Abstract
A growing body of research has examined the impact of childhood adversity on neural structure and function. Advances in our understanding of the neurodevelopmental consequences of adverse early environments require the identification of dimensions of environmental experience that influence neural development differently and mechanisms other than the frequently-invoked stress pathways. We propose a novel conceptual framework that differentiates between deprivation (absence of expected environmental inputs and complexity) and threat (presence of experiences that represent a threat to one’s physical integrity) and make predictions grounded in basic neuroscience principles about their distinct effects on neural development. We review animal research on fear learning and sensory deprivation as well as human research on childhood adversity and neural development to support these predictions. We argue that these previously undifferentiated dimensions of experience exert strong and distinct influences on neural development that cannot be fully explained by prevailing models focusing only on stress pathways. Our aim is not to exhaustively review existing evidence on childhood adversity and neural development, but to provide a novel framework to guide future research.
Keywords: childhood adversity, neural development, adverse childhood experience, deprivation, trauma, threat, fear learning
There has been a veritable explosion of research in the last decade into the long-term consequences of exposure to childhood adversity. The terms ‘childhood adversity,’ ‘adverse childhood experience,’ and ‘early life stress’ have been used to refer to a broad set of negative exposures during childhood, ranging from physical and sexual abuse to institutional rearing and chronic poverty (Anda et al., 2006; Burghy et al., 2012; Cohen et al., 2013). Evidence from population-based epidemiological studies indicates that childhood adversity is common and associated strongly with the subsequent onset of psychopathology not only in childhood, but also in adolescence and adulthood (Cohen et al., 2001; Green et al., 2010; Kessler et al., 1997; McLaughlin et al., 2012). Individuals who have been exposed to adverse childhood experiences are at elevated risk of developing a wide range of mental disorders, including mood, anxiety, behavior, and substance use disorders. Importantly, exposure to childhood adversity has been shown to explain more than 30% of mental disorders in the U.S. population (Green et al., 2010; McLaughlin et al., 2012), underscoring the significance of these experiences in shaping population-level mental health.
The strong and pervasive relationship between adverse childhood experiences and psychopathology has generated considerable interest in identifying the underlying mechanisms that explain these associations. However, identifying central mechanisms has proved difficult, because different types of adverse experiences frequently co-occur, meaning that most individuals exposed to childhood adversity have experienced multiple adverse experiences (Dong et al., 2004; Finkelhor et al., 2007; Green et al., 2010; McLaughlin et al., 2012). Recognition of the co-occurring nature of adverse childhood experiences has resulted in a shift from focusing on single types of adversity, such as parental death, divorce, abuse, and neglect (Chase-Lansdale et al., 1995; Dubowitz et al., 2002; Fristad et al., 1993; Mullen et al., 1993; Wolfe et al., 1994), to examining the associations between number of adverse childhood experiences and psychopathology (Arata et al., 2007; Dube et al., 2003; Edwards et al., 2003; but see also Humphreys & Zeanah, 2014 for a recent alternative approach). The fundamental lesson from this research has been that as childhood adversities increase, the likelihood of psychopathology increases. While this has proved valuable for identifying children in need of intervention, it has led to an oversimplification of the boundaries between distinct types of environmental experience and has done little to uncover the core underlying mechanisms through which adversity increases risk for psychopathology.
Here we propose that cognitive neuroscience provides a powerful set of tools that will allow us to most fruitfully identify the developmental pathways linking childhood adversity to psychopathology and that examining the imprint of environmental experience on neural structure and function will help to resolve some of the challenges inherent in studying complex and co-occurring exposures. Indeed, one of the basic principals of neuroscience, developed and elaborated over the last half century, is that early experience shapes the structure and subsequent function of the brain. A small but rapidly growing body of work has begun to examine the impact of childhood adversity on neural development (Hackman and Farah, 2009; Hart and Rubia, 2012). However, to date most existing work has conceptualized adverse childhood experiences purely within a stress perspective, which has hindered the identification underlying dimensions of environmental experience that might influence neural structure and function in distinct ways (but see Rao, et al., 2011 for a counter example). Here we argue that the distinct neural effects of different dimensions of experience have often been oversimplified or ignored. Extant research has almost universally defined childhood adversity according to broad descriptive categories (i.e., abuse, neglect, institutionalization, poverty) or has examined even broader constructs that combine diverse forms of adversity together, often referred to as ‘early life stress’ (Burghy et al., 2012; Cohen et al., 2006; Gatt et al., 2009). This term has been used to refer to such disparate experiences as parental psychopathology, abuse, poverty, marital conflict, and institutional rearing. This approach not only obscures meaningful differences between these types of experiences that are likely to have important implications for understanding their effects on neural development but also implicitly suggests that very different types of environmental experiences influence brain development through the same underlying mechanisms. This lack of specificity both with regard to the measurement of environmental experience and the impacts on brain development constitutes a critical barrier to identifying the pathways through which childhood adversity impacts neural development and, ultimately, psychopathology.
Current conceptualization of the impact of childhood adversity on neural development has focused almost exclusively on stress pathways and allostatic load (Burghy et al., 2012; Cohen et al., 2013). The stress model has been described in detail in numerous previous papers (see McEwen, 2012). Briefly, activation of the hypothalamic-pituitary-adrenal (HPA) axis results in the release of glucocorticoids, which can lead to structural and functional changes in brain regions with high concentrations of glucocorticoid receptors, including the hippocampus, amygdala, and prefrontal cortex (PFC) (McEwen, 2012). The HPA axis is a plastic system and exposure to extreme or chronic stress can lead to changes in the functioning of this system, resulting in excessive or blunted glucocorticoid release and related downstream structural consequences in the brain (McEwen, 1998, 2012). Extensive evidence suggests that early exposure to adverse environments can disrupt the development and functioning of the HPA axis (Gunnar and Quevedo, 2007), and this is the primary mechanism through which it is often argued that adverse experiences shape neural structure and function. Focusing only on this mechanism is problematic as adversity sometimes appears to have a remarkably broad impact on neurodevelopment. For example, children exposed to institutional rearing exhibit widespread cortical thinning in the superior and inferior parietal cortex (McLaughlin et al., 2013), and children exposed to neglect and poverty often have deficits in language abilities (Farah et al., 2006; Hildyard and Wolfe, 2002) and accompanying differences in neural function supporting language function (Raizada, Richards, Meltzoff, & Kuhl, 2008). Neither of these patterns is an obvious consequence of HPA axis activation or cortisol.
However, this evidence is by no means conclusive. Glucocorticoids are only one of many mediators that work together to modulate brain development following stress. The coordinated actions of these mediators are dependent on the state of differentiation of each brain region and are highly region and cell type specific when stress occurs. Indeed, a host of mechanisms of hormone action reveal that the whole brain is a target for the modulatory effects of stress, sex and other hormones via genomic and non-genomic receptors (Liston et al., 2013; McEwen, 2010; Popoli et al., 2012) As such, it is important to acknowledge that the effects of stress are not fully mediated by cortisol (the most common marker of HPA axis activation in human research) and that cortisol actions on their own do not explain how stress affects gene expression or neuronal plasticity (Gray et al., 2013). Thus, although it is possible, given the potentially wide variety of effects that stress can have on the brain, that the changes described above are the downstream effect of stress exposure, it is also possible—and we argue, likely—that alternative mechanisms explain these effects of childhood adversity on neural development. Investigating these mechanisms first requires a novel method of describing and measuring different forms of childhood adversity.
We argue here that the field must move beyond the prevailing approach to one that attempts to distill complex adverse experiences into their core underlying dimensions, and we propose a conceptual framework for doing so. Specifically, our model differentiates between experiences of deprivation (i.e., the absence of expected environmental inputs and complexity) and threat (i.e., the presence of experiences that represent a threat to one’s physical integrity) and provides predictions and preliminary evidence grounded in basic neuroscience principles and mechanisms drawn from animal research on sensory deprivation and fear learning about the expected effects of each of these dimensions of experience on neural structure and function. Our aim is not to exhaustively review existing evidence on early adversity and neural development in humans or animals, but to provide a novel conceptual framework to guide future research.
Importantly, we do not propose that deprivation and threat are the only dimensions of early experience that are important or that all types of childhood adversity can be conceptualized solely along these dimensions. For example, institutional rearing involves the complete absence of an attachment figure early in development, (Tottenham, 2012). This lack of species-typical expectations of the presence of an attachment figure in early development is a dimension not fully captured by either deprivation or threat. Rather, we propose that these are two dimensions of experience that have not previously been clearly differentiated or explained by prevailing models focused on stress pathways and argue that these dimensions of experience exert strong and distinct influences on neural development.
