Abstract
Africa is the birthplace of anatomically modern humans, and is the geographic origin of human migration across the globe within the last 100,000 years. The history of African populations has consisted of a number of demographic events that have influenced patterns of genetic and phenotypic variation across the continent. With the increasing amount of genomic data and corresponding developments in computational methods, researchers are able to explore long-standing evolutionary questions, expanding our understanding of human history within and outside of Africa. This review will summarize some of the recent findings regarding African demographic history, including the African Diaspora, and will briefly explore their implications for disease susceptibility in populations of African descent.
Introduction
Current paleontological and genetic evidence indicates that anatomically modern humans (AMHs) arose in Africa ~200 thousand years ago (kya) and have lived continuously on the African continent longer than in any other geographic region. African populations are characterized by higher levels of within-population and between-population genetic diversity relative to non-Africans consistent with a larger long-term effective population size of ancestral African populations [1–3]. The history of Africans has encompassed other demographic events such as population structure, admixture, long-range and short-range migration that have shaped patterns of genetic variation in modern populations [1,4]. In recent years, the resequencing of large portions of the genome and their analysis with new computational methods have increased power to infer past demographic events at an unprecedented resolution. Most notably, the recent finding that non-African populations share ancestry with Neanderthals, consistent with a model of archaic introgression, has provided additional insights into human evolutionary history. However, the extent of archaic admixture in diverse African populations still remains unclear. Given the central role of Africa in human evolution, characterizing extant genomic variation in diverse Africans will be important for reconstructing both ancient and recent demographic events, and for identifying variants that play a role in disease susceptibility in African populations. Here, we summarize our current knowledge of modern human origins and patterns of genetic diversity in populations of African descent, as well as explore their implications for the risk of complex disease.
The origin of anatomically modern humans in Africa
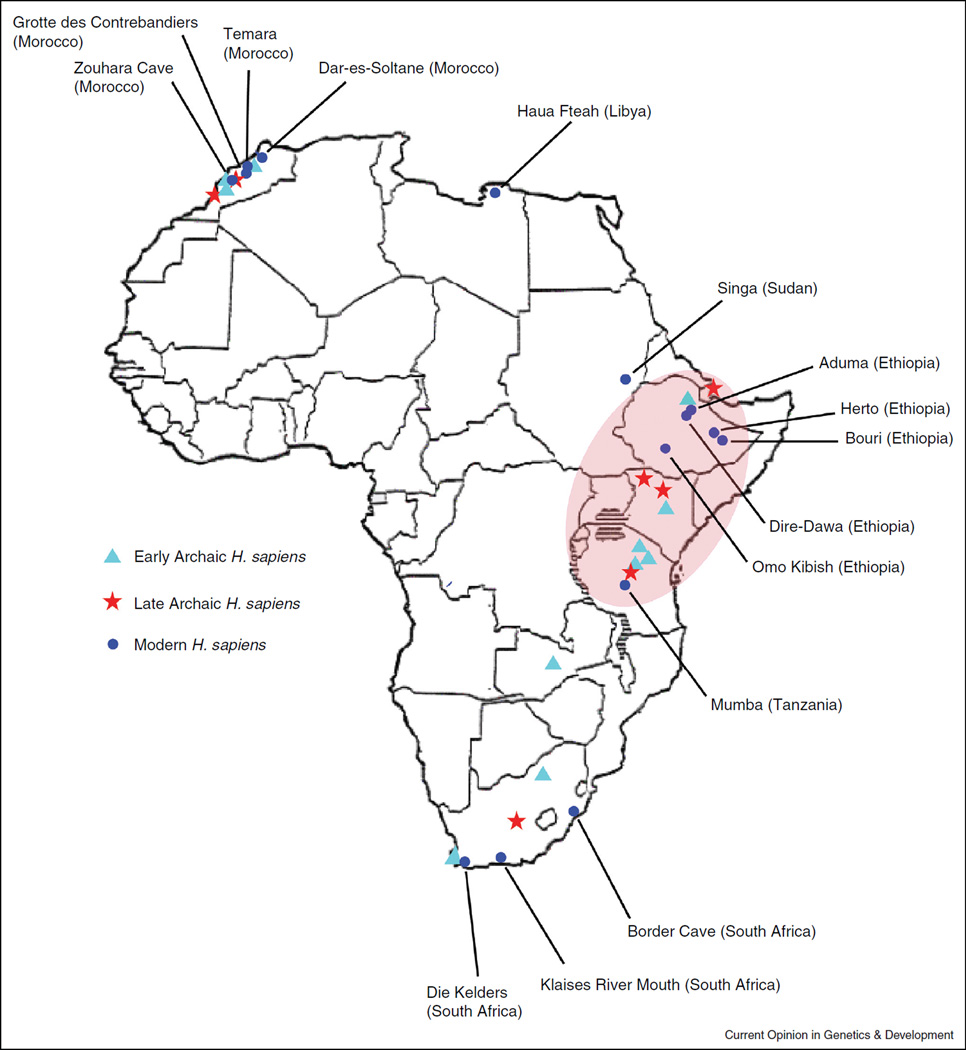
The earliest suite of derived morphological traits associated with AMHs was identified in fossils from Ethiopia dating to ~160–195 kya (Figure 1; Table 1) [5–7]. Other early AMHs displaying modern features were also found in Ethiopia, Sudan, Tanzania and South Africa dating to >100 kya and in the Middle East dating to ~100 kya (Figure 1; Table 1) [8–12]. Although eastern Africa has often been considered the geographic location of modern human origins ~200 kya, some have argued that South Africa is the site where AMHs originated. Indeed, a recent study suggested that the geographic distribution of genetic diversity in Africa, as measured by linkage disequilibrium (LD), is more consistent with a South African origin of modern humans [13]. However, this inference does not account for the possibility that the geographic location of populations in the present may have differed in the past. Furthermore, a large-scale analysis of southern African populations demonstrated the difficulty of localizing the origin of modern humans using summary statistics of diversity, such as LD [14••]. Nevertheless, regardless of the precise location of origin, paleontological and genetic evidence indicates that AMHs evolved on the African continent.
Figure 1.
The geography of major Homo sapiens (H. sapiens) fossil sites. This map, adapted from [8], illustrates the geographic distribution of sites in Africa where early archaic, late archaic and modern H. sapiens have been found. The labeled sites are the names of fossil remains that have been designated as modern H. sapiens. A more detailed description of the ‘modern’ features of these fossils is given in Table 1.
Table 1.
Description of modern human fossils in Africa. Here we outline some of the major morphological features identified in anatomically modern humans found in Africa, along with the remains recovered from each site, and the inferred age. This description corresponds to the fossils/geographic sites listed in Figure 1.
| Specimen | Recovered remains | Major anatomical features | Age (in years) |
|---|---|---|---|
| Omo 1 Omo2 |
Partial cranium and mandible (Omo 1) Partial cranium (Omo 2) |
The cranial vault of Omo 1 is high and globular, with a nearly vertical frontal profile, rounded occipital, and pronounced parietal bosses. The mandible has a slight chin. The postcranium is human-like in overall morphology [7] Omo 2 has a mosaic of modern (high vault) and archaic traits (strong sagittal keeling and angled occipital) [7]. |
195,000 |
| Herto | Crania | High cranial vault with a cranial capacity of 1450 cm3, at the high end of the human range. Some archaic morphology includes projecting supraorbital and flexed occipital tori [6,7]. | 154,000–160,000 |
| Singa | Partial cranium | Vaulted forehead and reduced supraorbital morphology; cranial capacity estimated to be 1340 cm3 [124]. | >133,000 |
| Mumba | Teeth | Size and shape of molars are consistent with AMHs | 130,000 |
| Klasies | Partial cranium, mandible | Maxilla and mandible are metrically within the range of modern humans; overall, there is a reduction in tooth size consistent with AMH morphology [8]. | 120,000 |
| Border Cave | Partial cranium, mandible | Cranium has a high curved frontal bone and the supraorbital bone is slightly protrusive [8]. | 90,000 |
| Aduma/Bouri | Cranial fragments | Characterized by a high vault profile, well-curved parietals and the absence of an occipital torus. Cranial dimensions cluster with AMHs [11]. | 79,000–105,000 |
| Dire-Dawa | Partial mandible | Size and shape of mandible are consistent with AMH [124]. | 61,000–77,000 |
| Die Kelders Cave | Isolated teeth | There is an overall reduction in the size of the crowns compared to archaic populations and overall morphological features resemble modern sub-Saharan Africans [125]. | 60,000–80,000 |
| Zouhra Cave at El Harhoura | Teeth | Size of the upper and lower molars, and enamel thickness are similar to AMH [126]. | Poorly dated |
| Haua Fteah | Partial mandibles | Absence of derived Neanderthal traits [124,127]. | 65,00–73,000 |
| Temara | Cranial and mandibular fragments | Morphology and metrics of occipital are similar to AMH; no discernible presence of a supraorbital torus [128]. | Poorly dated |
| Grotte des Contrebandiers | Teeth and mandibular fragments Reduced | Reduced anterior dentition relative to molars and reduced bucco-linguial expansion of front teeth contrary to Neanderthal specimens [126]. | Poorly dated |
| Dar es-Soltane | Partial cranium and mandible | A flattened mid-face and presence of a chin consistent with AMHs [8]. | Poorly dated |
Recent archaeological data also showed that modern behavior (such as symbolic culture and complex tool production) arose at a relatively early stage of human evolution, contrary to prior studies that argued for the later development of complex cognition ~45 kya [15,16]. In particular, technological advances in the form of heat-treated microlith stone tools were found in southern Africa dating to ~71 [17•,18]. The use of pigment, art and ornamental shells, indicative of artistic expression, was also documented as early as 164 kya in South Africa [19,20] and around 87 kya in northern Africa [21]. In addition, it has been suggested that the highest levels of linguistic diversity occur in Africa and that linguistic diversity decreased as modern humans migrated across the globe from Africa ~50–70 kya [22•,23]. Thus, key behavioral and morphological traits that define modern Homo sapiens may have evolved fairly closely together in Africa over the last 200,000 years.
