Abstract
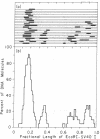
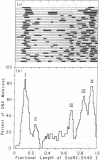
Sequences with 2-fold axes of symmetry have been detected and mapped on the simian virus 40 (SV40) genome by their ability to form hairpin turns in single-stranded SV40 DNA. Supercoiled SV40 DNA (SV40 I) was digested with restriction enzymes EcoRI and HpaII. The resulting linear DNA molecules with lengths of the complete SV40 genome were then denatured and photochemically reacted with 4,5',8-trimethylpsoralen (trioxsalen) at 16.0 +/- 0.5 degrees and different ionic strengths. Secondary structures on the single-stranded SV40 DNA were crosslinked and their positions analyzed by electron microscopy. There were no observable hairpin turns on the denatured SV40 DNA when it was photoreacted in 1 mM Tris-HCl/0.1 mM EDTA at pH 7.0. In 20 mM NaCl, one specific hairpin turn was detected at 0.17 +/- 0.02 map units on the map of EcoRI-digested SV40 DNA, where the 3' ends of early 19S mRNA, late 19S mRNA, and 16S mRNA of SV40 have been mapped. In 30 mM NaCl there are five more major hairpin turns besides the most stable one. The centers of four of these specific hairpin turns were mapped at 0.26 +/- 0.02, 0.68 +/- 0.03, 0.84 +/- 0.02, and 0.94 +/- 0.01 units on the map of EcoRI-digested SV40. The fifth one is at or near the unique EcoRI cleavage site on SV40 DNA. The possible functions of these sequences are discussed in terms of the nature of the promoter sites, the replication origin, the processing of RNA precursors, and regulation at the translational level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acheson N. H. Transcription during productive infection with polyoma virus and Simian virus 40. Cell. 1976 May;8(1):1–12. doi: 10.1016/0092-8674(76)90179-3. [DOI] [PubMed] [Google Scholar]
- Baltimore D., Jacobson M. F., Asso J., Huang A. S. The formation of poliovirus proteins. Cold Spring Harb Symp Quant Biol. 1969;34:741–746. doi: 10.1101/sqb.1969.034.01.083. [DOI] [PubMed] [Google Scholar]
- Büsen W., Hausen P. Distinct ribonuclease H activities in calf thymus. Eur J Biochem. 1975 Mar 3;52(1):179–190. doi: 10.1111/j.1432-1033.1975.tb03985.x. [DOI] [PubMed] [Google Scholar]
- Chou J. Y., Martin R. G. Complementation analysis of simian virus 40 mutants. J Virol. 1974 May;13(5):1101–1109. doi: 10.1128/jvi.13.5.1101-1109.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R. S. Light-induced cross-linking of DNA in the presence of a furocoumarin (psoralen). Studies with phage lambda, Escherichia coli, and mouse leukemia cells. Biochim Biophys Acta. 1970 Sep 17;217(1):30–39. doi: 10.1016/0005-2787(70)90119-x. [DOI] [PubMed] [Google Scholar]
- Cole R. S. Psoralen monoadducts and interstrand cross-links in DNA. Biochim Biophys Acta. 1971 Nov 29;254(1):30–39. doi: 10.1016/0005-2787(71)90111-0. [DOI] [PubMed] [Google Scholar]
- Dall'Acqua F., Marciani S., Vedaldi D., Rodighiero G. Studies on the photoreactions (365 nm) between DNA and some methylpsoralens. Biochim Biophys Acta. 1974 Jul 11;353(3):267–273. doi: 10.1016/0005-2787(74)90019-7. [DOI] [PubMed] [Google Scholar]
- Danna K. J., Nathans D. Bidirectional replication of Simian Virus 40 DNA. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3097–3100. doi: 10.1073/pnas.69.11.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., Weissman S. M., Zain B. S., Pan J., Lewis A. M., Jr The nucleotide sequence preceding an RNA polymerase initiation site on SV40 DNA. Part 2. The sequence of the early strand transcript. Nucleic Acids Res. 1974 Apr;1(4):595–611. doi: 10.1093/nar/1.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson R. C., Abelson J., Barnes W. M., Reznikoff W. S. Genetic regulation: the Lac control region. Science. 1975 Jan 10;187(4171):27–35. doi: 10.1126/science.1088926. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs and Escherichia coli ribosomal RNAs are cut from large precursor RNAs in vivo by ribonuclease 3. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3296–3300. doi: 10.1073/pnas.70.12.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareed G. C., Garon G. F., Salzman N. P. Origin and direction of simian virus 40 deoxyribonucleic acid replication. J Virol. 1972 Sep;10(3):484–491. doi: 10.1128/jvi.10.3.484-491.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert W., Maxam A. The nucleotide sequence of the lac operator. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3581–3584. doi: 10.1073/pnas.70.12.3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jay E., Roychoudhury R., Wu R. Nucleotide sequence with elements of an unusual two-fold rotational symmetry in the region of origin of replication of SV40 DNA+. Biochem Biophys Res Commun. 1976 Apr 5;69(3):678–686. doi: 10.1016/0006-291x(76)90929-3. [DOI] [PubMed] [Google Scholar]
