Abstract
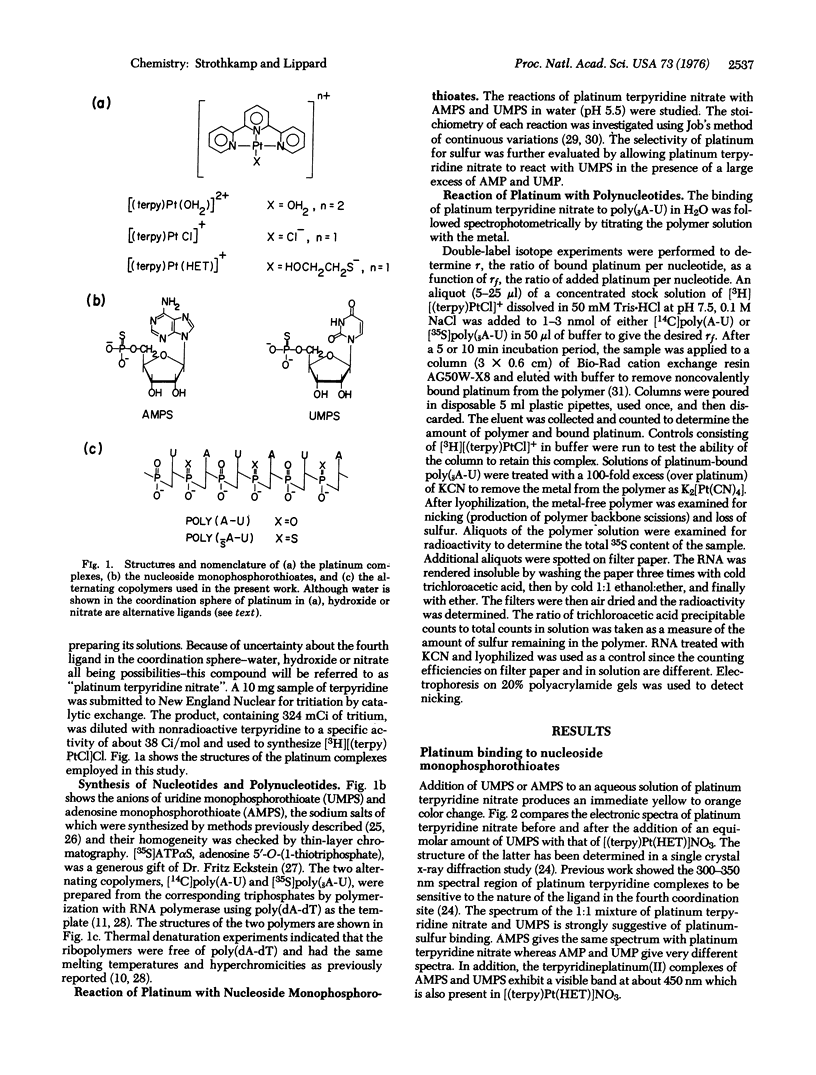
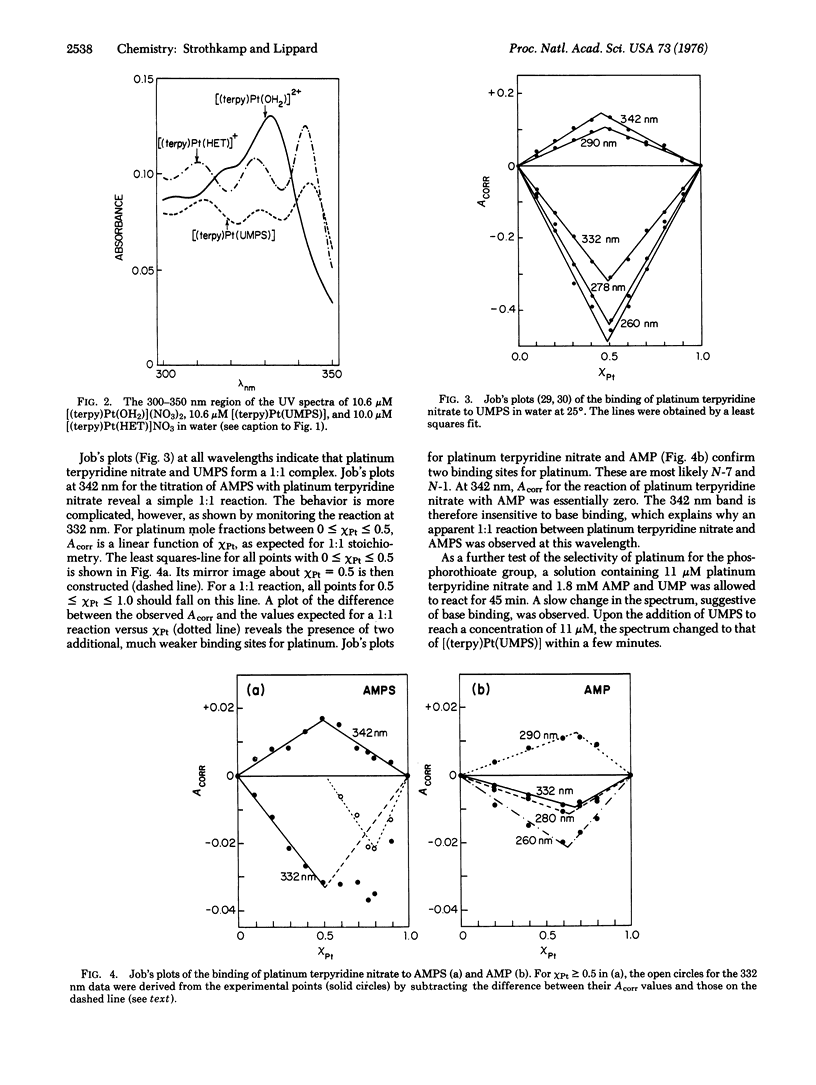
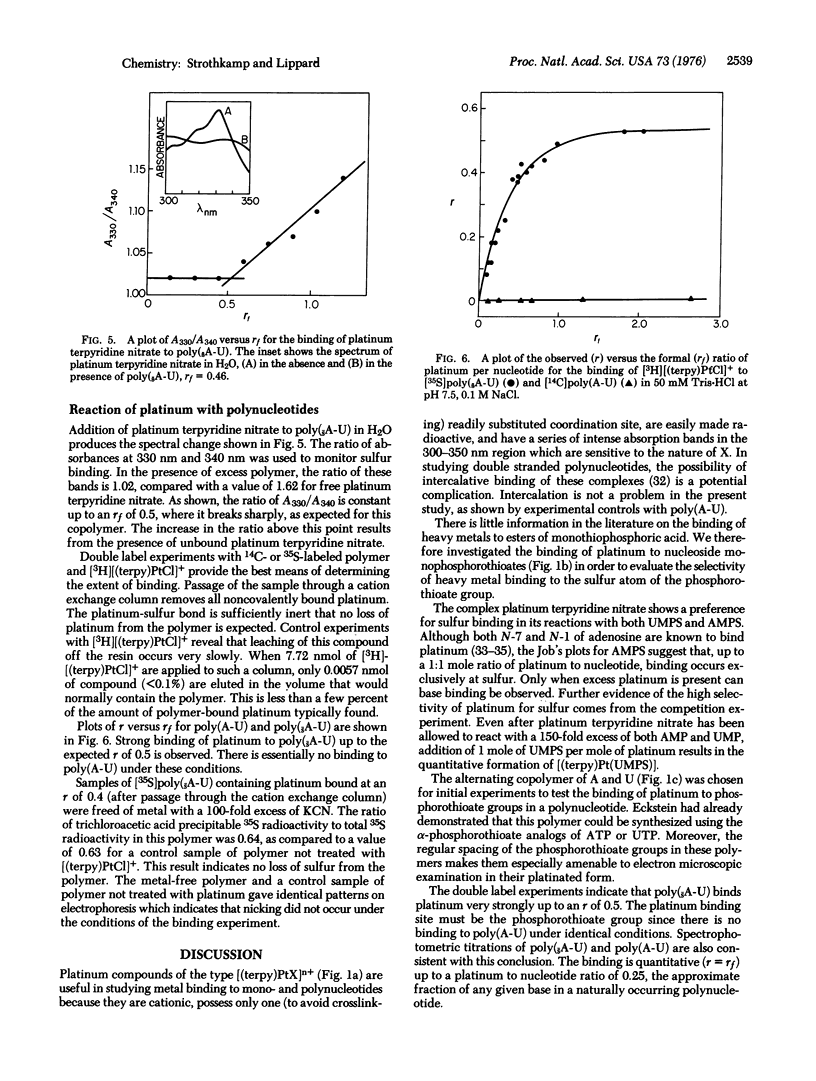
Platinum binding to nucleoside phosphorothioates has been examined to determine their suitability as heavy metal labeling sites for the potential electron microscopic sequencing of nucleic acids. The complex platinum terpyridine nitrate forms a 1:1 adduct with either adenosine or uridine monophosphorothioate. Spectroscopic evidence strongly indicates the presence of platinum-sulfur bonds. Both platinum terpyridine nitrate and chloroterpyridineplatinum(II) bind to poly(sA-U), a polymer prepared from adenosine 5'-O-(1-thiotriphosphate) and UTP. Binding to the sulfur atoms of the phosphorothioate groups is quantitative, as shown by double label experiments using [35S]poly(sA-U) and [3H]chloroterpyridine-platinum(II). Similar experiments with [14C]poly(A-U) indicated no platinum binding. No evidence of nicking or loss of sulfur from poly(sA-U) could be detected after platinum binding. The phosphorothioate group is a strong, highly selective binding site for platinum in polynucleotides. Previous studies have demonstrated quantitative enzymatic incorporation of phosphorothioate groups into a polynucleotide adjacent to a specific base [Matzura, H. & Eckstein, F. (1968) Eur. J. Biochem. 3, 448-452]. The use of heavy metal-labeled phosphorothioate groups for the sequencing of nucleic acids by electron microscopy therefore appears feasible.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEER M., MOUDRIANAKISEN Determination of base sequence in nucleic acids with the electron microscope: visibility of a marker. Proc Natl Acad Sci U S A. 1962 Mar 15;48:409–416. doi: 10.1073/pnas.48.