Abstract
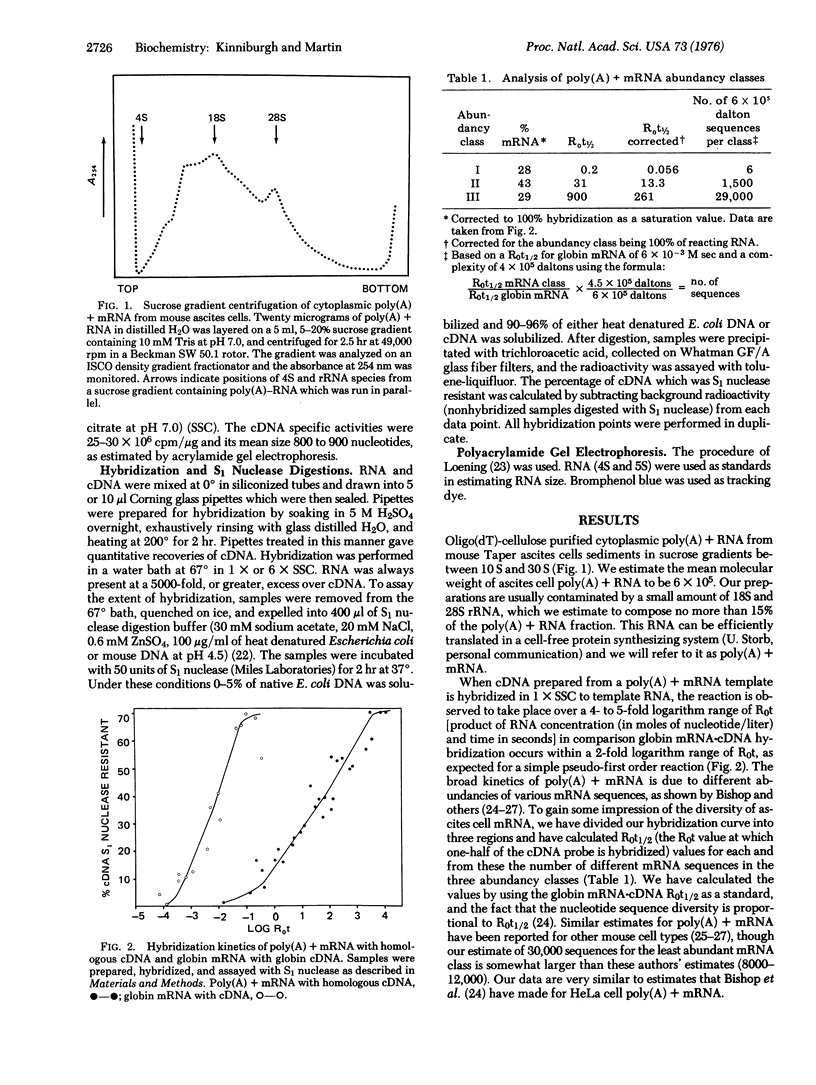
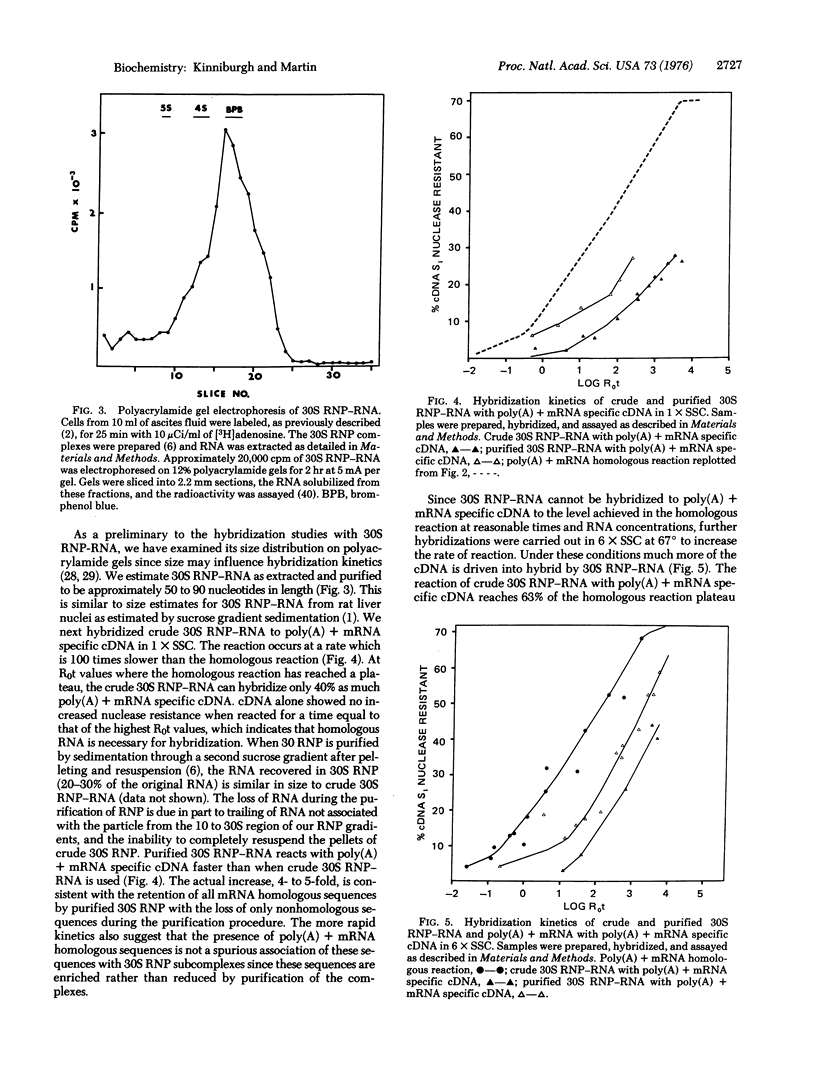
RNA from nuclear 30S ribonucleoprotein (RNP) complexes of mouse ascites cells has been shows to contain sequences homologous to poly(A) + mRNA by its ability to hybridize with complementary DNA prepared from poly(A) + mRNA template. Analysis of the hybridization kinetics of poly(A) + mRNA with its own complementary DNA revealed several abundancy classes. The total complexity of poly(A) + mRNA from ascites cells was estimated to be approximately 30,000 sequences of average molecular weight (6 X 10(5)). When the hybridization reaction of 30S RNP-RNA with mRNA-specific cDNA was compared to the homologous reaction the majority, and most probably all, of the poly(A) + mRNA sequences were found to be present in the RNA. The kinetics of hybridization suggest that 10-15% of the RNA in this RNP complex is homologous to poly(A) + mRNA. The 30S RNP subcomplexes therefore contain nuclear poly(A) + mRNA sequences as well as the bulk of heterogeneous RNA.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachenheimer S., Darnell J. E. Adenovirus-2 mRNA is transcribed as part of a high-molecular-weight precursor RNA. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4445–4449. doi: 10.1073/pnas.72.11.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnie G. D., MacPhail E., Young B. D., Getz M. J., Paul J. The diversity of the messenger RNA population in growing Friend cells. Cell Differ. 1974 Nov;3(4):221–232. doi: 10.1016/0045-6039(74)90005-0. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Morton J. G., Rosbash M., Richardson M. Three abundance classes in HeLa cell messenger RNA. Nature. 1974 Jul 19;250(463):199–204. doi: 10.1038/250199a0. [DOI] [PubMed] [Google Scholar]
- Blobel G. A protein of molecular weight 78,000 bound to the polyadenylate region of eukaryotic messenger RNAs. Proc Natl Acad Sci U S A. 1973 Mar;70(3):924–928. doi: 10.1073/pnas.70.3.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo M. S., Bishop J. O. Two classes of messenger RNA in cultured rat cells: repetitive sequence transcripts and unique sequence transcripts. J Mol Biol. 1974 Dec 25;90(4):649–663. doi: 10.1016/0022-2836(74)90530-0. [DOI] [PubMed] [Google Scholar]