Distinguishing between Deprivation and Threat
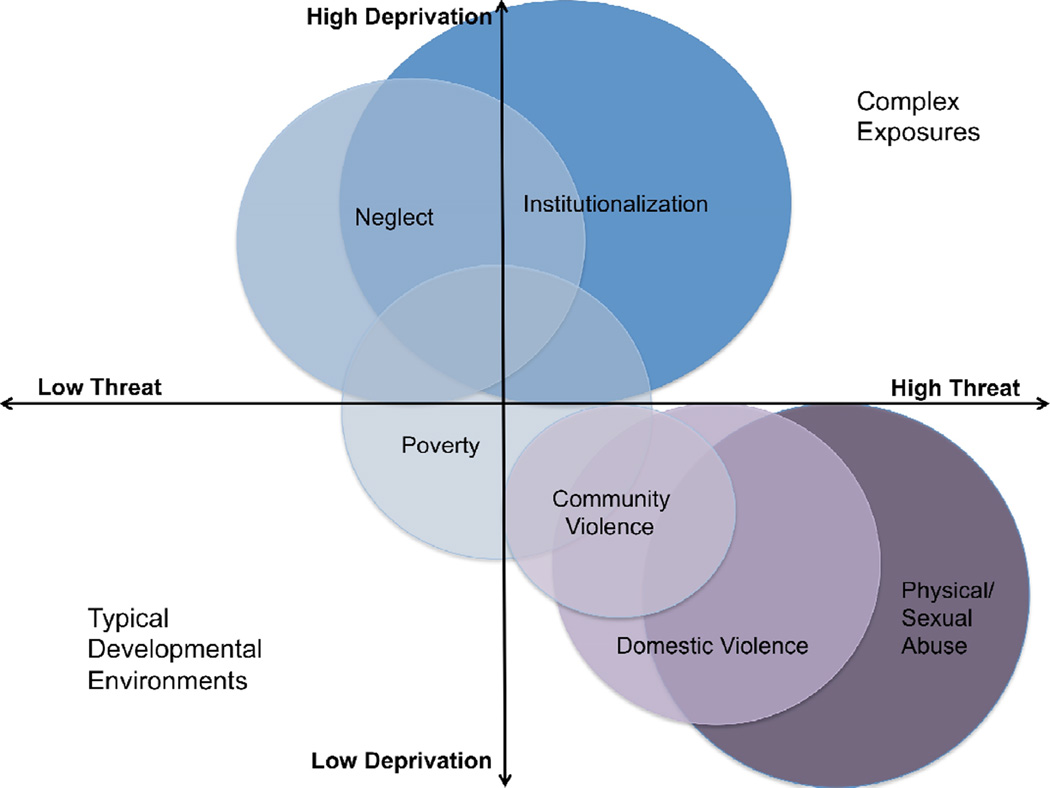
The framework we propose here distinguishes between core dimensions of environmental experience that underlie different forms of childhood adversity and describes their distinct impacts on neural development. The central distinction we make is between experiences of deprivation and experiences of threat. We suggest that these dimensions of experience can be assessed across different forms of childhood adversity (e.g., physical and sexual abuse, domestic violence, institutionalization, neglect) and will differentially predict aspects of neurodevelopment and ultimately behavior. Experiences of deprivation involve the absence of expected cognitive and social inputs as well as the absence of species- and age-typical complexity in environmental stimuli. The impact of the lack of cognitive complexity on cortical development has been well studied in animal models of sensory and global deprivation and is conserved across species (Diamond et al., 1972; Leporé et al., 2010; O'Kusky, 1985). The dimension of deprivation is central for children exposed to institutionalization, neglect, and poverty (Figure 1). In contrast, experiences of threat include events that involve actual or threatened death, serious injury, sexual violation, or other harm to one’s physical integrity. Threat experiences are conceptually similar to events defined as traumatic in the Diagnostic and Statistical Manual of Mental Disorders (American Psychiatric Association, 2013). Events involving threat of serious harm result in strong learning mediated by emotional learning networks that have been well characterized in animals and are conserved across species (Johansen et al., 2011; LeDoux, 2003). Threat is a primary dimension of experience for children exposed to physical and sexual abuse, domestic violence, and other types of interpersonal violence (Figure 1). Critically, we do not propose that exposure to deprivation and threat experiences occurs independently for children, as most of the exposures described above co-occur. Instead we propose that they can be measured separately and have unique effects on neurodevelopment.
Figure 1. Dimensions of threat and deprivation associated with commonly occurring adverse childhood experiences (ACEs).
1 Importantly, we argue that threat and deprivation are dimensions of experience that can be measured among children exposed to a wide ranges of ACEs, both those that occur in isolation (e.g., a single incident of community violence exposure) and those that are co-occurring (e.g., physical abuse and physical neglect). We use the term complex exposures to refer to experiences that in most cases involve aspects of both threat and deprivation. Institutionalization is one such exposure, which involves deprivation in both cognitive and social inputs—consistent with our definition of deprivation—as well as the absence of a primary attachment figure, which is an atypical experience that can represent a significant threat to safety and survival for an infant in the extreme absence of care. Note that institutional rearing also involves lack of species-typical expectations of the presence of an attachment figure in early development (Tottenham, 2012), a dimension not fully captured by either deprivation and threat.
2 Poverty differs in fundamental ways from the other exposures we describe. Critically, poverty does not inherently involve dimensions of either threat or deprivation (i.e., it is possible to be poor and to have no exposure to threatening experiences and typical exposure to cognitive, social, and environmental complexity). However, poverty is often a marker of exposure to both threat and deprivation, particularly deprivation in exposure to enriching and cognitively complex environments. Because the degree of threat and deprivation exposure associated with poverty is heterogeneous, this could be one reason that the findings with regard to poverty and neural development have been inconsistent across studies.
Below we separately describe deprivation and threat and their distinct impact on neural structure and function. Within each section we first review mechanisms of neural development from animal neuroscience (see Table 1 for a summary of animal paradigms included), describe how neuroimaging and neuropsychological measures in humans may reflect these processes, and review how exposure to deprivation and threat may shape these aspects of neural development in light of evidence from animal studies and emerging human research. Throughout we identify plausible mechanisms through which commonly studied forms of adversity (e.g., maltreatment, institutionalization) may come to affect neural development, leading to our novel model of environmental experience. We end by proposing directions for future research into the impact of adversity on neural development that will confirm or disprove these hypothesized pathways.
Table 1.
Dimensions of deprivation and threat associated with commonly used animal paradigms of early adversity
| Paradigm | Description | Primary dimension |
|---|---|---|
| Dark Rearing | Animals that typically develop ocular dominance columns (e.g., cats) are deprived of visual input via rearing in complete darkness. Animals are not deprived of other forms of sensory input (sound, tactile, taste, smell) but are raised without visual input until they are post-pubertal. | Deprivation |
| Individual rearing | Rodents are single-housed after weaning to reduce visual, auditory, and olfactory communication and to prevent physical interactions with littermates housed in separate cages in the same room. | Deprivation |
| Repetitive foot shock1 | Rodents are exposed to a series of aversive foot shocks in a closed chamber. The series of shocks is repeated daily for a number of consecutive days. | Threat |
| Chronic restraint | Rodents are physically restrained for a specified number of hours. Restraint is repeated daily for a number of consecutive days. | Threat |
| Predator odor | Rodents are exposed to a natural predator odor in a closed chamber for a specified number of hours. Exposure is repeated daily for a number of consecutive days. | Threat |
| Minimal bedding | Rodent dam and litter are housed with a minimal amount of nesting and bedding materials for a specified number of days prior to weaning. Minimal bedding is associated with rough handling of and stepping on pups as well as inconsistent and fragmented dam-pup interactions (Raineki et al., 2012). | Threat |
| Chronic maternal separation | Litter is removed from rodent dam and placed in an incubator for a specified number of hours. Separation is repeated daily for a number of consecutive days prior to weaning. | Deprivation and Threat |
Foot shock is the most commonly used stimulus in fear conditioning rodent models, which can also be used as models of early adversity (Raineki et al., 2010; Raineki et al., 2012; Sarro et al., 2013; Sevelinges et al., 2011).
Deprivation
Predictions based on Animal Literature
One of the areas where the impact of experience on neural development has been most clearly documented is in the pruning of synaptic connections during development in the central nervous system. These principals were first examined in studies employing sensory deprivation (Wiesel and Hubel, 1965b). Studies of deprived or anomalous sensory input during development illustrated that one of the primary mechanisms through which early experience shapes neural structure and function is by pruning initially over-produced synaptic connections (Huttenlocher et al., 1982), described more than three decades ago as the selective-elimination hypothesis (Changeux and Danchin, 1976; Petanjek et al., 2011; Purves and Lichtman, 1980). Here we propose that the same mechanisms through which sensory deprivation or anomalous sensory environments shape primary sensory cortex in animals may also be the mechanism through which broader social-cognitive deprivation shapes association cortex in humans. We argue that we can use basic principals of sensory deprivation to make predictions about the way that decreased exposure to cognitive and social stimulation affects neural development. Specifically, we suggest that an early environment without cognitive enrichment will yield a neural structure designed to deal with low complexity environments. We predict that exposure to cognitive and social deprivation in children results in a) age-specific reductions in thickness and volume of association cortex, as measured in vivo using MRI, due in part to early or over-pruning of synaptic connections, lower numbers of synaptic connections, and reduced dendritic branching; and b) reduced performance on tasks that depend on these areas (e.g., complex cognitive tasks). We expect that reductions in cortical thickness should be most pronounced in regions of association cortex that are recruited for processing complex social and cognitive inputs, including prefrontal cortex (PFC), superior and inferior parietal cortex, and superior temporal cortex.
We limit our argument to the development of association cortex simply because association cortex has a prolonged developmental trajectory relative to most areas of primary sensory cortex (Gogtay et al., 2004; Huttenlocher, 1979) and because social and cognitive inputs likely shape areas of cortex involved in complex social and cognitive processing. The term association cortex refers to lateral and medial prefrontal, parietal, and temporal areas of cortex that are not primarily involved in processing sensory stimuli or motoric responding but instead are activated in response stimuli that require cognitive processing (Goldman-Rakic, 1988; Mountcastle et al., 1975). These regions are considered amodal in that they respond to and process stimuli across multiple sensory modalities. While association cortex is likely to be organized along governing principals (Badre and D'Esposito, 2009), these principals continue to be investigated and are thought to be different to the organizational principals of primary sensory cortex (e.g., retinotopy). Generally it is understood that association cortex is necessary for higher-level cognitive processes such as executive function, language, or spatial navigation. We acknowledge that association cortex refers to a large area of cortex, making our predictions relatively non-specific. However, greater specificity in these predictions requires improvements in our measurement of deprivation in both animal and human models, and greater understanding of the specific types of social and cognitive inputs that are required for diverse regions of association cortex to develop normally. As we review below, the hypothesis that exposure to deprivation preferentially affects association cortex is born out in the current data on the association between exposure to environments characterized by deprivation and neural structure and function in humans.
Proliferation and Pruning
Early in the study of neural structure, it was hypothesized that synaptic connections emerged following a genetic blue print (Sperry, 1963). However, the “preformist” theory rapidly gave way to evidence in favor of the selective-elimination hypothesis that emphasized the critical role that environmental inputs played in shaping neural structure (Changeux and Danchin, 1976). Since that time, decades of work have documented that central and peripheral nervous system development contains two distinct phases of synaptic growth, which ultimately shape adult neural structure and function: proliferation and pruning. Synaptic proliferation occurs in a period beginning during the third trimester, peaking about three months after birth, and ending before the second year of life (Huttenlocher and Dabholkar, 1997; Petanjek et al., 2011). During this period, there are rapid increases in the ratio of asymmetrical to symmetrical synapses (an index of newly formed synaptic connections), synaptic density, and total number of synapses (Huttenlocher and de Courten, 1987; Rakic et al., 1986). Following synaptic proliferation, a period of pruning of synaptic connections occurs and continues for an extended period through childhood and adolescence. In humans, this synaptic elimination occurs earlier for primary sensory cortex and later for association cortex, although the final density of synapses in adulthood across areas of cortex is not different (Huttenlocher, 1979; Huttenlocher and Dabholkar, 1997; Huttenlocher and de Courten, 1987).