Ancient population structure in Africa
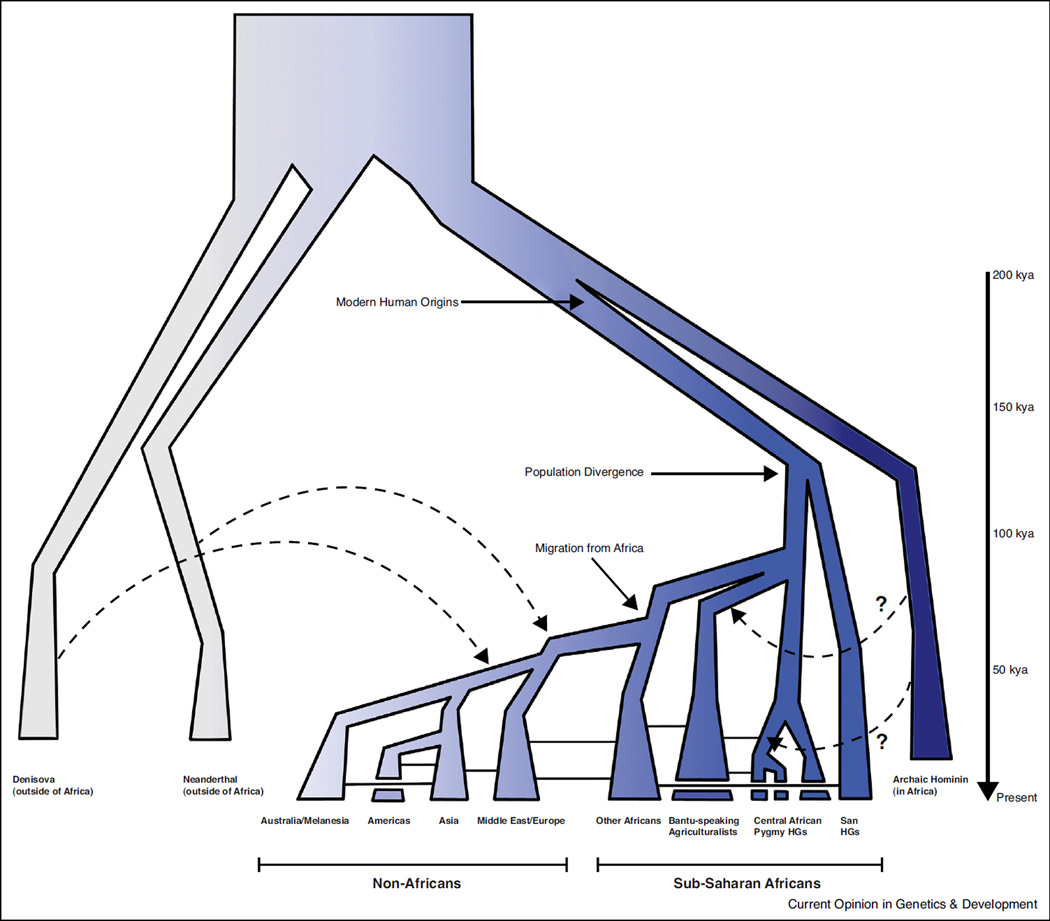
Several studies suggest that ancestral African populations were genetically differentiated before the expansion of modern humans from Africa ~50–100 kya. In particular, analyses of autosomal loci inferred divergence between the ancestors of Khoesan-speaking San hunter-gatherers and other African populations >100 kya [14••,24•,25–29]. This inference is in agreement with mtDNA and Y-chromosome studies that estimated divergence between the ancestors of Khoesan-speakers and other sub-Saharan Africans at >90 kya [14••,30–32]. Analyses have also detected substantial genetic differentiation in Central Africa, particularly between Pygmy and nonPygmy populations that are inferred to have separated ~60–70 kya (Figure 2) [30,33,34]. These estimates of divergence suggest an ancient origin of Khoesan-speaking and Pygmy hunter-gatherer genetic lineages, and provide evidence for deep genetic structure in Africa.
Figure 2.
A model of divergence and admixture in Africa. This figure illustrates some of the proposed divergence events in Africa, for example the divergence of San Khoesan-speaking hunter-gatherers (HGs) ancestors >100 kya [25,29], and the differentiation of the ancestor of Pygmy HGs from a non-Pygmy population ~60–70 kya [30,33,34]. Genetic substructure has also been detected among western Pygmies who also show evidence for admixture with Central African Bantu-speaking agriculturalists [30,33,34,90–93]. Solid lines indicate gene flow between the ancestors of modern populations, and the dashed arrows indicate archaic introgression. Studies have reported evidence for archaic introgression from an unknown archaic species into several populations including the Biaka Pygmy and the Yoruba [46,51,53] shown here by the dashed arrows and question marks (which indicate that additional studies of African populations are needed to understand the extent and timing of archaic admixture in Africa). Lastly, the decreasing intensity of the blue color within the modern human lineage represents the loss of diversity as AMHs migrated across the globe from Africa within the last 100,000 years.
In addition, a genome-wide study of a larger set of diverse Africans detected even more extensive population structure within Africa. Specifically, an analysis of 848 short tandem repeat polymorphisms (STRPs), 476 insertion-deletions (INDELs) and 3 single nucleotide polymorphisms (SNPs) genotyped in ~2400 individuals from 121 geographically diverse populations indicated 14 genetically divergent ancestral population clusters in Africa [3]. Each cluster consisted of populations that shared genetic similarity, as well as cultural and/or linguistic properties (e.g. Pygmies, Khoesan-speaking hunter-gatherers, Bantu-speakers, Cushitic-speakers). Thus, populations that speak languages belonging to the same linguistic family, for example, tend to have high levels of genetic relatedness. However, in some cases, there is discordance between linguistic and genetic affiliation due to a language shift, which can occur when the language of an expanding population is adopted by another population with little accompanying gene flow [3,35]. In addition, some linguistically-defined groups have shown evidence of fine-scale genetic differentiation, such as the northwestern and southeastern Khoesan-speakers in the Kalahari who are proposed to have separated within the last 30,000 years, as well as the Bantu and non-Bantu Niger-Kordofanian-speakers in western Africa [3,36,37•]. Overall, the observed population sub-division in Africa could have been facilitated by a number of factors, including physical barriers such as mountains and desert, as well as past climatic shifts that may have isolated sub-populations for periods of time followed by limited contact between groups [38,39].
Archaic admixture in Africa
With the recent increase in whole genome sequence data from fossil remains, a number of studies have identified regions of the genome in non-African populations that likely originated from archaic hominins, such as Neanderthals and Denisova (Figure 2) [24•,40–44,45••,46,47, 48•,49]. As more researchers explore the possibility of introgression in Africa, evidence for archaic admixture in African populations is emerging [50–53]. For example, a resequencing study of 61 autosomal intergenic regions detected longer blocks of LD than expected under a model of no admixture in western Biaka Pygmy hunter-gatherers, and suggested that these divergent haplo-types may have been introduced into the ancestors of the Biaka Pygmies by an unknown hominin species in Central Africa (Figure 2) [51]. A whole genome sequence analysis also identified overlapping regions of inferred introgression among Hadza, Sandawe, and Pygmy hunter-gatherers consistent with an admixture event with an archaic hominin in Africa predating the divergence of these populations [26••]. Additionally, a recent comparative study of African and Neanderthal genomes reported Neanderthal ancestry in East African and African American populations [47,54••,55•]. However, it was inferred that this introgression was likely due to recent admixture with non-Africans who could have introduced archaic DNA into these populations of African descent [47,55•]. Interestingly, a survey of Y-chromosome variation found a lineage (A00) that is highly divergent from other known lineages in an African American individual which could have an archaic origin [56]. Additional analyses are needed, however, to determine whether or not the presence of this unusual genetic lineage arose in humans through ancient population structure or archaic introgression.
Given the poor preservation of DNA in African fossils, direct comparison between modern and archaic African genomes, analogous to analyses of archaic admixture in non-Africans, is not currently feasible [1]. Therefore, until high quality archaic DNA is recovered in Africa, future studies will need to rely on robust computational methods, together with additional African genomic data, to further explore this question of ancient admixture, including the timing and location of admixture events. Intriguingly, the presence of archaic DNA in African populations also raises the possibility that the higher levels of diversity in sub-Saharan Africans compared to non-African populations could partially be the result of archaic admixture [57].