- Cornudella L., Faiferman I., Pogo A. O. Polyadenylic acid sequences in ascites cells nuclear particles and membrane-bound messenger RNA. Biochim Biophys Acta. 1973 Feb 4;294(1):541–546. doi: 10.1016/0005-2787(73)90113-5. [DOI] [PubMed] [Google Scholar]
- Daneholt B., Hosick H. The transcription unit in Balbiani ring 2 of Chironomus tentans. Cold Spring Harb Symp Quant Biol. 1974;38:629–635. doi: 10.1101/sqb.1974.038.01.067. [DOI] [PubMed] [Google Scholar]
- Firtel R. A., Pederson T. Ribonucleoprotein particles containing heterogeneous nuclear RNA in the cellular slime mold Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1975 Jan;72(1):301–305. doi: 10.1073/pnas.72.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. R., Wetmur J. G. Renaturation of bacteriophage phiX174 DNA-RNA hybrid: RNA length effect and nucleation rate constant. J Mol Biol. 1973 Jul 15;77(4):495–500. doi: 10.1016/0022-2836(73)90218-0. [DOI] [PubMed] [Google Scholar]
- Imaizumi T., Diggelmann H., Scherrer K. Demonstration of globin messenger sequences in giant nuclear precursors of messenger RNA of avian erythroblasts. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1122–1126. doi: 10.1073/pnas.70.4.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin D., Kumar A., Malt R. A. Messenger ribonucleoprotein complexes isolated with oligo(dT)-cellulose chromatography from kidney polysomes. Cell. 1975 Feb;4(2):157–165. doi: 10.1016/0092-8674(75)90122-1. [DOI] [PubMed] [Google Scholar]
- Kish V. M., Pederson T. Ribonucleoprotein organization of polyadenylate sequences in HeLa cell heterogeneous nuclear RNA. J Mol Biol. 1975 Jun 25;95(2):227–238. doi: 10.1016/0022-2836(75)90392-7. [DOI] [PubMed] [Google Scholar]
- Kumar A., Pederson T. Comparison of proteins bound to heterogeneous nuclear RNA and messenger RNA in HeLa cells. J Mol Biol. 1975 Aug 15;96(3):353–365. doi: 10.1016/0022-2836(75)90165-5. [DOI] [PubMed] [Google Scholar]