- Khoury G., Howley P., Nathans D., Martin M. Posttranscriptional selection of simian virus 40-specific RNA. J Virol. 1975 Feb;15(2):433–437. doi: 10.1128/jvi.15.2.433-437.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Martin M. A., Lee T. N., Danna K. J., Nathans D. A map of simian virus 40 transcription sites expressed in productively infected cells. J Mol Biol. 1973 Aug 5;78(2):377–389. doi: 10.1016/0022-2836(73)90123-x. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. A map of temperature-sensitive mutants of simian virus 40. Virology. 1975 Jul;66(1):70–81. doi: 10.1016/0042-6822(75)90179-8. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Ptashne M., Barrell B. G., Donelson J. Sequence of a repressor-binding site in the DNA of bacteriophage lamda. Nature. 1974 Aug 2;250(465):394–397. doi: 10.1038/250394a0. [DOI] [PubMed] [Google Scholar]
- Morrow J. F., Berg P. Cleavage of Simian virus 40 DNA at a unique site by a bacterial restriction enzyme. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3365–3369. doi: 10.1073/pnas.69.11.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder C., Delius H. Specificity of the break produced by restricting endonuclease R 1 in Simian virus 40 DNA, as revealed by partial denaturation mapping. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3215–3219. doi: 10.1073/pnas.69.11.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musajo L., Bordin F., Caporale G., Marciani S., Rigatti G. Photoreactions at 3655 Angstrom between pyrimidine bases and skin-photosensitizing furocoumarins. Photochem Photobiol. 1967 Oct;6(10):711–719. doi: 10.1111/j.1751-1097.1967.tb08736.x. [DOI] [PubMed] [Google Scholar]
- Pribnow D. Bacteriophage T7 early promoters: nucleotide sequences of two RNA polymerase binding sites. J Mol Biol. 1975 Dec 15;99(3):419–443. doi: 10.1016/s0022-2836(75)80136-7. [DOI] [PubMed] [Google Scholar]
- Prives C. L., Aviv H., Gilboa E., Revel M., Winocour E. The cell-free translation of SV40 messenger RNA. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):309–316. doi: 10.1101/sqb.1974.039.01.041. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Martin R. G. Genetic analysis of simian virus 40. 3. Characterization of a temperature-sensitive mutant blocked at an early stage of productive infection in monkey cells. J Virol. 1972 Jun;9(6):956–968. doi: 10.1128/jvi.9.6.956-968.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J., Sugden B., Keller W., Sharp P. A. Transcription of simian virus 40. 3. Mapping of "early" and "late" species of RNA. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3711–3715. doi: 10.1073/pnas.70.12.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller H., Gray C., Herrmann K. Nucleotide sequence of an RNA polymerase binding site from the DNA of bacteriophage fd. Proc Natl Acad Sci U S A. 1975 Feb;72(2):737–741. doi: 10.1073/pnas.72.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiua T., Ormondt H. V., Khorana H. G. The nucleotide sequence in the promoter region of the fene for an Escherichia coli tyrosine transfer ribonucleic acid. J Biol Chem. 1975 Feb 10;250(3):1087–1098. [PubMed] [Google Scholar]
- Sharp P. A., Sugden B., Sambrook J. Detection of two restriction endonuclease activities in Haemophilus parainfluenzae using analytical agarose--ethidium bromide electrophoresis. Biochemistry. 1973 Jul 31;12(16):3055–3063. doi: 10.1021/bi00740a018. [DOI] [PubMed] [Google Scholar]
- Shen C. J., Wiesehahn G., Hearst J. E. Cleavage patterns of Drosophila melanogaster satellite DNA by restriction enzymes. Nucleic Acids Res. 1976 Apr;3(4):931–951. doi: 10.1093/nar/3.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C. K., Hearst J. E. Psoralen-crosslinked secondary structure map of single-stranded virus DNA. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2649–2653. doi: 10.1073/pnas.73.8.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Ben-Ishai Z., Newbold J. E. Simian virus 40 transcription in productively infected and transformed cells. J Virol. 1974 Jun;13(6):1263–1273. doi: 10.1128/jvi.13.6.1263-1273.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg R. A., Warnaar S. O., Winocour E. Isolation and characterization of simian virus 40 ribonucleic acid. J Virol. 1972 Aug;10(2):193–201. doi: 10.1128/jvi.10.2.193-201.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zain B. S., Weissman S. M., Dhar R., Pan J. The nucleotide sequence preceding an RNA polymerase initiation site on SV40 DNA. Part 1. The sequence of the late strand transcript. Nucleic Acids Res. 1974 Apr;1(4):577–594. doi: 10.1093/nar/1.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]