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEER M., ZOBEL C. R. Electron stains. II: Electron microscopic studies on the visibility of stained DNA molecules. J Mol Biol. 1961 Dec;3:717–726. doi: 10.1016/s0022-2836(61)80076-4. [DOI] [PubMed] [Google Scholar]
- Beer M., Stern S., Carmalt D., Mohlhenrich K. H. Determination of base sequence in nucleic acids with the electron microscope. V. The thymine-specific reactions of osmium tetroxide with deoxyribonucleic acid and its components. Biochemistry. 1966 Jul;5(7):2283–2288. doi: 10.1021/bi00871a017. [DOI] [PubMed] [Google Scholar]
- Bond P. J., Langridge R., Jennette K. W., Lippard S. J. X-ray fiber diffraction evidence for neighbor exclusion binding of a platinum metallointercalation reagent to DNA. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4825–4829. doi: 10.1073/pnas.72.12.4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale R. M., Livingston D. C., Ward D. C. The synthesis and enzymatic polymerization of nucleotides containing mercury: potential tools for nucleic acid sequencing and structural analysis. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2238–2242. doi: 10.1073/pnas.70.8.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein F., Goody R. S. Synthesis and properties of diastereoisomers of adenosine 5'-(O-1-thiotriphosphate) and adenosine 5'-(O-2-thiotriphosphate). Biochemistry. 1976 Apr 20;15(8):1685–1691. doi: 10.1021/bi00653a015. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. J Am Chem Soc. 1970 Jul 29;92(15):4718–4723. doi: 10.1021/ja00718a039. [DOI] [PubMed] [Google Scholar]
