Abstract
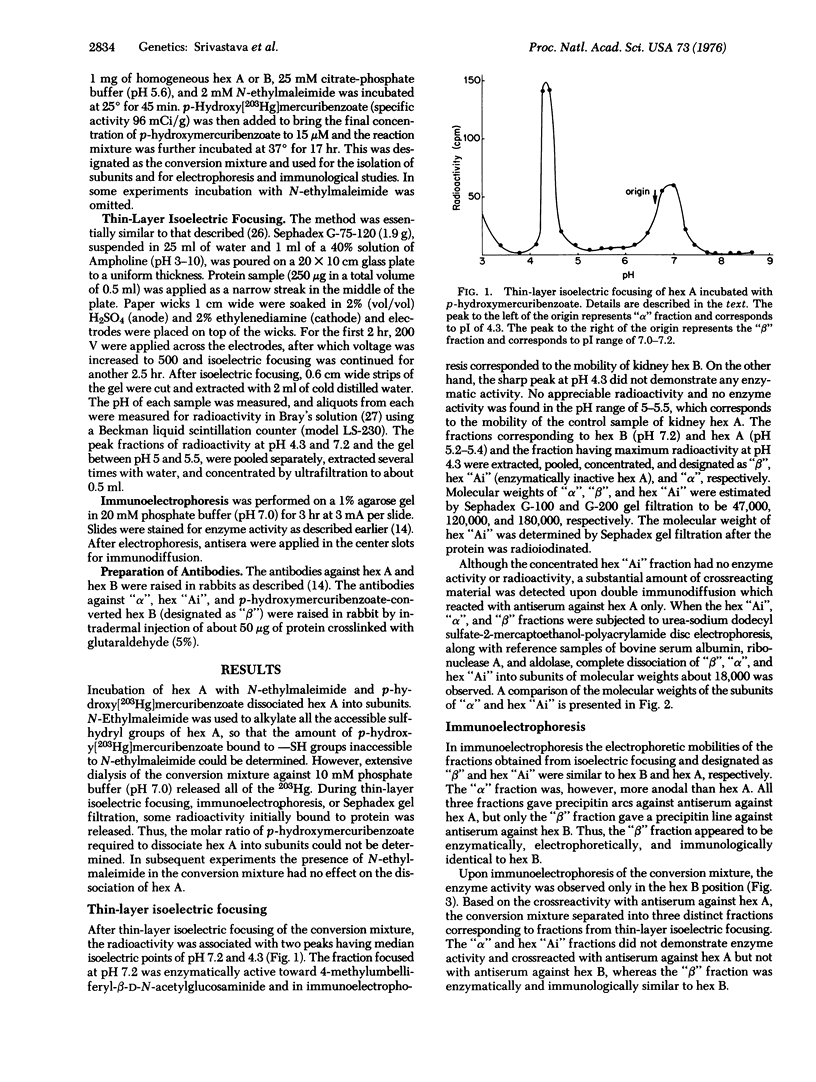
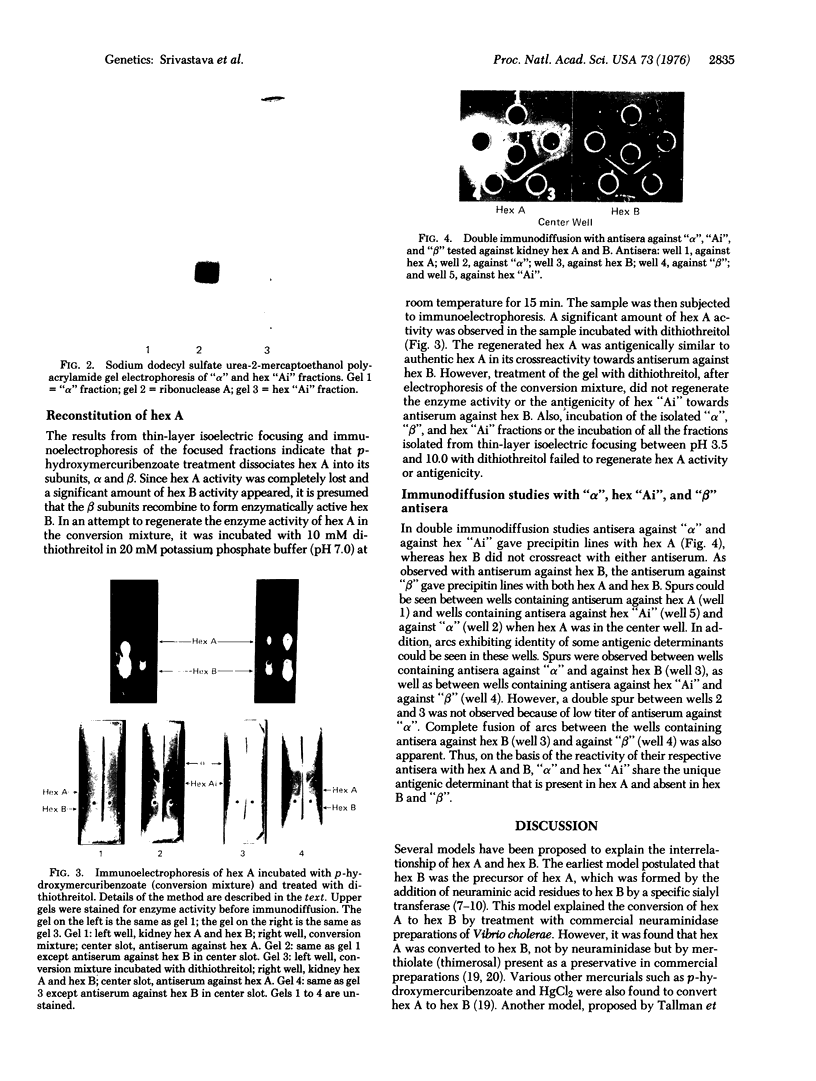
Human kidney hexosaminidase A (beta-N-acetylglucosaminidase; 2-acetamido-2-deoxy-beta-D-glucoside acetamidodeoxyglucohydrolase; EC 3.2.1.30) is a heteropolymer of two immunologically distinct subunits designated as alpha and beta. Hexosaminidase B, however, is a homopolymer comprised entirely of beta subunits. When human kidney hexosaminidase A was dissociated into its subunits by p-hydroxymercuribenzoate, three distinct proteins having isoelectric points of pH 7.2.5.4, and 4.3 were isolated. The fraction having an isoelectric point of pH 7.2, designated as beta fraction, was electrophoretically and immunologically identical to hexosaminidase B and was enzymatically active. The proteins having isoelectric points of pH 5.4 and 4.3, designated as hexosaminidase Ai and alpha fractions, respectively, were enzymatically inactive and crossreacted with antiserum against hexosaminidase A and not with antiserum against hexosaminidase B. Upon incubation of p-hydroxymercuribenzoate-treated hexosaminidase A with dithiothreitol,, hexosaminidase A activity, as well as antigenicity, was regenerated, indicating that alpha and beta subunits hybridize to form hexosaminidase A. Antibodies raised in rabbits against beta fractions reacted with both hexosaminidase A and B, whereas the antibodies against alpha and hexosaminidase Ai fractions reacted only against hexosaminidase A. This would indicate that both fractions are composed only of subunits unique to hexosaminidase A. The molecular weights of alpha,beta, and hexosaminidase Ai fractions were estimated to be 47,000, 120,000, and 180,000 respectively, by Sephadex gel filtration. Upon urea-sodium dodecyl sulfate polyacrylamide electrophoresis, each of the three fractions dissociated into a single polypeptide having a molecular weight of approximately 18,000. It is concluded that p-hydroxymercuribenzoate dissociates hexosaminidase A, (alphabeta)3, into its subunits, and the beta subunits can reassociate to form relatively stable hexosaminidase B, (betabeta)3, while the alpha subunits reassociate in both the dimeric state, alpha2, and a polymeric state, alpha8.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERS G. K. MOLECULAR EXCLUSION AND RESTRICTED DIFFUSION PROCESSES IN MOLECULAR-SIEVE CHROMATOGRAPHY. Biochemistry. 1964 May;3:723–730. doi: 10.1021/bi00893a021. [DOI] [PubMed] [Google Scholar]