- Lindberg U., Sundquist B. Isolation of messenger ribonucleoproteins from mammalian cells. J Mol Biol. 1974 Jun 25;86(2):451–468. doi: 10.1016/0022-2836(74)90030-8. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukanidin E. M., Olsnes S., Pihl A. Antigenic difference between informofers and protein bound to polyribosomal mRNA from rat liver. Nat New Biol. 1972 Nov 15;240(98):90–92. doi: 10.1038/newbio240090a0. [DOI] [PubMed] [Google Scholar]
- Martin T. E. A simple general method to determine the proportion of active ribosomes in eukaryotic cells. Exp Cell Res. 1973 Aug;80(2):496–498. doi: 10.1016/0014-4827(73)90333-9. [DOI] [PubMed] [Google Scholar]
- Martin T. E., McCarthy B. J. Synthesis and turnover of RNA in the 30-S nuclear ribonucleoprotein complexes of mouse ascites cells. Biochim Biophys Acta. 1972 Aug 25;277(2):354–367. doi: 10.1016/0005-2787(72)90417-0. [DOI] [PubMed] [Google Scholar]
- Martin T., Billings P., Levey A., Ozarslan S., Quinlan T., Swift H., Urbas L. Some properties of RNA:protein complexes from the nucleus of eukaryotic cells. Cold Spring Harb Symp Quant Biol. 1974;38:921–932. doi: 10.1101/sqb.1974.038.01.094. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Schimke R. T. Ovalbumin messenger RNA: evidence that the initial product of transcription is the same size as polysomal ovalbumin messenger. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4327–4331. doi: 10.1073/pnas.71.11.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milcarek C., Price R., Penman S. The metabolism of a poly(A) minus mRNA fraction in HeLa cells. Cell. 1974 Sep;3(1):1–10. doi: 10.1016/0092-8674(74)90030-0. [DOI] [PubMed] [Google Scholar]
- Morel Carlos, Kayibanda Boniface, Scherrer Klaus. Proteins associated with globin messenger RNA in avian erythroblasts: Isolation and comparison with the proteins bound to nuclear messenger-likie RNA. FEBS Lett. 1971 Oct 15;18(1):84–88. doi: 10.1016/0014-5793(71)80413-1. [DOI] [PubMed] [Google Scholar]
- Pederson T. Proteins associated with heterogeneous nuclear RNA in eukaryotic cells. J Mol Biol. 1974 Feb 25;83(2):163–183. doi: 10.1016/0022-2836(74)90386-6. [DOI] [PubMed] [Google Scholar]
- Perry R. P., La Torre J., Kelley D. E., Greenberg J. R. On the lability of poly(A) sequences during extraction of messenger RNA from polyribosomes. Biochim Biophys Acta. 1972 Mar 14;262(2):220–226. doi: 10.1016/0005-2787(72)90236-5. [DOI] [PubMed] [Google Scholar]
