Abstract
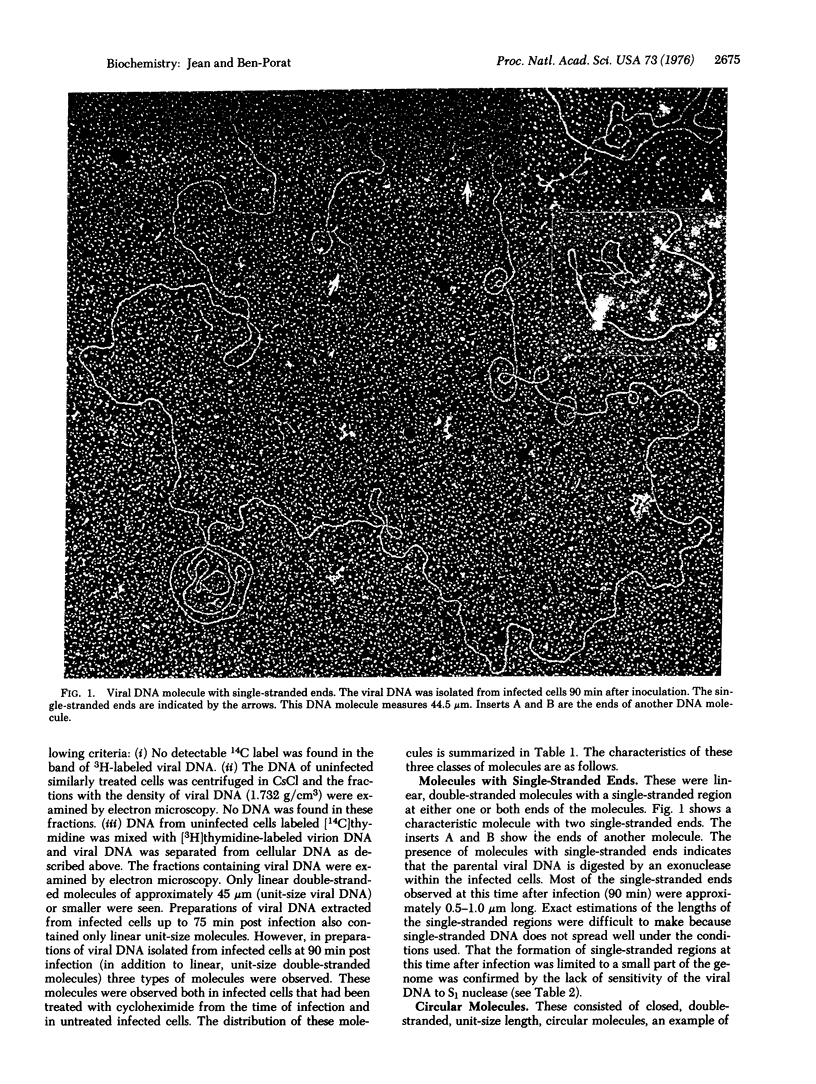
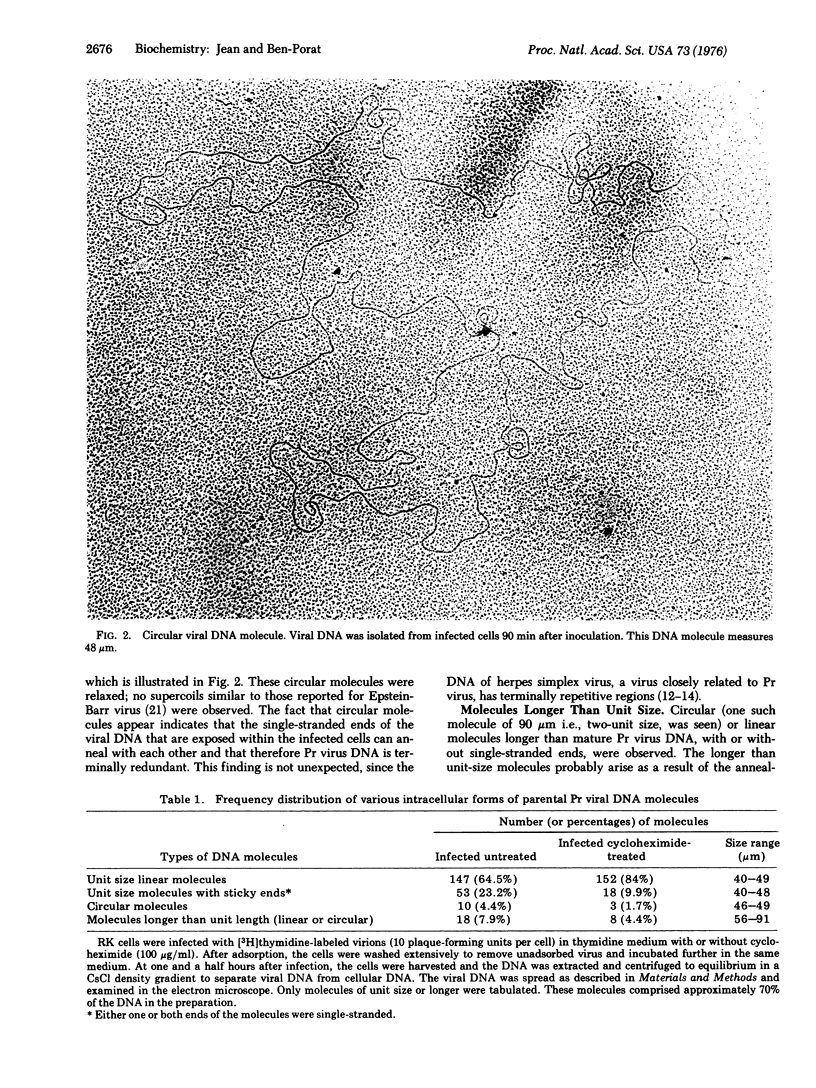
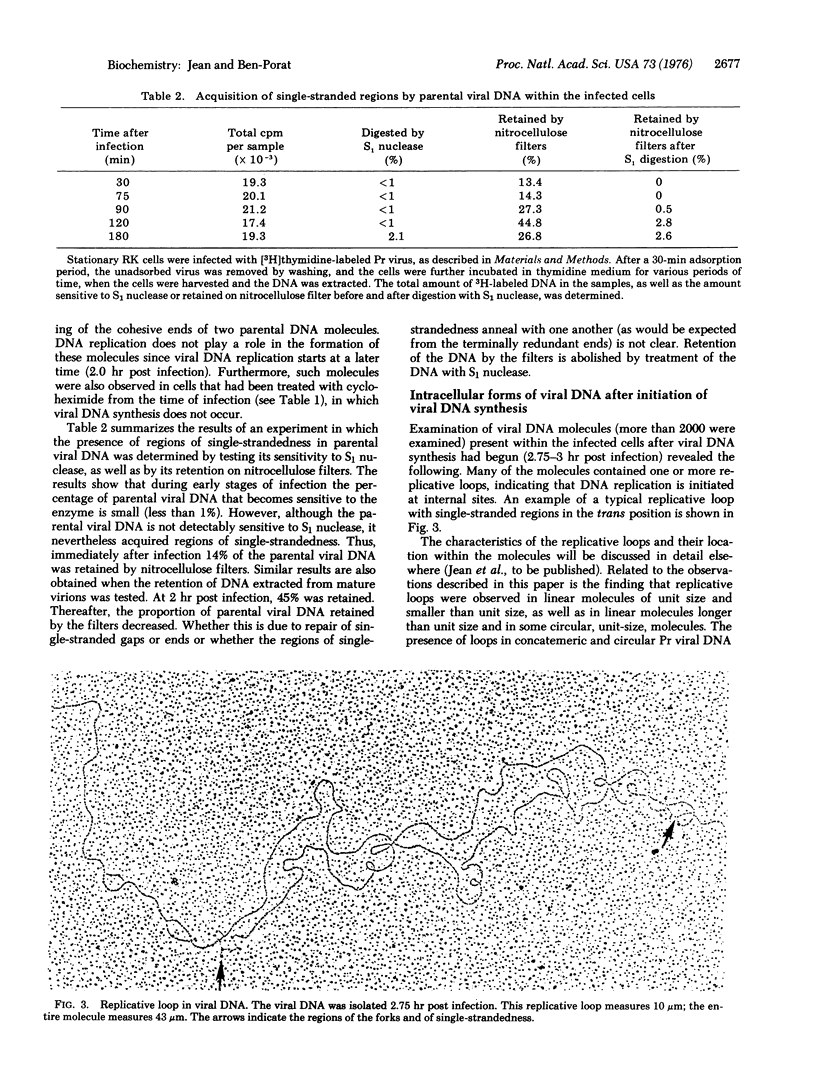
Intracellular forms of pseudorabies virus parental DNA were examined before and after the onset of viral DNA synthesis. Before initiation of synthesis, parental viral DNA acquires single-stranded ends. Circular and concatemeric molecules are also observed, indicating that the single-stranded ends are complementary. Viral DNA replication is initiated at an internal site within the DNA molecule, giving rise to characteristic replicative loops with single-stranded regions in the trans position. Such replicative loops were seen in unit-size (and smaller than unit-size) linear molecules as well as in circular and concatemeric molecules. These results show that the parental viral DNA molecules that acquire single-stranded ends, and consequently are able to form circles and concatemers, proceed to replicate.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEN-PORAT T., KAPLAN A. S. The synthesis and fate of pseudorabies virus DNA in infected mammalian cells in the stationary phase of growth. Virology. 1963 Jun;20:310–317. doi: 10.1016/0042-6822(63)90120-x. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Kaplan A. S., Stehn B., Rubenstein A. S. Concatemeric forms of intracellular herpesvirus DNA. Virology. 1976 Feb;69(2):547–560. doi: 10.1016/0042-6822(76)90484-0. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Stehn B., Kaplan A. S. Fate of parental herpesvirus DNA. Virology. 1976 Jun;71(2):412–422. doi: 10.1016/0042-6822(76)90369-x. [DOI] [PubMed] [Google Scholar]
