Abstract
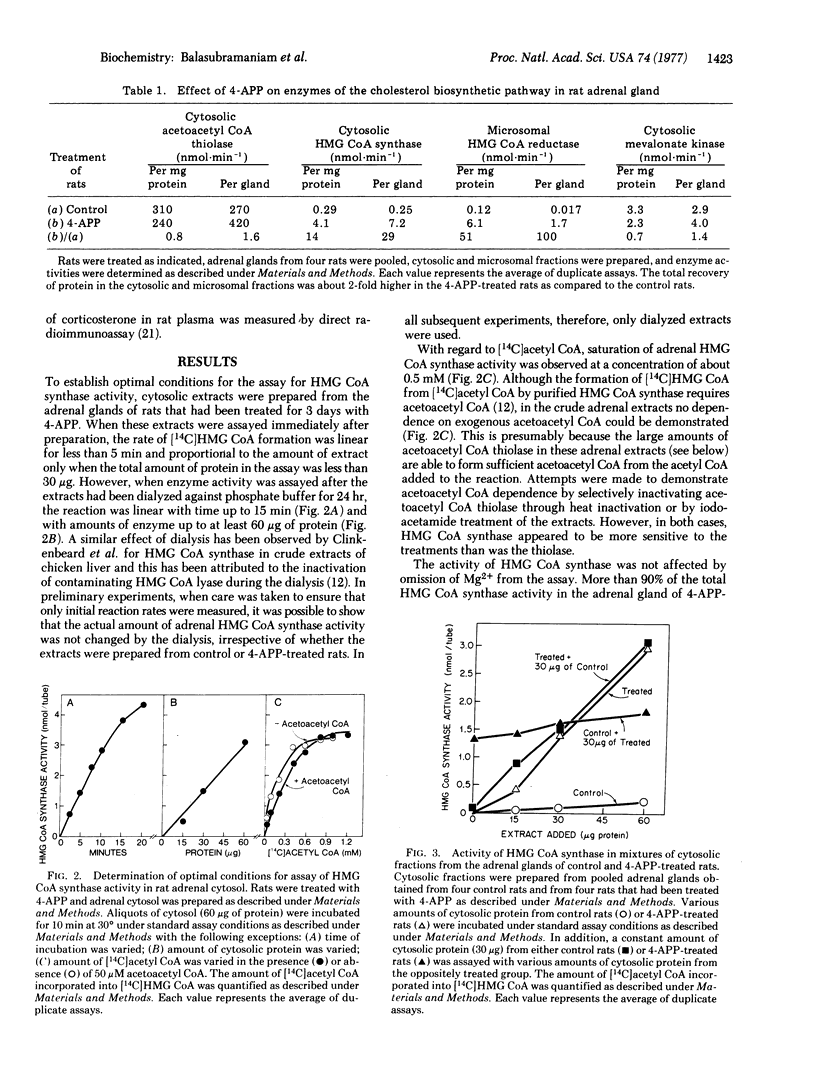
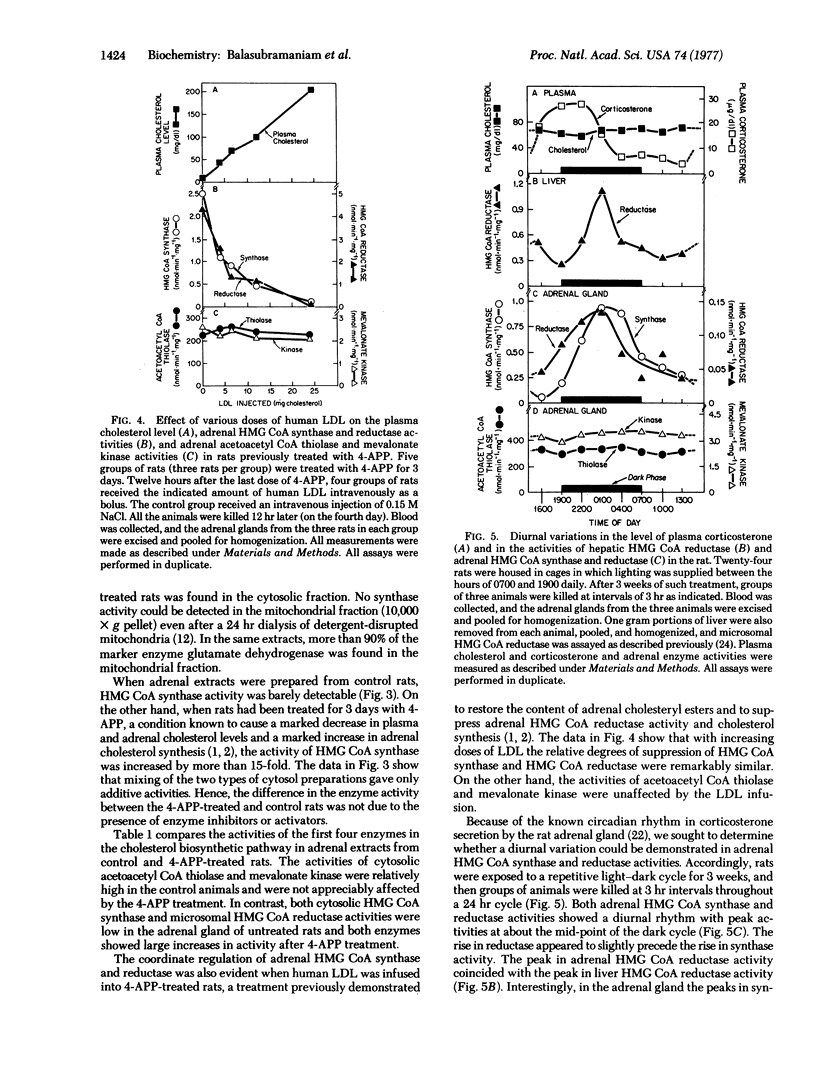
The activities of cytosolic 3-hydroxy-3-methylglutaryl coenzyme A synthase [3-hydroxy-3-methylglutaryl-CoA acetoacetyl-CoA-lyase (CoA-acylating), EC 4.1.3.5] and microsomal 3-hydroxy-3-methylglutaryl coenzyme A reductase[mevalonate:NADP+ oxidoreductase (CoA-acylating), EC 1.1.1.34], two sequential enzymes in the cholesterol biosynthetic pathway, were shown to be regulated coordinately in the adrenal gland of the rat. When the plasma cholesterol level was lowered by administration of 4-aminopyrazolopyrimidine, a treatment known to enhance cholesterol synthesis in the adrenal, synthase activity in the gland rose by 14- to 29-fold and reductase activity rose by 50- to 100-fold. The subsequent intravenous infusion of low density lipoprotein restored the plasma cholesterol level and suppressed synthase and reductase activities in parallel. The activities of adrenal 3-hydroxy-3-methylglutaryl coenzyme A synthase and reductase were also shown to exhibit a coordinate pattern of diurnal variation with peaks in both enzymes achieved at the mid-point of the dark cycle. The activity of adrenal acetoacetyl coenzyme A thiolase (acetyl CoA acetyltransferase; acetyl-CoA:acetyl-CoA C-acetyltransferase, EC 2.3.1.9), the enzyme preceding the synthase in the cholesterol biosynthetic pathway, and the activity of adrenal mevalonate kinase (ATP:mevalonate 5-phosphotransferase, EC 2.7.1.36), the enzyme following the reductase, were not enhanced by cholesterol deprivation, and neither exhibited a pattern of diurnal variation. The coordinate control of 3-hydroxy-3-methylglutaryl CoA synthase and reductase in rat adrenal gland provides a model system to study the biochemical mechanism for the regulation of cholesterol synthesis in a tissue that uses cholesterol for the synthesis of steroid hormones.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J. M., Dietschy J. M. Cholesterogenesis: derepression in extrahepatic tissues with 4-aminopyrazolo (3,4-d) pyrimidine. Science. 1976 Sep 3;193(4256):903–905. doi: 10.1126/science.948751. [DOI] [PubMed] [Google Scholar]