Migration and admixture
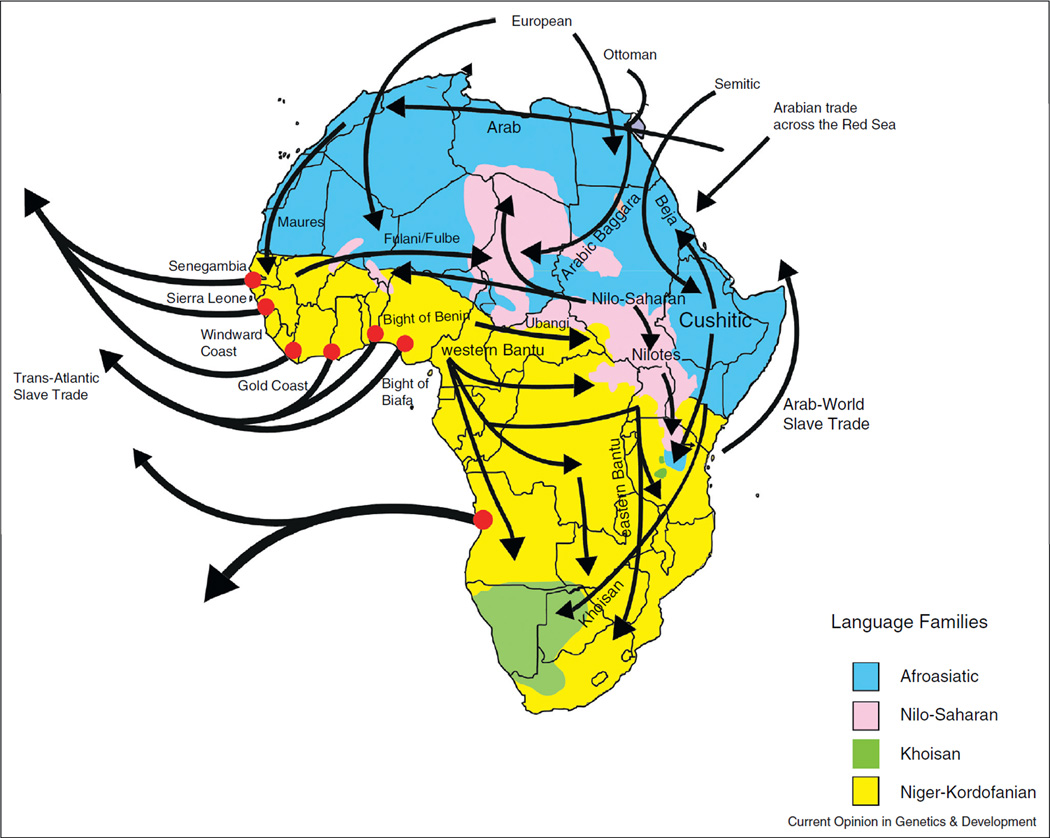
Although ancient admixture remains challenging to infer, more recent migration and admixture in Africa have become increasingly clear. One of the most significant migration events in recent history has been the expansion of Bantu-speaking agriculturalists first into the equatorial rainforests and then into eastern and southern Africa ~3–5 kya (Figure 3). Studies of autosomal and Y-chromosome loci have reported a relatively high level of shared variation among western Bantu Niger-Kordofanian-speakers as well as the presence of Bantu Niger-Kordofanian ancestry in many eastern and southern African populations [3,58], consistent with widespread migration across Africa. Furthermore, the highest frequency of the Y-chromosome lineage, E1b1a, typically associated with the Bantu expansion, occurred in western Africa and the frequency clinically decreased with geographic distance from this region, suggesting migration from an origin in western Africa [58,59]. These genetic results are congruent with linguistic data that proposed a West/West Central African origin for the spread of Bantu languages into East and South Africa [60–64]. Thus, the radiation of Bantu-speakers simultaneously involved the movement of people, language, and genes across the continent [35].
Figure 3.
The geography of major linguistic families and of historic migration events in Africa. Africa not only has the highest levels of genetic diversity, but a considerable amount of linguistic diversity is also found across the continent. Currently, more than 2000 distinct languages exist in Africa, representing about a third of the world’s languages, which can be classified into four major linguistic families: (1) Niger-Kordofanian is a family of languages (including Bantu) spoken primarily by agriculturalists across a wide geographic region in Africa; (2) Nilo-Saharan languages are spoken predominantly by pastoralists in Central and East Africa; (3) Afroasiatic languages are spoken mainly by pastoralists and agropastoralists in East and North Africa; (4) Khoesan, which consists of languages with click consonants, is spoken primarily by hunter-gatherer populations in East and South Africa. This map, adapted from [1,2], also shows a number of key migration events, most notably the geographic expansion of Bantu Niger-Kordofanian-speakers across Africa from a homeland near the Nigeria/Cameroon border, as well as the general geographic regions (shown here by the red circles) where enslaved Africans were transported from Africa to the New World based on historical records.
Other major migration events in Africa include the dispersal of Nilo-Saharan-speakers from Sudan both west-ward into Lake Chad ~8000 years ago and eastward to Kenya and Tanzania ~3000 years ago (Figure 3) [2,57]. Many Nilo-Saharan-speakers in East Africa also have high levels of Cushitic Afroasiatic ancestry, implying a long history of admixture between Nilo-Saharan and Cushitic-speakers, in agreement with archaeological data [2,3,65]. Recent data have also demonstrated the presence of the East African-specific mutation (C-14010) associated with lactose tolerance in southern Africa, suggesting gene flow between these geographic regions [66,67,68••,69]. Additionally, although present-day northern Africans are genetically differentiated from sub-Saharan Africans, populations in northern Africa have low levels of ancestry from western and eastern Africa [3,57,70–76], likely reflecting historic migration from these geographic regions into different parts of North Africa (Figure 3).
The genetic history of Africans has also been influenced by back-migration of non-African populations into Africa (Figure 3). For example, a genome-wide analysis detected substantial Maghrebi/Near Eastern ancestry in North African populations, resulting from ancient and recent migration of non-Africans into North Africa pre-sumably within the last 40,000 years [77]. Studies have also found a high proportion of non-African ancestry in Cushitic-speakers and Semitic-speakers from East Africa attributed to admixture >7 kya [78], as well as low levels of West Eurasian ancestry (European or Middle Eastern) in Khoe–Kwadi Khoesan-speakers in southern Africa [79]. The West Eurasian component present in southern African Khoe–Kwadi-speakers could have been acquired indirectly by these populations through admixture with migrating pastoralists from East Africa who have high levels of Cushitic ancestry [3,79,80]. This hypothesis of gene flow from eastern to southern Africa is further supported by other genetic and archaeological data documenting the spread of pastoralism from East to South Africa ~2 kya [37•,68••,79,81,82]. More recently, Europeans, South and East Asians have also migrated and admixed with local populations in southern Africa, giving rise to the modern-day ‘colored’ populations within this geographic region [3,57,83,84]. Overall, these above studies demonstrate that migration with subsequent admixture occurred at different points in time and over a wide geographic range, resulting in complex patterns of genetic variation in Africa.
Origin of African hunter-gatherer populations
Human populations practiced hunting-gathering/foraging strategies for much of their evolutionary history (Lee and Hitchcock, 2001). However, little is still known about the origin of African hunter-gatherer populations. Recent genome-wide SNP data indicated shared ancestry among East (Hadza and Sandawe) and South (San) African Khoesan-speaking hunter-gatherers [37•] consistent with the results of a previous mtDNA and Y-chromosome study [85]. Furthermore, mtDNA and Y-chromosome data suggested that these Khoesan-speakers likely shared a common ancestor ~35 kya and that the East African hunter-gatherers diverged from each other ~15 kya [85]. Interestingly, studies of genome-wide variation have found that Central African Pygmy and San hunter-gatherers share common ancestry, suggesting either an ancient common origin or gene flow among these populations [3,86]. These studies are congruent with Y-chromosome data showing uniquely shared lineages between Pygmy and Khoesan-speaking populations [30]. In addition, other analyses have inferred common ancestry among San, Hadza, Sandawe and Pygmy hunter-gatherers, implying a deep link between these populations [3,26••]. However, these latter results are also consistent with the possibility that shared variation among African hunter-gatherers could have arisen through gene flow between the ancestors of the San and Pygmy populations or by the loss of shared alleles in the ancestors of the Hadza and Sandawe [26••].
Among hunter-gatherer populations, the ancestors of San Khoesan-speakers are inferred to have separated from other Africans >100 kya, representing the earliest population split in the modern human lineage (Figure 2) [25,29,88]. Furthermore, studies of African mtDNA and autosomal diversity have suggested a deep time of divergence between the ancestors of Central African Pygmy and non-Pygmy populations ~60–70 kya [30,33,34] and a later divergence between ancestral western and eastern Pygmy populations >18 kya [29,30,33,34,89]. Fine-scale substructure was also observed among western Pygmies who diversified ~ 2.8 kya, possibly due to recent geo-graphic isolation, genetic drift, and differential levels of admixture between Pygmies and neighboring Bantu-speaking agriculturalists [30,33,34,90–93]. In addition, other analyses of autosomal and mtDNA variation inferred a higher effective population size (Ne) for the ancestors of Bantu-speaking agriculturalists with respect to Pygmy hunter-gatherers. These differences in Ne likely reflect the recent population expansion of ancestral Bantu-speakers associated with the emergence of agriculture and strong bottleneck events, occurring as early as 20 kya, in ancestral Pygmy populations [29,30,33,92,93].