Pruning of synaptic connections occurs in all six layers of cortex, but occurs primarily for synaptic connections on dendritic spines as compared to other classes of synapses on dendritic shafts or cell somas (Rakic et al., 1986). This is likely a corollary of the finding that presynaptic neurons rely on trophic factors released from their post-synaptic targets, and thus co-activation, for survival (Purves and Lichtman, 1980). That is, as two cells co-activate, the association between the cells strengthens, trophic factors are transmitted, and it becomes more likely that this synaptic connection will survive. In contrast, if a synaptic connection is infrequently activated, this connection becomes weaker over time and is likely to be pruned. Conceptually, the emergent system reflects the relative effectiveness of various pathways, theoretically yielding the most efficient system where only the most effective and environmentally relevant connections remain. Thus, through the interaction of pre and post-synaptic cell interactions, the elimination of synapses during development gives rise to the adult organization of the peripheral and central nervous system.
Visual Deprivation as Early Experience
Much of the early work concerning the effect of experience on neural structure and function comes from investigations of the effect of visual deprivation on visual cortex structure and function. In animals this has been observed through experimental manipulation of visual input during development. In initial studies of monocular deprivation, kittens were deprived of visual input to one eye during development, leading to irreversible changes in ocular dominance columns. In contrast, monocular deprivation in adult cats leads to no such irreversible effects (Wiesel and Hubel, 1965a, b). Importantly, where early monocular deprivation leads to changes in visual cortex organization, complete visual deprivation, or “dark rearing” leads to radical reductions in synapses in primary visual cortex (V1) and irreversible decreases in visual acuity (O’Kusky, 1985a). These and other findings ultimately led to the concept of developmental plasticity: the understanding that early experience has a preferentially permanent impact on neural structure, in particular during specific periods termed sensitive or critical periods when environmental stimuli have a more pronounced impact on neural structure and function (Hensch, 2005; Morishita and Hensch, 2008; O'Kusky, 1985).
In humans, the impact of sensory deprivation on neural development has been studied in individuals with congenital and late-onset blindness. Congenital blindness is associated with increased use of visual cortex to process auditory stimuli and the spatial relationships between auditory stimuli (Voss et al., 2008). In addition, congenitally blind individuals activate these cortical areas during tasks requiring the processing of auditory or tactile stimuli and when performing complex cognitive tasks (Collignon et al., 2013). This pattern of re-organization appears to reflect the fact that congenitally blind individuals may be able to use the inherent organizational structure of extrastriate cortex to process complex perceptual stimuli in other sensory modalities. Moreover, congenitally blind individuals have thinner primary visual cortex compared to sighted or late-blind participants (Collignon et al., 2013; Leporé et al., 2010), indicating that the reduction of inputs into primary visual cortex results in reductions in cortex and thus reduced processing capacity.
Thus it appears in humans and rodents that, (a) reduction in sensory inputs during periods of developmental plasticity leads to thinner cortex in primary sensory areas, due at least in part to increased synaptic pruning; and (b) non-primary sensory areas, such as extra-striate cortex, can be ‘colonized’ by other sensory and cognitive processes when typical inputs are absent. In humans, this appears to result in more diffuse patterns of activation in response to task demands. However, even in the context of cortical ‘colonization,’ cortical thinning occurs within primary sensory areas, indicating that the synaptic connections associated with reduced or absent environmental input may be observable using neuroimaging techniques.
Global Deprivation in Animal Models
A second literature in rodents has investigated the impact of a more general developmental experience, that of global deprivation and enrichment. It has been amply demonstrated in animal models that global deprivation due to single rodent housing results in widespread decreases in dendritic arborization, spines, and overall brain volume (Bennett et al., 1996; Bennett et al., 1974; Diamond et al., 1966; Diamond et al., 1972; Globus et al., 1973). This change in cortical structure has been observed following random assignment to individual housing with decreased visual, auditory, and social inputs for pre-pubertal and peri-pubertal animals (Bennett et al., 1996). Changes in dendritic morphology are marked throughout cortex (Diamond et al., 1975), are stronger and more persistent if the duration of exposure is longer (Bennett et al., 1974), and decline slowly following a transition to a new environment. These changes appear to be at least partially reversible through exposure to enriching, cognitively stimulating environments following deprivation (Diamond et al., 1972). Because the current evidence from rodent models of deprivation and enrichment are not tied specifically to the developmental stage at which exposures occur, it is difficult to know if such exposures would have a larger and less malleable impact if they occurred earlier or if they would have a smaller and more reversible impact if they occurred later.
In sum, evidence from the animal literature indicates that decreases in environmental input within a single modality (e.g., vision) during development can disrupt cortical organization and decrease dendritic arborization and number of synapses within corresponding sensory cortex regions. In animals exposed to multifaceted deprivation, a general lack of stimulation, general decreases in cortical thickness are observed due to decreases in dendritic arborization, neuronal depth, and glia cells. An obvious next step is to determine whether similar patterns of neural outcome are observed in children exposed to cognitive and social deprivation.
Consistency with Evidence from Human Studies
We next review evidence from studies of children exposed to diverse environments that share the characteristic of lacking complexity in cognitive and social inputs. These include institutionalization, low socio-economic status (SES), and neglect, each of which are characterized by deprivation in cognitive inputs (e.g., language), social stimulation, and in the case of institutionalization, the absence of a primary attachment figure (Hart and Risley, 1995; Hildyard and Wolfe, 2002; Smyke et al., 2007). Given observed neural changes following sensory and global deprivation in rodents, it is likely that these forms of cognitive and social deprivation will result in changes in thickness and volume in humans, as measured using MRI. We predict that exposure to these diverse forms of deprivation will be associated with age-specific reductions in cortical thickness as a result of decreases in dendritic arborization, spines, and density. Moreover, we expect that reductions in cortical thickness will be most pronounced in regions of association cortex that are recruited for processing complex social and cognitive inputs, including the PFC, superior parietal cortex, and superior temporal cortex. As shown below, existing evidence supports these predictions. Critically, however, because fine-grained measurement of the dimensions of deprivation and threat have not typically been undertaken in human studies of neurodevelopment and because prior studies have often focused on specific types of exposure (e.g., abuse) without measuring or reporting co-occurring exposures (e.g., neglect), any conclusions regarding the consistency of existing human work with our proposed framework are necessarily tentative.
Institutionalization in early childhood is a well-studied phenomenon involving exposure to profoundly deprived environments in early childhood. This exposure is complex and heterogeneous. However, most children in institutions are clearly deprived of species-expectant early experiences of two types. First, both the ratio of caregivers-to-children and caregiver investment in children are low (McCall et al., 2012; Zeanah et al., 2003). This lack of an early attachment figure, a central feature of early human experience, has been investigated and reviewed extensively by Tottenham and colleagues (Gee et al., 2013; Tottenham, 2012; Tottenham et al., 2010), and as such we do not review these effects in depth. However, briefly, lack of an early attachment figure results in increased susceptibility to anxiety that appear to be mediated by changes in amygdala structure and function (Tottenham, 2012). These findings are consistent with animal studies investigating deprivation of early maternal care (Eiland and McEwen, 2010; Tottenham and Sheridan, 2009).
Second, institutional rearing is associated with decreased cognitive and social inputs of numerous kinds. Children raised in institutions are less likely than children raised in families to be exposed to all forms of language, interactions with adults, variation in daily routines and experiences, novel and age-appropriate enriching cognitive stimuli (e.g., books, toys), opportunities for peer interaction, and a wide range of other types of environmental stimulation (Nelson et al., 2009; Smyke et al., 2007; Zeanah et al., 2003). Likely as a result of this profound cognitive and social deprivation, children raised in institutions are more likely than children raised in families to have deficits in cognitive function (Nelson et al., 2007; O'Connor et al., 2000) and in language production and comprehension (Albers et al., 1997; Windsor et al., 2011). Relatedly, children reared in institutional settings have a wide range of developmental problems including markedly elevated rates of attention-deficit/hyperactivity disorder (ADHD) (Kreppner et al., 2001; Zeanah et al., 2009). Several recent studies document associations between institutionalization and grey matter volume and thickness. In addition to being associated with global changes in cortical function (Chugani et al., 2001; Marshall et al., 2008; McLaughlin et al., 2010), institutionalization is associated with overall decreases in grey matter volume and thickness (McLaughlin et al., 2014; Mehta et al., 2009; Sheridan et al., 2012a), with the most pronounced reductions in areas of association cortex supporting complex cognitive and social processing including the PFC, superior and inferior parietal cortex, and superior temporal cortex (McLaughlin et al., in press). In some areas of cortex these decreases in thickness mediate the association between institutionalization and atypical cognition function (e.g., ADHD symptoms; McLaughlin et al., in press).
Although low parental SES is not as clear-cut or extreme an exposure as institutionalization, it similarly confers risk for less complex cognitive inputs during childhood. Low parental SES is associated with decreased complexity and amount of linguistic inputs (Hart and Risley, 1995), lower exposure to enriching cognitive experiences in the home (Bradley et al., 2001a; Bradley et al., 2001b) and in the school environment (Sirin, 2005), including decreased access to books and extracurricular experiences. Unsurprisingly, given this difference in exposure to complex cognitive stimuli, low SES is associated with decreased performance on complex cognitive tasks, including those tapping executive function and long-term memory (Evans and Schamberg, 2009; Farah et al., 2006; Hackman et al., 2010; Kishiyama et al., 2009; Noble et al., 2007), language ability (Fernald et al., 2013; Weisleder and Fernald, 2013), and overall cognitive and academic achievement (Brooks-Gunn and Duncan, 1997; Duncan et al., 1998; Jokela et al., 2009; Sirin, 2005). These differences in developmental outcomes are mediated by lack of exposure to complex and enriching activities in childhood (Bradley et al., 2001a; Bradley et al., 2001b; Linver et al., 2002; Yeung et al., 2002). In addition to the well-documented associations between low SES and cognitive function, low SES is additionally associated with decreased volume and volume by age in association cortex, particularly the PFC (Noble, Houston, Kan, & Sowell, 2012; Sheridan et al, in prep). Additionally, low SES is associated with increased levels and more diffuse patterns of activation in association cortex to support performance on language and executive functioning tasks in both children (Raizada and Kishiyama, 2010; Raizada et al., 2008; Sheridan et al., 2012b) and adults (Gianaros et al., 2008; Gianaros and Manuck, 2010; Gianaros et al., 2011). Further, in at least one instance the impact of low SES on neural function was explained by lack exposure to complex cognitive experiences, including language (Sheridan et al., 2012b).
