
- Dong W., Teraoka H., Tsujimoto Y., Stegeman J. J., Hiraga T. (2004). Role of aryl hydrocarbon receptor in mesencephalic circulation failure and apoptosis in zebrafish embryos exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 77, 109–116. [DOI] [PubMed] [Google Scholar]
- Ejiri N., Katayama K., Kiyosawa N., Baba Y., Doi K. (2005). Microarray analysis on CYPs expression in pregnant rats after treatment with pregnenolone-16alpha-carbonitrile and phenobarbital. Exp. Mol. Pathol. 78, 71–77. [DOI] [PubMed] [Google Scholar]
- Ekins S., Chang C., Mani S., Krasowski M. D., Reschly E. J., Iyer M., Kholodovych V., Ai N., Welsh W. J., Sinz M., et al. (2007). Human pregnane X receptor antagonists and agonists define molecular requirements for different binding sites. Mol. Pharmacol. 72, 592–603. [DOI] [PubMed] [Google Scholar]
- Ekins S., Reschly E. J., Hagey L. R., Krasowski M. D. (2008). Evolution of pharmacologic specificity in the pregnane X receptor. BMC Evol. Biol. 8, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elskus A. A., Stegeman J. J. (1989). Further consideration of phenobarbital effects on cytochrome P-450 activity in the killifish, Fundulus heteroclitus. Comp. Biochem. Physiol. C 92, 223–230. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero P. M., Hilbert D. M., Rudikoff S., Ward J. M., Gonzalez F. J. (1996). Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol. Appl. Pharmacol. 140, 173–179. [DOI] [PubMed] [Google Scholar]
- Flicek P., Ahmed I., Amode M. R., Barrell D., Beal K., Brent S., Carvalho-Silva D., Clapham P., Coates G., Fairley S., et al. (2013). Ensembl 2013. Nucleic Acids Res. 41, D48–D55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa-Sehara A., Sogawa K., Yamane M., Fujii-Kuriyama Y. (1987). Characterization of xenobiotic responsive elements upstream from the drug-metabolizing cytochrome P-450c gene: a similarity to glucocorticoid regulatory elements. Nucleic Acids Res. 15, 4179–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goksoyr A., Andersson T., Hansson T., Klungsoyr J., Zhang Y., Forlin L. (1987). Species characteristics of the hepatic xenobiotic and steroid biotransformation systems of two teleost fish, Atlantic cod (Gadus morhua) and rainbow trout (Salmo gairdneri). Toxicol. Appl. Pharmacol. 89, 347–360. [DOI] [PubMed] [Google Scholar]
- Goldstone J. V., McArthur A. G., Kubota A., Zanette J., Parente T., Jönsson M. E., Nelson D. R., Stegeman J. J. (2010). Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genomics 11, 643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin B., Redinbo M. R., Kliewer S. A. (2002). Regulation of cyp3a gene transcription by the pregnane x receptor. Annu. Rev. Pharmacol. Toxicol. 42, 1–23. [DOI] [PubMed] [Google Scholar]
- Handley-Goldstone H. M., Grow M. W., Stegeman J. J. (2005). Cardiovascular gene expression profiles of dioxin exposure in zebrafish embryos. Toxicol. Sci. 85, 683–693. [DOI] [PubMed] [Google Scholar]
- Hasegawa M., Kapelyukh Y., Tahara H., Seibler J., Rode A., Krueger S., Lee D. N., Wolf C. R., Scheer N. (2011). Quantitative prediction of human pregnane X receptor and cytochrome P450 3A4 mediated drug-drug interaction in a novel multiple humanized mouse line. Mol. Pharmacol. 80, 518–528. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Kitajima S., Aisaki K., Tanemura K., Taquahashi Y., Moriyama N., Ikeno E., Matsuda N., Saga Y., Blumberg B., et al. (2012). Development of humanized steroid and xenobiotic receptor mouse by homologous knock-in of the human steroid and xenobiotic receptor ligand binding domain sequence. J. Toxicol. Sci. 37, 373–380. [DOI] [PubMed] [Google Scholar]
- Jenny M. J., Karchner S. I., Franks D. G., Woodin B. R., Stegeman J. J., Hahn M. E. (2009). Distinct roles of two zebrafish AHR repressors (AHRRa and AHRRb) in embryonic development and regulating the response to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 119, 426–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S. A., Moore L. B., Shenk J. L., Wisely G. B., Hamilton G. A., McKee D. D., Tomkinson N. C., LeCluyse E. L., Lambert M. H., Willson T. M., et al. (2000). The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol. Endocrinol. 14, 27–39. [DOI] [PubMed] [Google Scholar]