Signatures of the trans-Atlantic movement
Africa is the geographic origin of millions of individuals of recent African descent in the United States and Caribbean whose ancestors were forcibly brought to the New World as slaves. Historical records have documented the movement of Africans into this region of the world primarily from locations along the western coast of Africa (from Senegal to Angola) (Figure 3) [94]. Subsequent to migration of indigenous Africans, there was considerable admixture with Europeans with a smaller contribution from indigenous American populations. Specifically, Afro-Caribbean populations are estimated to have ~65– 95% West African, ~4–27% European, and ~0–6% Native American ancestry [95–99]. Although pooled individuals from the Caribbean have a high proportion of African ancestry, fine-scale genetic structure has been observed within and between islands (particularly, Dominica, Grenada, St. Kitts, St. Lucia, St. Thomas, St. Vincent, Jamaica, and Trinidad) due to regional differences in levels of African and/or European ancestry [100•]. Similarly, a study of genetic admixture within Puerto Rico showed that levels of African ancestry varied geographically with the highest proportion occurring in the eastern part of the island where African slaves and their descendants historically engaged in sugar pro-duction [101]. In addition, genome-wide data have suggested that patterns of genetic ancestry in Cuba, Puerto Rico and Hispaniola (the Greater Antilles) were consistent with a model of two migration events from different regions of western Africa, implying that Afro-Caribbean populations have mixed African ancestry [102•]. These results are also congruent with a Y-chromosome study that found diverse haplotypes in Afro-Caribbeans from the Bahamas that were inferred to originate from different ethnic groups within West Central Africa [103]. Furthermore, isotope data from skeletal remains of enslaved Africans in Barbados suggested that first generation captives had different dietary histories likely due to differences in their geographic origins in Africa [104]. During the slave trade, the Caribbean has been an end-point of migration for hundreds of years, resulting in diverse genetic patterns. Because of the complexity of past migration events, additional studies across a broader geographic range of the Caribbean are needed to fully understand the extent of genetic variability and the different demographic processes that have contributed to it in Afro-Caribbean populations.
African Americans also have a high proportion of ancestry originating from western Africa, particularly Bantu and non-Bantu Niger-Kordofanian ancestry [3,36,105]. However, African Americans are characterized by genetic variability between populations living in different regions of the United States. An analysis of Y-chromosome loci genotyped in ~1300 individuals from Africa, the Caribbean, the District of Columbia (DC) and South Carolina (SC) detected genetic differentiation among African Americans that was largely attributed to geo-graphic differences in levels of European admixture [106•,107]. Specifically, a low proportion of European admixture was observed in individuals from SC compared to DC. These findings are in agreement with a prior study that also found low levels of European ancestry in SC, particularly among the Gullah Islanders [107,108]. Genome-wide data also demonstrated that individuals who self-identified as African American have a range of genetic ancestry with some individuals showing close to no West African ancestry, while others have almost complete West African ancestry [36]. Indeed, these studies indicate that populations of African descent have a complex history resulting in genetic heterogeneity. In the future, African Americans could potentially become more genetically diverse. Particularly, this pattern could emerge as individuals migrate from regions of Africa, not originally represented in the African Diaspora, into the United States contributing ancestry to subsequent generations of individuals who may self-identify as African Americans.
Implications of genetic structure and admixture for disease susceptibility mapping
Given the complex population history in the United States and Caribbean, it is not surprising that populations of recent African origin are genetically heterogeneous. This pattern of diversity has implications for traditional mapping studies of disease loci, which rely on accurate knowledge of population structure in cases and controls to avoid erroneous associations [1,109,110]. An alternative strategy specifically aimed at identifying variants associated with differential disease risk in admixed populations is mapping admixture by linkage disequilibrium (MALD) or ‘admixture mapping’. This approach uses admixture information to localize disease-associated polymorphisms that are divergent in frequency in the parental populations that have contributed to the population under study. MALD assumes that the genomic region containing disease-susceptibility alleles will be enriched for ancestry from the parental population in which disease risk is more prevalent [111]. Thus, MALD can be used to identify regions of the genome that potentially contain loci associated with differential disease susceptibility. Indeed, recent successes using this approach include the identification of loci underlying hypertension-attributed kidney disease [112,113] and prostate cancer [114,115], which disproportionately affect individuals of African descent.
Evolutionary history has influenced patterns of genetic variation, including the frequency and/or distribution of disease-susceptibility alleles in human populations, which could have implications for the onset of disease. For example, alleles at several genes associated with age-related macular degeneration (AMD), which is a break-down of tissue at the back of the eyes responsible for fine-scale vision, have been observed at different frequencies in human populations. In particular, the G-allele at SNP rs2230199, correlated with increased risk for AMD, is found at much higher frequency in European populations compared to African and Asian populations [116]. A number of studies have also reported higher mortality rates for several types of cancers, including breast, ovarian and prostate, in individuals of African ancestry compared to individuals of European or Asian descent [117–120]. Although environmental factors such as diet and access to health care play a key role in differential disease risk, genetic variation also contributes to differences in cancer susceptibility between populations. Recently, micro-RNAs (miRNAs) have been correlated with the onset, progression, and/or metastasis of cancers with known health disparities among populations. For example, the T-allele (rs12355840) within miRNA hsa-mir-202 has been shown to down-regulate expression of known cancer genes, and to be protective against breast cancer mortality [121]. A recent analysis of global miRNA variation demonstrated that African and African American populations have a lower frequency of the hsa-mir-202 T-allele compared to non-Africans [122], raising the possibility that differences in allele frequency at this locus could potentially contribute to current disparities in breast cancer mortality.
Whether these between-population differences in the frequency of alleles associated with disease susceptibility are due to demographic history or natural selection requires more detailed analyses. However, it is clear that differences in the frequency of alleles correlated with disease exist among human populations. A recent con-sequence of this finding has been the emergence of ‘racialized medicine’ to treat diseases that disproportionately affect a given population. This strategy assumes that the frequencies of genetic variants influencing drug metabolism and/or the onset of disease are different between ‘races’ (i.e. the categorization of individuals into discrete groups based on shared physical and/or cultural characteristics) but similar among individuals within the same ‘race’ [123]. However, members of a self-identified ‘race’ may not necessarily be genetically homogenous as previously discussed for populations of recent African descent. For admixed populations, like African Americans, it may be more beneficial to determine individual ancestry and to devise treatments based on personalized genomic variation.
Conclusions and future directions
Over the last several years, genetic analyses of ever-increasing numbers of genomes have provided significant insight into human evolutionary history. However, a continued challenge has been the inclusion of diverse African populations in studies aimed at investigating fine-scale population structure and ancient demographic pat-terns, including archaic admixture in Africa. Given the complex human population history in Africa, putative evidence for archaic admixture will need to be weighed against alternative scenarios, such as ancient population structure, that could give rise to similar patterns. To date, the genomes of a small fraction of the 2000 ethno-linguistic groups in Africa have been sequenced. As the cost of whole genome sequencing decreases, it will become feasible to conduct large-scale genomic sequencing of ethnically and geographically diverse Africans for the more detailed study of human population history. Furthermore, the integration of genomic information with phenotypic data, including health-related traits and tissue-specific gene expression, will be beneficial for identifying novel variation underlying complex disease in populations of African descent. Overall, these studies will shed light on modern human origins, African population history, and the genetic basis of complex traits, including disease susceptibility.
Acknowledgements
We thank Alessia Ranciaro and Renata Rawlings-Goss for crucial review of the manuscript and/or figures. The authors are funded by the US National Institutes of Health grant 5DP1ES022577 05 to S.A.T.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
Uncited reference
[87].
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Campbell MC, Tishkoff SA. African genetic diversity: implications for human demographic history, modern human origins, and complex disease mapping. Annu Rev Genomics Hum Genet. 2008;9:403–433. doi: 10.1146/annurev.genom.9.081307.164258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell MC, Tishkoff SA. The evolution of human genetic and phenotypic variation in Africa. Curr Biol. 2010;20:R166–R173. doi: 10.1016/j.cub.2009.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tishkoff SA, Reed FA, Friedlaender FR, Ehret C, Ranciaro A, Froment A, Hirbo JB, Awomoyi AA, Bodo JM, Doumbo O, et al. The genetic structure and history of Africans and African Americans. Science. 2009;324:1035–1044. doi: 10.1126/science.1172257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tishkoff SA, Verrelli BC. Patterns of human genetic diversity: implications for human evolutionary history and disease. Annu Rev Genom Hum Genet. 2003;4:293–340. doi: 10.1146/annurev.genom.4.070802.110226. [DOI] [PubMed] [Google Scholar]
- 5.McDougall I, Brown FH, Fleagle JG. Stratigraphic placement and age of modern humans from Kibish, Ethiopia. Nature. 2005;433:733–736. doi: 10.1038/nature03258. [DOI] [PubMed] [Google Scholar]
- 6.White TD, Asfaw B, DeGusta D, Gilbert H, Richards GD, Suwa G, Howell FC. Pleistocene Homo sapiens from Middle Awash, Ethiopia. Nature. 2003;423:742–747. doi: 10.1038/nature01669. [DOI] [PubMed] [Google Scholar]
- 7.Wood B, Leakey M. The Omo-Turkana Basin fossil hominins and their contribution to our understanding of human evolution in Africa. Evol Anthropol. 2011;20:264–292. doi: 10.1002/evan.20335. [DOI] [PubMed] [Google Scholar]
- 8.Brauer G. The origin of modern anatomy: by speciation or intraspecific evolution? Evol Anthropol. 2008;17:22–37. [Google Scholar]
- 9.Foley RA, Lahr MM. The evolution of the diversity of cultures. Philos Trans R Soc Lond B Biol Sci. 2011;366:1080–1089. doi: 10.1098/rstb.2010.0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grine FE. Observations on Middle Stone Age human teeth from Klasies River Main Site, South Africa. J Hum Evol. 2012;63:750–758. doi: 10.1016/j.jhevol.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Haile-Selassie Y, Asfaw B, White TD. Hominid cranial remains from Upper Pleistocene deposits at Aduma, Middle Awash, Ethiopia. Am J Phys Anthropol. 2004;123:1–10. doi: 10.1002/ajpa.10330. [DOI] [PubMed] [Google Scholar]
- 12.Rightmire GP. Out of Africa: modern human origins special feature: middle and later Pleistocene hominins in Africa and Southwest Asia. Proc Natl Acad Sci U S A. 2009;106:16046–16050. doi: 10.1073/pnas.0903930106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henn BM, Gignoux CR, Jobin M, Granka JM, Macpherson JM, Kidd JM, Rodriguez-Botigue L, Ramachandran S, Hon L, Brisbin A, et al. Hunter-gatherer genomic diversity suggests a southern African origin for modern humans. Proc Natl Acad Sci U S A. 2011;108:5154–5162. doi: 10.1073/pnas.1017511108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schlebusch CM, Skoglund P, Sjodin P, Gattepaille LM, Hernandez D, Jay F, Li S, De Jongh M, Singleton A, Blum MG, et al. Genomic variation in seven KhoeSan groups reveals adaptation and complex African history. Science. 2012;338:374–379. doi: 10.1126/science.1227721. This study describes genome-wide SNP variation in a large sample of linguistically diverse southern African populations. The authors show evidence of ancient (>30 kya) population substructure among Khoesan-speaking populations and signatures of natural selection at genes associated with muscle function and infectious disease, among others.