Neglect refers to inadequate care on the part of parents for their offspring. This can include a lack of provision for basic needs such as food, shelter, and clothing or a lack of provision for the emotional needs of a child. Because neglect inherently involves a lack of parental care, it constitutes an obvious form of early deprivation. When neglect is directly compared to abuse, children exposed to neglect are at greater risk for cognitive deficits than children exposed to abuse (Hildyard & Wolfe, 2002) and these deficits are similar to those observed in severe poverty and institutionalization (Dubowitz et al., 2002; Spratt et al., 2012), consistent with our conceptualization of neglect as a form of deprivation. Moreover, childhood emotional neglect predicts poor performance on a cognitive control task and a more widespread pattern of dorsolateral PFC activation during trials requiring inhibitory control (Mueller et al., 2010).
Threat
Predictions based on Animal Literature
Evidence from animal and human studies demonstrates consistently that early exposure to threat is associated with long-term changes in neural circuits that underlie emotional learning. Based on this evidence, we argue that early threat exposure impacts the structure, function, and coupling of the hippocampus, amygdala, and ventromedial prefrontal cortex (vmPFC). First, we predict that early threat exposure leads to changes in hippocampal morphology and function, including reduced dendritic spines and arborization and poor function in hippocampus-dependent learning and memory tasks. These predictions are based on extensive evidence that potential threats activate the hypothalamic-pituitary-adrenal (HPA) axis, leading to enhanced expression of corticotropin releasing hormone (CRH) in the hippocampus and damage to hippocampal neurons (Brunson et al., 2001; Ivy et al., 2010).
Second, we argue that early exposure to threat leads to changes in amygdala function. The amygdala detects and processes salient environmental stimuli, particularly stimuli that have emotional significance, such as facial displays of emotion (Adolphs, 2010; Davis and Whalen, 2001; Fitzgerald et al., 2006; Liberzon et al., 2003). Although the amygdala responds to both positive and negative emotional stimuli (Somerville et al., 2004), evidence suggests that it is centrally involved in detection of potential threats (Isenberg et al., 1999; Ohman, 2005) and required for the acquisition and expression of learned fear (Johansen et al., 2011; LeDoux, 2003). We suggest that early threat exposure leads to novel learning, resulting in the pairing of threat cues with previous neutral stimuli, a reduced threshold for experiencing fear, and heightened vigilance to detect other potential threats (van Marle et al., 2009), all of which are adaptive responses to potential danger. Together, these changes result in elevated amygdala activation to emotional stimuli due to the increased salience of such information, and potentially as a result of up-regulation of CRH receptors in the amygdala (Hatalski et al., 1998). Behaviorally, this results in heightened attention to threat-related cues, generalization of learned fear to previously neutral stimuli, and elevated emotional responses to a wide range of emotional cues. For a review of how institutionalization, a specific form of childhood adversity associated with high degrees of both threat and deprivation, influences amygdala development, please see Tottenham (2012).
Finally, we propose that chronic experiences of threat have additional influences on neural systems involved in modulating the amygdala and hippocampus. As a result of fear extinction mechanisms, exposure to consistently safe environments following early threats will result in new learning that inhibits previously acquired fear responses to threatening cues, termed extinction learning. The vmPFC is activated during retrieval of extinction learning and down-regulates the amygdala (Milad and Quirk, 2012; Milad et al., 2007; Quirk et al., 2003; Quirk et al., 2000). The vmPFC is thus essential for retention of extinction learning and inhibition of fear (Phelps et al., 2004; Quirk et al., 2000). However, learned fear continues to be represented in the amygdala and hippocampus, and previously extinguished fear can be re-activated following exposure to situations where fear learning initially occurred or to other threatening contexts (Bouton, 2002; Bouton et al., 2006; Rescorla, 2004). As such, we predict that chronic threat exposure will result in stronger representations of conditioned fear than extinction memories, lowering recruitment of the vmPFC in multiple forms of emotional processing. Over time, this reduced vmPFC recruitment will lead to accelerated pruning, resulting in reduced vmPFC thickness, and poor vmPFC-amygdala coupling (i.e., low structural and functional connectivity between these regions).
To justify our predictions regarding early threat and emotional learning networks, we review evidence from the animal literature on mechanisms underlying fear learning and extinction given substantial existing knowledge of the neural circuitry underlying these mechanisms and the consistency of that circuitry across species. Importantly, some (i.e., effects on hippocampus), but not all (i.e., effects on vmPFC), of the predictions outlined above have previously been articulated within the literature on stress and neural development (Tottenham and Sheridan, 2009). Indeed, the pathways we describe with regard to the hippocampus and amygdala have frequently been invoked as mechanisms through which stress influences the brain. We review this literature nonetheless as we expand upon prior predictions, identify other mechanisms (i.e., fear learning) that might alter hippocampus and amygdala development following threatening or traumatic experiences, and highlight the distinction between neural systems influenced by threat as compared to deprivation.
Fear Learning
Fear is a defensive mechanism that activates behavioral and neurobiological responses to danger that promote survival, including freezing and activation of sympathetic nervous system and HPA axis, generating downstream hormonal and metabolic changes (LeDoux, 2003). An extensive literature in rodents has characterized the neural circuitry that underlies fear learning using Pavlovian fear conditioning tasks (Johansen et al., 2011; Kim and Jung, 2006; LeDoux, 2003). In Pavlovian conditioning a previously innocuous stimulus (conditioned stimulus, CS) is paired with an aversive or threatening unconditioned stimulus (US). After repeated contingent pairings, the CS begins to elicit the behavioral and neurobiological responses associated with the US. Fear conditioning happens without effort, allowing threats to quickly elicit defensive responses that promote safety. In the animal literature, the amygdala and hippocampus contribute differentially to aspects of fear learning. The amygdala is necessary for both the acquisition and expression of conditioned fear in paradigms involving a cued CS (i.e., a simple sensory stimulus, like a tone or light) and a contextual CS (i.e., a more complex polymodal stimulus, such as the place where cue conditioning occurs; (Phillips and LeDoux, 1992). In contrast, the hippocampus is involved only in the acquisition of conditioned fear to complex contextual stimuli (Phillips and LeDoux, 1992). Lesions of the amygdala prevent fear acquisition and expression to both cued and contextual CS, while hippocampal lesions prevent fear acquisition only to contextual CS (Anagnostars et al., 1999; Cousens and Otto, 1998; Hitchcock and Davis, 1986; Phillips and LeDoux, 1994).
Learned fear is not immutable; conditioned fear generally abates with the passage of time as a result of extinction processes. Extensive evidence suggests that fear extinction involves novel learning of an association between the CS and absence of the US, rather than a loss of the initial CS-US association (Quirk, 2002). Because the CS-US pairing remains intact, extinguished fear can be re-activated through a variety of processes, including spontaneous recovery, exposure to novel threats, exposure to the CS in novel contexts, or re-exposure to the US, highlighting the context-dependent nature of extinction learning (Bouton, 2002, 2004; Bouton and King, 1983; Rescorla, 2004). Extinction of conditioned fear initially requires the amygdala, whereas retrieval of extinction memory on subsequent days additionally requires the vmPFC (Falls et al., 1992; Morgan et al., 1993; Quirk et al., 2000), which has direct projections to the amygdala (Hurley et al., 1991). vmPFC activation during recall of extinction memory inhibits the amygdala and dampens fear expression (Knapska and Maren, 2009; Milad and Quirk, 2002). Thus, successful fear extinction requires functional coupling of the vmPFC and amygdala (Quirk et al., 2003).
Effects of Early Threat on Fear Learning Circuits
Exposure to threatening stimuli early in development has consistently been shown to alter the neural circuitry underlying fear conditioning and extinction. Here we focus on paradigms that specifically elicit fear, including repeated foot shock, physical stress (e.g., restraint), and predator odor (see Table 1). We also highlight findings from minimal bedding paradigms that result in erratic, inconsistent maternal care provided to pups (Rice et al., 2008), as well as increases in rough handling of pups by the dam (Raineki et al., 2012; Roth and Sullivan, 2005). In the service of focusing explicitly on models of threat, we do not review paradigms that elicit more complex emotional and neural responses, including early maternal separation (Liu et al., 1997), which involves both high threat as well as high degrees of deprivation resulting from isolation and lack of both cognitive and social inputs during separation.
An extensive literature documents that acute and uncontrollable stressors in adulthood result in reduced dendritic length and branching as well as lower plasticity and long-term potentiation in the hippocampus, and impairments in hippocampus-dependent learning and memory (Kim et al., 2006; McEwen, 1999). Early threat exposure results in similar shifts in adult hippocampal structure and function in studies using a wide range of paradigms. Chronic restraint stress during pre-adolescence is associated with reduced apical dendritic length and branching of pyramidal neurons in the hippocampus and mPFC (Eiland et al., 2012). Dendritic atrophy, reduced long-term potentiation in the hippocampus, and deficits in hippocampusdependent learning and memory have also been observed in adult rats following early exposure to minimal bedding (Brunson et al., 2005; Ivy et al., 2010; Rice et al., 2008). Notably, some evidence suggests that the effects of early threat exposure on hippocampal morphology and cellular function do not emerge until adulthood (Isgor et al., 2004; Tsoory et al., 2008) and the effects of threat on hippocampal function appear to be larger when exposure occurs in childhood as compared to adulthood (Chen et al., 2006). Early threat exposure also appears to influence hippocampus-dependent aspects of fear conditioning. For example, in multiple studies, early exposure to repeated foot shock stress predicts attenuated extinction of fear-related freezing behavior during exposure to a contextual CS in adulthood, but no differences in the response to initial conditioning (Ishikawa et al., 2012; Matsumoto et al., 2008). Similarly, impaired extinction recall of context-dependent fear extinction following early threat results from disruptions in signaling in the vmPFC-hippocampus circuit, including poor synaptic transmission between these regions (Toledo-Rodriguez and Sandi, 2007). Multiple studies have found that the effects of early threat experiences on hippocampal development are mediated by excess levels of CRH and activation of CRH receptors in the hippocampus (Brunson et al., 2001; Ivy et al., 2010). These effects have sometimes been found to vary by sex. For example, exposure to predator odor followed by placement on an elevated platform in pre-adolescence is associated with impaired contextual learning in females during adolescence, as evidenced by reduced freezing to a contextual CS. In contrast, males exhibit enhanced fear conditioning to a cued CS in adolescence and reduced extinction to the cued CS in adulthood following this exposure (Toledo-Rodriguez and Sandi, 2007).