- Gal-Or L., Mellema J. E., Moudrianakis E. N., Beer M. Electron microscopic study of base sequence in nucleic acids. VII. Cytosine-specific addition of acyl hydrazides. Biochemistry. 1967 Jul;6(7):1909–1915. doi: 10.1021/bi00859a006. [DOI] [PubMed] [Google Scholar]
- Jennette K. W., Lippard S. J., Vassiliades G. A., Bauer W. R. Metallointercalation reagents. 2-hydroxyethanethiolato(2,2',2'-terpyridine)-platinum(II) monocation binds strongly to DNA by intercalation. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3839–3843. doi: 10.1073/pnas.71.10.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A. S., Walker R. T., Youngs V. Preparation of a stable mercury derivative of tyrosine transfer RNA. Biochim Biophys Acta. 1973 Mar 19;299(2):293–299. doi: 10.1016/0005-2787(73)90352-3. [DOI] [PubMed] [Google Scholar]
- Kong P. C., Theophanides T. Platinum complexes of nucleotides. Bioinorg Chem. 1975;5(1):51–58. doi: 10.1016/s0006-3061(00)80220-3. [DOI] [PubMed] [Google Scholar]
- MOUDRIANAKIS E. N., BEER M. DETERMINATION OF BASE SEQUENCE IN NUCLEIC ACIDS WITH THE ELECTRON MICROSCOPE. II. THE REACTION OF A GUANINE-SELECTIVE MARKER WITH THE MONONUCLEOTIDES. Biochim Biophys Acta. 1965 Jan 11;95:23–39. doi: 10.1016/0005-2787(65)90207-8. [DOI] [PubMed] [Google Scholar]
- Mansy S., Rosenberg B., Thomson A. J. Binding of cis- and trans-dichlorodiammineplatinum(II) to nucleosides. I. Location of the binding sites. J Am Chem Soc. 1973 Mar 7;95(5):1633–1640. doi: 10.1021/ja00786a045. [DOI] [PubMed] [Google Scholar]
- Matzura H., Eckstein F. A polyribonucleotide containing alternation P=O and P=S linkages. Eur J Biochem. 1968 Feb;3(4):448–452. doi: 10.1111/j.1432-1033.1967.tb19551.x. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Atkinson M. R. Adenosine 5'-phosphorothioate. A nucleotide analog that is a substrate, competitive inhibitor, or regulator of some enzymes that interact with adenosine 5'-phosphate. Biochemistry. 1968 Nov;7(11):4023–4029. doi: 10.1021/bi00851a032. [DOI] [PubMed] [Google Scholar]
