Abstract
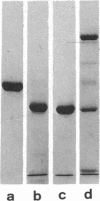
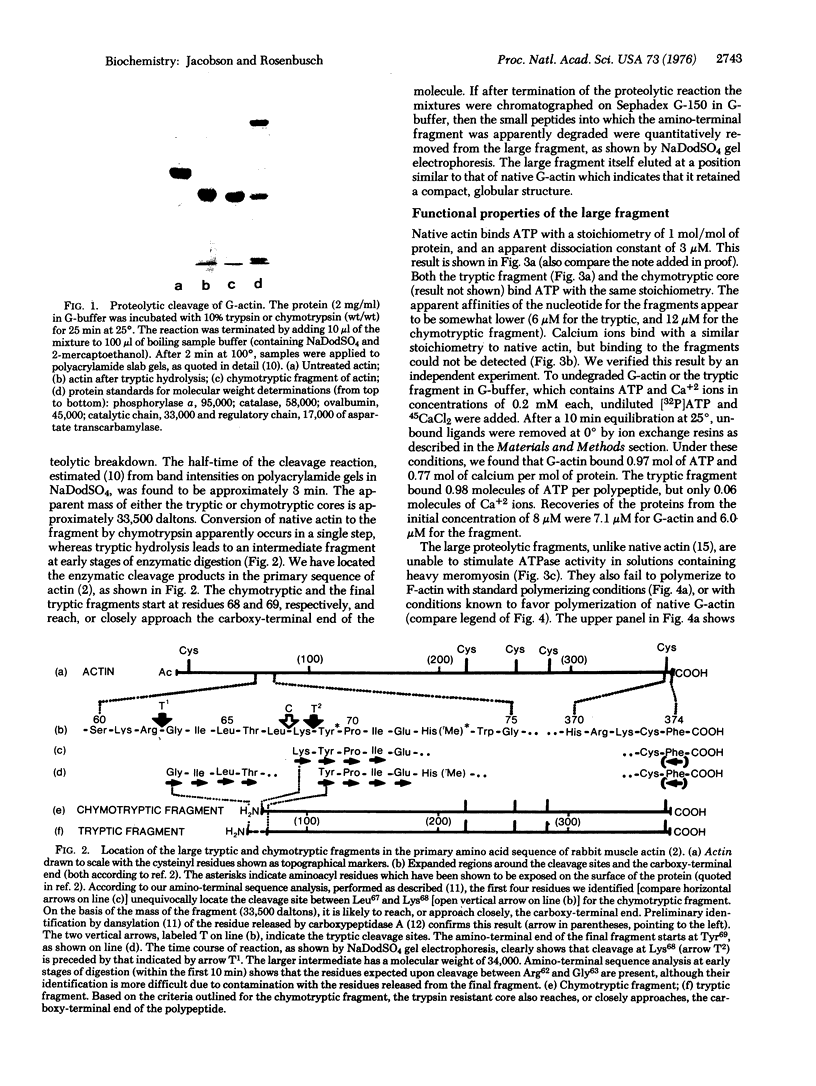
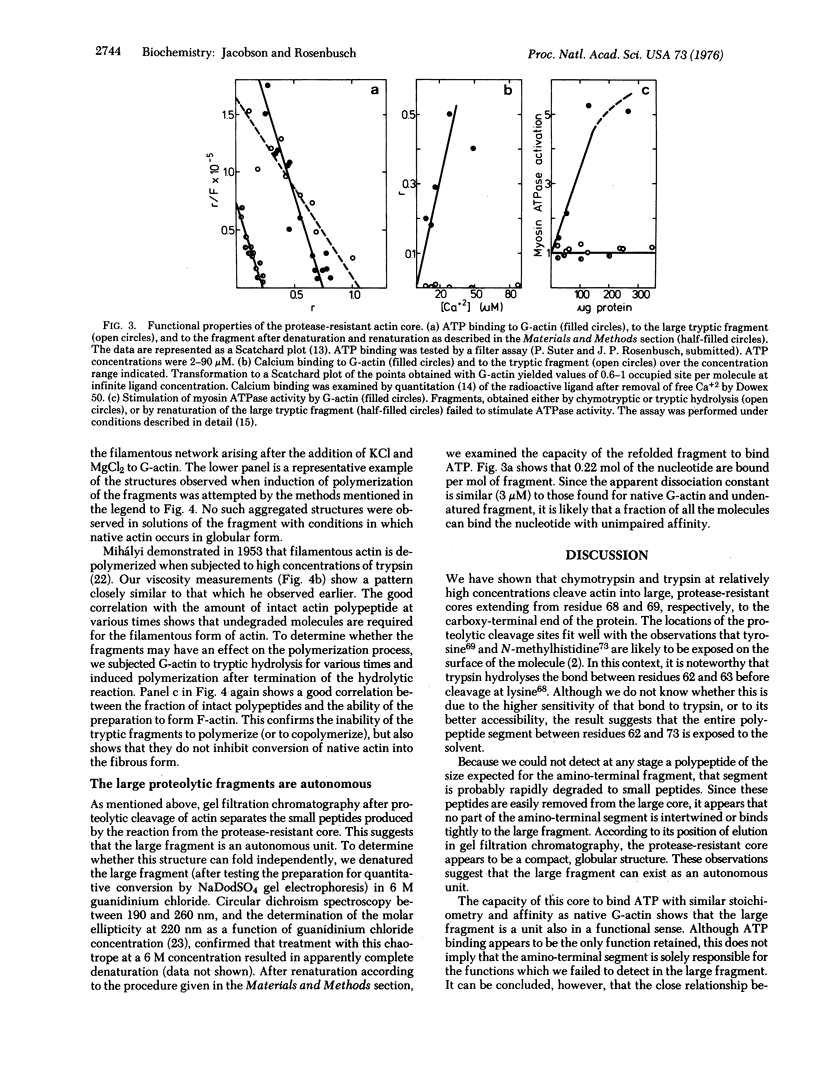
Actin can be cleaved by trypsin or chymotrypsin into a large, autonomous fragment with approximately 80% of the mass of the undegraded polypeptide. The protease-resistant cores obtained with either enzyme are very similar. Although the fragment does not bind calcium ions and fails to polymerize to the filamentous form of actin or to stimulate myosin adenosine triphosphatase (ATP phosphohydrolase, EC 3.6.1.3) activity, it retains the full capacity to bind ATP. This observation suggests that it represents an independent functional unit. Cleavage of globular actin with either trypsin or chymotrypsin occurs with half-times of 3 min, while that of filamentous actin proceeds with reaction half-times of 20 min for trypsin and nearly 2 hr for chymotrypsin. Denaturation and renaturation of the trypsin-resistant core shows that approximately 20% of the molecules refold to functional forms which indicates that the fragment can be considered as an independent unit of folding as well.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Elzinga M., Collins J. H., Kuehl W. M., Adelstein R. S. Complete amino-acid sequence of actin of rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2687–2691. doi: 10.1073/pnas.70.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J. H., Rosenbusch J. P., Weber K. K., Blout E. R. Conformational changes in aspartate trancarbamylase. I. Studies of ligand binding and of subunit interactions by circular dichroism spectroscopy. J Biol Chem. 1972 Oct 25;247(20):6482–6490. [PubMed] [Google Scholar]
- Kasai M. Thermodynamical aspect of G-F transformations of actin. Biochim Biophys Acta. 1969 Jun 24;180(2):399–409. doi: 10.1016/0005-2728(69)90124-8. [DOI] [PubMed] [Google Scholar]
- Kirschner K., Bisswanger H. Multifunctional proteins. Annu Rev Biochem. 1976;45:143–166. doi: 10.1146/annurev.bi.45.070176.001043. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lengsfeld A. M., Löw I., Wieland T., Dancker P., Hasselbach W. Interaction of phalloidin with actin. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2803–2807. doi: 10.1073/pnas.71.7.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowey S., Slayter H. S., Weeds A. G., Baker H. Substructure of the myosin molecule. I. Subfragments of myosin by enzymic degradation. J Mol Biol. 1969 May 28;42(1):1–29. doi: 10.1016/0022-2836(69)90483-5. [DOI] [PubMed] [Google Scholar]