Early exposure to threatening stimuli also leads to long-term changes in the structure and function of the amygdala. In paradigms involving uncontrollable shock delivered to pups, early threat exposure is associated with persistent anxiety and depression-like behaviors, absence of paired-pulse inhibition in the amygdala—reflecting deficits in inhibitory pathways regulating amygdala activity, and widespread changes in gene expression in the amygdala, particularly in genes that regulate serotonin and GABA (Sarro et al., in press; Sevelinges et al., 2011). In addition, chronic threat exposure leads to increased expression of CRH mRNA in the amygdala, potentially lowering the threshold for the expression of fear (Hatalski et al., 1998). Pre-adolescent chronic restraint stress is associated with atypical dendritic morphology, including increased spines, in the amygdala (Eiland et al., 2012). Similar effects on the amygdala have been observed in rodents exposed to the maternal minimal bedding paradigm. These include persistent elevations in c-Fos expression in the amygdala that increase with development (Cohen et al., 2013) and elevated amygdala activity to a forced swim test in adolescence that mediates depression-like responses to the stressor (Raineki et al., 2012).
Taken together, the rodent literature suggests that early exposure to uncontrollable threat results in long-term changes in hippocampal and amygdala structure and function as well as deficits in inhibitory control of these regions by the mPFC. Specifically, early threat predicts, a) reduced dendritic length and arborization in the hippocampus in adulthood, b) dampened long-term potentiation in the hippocampus, d) poor performance on hippocampus-dependent learning and memory tasks, e) increased dendritic length in the amygdala; f) elevations in basal amygdala activity as well as amygdala response to novelty and stress, g) increased anxiety and depression-like behaviors mediated by amygdala over-activity; and h) deficits in the functional coupling of the mPFC with the hippocampus and amygdala, as evidenced by reduced synaptic transmission and poor recall of extinction learning.
Consistency with Human Studies
The learning mechanisms and neural circuitry underlying fear conditioning and extinction are highly conserved across species. As in the animal model, amygdala activation is associated with fear acquisition and expression during conditioning (LaBar et al., 1998; Phelps et al., 2004). Likewise, the vmPFC is activated during extinction recall (Milad et al., 2007; Phelps et al., 2004), and increased vmPFC activity during such recall is associated with dampened amygdala activity (Milad et al., 2009; Milad and Quirk, 2012; Milad et al., 2007). The vmPFC is thus essential for retention of extinction learning and plays a central role in modulating the amygdala. Because acquiring neuroimaging data requires participants to be in a highly salient and novel context, the role of the hippocampus in contextual fear conditioning has been difficult to study in humans.
Despite the consistency with which fear learning mechanisms have been specified across animal and human models, there is a surprising lack of human research on how exposure to early experiences of threat influences fear conditioning across development. Although numerous studies have examined fear conditioning processes in adults with post-traumatic stress disorder (Orr et al., 2000; Peri et al., 2000), research on early threat exposure and fear learning in youths or adults is absent in the current literature. One of the challenges is developing paradigms that can be used ethically in children and adolescents, given that effective adult fear conditioning paradigms utilize shock as the US (Pine et al., 2001). A recently-developed task pairing emotional faces with a human scream as the US holds promise in this regard (Lau et al., 2011), but has yet to be applied to the study of early threat exposure. We review existing human studies examining associations between early threat and structure and function of the hippocampus, amygdala, and vmPFC, although we note that direct comparisons between animal and humans studies should be made with caution given the lack of studies examining early threat and fear learning in humans.
Based on the animal literature, we predict that early threat exposure leads to parallel changes in the structure and function of the hippocampus, amygdala, and vmPFC observed in animals. We review evidence for these predictions from studies of children exposed to threatening environments, including physical and sexual abuse, domestic violence, and other types of interpersonal violence. These environments share the characteristic of being significant threats to survival and therefore activate the neural circuitry underlying fear learning. Importantly, as noted above, because of the high co-occurrence of threat and deprivation exposure and because few studies have examined both dimensions within the same sample of children, the specificity of these effects remains to be confirmed in future studies.
Although early threat exposure has consistently been associated with reduced hippocampal volume in adults (Andersen and Teicher, 2008; Hart and Rubia, 2012; Teicher et al., 2012), studies of children have generally not found a relationship between threat exposure and hippocampal volume (De Bellis et al., 2001; Woon and Hedges, 2008). This pattern has led some to suggest that the effects of early threat on hippocampal development are not evident until adulthood (Tottenham and Sheridan, 2009), consistent with evidence from animal studies (Isgor et al., 2004), although the exact mechanisms explaining this developmental pattern remain to be identified. Atypical hippocampal function associated with early threat has been observed, however. For example, children with maltreatment-related PTSD symptoms exhibit less hippocampal activation during retrieval in a verbal declarative memory task than non-maltreated children (Carrion et al., 2010).
Differences in the volume of the amygdala as a function of threat exposure have generally not been found in children (De Bellis et al., 2001). However, atypical processing of emotional information—particularly facial emotion—has been observed consistently. Children exposed to threat exhibit amplified neural response to angry faces in ERP studies (Pollak et al., 1997; Pollak et al., 2001), and heightened amygdala activation to angry faces (McCrory et al., 2011) even when faces are presented pre-attentively (McCrory et al., 2013). These alterations in neural processing of facial emotion are consistent with behavioral findings suggesting that children with early threat exposure identify facial displays of anger more quickly and with less sensory information than non-exposed children (Pollak and Sinha, 2002), suggesting attention biases that facilitate the identification of anger.
Consistent with animal literature demonstrating differences in the development of the vmPFC following early threat, multiple recent studies observe that threat exposure in childhood is associated with reduced volume and/or thickness of the vmPFC (De Brito et al., 2013; Edmiston et al., 2010; Hanson et al., 2010; Kelly et al., 2013), consistent with our prediction that low recruitment of vmPFC will lead to accelerated pruning in this region. In addition, a recent study documents reduced resting-state amygdala-vmPFC connectivity in adolescent females exposed to child abuse (Herringa et al., 2013).
Recommendations for Future Research
The exposures that give rise to experiences of deprivation and threat co-occur at high rates in children and adolescents (Green et al., 2010; McLaughlin et al., 2012). This co-occurrence has generated many of the methodological and conceptual challenges in identifying dimensions of experience that influence specific aspects of neural development. We do not advocate that future research attempt to identify children who experience only one specific form of adversity, as that would surely result in conclusions that are not generalizable to most children exposed to adverse developmental environments. Instead, we propose the following concrete recommendations for future research. First, future studies examining neural development in children exposed to adverse early environments should measure the underlying dimensions of experience described here in addition to the traditional categories of exposure (e.g., physical and sexual abuse, neglect, poverty) to determine whether deprivation and threat are indeed associated with the predicted patterns of neural development proposed here. Dimensional measures of trauma exposure frequency and severity are widely available (Bernstein et al., 2003), and measures of environmental enrichment have been developed for young children (Caldwell and Bradley, 1984). Developing more extensive measures of cognitive and social inputs and environmental complexity that can be used over a wider range of development would facilitate this endeavor.
Relatedly, a second central challenge in characterizing the impact of deprivation on neural development involves determining the specific types of cognitive and social inputs that are required for the brain to develop normally. Characterizing the inputs necessary to facilitate sensory development is relatively straightforward, but this is far more challenging in the domain of more complex cognitive and social skills (e.g., executive functioning) and the cortical regions that support these types of functions, perhaps with the exception of language development (Kuhl, 2004). Determining the specific environmental inputs that are necessary to scaffold the development of these skills is critical to understand how deprivation in exposure to these types of experiences shapes brain development.
Third, experimental manipulation of specific aspects of experience would allow our predictions about deprivation and neural development to be tested in a more rigorous way. For example, a variety of experimental improvements in environmental context for children who are institutionalized would shed light on the veracity of our model. Ideally, all children could be removed from institutions and placed into family care; however, in societies where that is currently impossible, providing enhanced complexity of care could be advantageous. Potential environmental manipulations include increasing enriching cognitive experiences (e.g., greater access to complex toys and games, greater exposure to complex language, and more opportunities for adult instruction), reducing caregiver-to-child ratios, and staff schedules that ensure consistent caregiving of particular children by the same adults over time to facilitate the formation of more selective attachments as well as the provision of greater opportunities for cognitive enrichment. Such an approach was used by the St. Petersburg-USA Orphanage Research Team, which attempted to improve the institutional environment by reducing caregiver-to-child ratios and providing more consistent and responsive caregiving (The St. Petersburg-USA Orphanage Research Team, 2008). Although this intervention resulted in improvements in general cognitive ability, the effects on neural development are unknown. Collection of neuropsychological and neuroimaging data following this type of experimental design is an important next step in identifying causal pathways through which the absence of species-typical cognitive and social experience shapes neurodevelopment.
Fourth, despite consistencies in the neural circuitry underlying fear learning in animals and humans, there is a surprising lack of human research on how early threat influences fear learning across development. As noted above, only recently have developmentally appropriate and effective fear learning paradigms been developed for children and adolescents (Glenn et al., 2012; Lau et al., 2011). Examining the effects of early threat exposure on fear acquisition, expression, extinction, and generalization as well as the underlying neural circuitry supporting these learning processes represents a critical area for future research.