- Pal B. C., Shugart L. R., Isham K. R., Stulberg M. P. Modification of 4-thiouridine and phenylalanine transfer RNA with parachlormercuribenzoate. Arch Biochem Biophys. 1972 May;150(1):86–90. doi: 10.1016/0003-9861(72)90013-6. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Molecular weight estimation and separation of ribonucleic acid by electrophoresis in agarose-acrylamide composite gels. Biochemistry. 1968 Feb;7(2):668–674. doi: 10.1021/bi00842a023. [DOI] [PubMed] [Google Scholar]
- Pearson R. G. Acids and bases. Science. 1966 Jan 14;151(3707):172–177. doi: 10.1126/science.151.3707.172. [DOI] [PubMed] [Google Scholar]
- Scheit K. H., Faerber P. The interactions of 2-thiopyrimidine bases with hydroxymercurybenzene sulfonate. Eur J Biochem. 1973 Mar 15;33(3):545–550. doi: 10.1111/j.1432-1033.1973.tb02714.x. [DOI] [PubMed] [Google Scholar]
- Sunshine H. R., Lippard S. J. Mercury(II) binding to s4U in E. coli tRNA(Val). Nucleic Acids Res. 1974 May;1(5):673–688. doi: 10.1093/nar/1.5.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall J., Langmore J., Isaacson M., Crewe A. V. Scanning transmission electron microscopy at high resolution. Proc Natl Acad Sci U S A. 1974 Jan;71(1):1–5. doi: 10.1073/pnas.71.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting R. F., Ottensmeyer F. P. Heavy atoms in model compounds and nucleic acid imaged by dark field transmission electron microscopy. J Mol Biol. 1972 Jun 20;67(2):173–181. doi: 10.1016/0022-2836(72)90234-3. [DOI] [PubMed] [Google Scholar]