- Biswal N., Murray B. K., Benyesh-Melnick M. Ribonucleotides in newly synthesized DNA of herpes simplex virus. Virology. 1974 Sep;61(1):87–99. doi: 10.1016/0042-6822(74)90244-x. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Frenkel N., Roizman B. Separation of the herpesvirus deoxyribonucleic acid duplex into unique fragments and intact strand on sedimentation in alkaline gradients. J Virol. 1972 Oct;10(4):565–572. doi: 10.1128/jvi.10.4.565-572.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafstrom R. H., Alwine J. C., Steinhart W. L., Hill C. W. Terminal repetitions in herpes simplex virus type 1 DNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):679–681. doi: 10.1101/sqb.1974.039.01.081. [DOI] [PubMed] [Google Scholar]
- Huberman J. A. Visualization of replicating mammalian and T4 bacteriophage DNA. Cold Spring Harb Symp Quant Biol. 1968;33:509–524. doi: 10.1101/sqb.1968.033.01.059. [DOI] [PubMed] [Google Scholar]
- KAPLAN A. S., BEN-PORAT T. MODE OF REPLICATION OF PSEUDORABIES VIRUS DNA. Virology. 1964 May;23:90–95. doi: 10.1016/s0042-6822(64)80011-8. [DOI] [PubMed] [Google Scholar]
- KAPLAN A. S., VATTER A. E. A comparison of herpes simplex and pseudorabies viruses. Virology. 1959 Apr;7(4):394–407. doi: 10.1016/0042-6822(59)90068-6. [DOI] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Adams A., Lindahl T., Bornkamm G. W., Bjursell G., Klein G., Giovanella B. C., Singh S. Intracellular forms of Epstein-Barr virus DNA in human tumour cells in vivo. Nature. 1976 Mar 25;260(5549):302–306. doi: 10.1038/260302a0. [DOI] [PubMed] [Google Scholar]