Fifth, the issue of developmental timing of exposure and outcome measurement is of central importance to studying the impact of environmental experience on neural development (Hensch, 2005). Although sensitive periods in sensory and language development have been clearly identified, progress in identifying similar periods when the brain is particularly likely to be influenced by more complex cognitive and social environmental inputs has proved challenging due to measurement issues with regard to exposure timing as well as the more complicated neural circuitry that underlies higher-order cognition. We have learned the most about sensitive periods in cognitive and social development from studies of institutionalization, where precise information about the timing and duration of exposure is often available (McLaughlin et al., 2011; Nelson et al., 2007). Consistent with the above recommendations about the importance of experimental manipulation, random assignment to improved environments for children exposed to high deprivation or threat may hold the most promise for identifying how the impact of these environments varies according to timing and duration of exposure. An additional possibility rarely used in the human literature involves examining the differential impact of exposures occurring in childhood versus adulthood, which has frequently been used in animal models (Chen et al., 2006). Such an approach will determine whether early exposure to deprivation and threat influences the brain in ways that are either qualitatively or quantitatively different than adult exposure.
Sixth, longitudinal studies are needed to determine whether disruptions in typical patterns of neural development following deprivation and threat are, indeed, mechanisms linking these experiences to the onset of psychopathology. Given evidence for greater fear expression during conditioning paradigms, deficits in extinction learning, and disruptions in the neural circuitry underlying fear learning and extinction among both children and adults with anxiety disorders (Britton et al., 2013; Craske et al., 2008; Lau et al., 2008; Liberman et al., 2006), these pathways are likely involved in the association between threat exposure and anxiety pathology. The neural changes we argue to be a result of deprivation exposure—including age-specific reductions in cortical thickness, poor performance on complex cognitive tasks, and inefficient neural recruitment during such tasks—have also been linked to externalizing disorders in numerous studies (Anderson et al., 1999; Durston et al., 2003; Shaw et al., 2006). Moreover, age-specific cortical thinning in association cortex has been shown to link severe early-life deprivation to ADHD (McLaughlin et al., 2014). However, additional studies are needed to confirm these predictions.
Seventh, most adverse developmental environments are likely to include some degree of exposure to both deprivation and threat dimensions (Figure 1). As such, it is important to consider how neural consequences related to deprivation interact with those related to threat in shaping neural development. For example, the pattern of cortical thinning in lateral PFC and related deficits in inhibition that has been associated with exposure to deprivation (Farah et al., 2006; McLaughlin et al., 2014; Mueller et al., 2010; Noble et al., 2007) might interact with the heightened amygdala and emotional reactivity to emotional stimuli associated with exposure to threat (McCrory et al., 2013; McCrory et al., 2011) to produce deficits not only in automatic aspects of emotion regulation, such as fear extinction, but also in effortful emotion regulation processes, such as cognitive reappraisal. Effortful emotion regulation involves a more complex network including connectivity of the lateral PFC and superior temporal cortex, which alters the semantic representative of emotional stimuli and, in turn, inhibits the amygdala (Buhle et al., in press). The most pronounced deficits in these processes would likely result from exposure to both deprivation and threat dimensions, although this remains to be determined empirically. Understanding how these dimensions interact to influence neural development will be necessary to understand the wide range of negative developmental outcomes stemming from complex adverse experiences.
Finally, we have focused on two dimensions of experience that are particularly likely to impact neural development, but there are undoubtedly others. For example, the degree of environmental predictability has been argued to be central aspect of environmental experience that shapes the development and evolution of human life history strategies (Ellis et al., 2009), and unpredictability or chaos in childhood predicts psychological adjustment and early-onset sexual behavior (Belsky et al., 2012; Evans et al., 2005). Another dimension that is like to have relevance for early neural development is loss of an attachment figure. This is differentiated from the frequently studied exposure of institutional rearing, which involves complete absence of a preferential attachment figure in early life. Loss of such a figure has been consistently linked to a life-course persistent risk for major depression, which could be explained by changes in development of the ventral striatum and reward processing (Wacker et al., 2009). These highlight just two additional dimensions of early life experience that are likely to have meaningful effects on brain development. Future studies should identify of other key dimensions of experience and characterize their impact on the developing brain.
Conclusion
We propose a novel conceptual framework for understanding the impact of childhood adversity on neural development and argue that the field must move beyond the prevailing approach of examining the impact of complex and co-occurring exposures on brain development to distilling those complex experiences into their core underlying dimensions. Two important dimensions that appear to have distinct effects on neural development are deprivation and threat. Existing evidence from human studies provides preliminary support for our predictions about how these types of experiences influence neural development, although additional work is needed to ultimately determine the utility of our conceptual framework. We believe that such an approach will improve our understanding of how atypical experience influences the developing brain and, ultimately, confers risk for psychopathology.
Highlights.
-
-
Novel framework differentiating adverse early experiences of deprivation and threat
-
-
Deprivation, or the absence of expected inputs, impacts proliferation and pruning
-
-
Threat, or the presence of threats to one’s physical integrity, impacts fear learning
-
-
Predictions based on animal literature are supported by evidence from human studies
Acknowledgements
This research was funded by the National Institutes of Mental Health (K01-MH092625 to McLaughlin and K01-MH092555 to Sheridan).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial disclosures or conflicts of interest to report.
References
- Adolphs R. What does the amygdala contribute to social cognition. Annals of the New York Academy of Sciences. 2010;119:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers LH, Johnson DE, Hostetter MK, Iverson S, Miller LC. Health of children adopted from the former Soviet Union and Eastern Europe. Comparison with preadoptive medical records. JAMA. 1997;278:922–924. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th Edition (DSM-5) Washington, DC: American Psychiatric Press; 2013. [Google Scholar]
- Anagnostars SG, Maren S, Fanselow MS. Temporally graded retrograde amnesia of contextual fear after hippocampal damage in rats: within-subjects examination. Journal of Neuroscience. 1999;19:1106–1114. doi: 10.1523/JNEUROSCI.19-03-01106.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Felitti VJ, Bremmer JD, Walker JD, Whitfield CL, Perry BD, Dube SR, Giles WH. The enduring effect of abuse and related adverse experiences in childhood: A convergence of evidence from neurobiology and epidemiology. European Archives of Psychiatry and Clinical Neuroscience. 2006;256:174–186. doi: 10.1007/s00406-005-0624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends in Neuroscience. 2008;31:183–191. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Bechara A, Damasio H, Tranel D, Damasio AR. Impairment of social and moral behavior related to early damage in human prefrontal cortex. Nature Neuroscience. 1999;2:1032–1037. doi: 10.1038/14833. [DOI] [PubMed] [Google Scholar]
- Arata CM, Langhinrichsen-Roling J, Bowers D, O'Brien N. Differential correlates of multi-type maltreatment among urban youth. Child Abuse and Neglect. 2007;31:393–415. doi: 10.1016/j.chiabu.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Badre D, D'Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat. Rev. Neurosci. 2009;10:659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J, Schlomer GL, Ellis BJ. Beyond cumulative risk: Distinguishing harshness and unpredictability as determinants of parenting and early life history strategy. Developmental Psychology. 2012;48:662–673. doi: 10.1037/a0024454. [DOI] [PubMed] [Google Scholar]
- Bennett EL, Diamond MC, Krech D, Rosenzweig MR. Chemical and anatomical plasticity of brain. 1996. J Neuropsychiatry Clin Neurosci. 1964;8:459–470. doi: 10.1176/jnp.8.4.459. [DOI] [PubMed] [Google Scholar]
- Bennett EL, Rosenzweig MR, Diamond MC, Morimoto H, Hebert M. Effects of successive environments on brain measures. Physiol. Behav. 1974;12:621–631. doi: 10.1016/0031-9384(74)90212-1. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Stein JA, Newcomb MD, Walker E, Pogge D, Ahluvalia T, Stokes J, Handelsman L, Medrano M, Desmond D, Zule W. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse and Neglect. 2003;27:169–190. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, ambiguity, and unlearning: Sources of relapse after behavioral extinction. Biological Psychiatry. 2002;52:976–986. doi: 10.1016/s0006-3223(02)01546-9. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context and behavioral processes in extinction. Learning and Memory. 2004;11:485–494. doi: 10.1101/lm.78804. [DOI] [PubMed] [Google Scholar]
- Bouton ME, King DA. Contextual control of the extinctino of conditioned fear: tests for the associative value of the context. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9:248–265. [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: Behavioral and biological mechanisms. Biological Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Convyn RF, Burchinal M, McAdoo HP, Coll CG. The home environments of children in the United States part II: relations with behavioral development through age thirteen. Child development. 2001a;72:1868–1886. doi: 10.1111/1467-8624.t01-1-00383. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF, McAdoo HP, Coll CG. The home environments of children in the United States part I: variations by age, ethnicity, and poverty status. Child development. 2001b;72:1844–1867. doi: 10.1111/1467-8624.t01-1-00382. [DOI] [PubMed] [Google Scholar]