- 15.Bar-Yosef O. The Upper Paleolithic revolution. Annu Rev Anthropol. 2002;31:363–393. [Google Scholar]
- 16.Mellars P. The impossible coincidence. A single-species model for the origins of modern human behavior in Europe. Evol Anthropol. 2005;14:12–27. [Google Scholar]
- 17. Brown KS, Marean CW, Jacobs Z, Schoville BJ, Oestmo S, Fisher EC, Bernatchez J, Karkanas P, Matthews T. An early and enduring advanced technology originating 71,000 years ago in South Africa. Nature. 2012;491:590–593. doi: 10.1038/nature11660. This paper describes an advanced stone tool technology associated with modern human behavior dated to ~70 kya discovered in South Africa, The authors suggest that microlithic technology used to create composite tool components as part of advanced projectile weapons originated early in South Africa.
- 18.McBrearty S. Palaeoanthropology: sharpening the mind. Nature. 2012;491:531–532. doi: 10.1038/nature11751. [DOI] [PubMed] [Google Scholar]
- 19.Henshilwood CS, d’Errico F, van Niekerk KL, Coquinot Y, Jacobs Z, Lauritzen SE, Menu M, Garcia-Moreno R. A 100,000-year-old ochre-processing workshop at Blombos Cave, South Africa. Science. 2011;334:219–222. doi: 10.1126/science.1211535. [DOI] [PubMed] [Google Scholar]
- 20.Marean CW, Bar-Matthews M, Bernatchez J, Fisher E, Goldberg P, Herries AIR, Jacobs Z, Jerardino A, Karkanas P, Minichillo T, et al. Early human use of marine resources and pigment in South Africa during the Middle Pleistocene. Nature. 2007;449:905–U911. doi: 10.1038/nature06204. [DOI] [PubMed] [Google Scholar]
- 21.Bouzouggar A, Barton N, Vanhaeren M, d’Errico F, Collcutt S, Higham T, Hodge E, Parfitt S, Rhodes E, Schwenninger JL, et al. 82,000-year-old shell beads from North Africa and implications for the origins of modern human behavior. Proc Natl Acad Sci U S A. 2007;104:9964–9969. doi: 10.1073/pnas.0703877104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Atkinson QD. Phonemic diversity supports a serial founder effect model of language expansion from Africa. Science. 2011;332:346–349. doi: 10.1126/science.1199295. This study shows that global patterns of phoneme (units of sound that differentiate words) diversity are clinal with the highest level of diversity present in Africa. The authors argue that modern human languages originated in Africa and that a cultural founder effect (similar to a serial founder–effect model for genetic diversity) occurred during the expansion of modern humans from Africa ~50–70, kya, resulting in contemporary patterns of global language diversity.
- 23.Perreault C, Mathew S. Dating the origin of language using phonemic diversity. PLoS One. 2012;7:e35289. doi: 10.1371/journal.pone.0035289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Excoffier L, Dupanloup I, Huerta-Sanchez E, Sousa VC, Foll M. Robust demographic inference from genomic and SNP data. PLoS Genet. 2013;9:e1003905. doi: 10.1371/journal.pgen.1003905. This study describes a simulation-based method to infer demographic parameters and is suitable to the study of complex demographic models from large genomic datasets.
- 25.Gronau I, Hubisz MJ, Gulko B, Danko CG, Siepel A. Bayesian inference of ancient human demography from individual genome sequences. Nat Genet. 2011;43:1031–1034. doi: 10.1038/ng.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lachance J, Vernot B, Elbers CC, Ferwerda B, Froment A, Bodo JM, Lema G, Fu W, Nyambo TB, Rebbeck TR, et al. Evolutionary history and adaptation from high-coverage whole-genome sequences of diverse African hunter-gatherers. Cell. 2012;150:457–469. doi: 10.1016/j.cell.2012.07.009. This study of high coverage whole genome sequencing in three hunter-gatherer populations in Africa identifies millions of novel variants, demonstrating high levels of genetic variation in Africa. The authors detected population-specific signatures of adaptation at genes involved in immunity, metabolism, olfaction and taste perception. Furthermore, within the pygmy population, the authors identified highly differentiated loci that play a role in growth and anterior pituitary function and that were associated with height.
- 27.Schlebusch CM, Lombard M, Soodyall H. MtDNA control region variation affirms diversity and deep sub-structure in populations from southern Africa. BMC Evol Biol. 2013;13:56. doi: 10.1186/1471-2148-13-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuster SC, Miller W, Ratan A, Tomsho LP, Giardine B, Kasson LR, Harris RS, Petersen DC, Zhao F, Qi J, et al. Complete Khoisan and Bantu genomes from southern Africa. Nature. 2010;463:943–947. doi: 10.1038/nature08795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Veeramah KR, Wegmann D, Woerner A, Mendez FL, Watkins JC, Destro-Bisol G, Soodyall H, Louie L, Hammer MF. An early divergence of KhoeSan ancestors from those of other modern humans is supported by an ABC-based analysis of autosomal resequencing data. Mol Biol Evol. 2012;29:617–630. doi: 10.1093/molbev/msr212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Batini C, Ferri G, Destro-Bisol G, Brisighelli F, Luiselli D, Sanchez-Diz P, Rocha J, Simonson T, Brehm A, Montano V, et al. Signatures of the preagricultural peopling processes in sub-Saharan Africa as revealed by the phylogeography of early Y chromosome lineages. Mol Biol Evol. 2011;28:2603–2613. doi: 10.1093/molbev/msr089. [DOI] [PubMed] [Google Scholar]
- 31.Behar DM, Villems R, Soodyall H, Blue-Smith J, Pereira L, Metspalu E, Scozzari R, Makkan H, Tzur S, Comas D, et al. The dawn of human matrilineal diversity. Am J Hum Genet. 2008;82:1130–1140. doi: 10.1016/j.ajhg.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi W, Ayub Q, Vermeulen M, Shao RG, Zuniga S, van der Gaag K, de Knijff P, Kayser M, Xue Y, Tyler-Smith C. A worldwide survey of human male demographic history based on Y-SNP and Y-STR data from the HGDP-CEPH populations. Mol Biol Evol. 2010;27:385–393. doi: 10.1093/molbev/msp243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batini C, Lopes J, Behar DM, Calafell F, Jorde LB, van der Veen L, Quintana-Murci L, Spedini G, Destro-Bisol G, Comas D. Insights into the demographic history of African Pygmies from complete mitochondrial genomes. Mol Biol Evol. 2011;28:1099–1110. doi: 10.1093/molbev/msq294. [DOI] [PubMed] [Google Scholar]
- 34.Quintana-Murci L, Quach H, Harmant C, Luca F, Massonnet B, Patin E, Sica L, Mouguiama-Daouda P, Comas D, Tzur S, et al. Maternal traces of deep common ancestry and asymmetric gene flow between Pygmy hunter-gatherers and Bantu-speaking farmers. Proc Natl Acad Sci U S A. 2008;105:1596–1601. doi: 10.1073/pnas.0711467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diamond J, Bellwood P. Farmers and their languages: the first expansions. Science. 2003;300:597–603. doi: 10.1126/science.1078208. [DOI] [PubMed] [Google Scholar]
- 36.Bryc K, Auton A, Nelson MR, Oksenberg JR, Hauser SL, Williams S, Froment A, Bodo JM, Wambebe C, Tishkoff SA, et al. Genome-wide patterns of population structure and admixture in West Africans and African Americans. Proc Natl Acad Sci U S A. 2010;107:786–791. doi: 10.1073/pnas.0909559107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pickrell JK, Patterson N, Barbieri C, Berthold F, Gerlach L, Guldemann T, Kure B, Mpoloka SW, Nakagawa H, Naumann C, et al. The genetic prehistory of southern Africa. Nat Commun. 2012;3:1143. doi: 10.1038/ncomms2140. This study describes genome-wide SNP variation using a SNP array designed from unbiased detection of SNPs via sequencing in a large number of southern African populations as well as several other comparative African populations. The authors quantify levels of European and Bantu admixture in Khoesan-speaking populations and show evidence of population subdivision >30 kya. Their results also indicate that East African Hadza and Sandawe Khoesan-speaking populations have ancestry derived from admixture with a population related to southern Khoesan-speaking populations.