- Andersen J. M., Dietschy J. M. Regulation of sterol synthesis in adrenal gland of the rat by both high and low density human plasma lipoproteins. Biochem Biophys Res Commun. 1976 Oct 4;72(3):880–885. doi: 10.1016/s0006-291x(76)80214-8. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam S., Goldstein J. L., Faust J. R., Brown M. S. Evidence for regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and cholesterol synthesis in nonhepatic tissues of rat. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2564–2568. doi: 10.1073/pnas.73.8.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramaniam S., Goldstein J. L., Faust J. R., Brunschede G. Y., Brown M. S. Lipoprotein-mediated regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity and cholesteryl ester metabolism in the adrenal gland of the rat. J Biol Chem. 1977 Mar 10;252(5):1771–1779. [PubMed] [Google Scholar]
- Barth C., Hackenschmidt J., Ullmann H., Decker K. Inhibition of cholesterol synthesis by (-)-hydroxycitrate in perfused rat liver. Evidence for an extramitochondrial mevalonate synthesis from acetyl coenzyme A. FEBS Lett. 1972 May 15;22(3):343–346. doi: 10.1016/0014-5793(72)80266-7. [DOI] [PubMed] [Google Scholar]
- Borkowski A., Delcroix C., Levin S. Metabolism of adrenal cholesterol in man. I. In vivo studies. J Clin Invest. 1972 Jul;51(7):1664–1678. doi: 10.1172/JCI106968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd G. S., Trzeciak W. H. Cholesterol metabolism in the adrenal cortex: studies on the mode of action of ACTH. Ann N Y Acad Sci. 1973;212:361–377. doi: 10.1111/j.1749-6632.1973.tb47607.x. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Dietschy J. M., Siperstein M. D. 3-Hydroxy-3-methylglutaryl coenzyme A reductase. Solubilization and purification of a cold-sensitive microsomal enzyme. J Biol Chem. 1973 Jul 10;248(13):4731–4738. [PubMed] [Google Scholar]
- Brown M. S., Dana S. E., Goldstein J. L. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Feb 10;249(3):789–796. [PubMed] [Google Scholar]
- Clinkenbeard K. D., Reed W. D., Mooney R. A., Lane M. D. Intracellular localization of the 3-hydroxy-3-methylglutaryl coenzme A cycle enzymes in liver. Separate cytoplasmic and mitochondrial 3-hydroxy-3-methylglutaryl coenzyme A generating systems for cholesterogenesis and ketogenesis. J Biol Chem. 1975 Apr 25;250(8):3108–3116. [PubMed] [Google Scholar]