- Britton JC, Grillon C, Lissek S, Norcross M, Szuhany KL, Chen G, Ernst M, Nelson EE, Leibenluft E, Shechner T, Pine DS. Response to learned threat: An fMRI study in adolescent and adult anxiety. American Journal of Psychiatry. 2013 doi: 10.1176/appi.ajp.2013.12050651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J, Duncan GJ. The effects of poverty on children. The Future of Children. 1997;7:55–71. [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotrophin-releasing hormone reproduce the effects of early-life stress. Proceedings of the National Academy of Sciences. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunson KL, Kramar E, Lin B, Chen Y, Colgin LL, Yanagihara TK, Lynch G, Baram TZ. Mechanisms of late-onset cognitive decline after early-life stress. Journal of Neuroscience. 2005;25:9328–9338. doi: 10.1523/JNEUROSCI.2281-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle J, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cerebral Cortex. doi: 10.1093/cercor/bht154. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, Fox ME, Hayes AS, Kalin NH, Essex MJ, Davidson RJ, Birn RM. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience. 2012;15:1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell BM, Bradley RH. Home observation for measurement of the environment. University of Arizona at Little Rock, Little Rock. 1984 [Google Scholar]
- Carrion VG, Haas BW, Garrett A, Song S, Reiss AL. Reduced hippocampal activity in youth with posttraumatic stress symptoms: An fMRI study. Journal of Pediatric Psychology. 2010;35:559–569. doi: 10.1093/jpepsy/jsp112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux J-P, Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264:705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- Chase-Lansdale PL, Cherlin AJ, Kiernan KE. The long-term effects of parental divorce on the mental health of young adults: a developmental perspective. Child Development. 1995;66:1614–1634. [PubMed] [Google Scholar]
- Chen Y, Fenoglio KA, Dube CM, Grigoriadias DE, Baram TZ. Cellular and molecular mechanisms of hippocampal activation by acute stress are age-dependent. Molecular Psychiatry. 2006;11:992–1002. doi: 10.1038/sj.mp.4001863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani HT, Behen ME, Muzik O, Juhász C, Nagy F, Chugani DC. Local brain functional activity following early deprivation: a study of post institutionalized Romanian orphans. NeuroImage. 2001;14:1290–1301. doi: 10.1006/nimg.2001.0917. [DOI] [PubMed] [Google Scholar]
- Cohen MM, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proceedings of the National Academy of Sciences. 2013;110:18274–18278. doi: 10.1073/pnas.1310163110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Brown J, Smailes E. Child abuse and neglect and the development of mental disorders in the general population. Development and Psychopathology. 2001;13:981–999. [PubMed] [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, Paul RH, Sweet L, Tate D, Gunstad J, Stroud LR, McCaffery J, Hitsman B, Niaura R, Clark CR, MacFarlane A, Bryant RA, Gordon E, Williams LM. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological Psychiatry. 2006;59:975–982. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Collignon O, Dormal G, Albouy G, Vandewalle G, Voss P, Phillips C, Lepore F. Impact of blindness onset on the functional organization and the connectivity of the occipital cortex. Brain. 2013;136:2769–2783. doi: 10.1093/brain/awt176. [DOI] [PubMed] [Google Scholar]
- Cousens G, Otto T. Both pre-and post training excitotoxic lesions of the basolateral amygdala abolish the expression of olfactory and contextual fear conditioning. Behavioral Neuroscience. 1998;112:1092–1103. doi: 10.1037//0735-7044.112.5.1092. [DOI] [PubMed] [Google Scholar]
- Craske MG, Waters AM, Bergman RL, Naliboff B, Lipp OV, Negoro H, Ornitz EM. Is aversive learning a marker of risk for anxiety disorders in children? Behaviour Research and Therapy. 2008;46:954–967. doi: 10.1016/j.brat.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot londitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biological Psychiatry. 2001;50:305–309. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- De Brito SA, Viding E, Sebastian CL, Kelly PA, Mechelli A, Maris H, McCrory EJ. Reduced orbitofrontal and temporal gray matter in a community sample of maltreated children. Journal of Child Psychology and Psychiatry. 2013;54:105–112. doi: 10.1111/j.1469-7610.2012.02597.x. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Law F, Rhodes H, Lindner B, Rosenzweig MR, Krech D, Bennett EL. Increases in cortical depth and glia numbers in rats subjected to enriched environment. J. Comp. Neurol. 1966;128:117–126. doi: 10.1002/cne.901280110. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Lindner B, Johnson R, Bennett EL, Rosenzweig MR. Differences in occipital cortical synapses from environmentally enriched, impoverished, and standard colony rats. J. Neurosci. Res. 1975;1:109–119. doi: 10.1002/jnr.490010203. [DOI] [PubMed] [Google Scholar]
- Diamond MC, Rosenzweig MR, Bennett EL, Lindner B, Lyon L. Effects of environmental enrichment and impoverishment on rat cerebral cortex. J. Neurobiol. 1972;3:47–64. doi: 10.1002/neu.480030105. [DOI] [PubMed] [Google Scholar]
- Dong M, Anda RF, Felitti VJ, Dube SR, Williamson DF, Thompson TJ, Loo CM, Giles WH. The interrelatedness of multiple forms of childhood abuse, neglect, and household dysfunction. Child Abuse and Neglect. 2004;28:771–784. doi: 10.1016/j.chiabu.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Chapman DP, Giles WH, Anda RF. Childhood abuse, neglect, and household dysfunction and the risk of illicit drug use: the adverse childhood experiences study. Pediatrics. 2003;111:564–572. doi: 10.1542/peds.111.3.564. [DOI] [PubMed] [Google Scholar]
- Dubowitz H, Papas MA, Black MM, Starr RH., Jr Child neglect: Outcomes in high-risk urban preschoolers. Pediatrics. 2002;109:1100–1107. doi: 10.1542/peds.109.6.1100. [DOI] [PubMed] [Google Scholar]
- Duncan GJ, Yeung WJ, Brooks-Gunn J, Smith JR. How much does childhood poverty affect the life chances of children? American Sociological Review. 1998;63:406–423. [Google Scholar]
- Durston S, Tottenham N, Thomas KM, Davidson MC, Eigsti I-M, Yang Y, Ulug AM, Casey BJ. Differential patterns of striatal activation in young children with and without ADHD. Biological Psychiatry. 2003;53:871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, Blumberg H. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Archives of Pediatrics and Adolescent Medicine. 2010;165:1069–1077. doi: 10.1001/archpediatrics.2011.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: Results from the adverse childhood experiences study. American Journal of Psychiatry. 2003;160:1453–1460. doi: 10.1176/appi.ajp.160.8.1453. [DOI] [PubMed] [Google Scholar]
- Eiland L, McEwen BS. Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus. 2010 doi: 10.1002/hipo.20862. [DOI] [PubMed] [Google Scholar]
- Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37:39–47. doi: 10.1016/j.psyneuen.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Figueredo AJ, Brumbach BH, Schlomer GL. Fundamental dimensions of environmental risk: The impact of harsh versus unpredictable environments on the evolution and development of life history strategies. Human Nature. 2009;20:204–268. doi: 10.1007/s12110-009-9063-7. [DOI] [PubMed] [Google Scholar]
- Evans GW, Gonnella C, Mareynyszyn LA, Gentile L, Salpekar N. The role of chaos in poverty and children's socioemotional adjustment. Psychological Science. 2005;16:560–565. doi: 10.1111/j.0956-7976.2005.01575.x. [DOI] [PubMed] [Google Scholar]
- Evans GW, Schamberg MA. Childhood poverty, chronic stress, and adult working memory. Proc. Natl. Acad. Sci. U.S.A. 2009;106:6545–6549. doi: 10.1073/pnas.0811910106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJD, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. Journal of Neuroscience. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ, Shera DM, Savage JH, Betancourt L, Gianetta JM, Brodsky NL, Malmud EK, Hurt H. Childhood poverty: Specific associations with neurocognitive development. Brain Research. 2006;1110:166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Fernald A, Marchman VA, Weisleder A. SES differences in language processing skill and vocabulary are evident at 18 months. Developmental science. 2013;16:234–248. doi: 10.1111/desc.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelhor D, Ormrod R, Turner H. Polyvictimization and trauma in a national longitudinal cohort. Development and Psychopathology. 2007;19:149–166. doi: 10.1017/S0954579407070083. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DA, Angstadt M, Jelsone LM, Nathan PJ, Phan KL. Beyond threat: Amygdala reactivity across multiple expressions of facial affect. Neuroimage. 2006;30:1441–1448. doi: 10.1016/j.neuroimage.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Fristad MA, Jedel R, Weller RA, WEller EB. Psychosocial functioning in children after the death of a parent. American Journal of Psychiatry. 1993;150:511–513. doi: 10.1176/ajp.150.3.511. [DOI] [PubMed] [Google Scholar]
- Gatt JM, Nemeroff CB, Dobson-Stone C, Paul RH, Bryant RH, Bryant RA, Schofield PR, Gordon E, Kemp AH, Williams LM. Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety Molecular Psychiatry. 2009;14:681–695. doi: 10.1038/mp.2008.143. [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N. Early developmental emergence of human amygdalaprefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences. 2013;110:15638–15643. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Hariri AR, Sheu LK, Manuck SB, Matthews KA, Cohen S. Potential neural embedding of parental social standing. Social Cognitive and Affective Neuroscience. 2008;3:91–96. doi: 10.1093/scan/nsn003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Manuck SB. Neurobiological pathways linking socioeconomic position and health. Psychosomatic medicine. 2010;72:450–461. doi: 10.1097/PSY.0b013e3181e1a23c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Manuck SB, Sheu LK, Kuan DCH, Votruba-Drzal E, Craig AE, Hariri AR. Parental education predicts corticostriatal functionality in adulthood. Cerebral cortex. 2011;21:896–910. doi: 10.1093/cercor/bhq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn CR, Klein DN, Lissek S, Britton JC, Pine DS, Hajcak G. The development of fear learning and generalization in 8–13 year-olds. Developmental Psychobiology. 2012;54:675–684. doi: 10.1002/dev.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Globus A, Rosenzweig MR, Bennett EL, Diamond MC. Effects of differential experience on dendritic spine counts in rat cerebral cortex. J Comp Physiol Psychol. 1973;82:175–181. doi: 10.1037/h0033910. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd J, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen L, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: Parallel distributed networks in primate association cortex. Annual Review of Neuroscience. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Gray JD, Rubin TG, Hunter RG, McEwen BS. Hippocampal gene expression changes underlying stress sensitization and recovery. Molecular Psychiatry. 2013 doi: 10.1038/mp.2013.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JG, McLaughlin KA, Berglund P, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC. Childhood adversities and adult psychopathology in the National Comorbidity Survey Replication (NCS-R) I: Associations with first onset of DSM-IV disorders. Archives of General Psychiatry. 2010;62:113–123. doi: 10.1001/archgenpsychiatry.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnar MR, Quevedo K. The neurobiology of stress and development. Annual Review of Psychology. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends in Cognitive Sciences. 2009;13:65–73. doi: 10.1016/j.tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ, Meaney MJ. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nat. Rev. Neurosci. 2010;11:651–659. doi: 10.1038/nrn2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson JRT, Pollak SD. Early stress is associated with alterations in the orbitofrontal cortex: A tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience. 2010;30:7466–7472. doi: 10.1523/JNEUROSCI.0859-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B, Risley TR. Meaningful differences in the everyday experiences of young American children. Baltimore: Paul H Brooks Publishing; 1995. [Google Scholar]