- Jonsson M. E., Jenny M. J., Woodin B. R., Hahn M. E., Stegeman J. J. (2007). Role of AHR2 in the expression of novel cytochrome P450 1 family genes, cell cycle genes, and morphological defects in developing zebra fish exposed to 3,3',4,4',5-pentachlorobiphenyl or 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 100, 180–193. [DOI] [PubMed] [Google Scholar]
- Jonsson M. E., Kubota A., Timme-Laragy A. R., Woodin B., Stegeman J. J. (2012). Ahr2-dependence of PCB126 effects on the swim bladder in relation to expression of CYP1 and cox-2 genes in developing zebrafish. Toxicol. Appl. Pharmacol. 265, 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karchner S. I., Franks D. G., Hahn M. E. (2005). AHR1B, a new functional aryl hydrocarbon receptor in zebrafish: tandem arrangement of ahr1b and ahr2 genes. Biochem. J. 392, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast H. R., Goodwin B., Tarr P. T., Jones S. A., Anisfeld A. M., Stoltz C. M., Tontonoz P., Kliewer S., Willson T. M., Edwards P. A. (2002). Regulation of multidrug resistance-associated protein 2 (ABCC2) by the nuclear receptors pregnane X receptor, farnesoid X-activated receptor, and constitutive androstane receptor. J. Biol. Chem. 277, 2908–2915. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Goodwin B., Willson T. M. (2002). The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr. Rev. 23, 687–702. [DOI] [PubMed] [Google Scholar]
- Kohle C., Bock K. W. (2009). Coordinate regulation of human drug-metabolizing enzymes, and conjugate transporters by the Ah receptor, pregnane X receptor and constitutive androstane receptor. Biochem. Pharmacol. 77, 689–699. [DOI] [PubMed] [Google Scholar]
- Krasowski M. D., Ai N., Hagey L. R., Kollitz E. M., Kullman S. W., Reschly E. J., Ekins S. (2011). The evolution of farnesoid X, vitamin D, and pregnane X receptors: insights from the green-spotted pufferfish (Tetraodon nigriviridis) and other non-mammalian species. BMC Biochem. 12, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasowski M. D., Yasuda K., Hagey L. R., Schuetz E. G. (2005). Evolution of the pregnane x receptor: adaptation to cross-species differences in biliary bile salts. Mol. Endocrinol. 19, 1720–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer X. C., Baldwin W. S. (2005). CAR and PXR: xenosensors of endocrine disrupters? Chem . Biol. Interact. 155, 111–128. [DOI] [PubMed] [Google Scholar]
- Kubota A., Bainy A. C. D., Woodin B. R., Goldstone J. V., Stegeman J. J. (2013). The cytochrome P450 2AA gene cluster in zebrafish (Danio rerio): expression of CYP2AA1 and CYP2AA2 and response to phenobarbital-type inducers. Toxicol. Appl. Pharmacol. 272, 172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota A., Stegeman J. J., Woodin B. R., Iwanaga T., Harano R., Peterson R. E., Hiraga T., Teraoka H. (2011). Role of zebrafish cytochrome P450 CYP1C genes in the reduced mesencephalic vein blood flow caused by activation of AHR2. Toxicol. Appl. Pharmacol. 253, 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Shah Y., Cheung C., Guo G. L., Feigenbaum L., Krausz K. W., Idle J. R., Gonzalez F. J. (2007). The PREgnane X receptor gene-humanized mouse: a model for investigating drug-drug interactions mediated by cytochromes P450 3A. Drug Metab. Dispos. 35, 194–200. [DOI] [PubMed] [Google Scholar]
- Maglich J. M., Stoltz C. M., Goodwin B., Hawkins-Brown D., Moore J. T., Kliewer S. A. (2002). Nuclear pregnane x receptor and constitutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic detoxification. Mol. Pharmacol. 62, 638–646. [DOI] [PubMed] [Google Scholar]
- Martin M. T., Dix D. J., Judson R. S., Kavlock R. J., Reif D. M., Richard A. M., Rotroff D. M., Romanov S., Medvedev A., Poltoratskaya N., et al. (2010). Impact of environmental chemicals on key transcription regulators and correlation to toxicity end points within EPA’s ToxCast program. Chem. Res. Toxicol. 23, 578–590. [DOI] [PubMed] [Google Scholar]