- MIHALYI E. Trypsin digestion of muscle proteins. II. The kinetics of the digestion. J Biol Chem. 1953 Mar;201(1):197–209. [PubMed] [Google Scholar]
- MIHASHI K., OOI T. LOCATION OF ABNORMAL TYROSINES IN ACTIN. Biochemistry. 1965 May;4:805–813. doi: 10.1021/bi00881a003. [DOI] [PubMed] [Google Scholar]
- Mihashi K. Alkylation of urea-denatured actin with iodoacetate: functional reorganization of actin. Biochim Biophys Acta. 1972 May 25;267(2):409–421. doi: 10.1016/0005-2728(72)90128-4. [DOI] [PubMed] [Google Scholar]
- Muszbek L., Gladner J. A., Laki K. The fragmentation of actin by thrombin. Isolation and characterization of the split products. Arch Biochem Biophys. 1975 Mar;167(1):99–103. doi: 10.1016/0003-9861(75)90445-2. [DOI] [PubMed] [Google Scholar]
- Muszbek L., Laki K. Cleavage of actin by thrombin. Proc Natl Acad Sci U S A. 1974 Jun;71(6):2208–2211. doi: 10.1073/pnas.71.6.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A. Biochemical and structural studies of actomyosin-like proteins from non-muscle cells. II. Purification, properties, and membrane association of actin from amoebae of Dictyostelium discoideum. J Biol Chem. 1974 Sep 25;249(18):6013–6020. [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Suter P., Rosenbusch J. P. Topology of binding sites for carbamyl phosphate in aspartate transcarbamylase from Escherichia coli. The use of pyridoxal phosphate as covalent probe. Eur J Biochem. 1975 May;54(1):293–299. doi: 10.1111/j.1432-1033.1975.tb04139.x. [DOI] [PubMed] [Google Scholar]
- Takacs B. J., Rosenbusch J. P. Modification of Escherichia coli membranes in the prereplicative phase of phage T4 infection. Specificity of association and quantitation of bound phage proteins. J Biol Chem. 1975 Mar 25;250(6):2339–2350. [PubMed] [Google Scholar]
- Tanford C. Protein denaturation. Adv Protein Chem. 1968;23:121–282. doi: 10.1016/s0065-3233(08)60401-5. [DOI] [PubMed] [Google Scholar]
- Weber K., Kuter D. J. Reversible denaturation of enzymes by sodium dodecyl sulfate. J Biol Chem. 1971 Jul 25;246(14):4504–4509. [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]
- Wetlaufer D. B. Nucleation, rapid folding, and globular intrachain regions in proteins. Proc Natl Acad Sci U S A. 1973 Mar;70(3):697–701. doi: 10.1073/pnas.70.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAGI K., MASE R., SAKAKIBARA I., ASAI H. FUNCTION OF HEAVY MEROMYOSIN IN THE ACCELERATION OF ACTIN POLYMERIZATION. J Biol Chem. 1965 Jun;240:2448–2454. [PubMed] [Google Scholar]