- Beutler E., Kuhl W., Comings D. Hexosaminidase isozyme in type O Gm2 gangliosidosis (Sandhoff-Jatzkewitz disease). Am J Hum Genet. 1975 Sep;27(5):628–638. [PMC free article] [PubMed] [Google Scholar]
- Beutler E., Kuhl W. Subunit structure of human hexosaminidase verified: interconvertibility of hexosaminidase isozymes. Nature. 1975 Nov 20;258(5532):262–264. doi: 10.1038/258262a0. [DOI] [PubMed] [Google Scholar]
- Beutler E., Villacorte D., Kuhl W., Guinto E., Srivastava S. Nonenzymatic conversion of human hexosaminidase A. J Lab Clin Med. 1975 Aug;86(2):195–203. [PubMed] [Google Scholar]
- Carmody P. J., Rattazzi M. C. Conversion of human hexosaminidase A to hexosaminidase "B" by crude Vibrio cholerae neuraminidase preparations: merthiolate is the active factor. Biochim Biophys Acta. 1974 Nov 5;371(1):117–125. doi: 10.1016/0005-2795(74)90160-3. [DOI] [PubMed] [Google Scholar]
- Gilbert F., Kucherlapati R., Creagan R. P., Murnane M. J., Darlington G. J., Ruddle F. H. Tay-Sachs' and Sandhoff's diseases: the assignment of genes for hexosaminidase A and B to individual human chromosomes. Proc Natl Acad Sci U S A. 1975 Jan;72(1):263–267. doi: 10.1073/pnas.72.1.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone A., Konecny P., Koenig H. Lysosomal hydrolases: Conversion of acidic to basic forms by neuraminidase. FEBS Lett. 1971 Feb 12;13(1):68–72. doi: 10.1016/0014-5793(71)80667-1. [DOI] [PubMed] [Google Scholar]
- Hultberg B. N-acetylhexosaminidase activities in Tay-Sachs disease. Lancet. 1969 Nov 29;2(7631):1195–1195. doi: 10.1016/s0140-6736(69)92520-3. [DOI] [PubMed] [Google Scholar]
- Ikonne J. U., Ellis R. B. N-acetyl-beta-D-hexosaminidase component A. Different forms in human tissues and fluids. Biochem J. 1973 Nov;135(3):457–462. doi: 10.1042/bj1350457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonne J. U., Rattazzi M. C., Desnick R. J. Characterization of Hex S, the major residual beta hexosaminidase activity in type O Gm2 gangliosidosis (Sandhoff-Jatzkewitz disease). Am J Hum Genet. 1975 Sep;27(5):639–650. [PMC free article] [PubMed] [Google Scholar]