- 38.Gasse F, Chalie F, Vincens A, Williams MAJ, Williamson D. Climatic patterns in equatorial and southern Africa from 30,000 to 10,000 years ago reconstructed from terrestrial and near-shore proxy data. Quaternary Sci Rev. 2008;27:2316–2340. [Google Scholar]
- 39.Kim SJ, Crowley TJ, Erickson DJ, Govindasamy B, Duffy PB, Lee BY. High-resolution climate simulation of the last glacial maximum. Clim Dyn. 2008;31:1–16. [Google Scholar]
- 40.Alves I, Sramkova Hanulova A, Foll M, Excoffier L. Genomic data reveal a complex making of humans. PLoS Genet. 2012;8:e1002837. doi: 10.1371/journal.pgen.1002837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Currat M, Excoffier L. Strong reproductive isolation between humans and Neanderthals inferred from observed patterns of introgression. Proc Natl Acad Sci U S A. 2011;108:15129–15134. doi: 10.1073/pnas.1107450108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green RE, Krause J, Briggs AW, Maricic T, Stenzel U, Kircher M, Patterson N, Li H, Zhai W, Fritz MH, et al. A draft sequence of the Neandertal genome. Science. 2010;328:710–722. doi: 10.1126/science.1188021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyer M, Kircher M, Gansauge MT, Li H, Racimo F, Mallick S, Schraiber JG, Jay F, Prufer K, de Filippo C, et al. A high-coverage genome sequence from an archaic Denisovan individual. Science. 2012;338:222–226. doi: 10.1126/science.1224344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reich D, Green RE, Kircher M, Krause J, Patterson N, Durand EY, Viola B, Briggs AW, Stenzel U, Johnson PL, et al. Genetic history of an archaic hominin group from Denisova Cave in Siberia. Nature. 2010;468:1053–1060. doi: 10.1038/nature09710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vernot B, Akey JM. Resurrecting surviving Neandertal lineages from modern human genomes. Science. 2014;343:1017–1021. doi: 10.1126/science.1245938. These authors, and Sankararman et al., 2014, identify archaic lineages in modern genomes and suggest that Neanderthal introgression into modern human populations outside of African occurred multiples times during human evolutionary history. Furthermore, the authors identify an excess of Neanderthal alleles at genes that may have been adaptive in modern humans. In addition, their results also show a deficit of Neanderthal ancestry in some regions of the human genome, such as chromosomal arms, suggesting that archaic alleles may have been deleterious in particular genomic regions.
- 46.Wall JD, Lohmueller KE, Plagnol V. Detecting ancient admixture and estimating demographic parameters in multiple human populations. Mol Biol Evol. 2009;26:1823–1827. doi: 10.1093/molbev/msp096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wall JD, Yang MA, Jay F, Kim SK, Durand EY, Stevison LS, Gignoux C, Woerner A, Hammer MF, Slatkin M. Higher levels of neanderthal ancestry in East Asians than in Europeans. Genetics. 2013;194:199–209. doi: 10.1534/genetics.112.148213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harris K, Nielsen R. Inferring demographic history from a spectrum of shared haplotype lengths. PLoS Genet. 2013;9:e1003521. doi: 10.1371/journal.pgen.1003521. This study compares the distribution of both simulated and empirical tracts of identity by state (IBS) in trio parents from the 1000 Genomes project to infer past human demographic events. Results indicate that ancestors of modern African and European populations diverged ~55 thousand year ago (kya) with extensive gene flow between ancestral Africans and Europeans after this initial date of divergence until 13 kya. Data also show that segments of modern European genomes originated from an archaic hominid species, indicating a complex population history of modern humans.
- 49.Yang MA, Malaspinas AS, Durand EY, Slatkin M. Ancient structure in Africa unlikely to explain Neanderthal and non-African genetic similarity. Mol Biol Evol. 2012;29:2987–2995. doi: 10.1093/molbev/mss117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Garrigan D, Mobasher Z, Severson T, Wilder JA, Hammer MF. Evidence for archaic Asian ancestry on the human X chromosome. Mol Biol Evol. 2005;22:189–192. doi: 10.1093/molbev/msi013. [DOI] [PubMed] [Google Scholar]
- 51.Hammer MF, Woerner AE, Mendez FL, Watkins JC, Wall JD. Genetic evidence for archaic admixture in Africa. Proc Natl Acad Sci U S A. 2011;108:15123–15128. doi: 10.1073/pnas.1109300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Plagnol V, Wall JD. Possible ancestral structure in human populations. PLoS Genet. 2006;2:e105. doi: 10.1371/journal.pgen.0020105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wall JD, Hammer MF. Archaic admixture in the human genome. Curr Opin Genet Dev. 2006;16:606–610. doi: 10.1016/j.gde.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 54. Sankararaman S, Mallick S, Dannemann M, Prufer K, Kelso J, Paabo S, Patterson N, Reich D. The genomic landscape of Neanderthal ancestry in present-day humans. Nature. 2014;507:354–357. doi: 10.1038/nature12961. This study, and Vernot et al., 2014, identifies archaic lineages in modern genomes and demonstrates that some regions of the genome have an excess or a deficit of archaic ancestry possibly due to natural selection. In particular, regions of the genome in non-Africans with a high frequency of Neanderthal alleles were found to be enriched for genes that play a role in skin morphology, which may have helped humans adapt to environments outside of Africa. Additionally, the authors also report evidence for negative selection against derived Neanderthal ancestry in regions of modern human genomes known to play a role in hybrid sterility, suggesting that the introduction of some Neanderthal alleles into modern human populations were not tolerated.
- 55. Wang S, Lachance J, Tishkoff SA, Hey J, Xing J. Apparent variation in Neanderthal admixture among African populations is consistent with gene flow from Non-African populations. Genome Biol Evol. 2013;5:2075–2081. doi: 10.1093/gbe/evt160. This study examines whole-genome data from 38 sub-Saharan Africans from eight populations and 25 non-African individuals from five populations. This study detected shared alleles between Neanderthal and some populations of African descent but argues that this signature of archaic admixture is likely due to recent admixture with non-African populations, refuting the hypothesis of an earlier introgression event within Africa between the ancestors of Neanderthals and modern African populations.
- 56.Mendez FL, Krahn T, Schrack B, Krahn AM, Veeramah KR, Woerner AE, Fomine FL, Bradman N, Thomas MG, Karafet TM, et al. An African American paternal lineage adds an extremely ancient root to the human Y chromosome phylogenetic tree. Am J Hum Genet. 2013;92:454–459. doi: 10.1016/j.ajhg.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gomez F, Hirbo J, Tishkoff SA. Genetic variation and adaptation in Africa: implications for human evolution and disease. Cold Spring Harb Perspect Biol. 2014;6:1–21. doi: 10.1101/cshperspect.a008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.de Filippo C, Barbieri C, Whitten M, Mpoloka SW, Gunnarsdottir ED, Bostoen K, Nyambe T, Beyer K, Schreiber H, de Knijff P, et al. Y-Chromosomal variation in sub-Saharan Africa: insights into the history of Niger-Congo groups. Mol Biol Evol. 2011;28:1255–1269. doi: 10.1093/molbev/msq312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hirbo JB, Rnciaro A, Campbell MC, Meskel D, Omar SA, Belay G, Ibrahim M, Lema G, Nyambo TB, Kivisild T, Lahr MM, Tishkoff SA. Evidence of ancient African substructure correlated with geography and language based on mitochondrial and Y chromosome data. unpublished data [Google Scholar]
- 60.Currie TE, Meade A, Guillon M, Mace R. Cultural phylogeography of the Bantu Languages of sub-Saharan Africa. Proc Biol Sci. 2013;280:20130695. doi: 10.1098/rspb.2013.0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ehret C. Bantu expansions: re-envisioning a central problem of early African history. Int J Afr Historical Studies. 2001;34:5–41. [Google Scholar]
- 62.Nurse D, Philippson G, editors. The Bantu Languages. Routledge; 2003. [Google Scholar]
- 63.Phillipson DW, editor. African Archaeology. Cambridge University Press; 2005. [Google Scholar]
- 64.Vansina J. Western Bantu expansion. J Afr History. 1984;25:129–145. [Google Scholar]
- 65.Blench R, editor. Archaeology, Language and the African Past. AltaMira Press; 2006. [Google Scholar]
- 66.Breton G, Schlebusch CM, Lombard M, Sjodin P, Soodyall H, Jakobsson M. Lactase persistence alleles reveal partial east African ancestry of southern African Khoe Pastoralists. Curr Biol. 2014;24:852–858. doi: 10.1016/j.cub.2014.02.041. [DOI] [PubMed] [Google Scholar]
- 67.Coelho M, Sequeira F, Luiselli D, Beleza S, Rocha J. On the edge of Bantu expansions: mtDNA, Y chromosome and lactase persistence genetic variation in southwestern Angola. BMC Evol Biol. 2009;9 doi: 10.1186/1471-2148-9-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ranciaro A, Campbell MC, Hirbo JB, Ko WY, Froment A, Anagnostou P, Kotze MJ, Ibrahim M, Nyambo T, Omar SA, et al. Genetic origins of lactase persistence and the spread of pastoralism in Africa. Am J Human Genet. 2014;94:496–510. doi: 10.1016/j.ajhg.2014.02.009. This study characterizes nucleotide and microsatellite variation at regions that play a role in regulating lactase gene expression in geographically and ethnically diverse Africans, and a comparative sample of non-Africans. The authors demonstrate association of several variants ~14,000 bp upstream of the lactase encoding gene (LCT) with the lactase persistence (LP) trait in East Africa and observe signatures of strong recent natural selection at these loci. They also reconstruct the evolutionary history of these variants and found that the East African LP-associated variant (C-14010) is present in southern Africa, reflecting historic migration events.
