Abstract
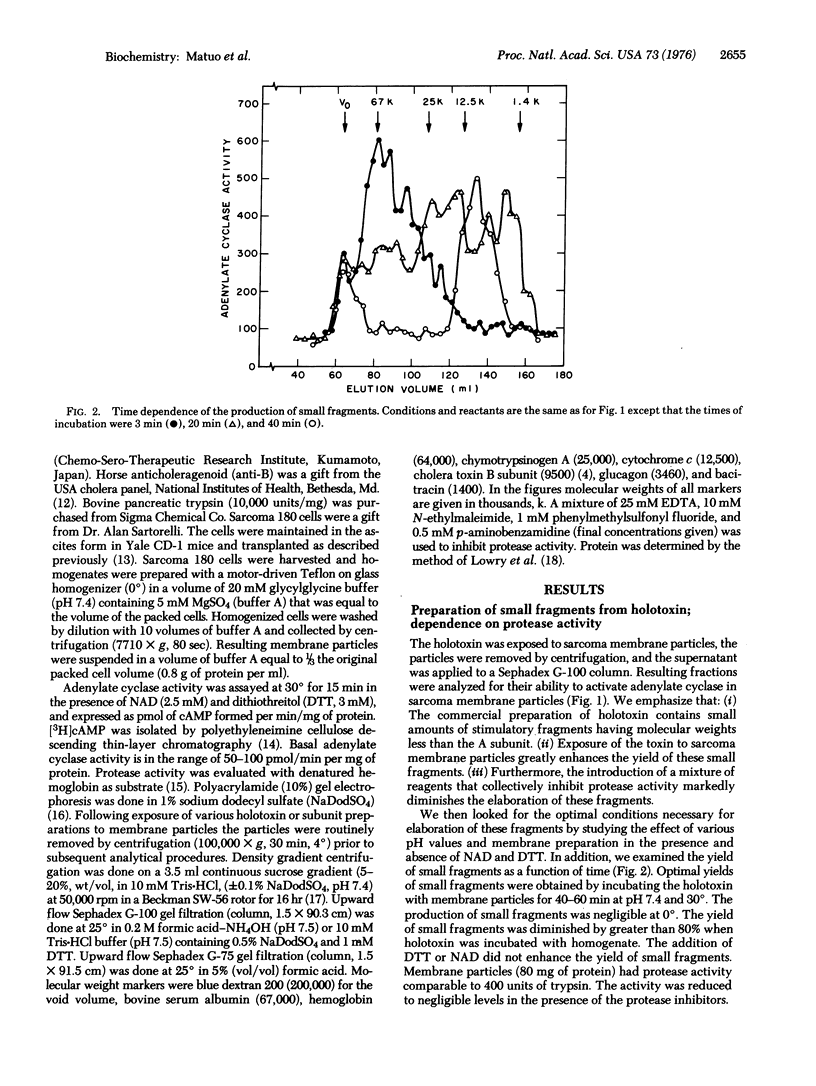
Exposure of cholera toxin to membrane particles prepared from sarcoma 180 cells gives rise to a variety of fragments which are capable of activating adenylate cyclase [ATP:pyrophosphate-lyase (cyclizing), EC 4.6.1.1]. A major component of these fragments has an apparent molecular weight in the 8,000-10,000 range. The smallest stimulatory fragment has a molecular weight of approximately 1400. The small size of the fragments is confirmed by Sephadex gel filtration, in the presence of either sodium dodecyl sulfate or formic acid. These fragments are produced from holotoxin or its A subunit by protease(s) found in sarcoma membrane particles. Production of fragments appears optimal in 40-60 min at 30 degrees and pH 7, and is prevented by protease inhibitors. The ability of the small fragments to activate adenylate cyclase is reversed by anti-holotoxin, but not anticholeragenoid, antibodies. These fragments require NAD for the activation of adenylate cyclase and are fully active after heating at 90 degrees for 5 min (pH 7).