- Klapper M. H. Rapid binding of PMB to non-SH groups on hemerythrin. Biochem Biophys Res Commun. 1970 Jan 6;38(1):172–179. doi: 10.1016/0006-291x(70)91100-9. [DOI] [PubMed] [Google Scholar]
- Kolodny E. H., Brady R. O., Volk B. W. Demonstration of an alteration of ganglioside metabolism in Tay-Sachs disease. Biochem Biophys Res Commun. 1969 Oct 22;37(3):526–531. doi: 10.1016/0006-291x(69)90947-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lalley P. A., Rattazzi M. C., Shows T. B. Human beta-D-N-acetylhexosaminidases A and B: expression and linkage relationships in somatic cell hybrids. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1569–1573. doi: 10.1073/pnas.71.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada S., O'Brien J. S. Tay-Sachs disease: generalized absence of a beta-D-N-acetylhexosaminidase component. Science. 1969 Aug 15;165(3894):698–700. doi: 10.1126/science.165.3894.698. [DOI] [PubMed] [Google Scholar]
- Radola B. J. Analytical and preparative isoelectric focusing in gel-stabilized layers. Ann N Y Acad Sci. 1973 Jun 15;209:127–143. doi: 10.1111/j.1749-6632.1973.tb47523.x. [DOI] [PubMed] [Google Scholar]
- Robinson D., Carroll M. Tay-Sachs disease: interrelation of hexosaminidases A and B. Lancet. 1972 Feb 5;1(7745):322–323. doi: 10.1016/s0140-6736(72)90332-7. [DOI] [PubMed] [Google Scholar]
- Robinson D., Stirling J. L. N-Acetyl-beta-glucosaminidases in human spleen. Biochem J. 1968 Apr;107(3):321–327. doi: 10.1042/bj1070321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SVENNERHOLM L. The chemical structure of normal human brain and Tay-Sachs gangliosides. Biochem Biophys Res Commun. 1962 Nov 27;9:436–441. doi: 10.1016/0006-291x(62)90030-x. [DOI] [PubMed] [Google Scholar]
- Sandhoff K., Andreae U., Jatzkewitz H. Deficient hexozaminidase activity in an exceptional case of Tay-Sachs disease with additional storage of kidney globoside in visceral organs. Life Sci. 1968 Mar 15;7(6):283–288. doi: 10.1016/0024-3205(68)90024-6. [DOI] [PubMed] [Google Scholar]
- Sandhoff K., Harzer K., Wässle W., Jatzkewitz H. Enzyme alterations and lipid storage in three variants of Tay-Sachs disease. J Neurochem. 1971 Dec;18(12):2469–2489. doi: 10.1111/j.1471-4159.1971.tb00204.x. [DOI] [PubMed] [Google Scholar]
