Abstract
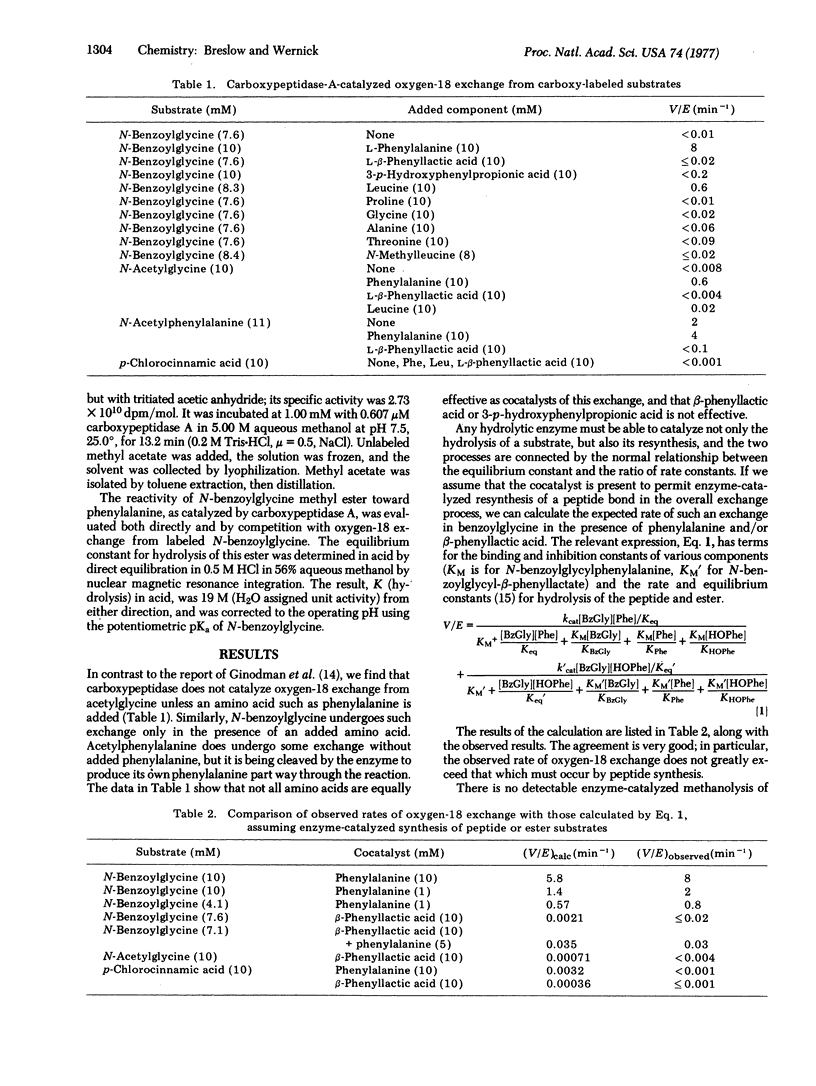
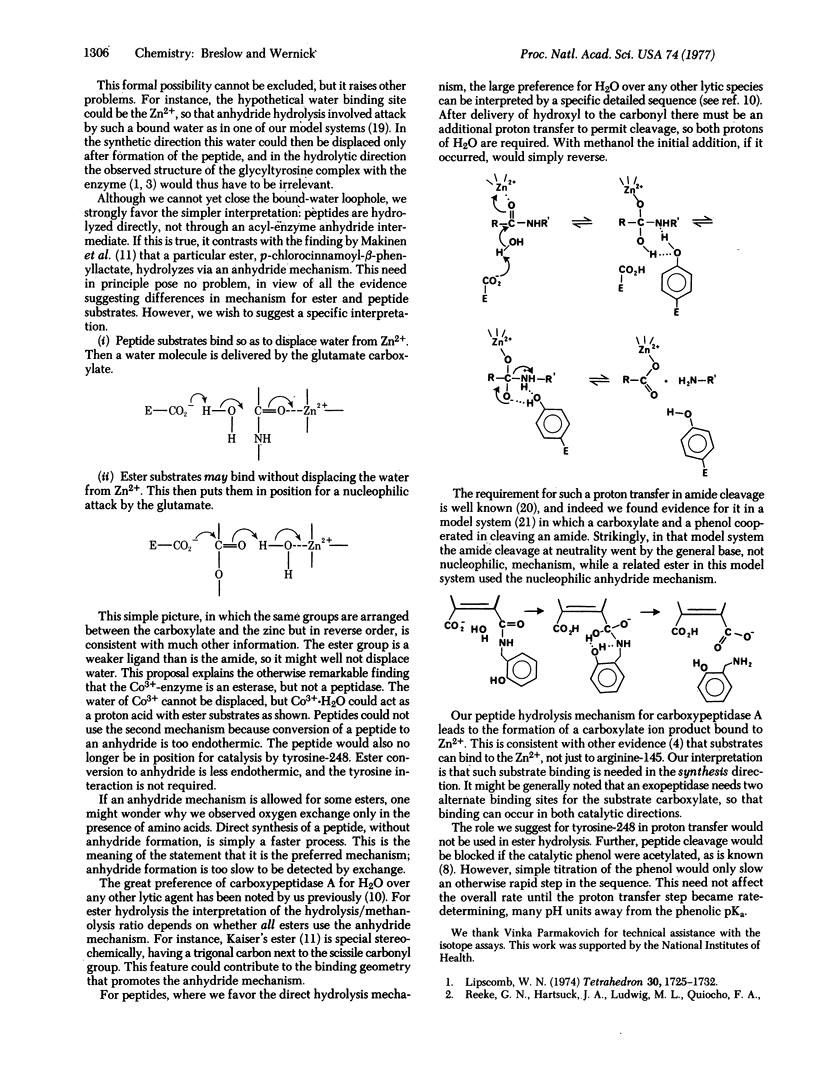
We have reported evidence that an anhydride intermediate is not involved in the hydrolysis of typical peptide substrates by carboxypeptidase A (peptidyl-L-amino-acid hydrolase, EC 3.4.12.2), and we describe further evidence here. Recently an anhydride intermediate has been detected in the hydrolysis of an ester substrate by this enzyme. Other evidence also suggests that esters and peptides may not be cleaved by the same type of mechanism. A possible explanation is that the substrate carbonyl and a water molecule are always aligned between glutamate-270 and the zinc atom of the enzyme, but not always in the same sequence. With peptides the carbonyl is coordinated to zinc, and the water is delivered by glutamate acting as a general base. Esters are weaker ligands, and in some cases the ester carbonyl may not displace water from zinc. This would lead to a nucleophilic mechanism, with glutamate-270 forming an anhydride while zinc-aquo serves as a Brönsted acid. This picture is consistent with other evidence on ester cleavage, and resolves the otherwise baffling discrepant data on peptide as compared to ester substrates.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auld D. S., Holmquist B. Carboxypeptidase A. Differences in the mechanisms of ester and peptide hydrolysis. Biochemistry. 1974 Oct 8;13(21):4355–4361. doi: 10.1021/bi00718a018. [DOI] [PubMed] [Google Scholar]
- Breslow R., McClure D. E., Brown R. S., Eisenach J. Letter: Very fast zinc-catalyzed hydrolysis of an anhydride. A model for the rate and mechanism of carboxypeptidase A catalysis. J Am Chem Soc. 1975 Jan 8;97(1):194–195. doi: 10.1021/ja00834a037. [DOI] [PubMed] [Google Scholar]
- Breslow R., McClure D. E. Letter: Cooperative catalysis of the cleavage of an amide by carboxylate and phenolic groups in a carboxypeptidase A model. J Am Chem Soc. 1976 Jan 7;98(1):258–259. doi: 10.1021/ja00417a054. [DOI] [PubMed] [Google Scholar]
- Breslow R., Wernick D. Letter: On the mechanism of catalysis by carboxypeptidase A. J Am Chem Soc. 1976 Jan 7;98(1):259–261. doi: 10.1021/ja00417a055. [DOI] [PubMed] [Google Scholar]