- Clinkenbeard K. D., Sugiyama T., Moss J., Reed W. D., Lane M. D. Molecular and catalytic properties of cytosolic acetoacetyl coenzyme A thiolase from avian liver. J Biol Chem. 1973 Apr 10;248(7):2275–2284. [PubMed] [Google Scholar]
- Dexter R. N., Fishman L. M., Ney R. L. Stimulation of adrenal cholesterol uptake from plasma by adrenocorticotrophin. Endocrinology. 1970 Nov;87(5):836–846. doi: 10.1210/endo-87-5-836. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M., McGarry J. D. Limitations of acetate as a substrate for measuring cholesterol synthesis in liver. J Biol Chem. 1974 Jan 10;249(1):52–58. [PubMed] [Google Scholar]
- Gomez-Sanchez C., Holland O. B., Higgins J. R., Kem D. C., Kaplan N. M. Circadian rhythms of serum renin activity and serum corticosterone, prolactin, and aldosterone concentrations in the male rat on normal and low-sodium diets. Endocrinology. 1976 Aug;99(2):567–572. doi: 10.1210/endo-99-2-567. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez C., Murry B. A., Kem D. C., Kaplan N. M. A direct radioimmunoassay of corticosterone in rat serum. Endocrinology. 1975 Mar;96(3):796–798. doi: 10.1210/endo-96-3-796. [DOI] [PubMed] [Google Scholar]
- HENDERSON J. F. STUDIES ON FATTY LIVER INDUCTION BY 4-AMINOPYRAZOLOPYRIMIDINE. J Lipid Res. 1963 Jan;4:68–74. [PubMed] [Google Scholar]
- Ishitani K., Niitsu Y., Listowsky I. Characterization of the different polypeptide components and analysis of subunit assembly in ferritin. J Biol Chem. 1975 Apr 25;250(8):3124–3128. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MORRIS M. D., CHAIKOFF I. L. The origin of cholesterol in liver, small intestine, adrenal gland, and testis of the rat: dietary versus endogenous contributions. J Biol Chem. 1959 May;234(5):1095–1097. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Ketogenesis and cholesterol synthesis in normal and neoplastic tissues of the rat. J Biol Chem. 1969 Aug 10;244(15):4251–4256. [PubMed] [Google Scholar]
- Reed W. D., Clinkenbeard D., Lane M. D. Molecular and catalytic properties of mitochondrial (ketogenic) 3-hydroxy-3-methylglutaryl coenzyme A synthase of liver. J Biol Chem. 1975 Apr 25;250(8):3117–3123. [PubMed] [Google Scholar]
- Shiff T. S., Roheim P. S., Eder H. A. Effects of high sucrose diets and 4-aminopyrazolopyrimidine on serum lipids and lipoproteins in the rat. J Lipid Res. 1971 Sep;12(5):596–603. [PubMed] [Google Scholar]