- Sandhoff K. Variation of beta-N-acetylhexosaminidase-pattern in Tay-Sachs disease. FEBS Lett. 1969 Aug;4(4):351–354. doi: 10.1016/0014-5793(69)80274-7. [DOI] [PubMed] [Google Scholar]
- Snyder P. D., Jr, Krivit W., Sweeley C. C. Generalized accumulation of neutral glycosphingolipids with G M2 ganglioside accumulation in the brain. J Lipid Res. 1972 Jan;13(1):128–136. [PubMed] [Google Scholar]
- Srivastava S. K., Awasthi Y. C., Yoshida A., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. I. Purification and properties. J Biol Chem. 1974 Apr 10;249(7):2043–2048. [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Antibody against purified human hexosaminidase B cross-reacting with human hexosaminidase A. Biochem Biophys Res Commun. 1972 May 26;47(4):753–759. doi: 10.1016/0006-291x(72)90556-6. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Hexosaminidase-A and hexosaminidase-B: studies in Tay-Sachs' and Sandhoff's disease. Nature. 1973 Feb 16;241(5390):463–463. doi: 10.1038/241463a0. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. 3. Biochemical genetics of Tay-Sachs and Sandhoff's diseases. J Biol Chem. 1974 Apr 10;249(7):2054–2057. [PubMed] [Google Scholar]
- Srivastava S. K., Wiktorowicz J., Klebe R., Awasthi Y. C. Studies on beta-D-N-acetylhexosaminidase. Various isozymes in tissues of normal subjects and Sandhoff's disease patients. Biochim Biophys Acta. 1975 Aug 26;397(2):428–436. doi: 10.1016/0005-2744(75)90132-1. [DOI] [PubMed] [Google Scholar]
- Srivastava S. K., Yoshida A., Awasthi Y. C., Beutler E. Studies on human beta-D-N-acetylhexosaminidases. II. Kinetic and structural properties. J Biol Chem. 1974 Apr 10;249(7):2049–2053. [PubMed] [Google Scholar]
- Tallman J. F., Brady R. O., Quirk J. M., Villalba M., Gal A. E. Isolation and relationship of human hexosaminidases. J Biol Chem. 1974 Jun 10;249(11):3489–3499. [PubMed] [Google Scholar]
- Thomas G. H., Taylor H. A., Miller C. S., Axelman J., Migeon B. R. Genetic complementation after fusion of Tay-Sachs and Sandhoff cells. Nature. 1974 Aug 16;250(467):580–582. doi: 10.1038/250580a0. [DOI] [PubMed] [Google Scholar]
- Young E. P., Ellis R. B., Lake B. D., Patrick A. D. Tay-sachs disease and related disorders: Fractionation of brain N-acetyl-beta-hexosaminidase on DEAE-cellulose. FEBS Lett. 1970 Jul 15;9(1):1–4. doi: 10.1016/0014-5793(70)80295-2. [DOI] [PubMed] [Google Scholar]