- Mathas M., Burk O., Qiu H., Nusshag C., Godtel-Armbrust U., Baranyai D., Deng S., Romer K., Nem D., Windshugel B., et al. (2012). Evolutionary history and functional characterization of the amphibian xenosensor CAR. Mol. Endocrinol. 26, 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milnes M. R., Garcia A., Grossman E., Grun F., Shiotsugu J., Tabb M. M., Kawashima Y., Katsu Y., Watanabe H., Iguchi T., et al. (2008). Activation of steroid and xenobiotic receptor (SXR, NR1I2) and its orthologs in laboratory, toxicologic, and genome model species. Environ. Health Perspect. 116, 880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore L. B., Maglich J. M., McKee D. D., Wisely B., Willson T. M., Kliewer S. A., Lambert M. H., Moore J. T. (2002). Pregnane X receptor (PXR), constitutive androstane receptor (CAR), and benzoate X receptor (BXR) define three pharmacologically distinct classes of nuclear receptors. Mol. Endocrinol. 16, 977–986. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Koymans L., Kamataki T., Stegeman J. J., Feyereisen R., Waxman D. J., Waterman M. R., Gotoh O., Coon M. J., Estabrook R. W., et al. (1996). P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics 6, 1–42. [DOI] [PubMed] [Google Scholar]
- Reschly E. J., Bainy A. C. D., Mattos J. J., Hagey L. R., Bahary N., Mada S. R., Ou J., Venkataramanan R., Krasowski M. D. (2007). Functional evolution of the vitamin D and pregnane X receptors. BMC Evol. Biol. 7, 222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe S. (1990). Polychlorinated biphenyls (PCBs), dibenzo-p-dioxins (PCDDs), dibenzofurans (PCDFs), and related compounds: environmental and mechanistic considerations which support the development of toxic equivalency factors (TEFs). Crit. Rev. Toxicol. 21, 51–88. [DOI] [PubMed] [Google Scholar]
- Sandelin A., Wasserman W. W. (2005). Prediction of nuclear hormone receptor response elements. Mol. Endocrinol. 19, 595–606. [DOI] [PubMed] [Google Scholar]
- Scheer N., Ross J., Rode A., Zevnik B., Niehaves S., Faust N., Wolf C. R. (2008). A novel panel of mouse models to evaluate the role of human pregnane X receptor and constitutive androstane receptor in drug response. J. Clin. Invest. 118, 3228–3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D., Yang D., Yan B. (2010). Dexamethasone transcriptionally increases the expression of the pregnane X receptor and synergistically enhances pyrethroid esfenvalerate in the induction of cytochrome P450 3A23. Biochem. Pharmacol. 80, 1274–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda J., Xie W., Rosenfeld J. M., Barwick J. L., Guzelian P. S., Evans R. M. (2002). Regulation of a xenobiotic sulfonation cascade by nuclear pregnane X receptor (PXR). Proc. Natl Acad. Sci. USA 99, 13801–13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueyoshi T., Negishi M. (2001). Phenobarbital response elements of cytochrome P450 genes and nuclear receptors. Annu. Rev. Pharmacol. Toxicol. 41, 123–143. [DOI] [PubMed] [Google Scholar]
- Tanguay R. L., Abnet C. C., Heideman W., Peterson R. E. (1999). Cloning and characterization of the zebrafish (Danio rerio) aryl hydrocarbon receptor. Biochim. Biophys. Acta 1444, 35–48. [DOI] [PubMed] [Google Scholar]
- Timsit Y. E., Negishi M. (2007). CAR and PXR: the xenobiotic-sensing receptors. Steroids 72, 231–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng H. P., Hseu T. H., Buhler D. R., Wang W. D., Hu C. H. (2005). Constitutive and xenobiotics-induced expression of a novel CYP3A gene from zebrafish larva. Toxicol. Appl. Pharmacol. 205, 247–258. [DOI] [PubMed] [Google Scholar]
- Waxman D. J. (1999). P450 gene induction by structurally diverse xenochemicals: central role of nuclear receptors CAR, PXR, and PPAR. Arch. Biochem. Biophys. 369, 11–23. [DOI] [PubMed] [Google Scholar]
- Willson T. M., Kliewer S. A. (2002). PXR, CAR and drug metabolism. Nat. Rev. Drug Discov. 1, 259–266. [DOI] [PubMed] [Google Scholar]
- Xie W., Barwick J. L., Downes M., Blumberg B., Simon C. M., Nelson M. C., Neuschwander-Tetri B. A., Brunt E. M., Guzelian P. S., Evans R. M. (2000). Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature 406, 435–439. [DOI] [PubMed] [Google Scholar]
- Zeruth G., Pollenz R. S. (2007). Functional analysis of cis-regulatory regions within the dioxin-inducible CYP1A promoter/enhancer region from zebrafish (Danio rerio). Chem. Biol. Interact. 170, 100–113. [DOI] [PubMed] [Google Scholar]
- Zhang B., Xie W., Krasowski M. D. (2008). PXR: a xenobiotic receptor of diverse function implicated in pharmacogenetics. Pharmacogenomics 9, 1695–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., LeCulyse E., Liu L., Hu M., Matoney L., Zhu W., Yan B. (1999). Rat pregnane X receptor: molecular cloning, tissue distribution, and xenobiotic regulation. Arch. Biochem. Biophys. 368, 14–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.