Full text
PDF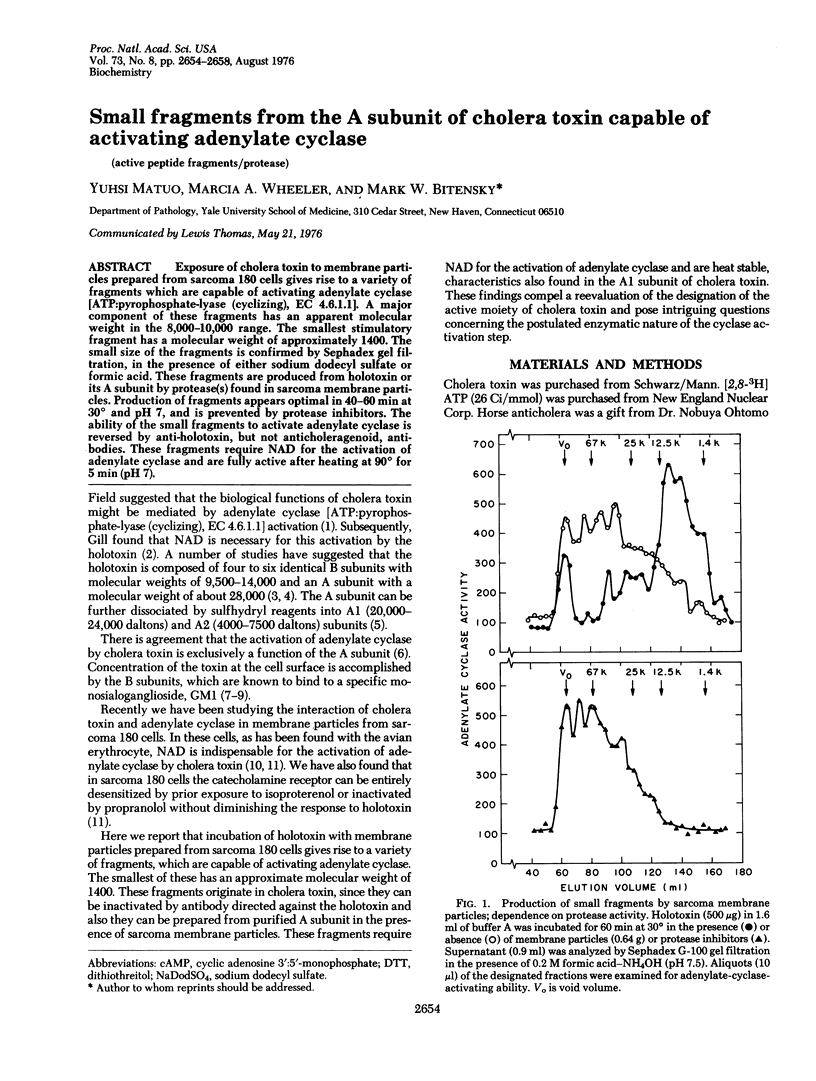

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal K. C., Booth B. A., Sartorelli A. C. Potential antitumor agents. I. A series of 5-substituted 1-formylisoquinoline thiosemicarbazones. J Med Chem. 1968 Jul;11(4):700–703. doi: 10.1021/jm00310a014. [DOI] [PubMed] [Google Scholar]
- Bitensky M. W., Wheeler M. A., Mehta H., Miki N. Cholera toxin activation of adenylate cyclase in cancer cell membrane fragments. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2572–2576. doi: 10.1073/pnas.72.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Gangliosides and membrane receptors for cholera toxin. Biochemistry. 1973 Aug 28;12(18):3558–3566. doi: 10.1021/bi00742a032. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Interaction of Vibrio cholerae enterotoxin with cell membranes. Biochemistry. 1973 Aug 28;12(18):3547–3558. doi: 10.1021/bi00742a031. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Vibrio cholerae choleragenoid. Mechanism of inhibition of cholera toxin action. Biochemistry. 1973 Aug 28;12(18):3577–3581. doi: 10.1021/bi00742a034. [DOI] [PubMed] [Google Scholar]
- Field M., Plotkin G. R., Silen W. Effects of vasopressin, theophylline and cyclic adenosine monophosphate on short-circuit current across isolated rabbit ileal mucosa. Nature. 1968 Feb 3;217(5127):469–471. doi: 10.1038/217469a0. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Boesman M., Neoh S. H., LaRue M. K., Delaney R. Dissociation and recombination of the subunits of the cholera enterotoxin (choleragen). J Immunol. 1974 Jul;113(1):145–150. [PubMed] [Google Scholar]
- Finkelstein R. A. Monospecific equine antiserum against cholera exo-enterotoxin. Infect Immun. 1970 Dec;2(6):691–697. doi: 10.1128/iai.2.6.691-697.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M. Involvement of nicotinamide adenine dinucleotide in the action of cholera toxin in vitro. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2064–2068. doi: 10.1073/pnas.72.6.2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M. Multiple roles of erythrocyte supernatant in the activation of adenylate cyclase by Vibrio cholerae toxin in vitro. J Infect Dis. 1976 Mar;133 (Suppl):55–63. doi: 10.1093/infdis/133.supplement_1.s55. [DOI] [PubMed] [Google Scholar]
- Keirns J. J., Wheeler M. A., Bitensky M. W. Isolation of cyclic AMP and cyclic GMP by thin-layer chromatography. Application to assay of adenylate cyclase, guanylate cyclase, and cyclic nucleotide phosphodiesterase. Anal Biochem. 1974 Oct;61(2):336–348. doi: 10.1016/0003-2697(74)90400-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Rifkind D., Frey J. A., Petersen E. A., Dinowitz M. Delayed hypersensitivity to fungal antigens in mice. II. Molecular classes in immunogenic RNA extracts that transfer delayed hypersensitivity. J Infect Dis. 1976 May;133(5):523–532. doi: 10.1093/infdis/133.5.523. [DOI] [PubMed] [Google Scholar]
- Steck T. L., Fairbanks G., Wallach D. F. Disposition of the major proteins in the isolated erythrocyte membrane. Proteolytic dissection. Biochemistry. 1971 Jun 22;10(13):2617–2624. doi: 10.1021/bi00789a031. [DOI] [PubMed] [Google Scholar]
- Van Heyningen S., King C. A. Short communications. Subunit A from cholera toxin is an activator of adenylate cyclase in pigeon erythrocytes. Biochem J. 1975 Jan;146(1):269–271. doi: 10.1042/bj1460269. [DOI] [PMC free article] [PubMed] [Google Scholar]