- 69.Torniainen S, Parker MI, Holmberg V, Lahtela E, Dandara C, Jarvela I. Screening of variants for lactase persistence/non-persistence in populations from South Africa and Ghana. Bmc Genetics. 2009:10. doi: 10.1186/1471-2156-10-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Atzmon G, Hao L, Pe’er I, Velez C, Pearlman A, Palamara PF, Morrow B, Friedman E, Oddoux C, Burns E, et al. Abraham’s Children in the Genome Era: Major Jewish Diaspora populations comprise distinct genetic clusters with shared middle eastern ancestry. Am J Hum Genet. 2010;86:850–859. doi: 10.1016/j.ajhg.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bray SM, Mulle JG, Dodd AF, Pulver AE, Wooding S, Warren ST. Signatures of founder effects, admixture, and selection in the Ashkenazi Jewish population. Proc Natl Acad Sci U S A. 2010;107:16222–16227. doi: 10.1073/pnas.1004381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Henn BM, Cavalli-Sforza LL, Feldman MW. The great human expansion. Proc Natl Acad Sci U S A. 2012;109:17758–17764. doi: 10.1073/pnas.1212380109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hunter-Zinck H, Musharoff S, Salit J, Al-Ali KA, Chouchane L, Gohar A, Matthews R, Butler MW, Fuller J, Hackett NR, et al. Population genetic structure of the people of Qatar. Am J Hum Genet. 2010;87:17–25. doi: 10.1016/j.ajhg.2010.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kopelman NM, Stone L, Wang CL, Gefel D, Feldman MW, Hillel J, Rosenberg NA. Genomic microsatellites identify shared Jewish ancestry intermediate between Middle Eastern and European populations. BMC Genet. 2009:10. doi: 10.1186/1471-2156-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li JZ, Absher DM, Tang H, Southwick AM, Casto AM, Ramachandran S, Cann HM, Barsh GS, Feldman M, Cavalli-Sforza LL, et al. Worldwide human relationships inferred from genome-wide patterns of variation. Science. 2008;319:1100–1104. doi: 10.1126/science.1153717. [DOI] [PubMed] [Google Scholar]
- 76.Rosenberg NA, Pritchard JK, Weber JL, Cann HM, Kidd KK, Zhivotovsky LA, Feldman MW. Genetic structure of human populations. Science. 2002;298:2381–2385. doi: 10.1126/science.1078311. [DOI] [PubMed] [Google Scholar]
- 77.Henn BM, Botigue LR, Gravel S, Wang W, Brisbin A, Byrnes JK, Fadhlaoui-Zid K, Zalloua PA, Moreno-Estrada A, Bertranpetit J, et al. Genomic ancestry of North Africans supports back-to-Africa migrations. PLoS Genet. 2012;8:e1002397. doi: 10.1371/journal.pgen.1002397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hodgson JA, Mulligan CJ, Al-Meeri A, Raaum RL. Early back-to-Africa migration into the Horn of Africa. PLoS Genet. 2014;10:e1004393. doi: 10.1371/journal.pgen.1004393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pickrell JK, Patterson N, Loh PR, Lipson M, Berger B, Stoneking M, Pakendorf B, Reich D. Ancient west Eurasian ancestry in southern and eastern Africa. Proc Natl Acad Sci U S A. 2014;111:2632–2637. doi: 10.1073/pnas.1313787111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirbo JB, Ranciaro A, Tishkoff SA. Population structure and migration in Africa: correlations between archeological, linguistic, and genetic data. In: Campbell B, Crawford MH, editors. Causes and Consequences of Migration. Cambridge University Press; 2012. pp. 134–171. [Google Scholar]
- 81.Smith AB. Origins and spread of pastoralism in Africa. Annu Rev Anthropol. 1992;21:125–141. [Google Scholar]
- 82.Henn BM, Gignoux C, Lin AA, Oefner PJ, Shen P, Scozzari R, Cruciani F, Tishkoff SA, Mountain JL, Underhill PA. Y-chromosomal evidence of a pastoralist migration through Tanzania to southern Africa. Proc Natl Acad Sci U S A. 2008;105:10693–10698. doi: 10.1073/pnas.0801184105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.de Wit E, Delport W, Rugamika CE, Meintjes A, Moller M, van Helden PD, Seoighe C, Hoal EG. Genome-wide analysis of the structure of the South African Coloured Population in the Western Cape. Hum Genet. 2010;128:145–153. doi: 10.1007/s00439-010-0836-1. [DOI] [PubMed] [Google Scholar]
- 84.Patterson N, Petersen DC, van der Ross RE, Sudoyo H, Glashoff RH, Marzuki S, Reich D, Hayes VM. Genetic structure of a unique admixed population: implications for medical research. Hum Mol Genet. 2010;19:411–419. doi: 10.1093/hmg/ddp505. [DOI] [PubMed] [Google Scholar]
- 85.Tishkoff SA, Gonder MK, Henn BM, Mortensen H, Knight A, Gignoux C, Fernandopulle N, Lema G, Nyambo TB, Ramakrishnan U, et al. History of click-speaking populations of Africa inferred from mtDNA and Y chromosome genetic variation. Mol Biol Evol. 2007;24:2180–2195. doi: 10.1093/molbev/msm155. [DOI] [PubMed] [Google Scholar]
- 86.Hellenthal G, Auton A, Falush D. Inferring human colonization history using a copying model. PLoS Genet. 2008;4:e1000078. doi: 10.1371/journal.pgen.1000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jakobsson M, Scholz SW, Scheet P, Gibbs JR, VanLiere JM, Fung HC, Szpiech ZA, Degnan JH, Wang K, Guerreiro R, et al. Genotype, haplotype and copy-number variation in worldwide human populations. Nature. 2008;451:998–1003. doi: 10.1038/nature06742. [DOI] [PubMed] [Google Scholar]
- 88.Petersen DC, Libiger O, Tindall EA, Hardie RA, Hannick LI, Glashoff RH, Mukerji M, Fernandez P, Haacke W, Schork NJ, et al. Complex patterns of genomic admixture within southern Africa. PLoS Genet. 2013;9:e1003309. doi: 10.1371/journal.pgen.1003309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Patin E, Laval G, Barreiro LB, Salas A, Semino O, Santachiara-Benerecetti S, Kidd KK, Kidd JR, Van der Veen L, Hombert JM, et al. Inferring the demographic history of African farmers and pygmy hunter-gatherers using a multilocus resequencing data set. PLoS Genet. 2009;5:e1000448. doi: 10.1371/journal.pgen.1000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Berniell-Lee G, Calafell F, Bosch E, Heyer E, Sica L, Mouguiama-Daouda P, van der Veen L, Hombert JM, Quintana-Murci L, Comas D. Genetic and demographic implications of the Bantu expansion: insights from human paternal lineages. Mol Biol Evol. 2009;26:1581–1589. doi: 10.1093/molbev/msp069. [DOI] [PubMed] [Google Scholar]
- 91.Patin E, Siddle KJ, Laval G, Quach H, Harmant C, Becker N, Froment A, Regnault B, Lemee L, Gravel S, et al. The impact of agricultural emergence on the genetic history of African rainforest hunter-gatherers and agriculturalists. Nat Commun. 2014;5:3163. doi: 10.1038/ncomms4163. [DOI] [PubMed] [Google Scholar]
- 92.Verdu P, Austerlitz F, Estoup A, Vitalis R, Georges M, Thery S, Froment A, Le Bomin S, Gessain A, Hombert JM, et al. Origins and genetic diversity of pygmy hunter-gatherers from western central Africa. Curr Biol. 2009;19:312–318. doi: 10.1016/j.cub.2008.12.049. [DOI] [PubMed] [Google Scholar]
- 93.Verdu P, Destro-Bisol G. African Pygmies, what’s behind a name? Hum Biol. 2012;84:1–10. doi: 10.3378/027.084.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lovejoy PE, editor. Identifying Enslaved Africans in the African Diaspora. Cassell Academic; 2000. [Google Scholar]
- 95.Deason ML, Salas A, Newman SP, Macaulay VA, St AMEY, Pitsiladis YP. Interdisciplinary approach to the demography of Jamaica. BMC Evol Biol. 2012;12:24. doi: 10.1186/1471-2148-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gaieski JB, Owings AC, Vilar MG, Dulik MC, Gaieski DF, Gittelman RM, Lindo J, Gau L, Schurr TG, Genographic C. Genetic ancestry and indigenous heritage in a Native American descendant community in Bermuda. Am J Phys Anthropol. 2011;146:392–405. doi: 10.1002/ajpa.21588. [DOI] [PubMed] [Google Scholar]
- 97.Murray T, Beaty TH, Mathias RA, Rafaels N, Grant AV, Faruque MU, Watson HR, Ruczinski I, Dunston GM, Barnes KC. African and non-African admixture components in African Americans and an African Caribbean population. Genet Epidemiol. 2010;34:561–568. doi: 10.1002/gepi.20512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Parra EJ, Marcini A, Akey L, Martinson J, Batzer MA, Cooper R, Forrester T, Allison DB, Deka R, Ferrell RE, et al. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet. 1998;63:1839–1851. doi: 10.1086/302148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Simms TM, Barrett DA, McCartney Q, Herrera RJ. Divergent genetic strata in five Bahamian islands. Forensic Sci Int Genet. 2012;6:81–90. doi: 10.1016/j.fsigen.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 100. Torres JB, Stone AC, Kittles R. An anthropological genetic perspective on Creolization in the Anglophone Caribbean. Am J Phys Anthropol. 2013;151:135–143. doi: 10.1002/ajpa.22261. This study examines autosomal variation using ancestry informative markers across several islands in the Caribbean and highlights some of the historical factors associated with the Trans-Atlantic slave trade that have shaped patterns of variation in contemporary Afro-Caribbean populations.