- Rubenstein A. S., Kaplan A. S. Electron microscopic studies of the DNA of defective and standard pseudorabies virions. Virology. 1975 Aug;66(2):385–392. doi: 10.1016/0042-6822(75)90211-1. [DOI] [PubMed] [Google Scholar]
- Sheldrick P., Berthelot N. Inverted repetitions in the chromosome of herpes simplex virus. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 2):667–678. doi: 10.1101/sqb.1974.039.01.080. [DOI] [PubMed] [Google Scholar]
- Shlomai J., Friedmann A., Becker Y. Replication intermediates of herpes simplex virus DNA. Virology. 1976 Feb;69(2):647–659. doi: 10.1016/0042-6822(76)90493-1. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]
- WARNER J. R., KNOPF P. M., RICH A. A multiple ribosomal structure in protein synthesis. Proc Natl Acad Sci U S A. 1963 Jan 15;49:122–129. doi: 10.1073/pnas.49.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth S., Hayward G. S., Roizman B. Anatomy of herpes simplex virus DNA. V. Terminally repetitive sequences. J Virol. 1976 Feb;17(2):503–512. doi: 10.1128/jvi.17.2.503-512.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadsworth S., Jacob R. J., Roizman B. Anatomy of herpes simplex virus DNA. II. Size, composition, and arrangement of inverted terminal repetitions. J Virol. 1975 Jun;15(6):1487–1497. doi: 10.1128/jvi.15.6.1487-1497.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie N. M. The synthesis and substructure of herpesvirus DNA: the distribution of alkali-labile single strand interruptions in HSV-1 DNA. J Gen Virol. 1973 Dec;21(3):453–467. doi: 10.1099/0022-1317-21-3-453. [DOI] [PubMed] [Google Scholar]