- Quinlan T. J., Billings P. B., Martin T. E. Nuclear ribonucleoprotein complexes containing polyadenylate from mouse ascites cells. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2632–2636. doi: 10.1073/pnas.71.7.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryffel G. U., McCarthy B. J. Complexity of cytoplasmic RNA in different mouse tissues measured by hybridization of polyadenylated RNA to complementary DNA. Biochemistry. 1975 Apr 8;14(7):1379–1385. doi: 10.1021/bi00678a006. [DOI] [PubMed] [Google Scholar]
- Samarina O. P., Lukanidin E. M., Molnar J., Georgiev G. P. Structural organization of nuclear complexes containing DNA-like RNA. J Mol Biol. 1968 Apr 14;33(1):251–263. doi: 10.1016/0022-2836(68)90292-1. [DOI] [PubMed] [Google Scholar]
- Soeiro R., Vaughan M. H., Warner J. R., Darnell J. E., Jr The turnover of nuclear DNA-like RNA in HeLa cells. J Cell Biol. 1968 Oct;39(1):112–118. doi: 10.1083/jcb.39.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens R. H., Williamson A. R. Specific IgG mRNA molecules from myeloma cells in heterogeneous nuclear and cytoplasmic RNA containing poly-A. Nature. 1972 Sep 15;239(5368):143–146. doi: 10.1038/239143a0. [DOI] [PubMed] [Google Scholar]
- Sutton W. D. A crude nuclease preparation suitable for use in DNA reassociation experiments. Biochim Biophys Acta. 1971 Jul 29;240(4):522–531. doi: 10.1016/0005-2787(71)90709-x. [DOI] [PubMed] [Google Scholar]
- Verma I. M., Temple G. F., Fan H., Baltimore D. In vitro synthesis of DNA complementary to rabbit reticulocyte 10S RNA. Nat New Biol. 1972 Feb 9;235(58):163–167. doi: 10.1038/newbio235163a0. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Penman S. The messenger RNA sequences in growing and resting mouse fibroblasts. Cell. 1975 Oct;6(2):197–206. doi: 10.1016/0092-8674(75)90010-0. [DOI] [PubMed] [Google Scholar]
- Williamson R., Drewienkiewicz C. E., Paul J. Globin messenger sequences in high molecular weight RNA from embryonic mouse liver. Nat New Biol. 1973 Jan 17;241(107):66–68. doi: 10.1038/newbio241066a0. [DOI] [PubMed] [Google Scholar]