- 101.Via M, Gignoux CR, Roth LA, Fejerman L, Galanter J, Choudhry S, Toro-Labrador G, Viera-Vera J, Oleksyk TK, Beckman K, et al. History shaped the geographic distribution of genomic admixture on the island of Puerto Rico. PLoS One. 2011;6:e16513. doi: 10.1371/journal.pone.0016513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Moreno-Estrada A, Gravel S, Zakharia F, McCauley JL, Byrnes JK, Gignoux CR, Ortiz-Tello PA, Martinez RJ, Hedges DJ, Morris RW, et al. Reconstructing the population genetic history of the Caribbean. PLoS Genet. 2013;9:e1003925. doi: 10.1371/journal.pgen.1003925. This genome-wide study reconstructs local ancestry across admixed genomes in populations to better understand the population genetic history of the Caribbean and South America. The authors indicate that Afro-Caribbean populations have mixed ancestry from different regions of Africa due to different waves of forced migration from Africa during the Trans-Atlantic slave trade. The study provides additional information regarding the general geographic origins of several Afro-Caribbean populations and sheds light on the complex patterns of past migration of indigenous Africans into the Caribbean.
- 103.Simms TM, Martinez E, Herrera KJ, Wright MR, Perez OA, Hernandez M, Ramirez EC, McCartney Q, Herrera RJ. Paternal lineages signal distinct genetic contributions from British loyalists and continental Africans among different Bahamian Islands. Am J Phys Anthropol. 2011;146:594–608. doi: 10.1002/ajpa.21616. [DOI] [PubMed] [Google Scholar]
- 104.Schroeder H, O’Connell TC, Evans JA, Shuler KA, Hedges RE. Trans-Atlantic slavery: isotopic evidence for forced migration to Barbados. Am J Phys Anthropol. 2009;139:547–557. doi: 10.1002/ajpa.21019. [DOI] [PubMed] [Google Scholar]
- 105.Zakharia F, Basu A, Absher D, Assimes TL, Go AS, Hlatky MA, Iribarren C, Knowles JW, Li J, Narasimhan B, et al. Characterizing the admixed African ancestry of African Americans. Genome Biol. 2009;10:R141. doi: 10.1186/gb-2009-10-12-r141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Jin WF, Wang SJ, Wang HF, Jin L, Xu SH. Exploring population admixture dynamics via empirical and simulated genome-wide distribution of ancestral chromosomal segments. Am J Hum Genet. 2012;91:849–862. doi: 10.1016/j.ajhg.2012.09.008. This large-scale genotype analysis explores models of admixture based on the distribution of ancestry on chromosomal segments across the genome in a number of populations including African Americans. This study reports patterns of variation in African American populations consistent with a model of population substructure, demonstrating that African Americans are not genetically homogeneous.
- 107.Torres JB, Doura MB, Keita SOY, Kittles RA. Y Chromosome lineages in men of West African descent. PLoS One. 2012;7:e29687. doi: 10.1371/journal.pone.0029687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Parra EJ, Kittles RA, Argyropoulos G, Pfaff CL, Hiester K, Bonilla C, Sylvester N, Parrish-Gause D, Garvey WT, Jin L, et al. Ancestral proportions and admixture dynamics in geographically defined African Americans living in South Carolina. Am J Phys Anthropol. 2001;114:18–29. doi: 10.1002/1096-8644(200101)114:1<18::AID-AJPA1002>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 109.Teo YY, Small KS, Kwiatkowski DP. Methodological challenges of genome-wide association analysis in Africa. Nat Rev Genet. 2010;11:149–160. doi: 10.1038/nrg2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tian C, Plenge RM, Ransom M, Lee A, Villoslada P, Selmi C, Klareskog L, Pulver AE, Qi L, Gregersen PK, et al. Analysis and application of European genetic substructure using 300 K SNP information. PLoS Genet. 2008;4:e4. doi: 10.1371/journal.pgen.0040004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shriner D. Overview of admixture mapping. Curr Protoc Hum Genet. 2013;Chapter 1(Unit 1):23. doi: 10.1002/0471142905.hg0123s76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Freedman BI, Kopp JB, Langefeld CD, Genovese G, Friedman DJ, Nelson GW, Winkler CA, Bowden DW, Pollak MR. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol. 2010;21:1422–1426. doi: 10.1681/ASN.2010070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Simino J, Rao DC, Freedman BI. Novel findings and future directions on the genetics of hypertension. Curr Opin Nephrol Hypertens. 2012;21:500–507. doi: 10.1097/MNH.0b013e328354e78f. [DOI] [PubMed] [Google Scholar]
- 114.Freedman ML, Haiman CA, Patterson N, McDonald GJ, Tandon A, Waliszewska A, Penney K, Steen RG, Ardlie K, John EM, et al. Admixture mapping identifies 8q24 as a prostate cancer risk locus in African-American men. Proc Natl Acad Sci U S A. 2006;103:14068–14073. doi: 10.1073/pnas.0605832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Murphy AB, Ukoli F, Freeman V, Bennett F, Aiken W, Tulloch T, Coard K, Angwafo F, Kittles RA. 8q24 risk alleles in West African and Caribbean men. Prostate. 2012;72:1366–1373. doi: 10.1002/pros.22486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ermini L, Wilson IJ, Goodship THJ, Sheerin NS. Complement polymorphisms: geographical distribution and relevance to disease. Immunobiology. 2012;217:265–271. doi: 10.1016/j.imbio.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 117.Al-Refaie WB, Tseng JF, Gay G, Patel-Parekh L, Mansfield PF, Pisters PWT, Yao JC, Feig BW. The impact of ethnicity on the presentation and prognosis of patients with gastric adenocarcinoma — results from the National Cancer Data Base. Cancer. 2008;113:461–469. doi: 10.1002/cncr.23572. [DOI] [PubMed] [Google Scholar]
- 118.Chornokur G, Amankwah EK, Schildkraut JM, Phelan CM. Global ovarian cancer health disparities. Gynecol Oncol. 2013;129:258–264. doi: 10.1016/j.ygyno.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karliner LS, Kerlikowske K. Ethnic disparities in breast cancer. Womens Health (Lond Engl) 2007;3:679–688. doi: 10.2217/17455057.3.6.679. [DOI] [PubMed] [Google Scholar]
- 120.Sun M, Abdollah F, Liberman D, Abdo A, Thuret R, Tian Z, Shariat SF, Montorsi F, Perrotte P, Karakiewicz PI. Racial disparities and socioeconomic status in men diagnosed with testicular germ cell tumors a survival analysis. Cancer. 2011;117:4277–4285. doi: 10.1002/cncr.25969. [DOI] [PubMed] [Google Scholar]
- 121.Hoffman AE, Liu R, Fu A, Zheng TZ, Slack F, Zhu Y. Targetome profiling, pathway analysis and genetic association study implicate miR-202 in lymphomagenesis. Cancer Epidemiol Biomarkers Prevention. 2013;22:327–336. doi: 10.1158/1055-9965.EPI-12-1131-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rawlings-Goss RA, Campbell MC, Tishkoff SA. Global population-specific variation in miRNA associated with cancer risk and clinical biomarkers. BMC Med Genom. 2014;7:53. doi: 10.1186/1755-8794-7-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lee SS. Racializing drug design: implications of pharmacogenomics for health disparities. Am J Public Health. 2005;95:2133–2138. doi: 10.2105/AJPH.2005.068676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pearson OM. Africa: the cradle of modern people. In: Smith FH, Ahern JC, editors. The Origins of Modern Humans: Biology Reconsidered. John Wiley & Sons; 2013. pp. 1–43. [Google Scholar]
- 125.Grine FE. Middle Stone Age human fossils from Die Kelders Cave 1, Western Cape Province, South Africa. J Hum Evol. 2000;38:129–145. doi: 10.1006/jhev.1999.0353. [DOI] [PubMed] [Google Scholar]
- 126.Hublin J-J, Verna C, Bailey S, Smith T, Olejniczak A, Sbihi-Alaoui FZ, Zouak M. Dental evidence from the Aterian human populations of Morocco. In: Hublin J-J, McPherron SP, editors. Modern Origins: A North African Perspective. Springer Science+Business Media; 2012. pp. 189–204. [Google Scholar]
- 127.Douka K, Jacobs Z, Lane C, Grun R, Farr L, Hunt C, Inglis RH, Reynolds T, Albert P, Aubert M, et al. The chronostratigraphy of the Haua Fteah cave (Cyrenaica, northeast Libya) J Hum Evol. 2014;66:39–63. doi: 10.1016/j.jhevol.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 128.Hublin JJ. Recent human evolution in northwestern Africa. Philos Trans R Soc Lond B Biol Sci. 1992;337:185–191. doi: 10.1098/rstb.1992.0096. [DOI] [PubMed] [Google Scholar]