- COLEMAN J. E., VALLEE B. L. Metallocarboxypeptidases: stability constants and enzymatic characteristics. J Biol Chem. 1961 Aug;236:2244–2249. [PubMed] [Google Scholar]
- Ginodman L. M., Mal'tsev N. I., Orekhovich V. N. Issledovanie sposobnosti karboksipeptidazy A katalizirovat' reaktsii izotopnogo obmena kisloroda i transpeptidatsii. Biokhimiia. 1966 Nov-Dec;31(6):1073–1078. [PubMed] [Google Scholar]
- Johansen J. T., Klyosov A. A., Vallee B. L. Circular dichroism-inhibitor titrations of arsanilazotyrosine-248 carboxypeptidase A. Biochemistry. 1976 Jan 27;15(2):296–303. doi: 10.1021/bi00647a009. [DOI] [PubMed] [Google Scholar]
- Kang E. P., Storm C. B., Carson F. W. Cobalt(III) carboxypeptidase A. J Am Chem Soc. 1975 Nov 12;97(23):6723–6728. doi: 10.1021/ja00856a021. [DOI] [PubMed] [Google Scholar]
- Lipscomb W. N., Hartsuck J. A., Reeke G. N., Jr, Quiocho F. A., Bethge P. H., Ludwig M. L., Steitz T. A., Muirhead H., Coppola J. C. The structure of carboxypeptidase A. VII. The 2.0-angstrom resolution studies of the enzyme and of its complex with glycyltyrosine, and mechanistic deductions. Brookhaven Symp Biol. 1968 Jun;21(1):24–90. [PubMed] [Google Scholar]
- Makinen M. W., Yammura K., Kaiser E. T. Mechanism of action of carboxypeptidase A in ester hydrolysis. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3882–3886. doi: 10.1073/pnas.73.11.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau H., Riordan J. F. Gas chromatography-mass spectrometry for probing the structure and mechanism of action of enzyme active sites. The role of Glu-270 in carboxypeptidase A. Biochemistry. 1975 Dec 2;14(24):5285–5294. doi: 10.1021/bi00695a009. [DOI] [PubMed] [Google Scholar]
- Quiocho F. A., Lipscomb W. N. Carboxypeptidase A: a protein and an enzyme. Adv Protein Chem. 1971;25:1–78. doi: 10.1016/s0065-3233(08)60278-8. [DOI] [PubMed] [Google Scholar]
- Reeke G. N., Hartsuck J. A., Ludwig M. L., Quiocho F. A., Steitz T. A., Lipscomb W. N. The structure of carboxypeptidase a, vi. Some results at 2.0-a resolution, and the complex with glycyl-tyrosine at 2.8-a resolution. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2220–2226. doi: 10.1073/pnas.58.6.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]