- Hart H, Rubia K. Neuroimaging of child abuse: a critical review. Frontiers in Human Neuroscience. 2012;6 doi: 10.3389/fnhum.2012.00052. Article 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatalski CG, Guirguis C, Baram TZ. Corticotropin releasing factor mRNA expression in the hypothalamic paraventricular nucleus and the central nucleus of the amygdala is modulated by repeated acute stress in the immature rat. Journal of Neuroendocrinology. 1998;10:663–669. doi: 10.1046/j.1365-2826.1998.00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch T. Critical period plasticity in local cortical circuits. Nature Reviews Neuroscience. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Herringa RJ, Birn RM, Ruttle PL, Stodola DE, Davidson RJ, Essex MJ. Childhood maltreatment is associated with altered fear circuitry and increased internalizing symptoms by late adolescence. Proceedings of the National Academy of Sciences. 2013;110:19119–19124. doi: 10.1073/pnas.1310766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildyard KL, Wolfe DA. Child neglect: developmental issues and outcomes. Child Abuse and Neglect. 2002;26:679–695. doi: 10.1016/s0145-2134(02)00341-1. [DOI] [PubMed] [Google Scholar]
- Hitchcock JM, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behavioral Neuroscience. 1986;100:11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. Journal of Computational Neurology. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain research. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, Dabholkar AS. Regional differences in symaptogenesis in human cerebral cortex. Journal of Comprehensive Neurology. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C. The development of synapses in striate cortex of man. Hum Neurobiol. 1987;6:1–9. [PubMed] [Google Scholar]
- Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H. Synaptogenesis in human visual cortex — evidence for synapse elimination during normal development. Neuroscience Letters. 1982;33:247–252. doi: 10.1016/0304-3940(82)90379-2. [DOI] [PubMed] [Google Scholar]
- Isenberg N, Silbersweig D, Engelien A, Emmerich S, Malavade K, Beattlie B, Leon AC, Stern E. Linguistic threat activates the human amygdala. Proceedings of the National Academy of Sciences. 1999;96:10456–10459. doi: 10.1073/pnas.96.18.10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period of hippocampal morphology and on cognitive and stress axis function in rats. Hippocampus. 2004;14:636–648. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Saito Y, Yanagawa Y, Otani S, Hiraide S, Shimamura K, Matsumoto M, Togashi H. Early postnatal stress alters extracellular signal-regulated kinase signaling in the corticolimbid system modulating emotional circuitry in adult rats. European Journal of Neuroscience. 2012;35:135–145. doi: 10.1111/j.1460-9568.2011.07921.x. [DOI] [PubMed] [Google Scholar]
- Ivy AS, Rex CS, Chen Y, Dube C, Maras PM, Grigoriadis DE, Gall CM, Lynch G, Baram TZ. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. Journal of Neuroscience. 2010;30:13005–13015. doi: 10.1523/JNEUROSCI.1784-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokela M, Elovainio M, Singh-Manoux A, Kivimäki M. IQ, socioeconomic status, and early death: The US National Longitudinal Survey of Youth. Psychosomatic Medicine. 2009;71:322–328. doi: 10.1097/PSY.0b013e31819b69f6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PA, Viding E, Wallace GL, Schaer M, De Brito SA, Robustelli B, McCrory EJ. Cortical thickness, surface area, and gyrification abnormalities in children exposed to maltreatment: Neural markers of vulnerability? Biological Psychiatry. 2013;74:845–852. doi: 10.1016/j.biopsych.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychological Medicine. 1997;27:1101–1119. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Jung MW. Neural circuits and mechanisms involved in Pavlovian fear conditioning: A critical review. Neuroscience and Biobehavioral Reviews. 2006;30:188–202. doi: 10.1016/j.neubiorev.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Song EY, Kosten TA. Stress effects in the hippocampus: Synaptic plasticity and memory. Stress. 2006;9:1–11. doi: 10.1080/10253890600678004. [DOI] [PubMed] [Google Scholar]
- Kishiyama MM, Boyce WT, Jimenez AM, Perry LM, Knight RT. Socioeconomic disparities affect prefrontal function in children. Journal of Cognitive Neuroscience. 2009;21:1106–1115. doi: 10.1162/jocn.2009.21101. [DOI] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learning and Memory. 2009;16:486–493. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreppner JM, O'Connor TG, Rutter M, Beckett C, Castle J, Croft C. Can inattention/overactivity be an institutional deprivation syndrome? Journal of Abnormal Child Psychology. 2001;29:513–528. doi: 10.1023/a:1012229209190. [DOI] [PubMed] [Google Scholar]
- Kuhl PK. Early language acquisition: cracking the speech code. Nature Reviews Neuroscience. 2004;5:831–843. doi: 10.1038/nrn1533. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lau JY, Britton JC, Nelson EE, Angold A, Ernst M, Goldwin M, Grillon C, Leibenluft E, Lissek S, Norcross M, Shiffrin ND, Pine DS. Distinct neural signatures of threat learning in adolescents and adults. Proceedings of the National Academy of Sciences. 2011;108:4500–4505. doi: 10.1073/pnas.1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Lissek SF, Nelson EE, Lee Y, Roberson-Nay R, Poeth K, Jenness J, Ernst M, Grillon C, Pine DS. Fear conditioning in adolescents with anxiety disorders: Results from a novel experimental paradigm. Journal of the American Academy of Child & Adolescent Psychiatry. 2008;47:94–102. doi: 10.1097/chi.0b01e31815a5f01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology. 2003;23:727–737. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leporé N, Voss P, Lepore F, Chou Y-Y, Fortin M, Gougoux F, Lee AD, Brun C, Lassonde M, Madsen SK, Toga AW, Thompson PM. Brain structure changes visualized in early-and late-onset blind subjects. NeuroImage. 2010;49:134–140. doi: 10.1016/j.neuroimage.2009.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman LC, Lipp OV, Spence SH, March S. Evidence for retarded extinction of aversive learning in anxious children. Behaviour Research and Therapy. 2006;44:1491–1502. doi: 10.1016/j.brat.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Liberzon I, Phan KL, Decker LR, Taylor SF. Extended amygdala and emotional salience: A PET activation study of positive and negative affect. Neuropsychopharmacology. 2003;28:726–733. doi: 10.1038/sj.npp.1300113. [DOI] [PubMed] [Google Scholar]
- Linver MR, Brooks-Gunn J, Kohen DE. Family processes as pathways from income to young children's development. Developmental psychology. 2002;38:719–734. [PubMed] [Google Scholar]
- Liston C, Cichon JM, Jeanneteau F, Jia Z, Chao MV, Gan WB. Circadian glucocorticoid oscillations promote learning-dependent synapse formation and maintenance. Nature Neuroscience. 2013;16:698–705. doi: 10.1038/nn.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, Sharma S, Pearson D, Plotsky PM, Meaney MJ. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Marshall PJ, Reeb BC, Fox NA, Nelson CA, Zeanah CH. Effects of early intervention on EEG power and coherence in previously institutionalized children in Romania. Development and Psychopathology. 2008;20:861–880. doi: 10.1017/S0954579408000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Togashi H, Konno K, Koseki H, Hirata R, Izumi T, Yamaguchi T, Yoshioka M. Early postnatal stress alters the extinction of context-dependent conditioned fear in adult rats. Pharmacology, Biochemistry and Behavior. 2008;89:247–252. doi: 10.1016/j.pbb.2007.12.017. [DOI] [PubMed] [Google Scholar]
- McCall R, Van Ijzendoorn MH, Juffer H, Groark CJ, Groza VK. Children Without Permanent Parents: Research, Practice, and Policy. 1 ed. Wiley-Blackwell; 2012. [Google Scholar]
- McCrory EJ, De Brito SA, Kelly PA, Bird G, Sebastian CL, Mechelli A, Samuel S, Viding E. Amygdala activation in matlreated children during pre-attentive emotional processing. British Journal of Psychiatry. 2013;202:269–276. doi: 10.1192/bjp.bp.112.116624. [DOI] [PubMed] [Google Scholar]
- McCrory EJ, De Brito SA, Sebastian CL, Mechelli A, Bird G, Kelly PA, Viding E. Heightened neural reactivity to threat in child victims of family violence. Current Biology. 2011;21:R947–R948. doi: 10.1016/j.cub.2011.10.015. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. New England Journal of Medicine. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annual Review of Neuroscience. 1999;22:105–122. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress, sex, neural adaptation to a changing environment: mechanisms of neuronal remodeling. Annals of the New York Academy of Sciences. 2010;1204:E38–E59. doi: 10.1111/j.1749-6632.2010.05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Brain on stress: How the social environment gets under the skin. Proceedings of the National Academy of Sciences. 2012;109:17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Fox NA, Zeanah CH, Nelson CA. Adverse rearing environments and neural development in children: The development of frontal EEG asymmetry. Biological Psychiatry. 2011;70:1008–1015. doi: 10.1016/j.biopsych.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Fox NA, Zeanah CH, Sheridan MA, Marshall PJ, Nelson CA. Delayed maturation in brain electrical activity explains the association between early environmental deprivation and symptoms of attention-deficit/hyperactivity disorder (ADHD) Biological Psychiatry. 2010;68:329–336. doi: 10.1016/j.biopsych.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky A, Kessler RC. Childhood adversities and first onset of psychiatric disorders in a national sample of adolescents. Archives of General Psychiatry. 2012;69:1151–1160. doi: 10.1001/archgenpsychiatry.2011.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, Nelson CA. Widespread reductions in cortical thickness following severe early-life deprivation: A neurodevelopmental pathway to ADHD. Biological Psychiatry. 2014;76:629–638. doi: 10.1016/j.biopsych.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta MA, Golembo NI, Nosarti C, Colvert E, Mota A, Williams SC, Rutter M, Sonuga-Barke EJS. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: The English and Romanian Adoptees Study Pilot. Journal of Child Psychology and Psychiatry. 2009;50:943–951. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: Ten years of progress. Annual Review of Psychology. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neuroscience Letters. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Morishita H, Hensch TK. Critical period revisited: impact on vision. Current Opinion in Neurobiology. 2008;18:101–107. doi: 10.1016/j.conb.2008.05.009. [DOI] [PubMed] [Google Scholar]
- Mountcastle VB, Lynch JC, Georgopoulos A, Sakata H, Acuna C. Posterior parietal association cortex of the monkey: command functions for operations within extrapersonal space. Journal of Neurophysiology. 1975;38:871–908. doi: 10.1152/jn.1975.38.4.871. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, Pine DS, Ernst M. Early-life stress is associated with impairment in cognitive control in adolescence: an fMRI study. Neuropsychologia. 2010;48:3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen PE, Martin JM, Anderson J, Romans SE, Herbison GP. Childhood sexual abuse and mental health in adult life. British Journal of Psychiatry. 1993;163:721–732. doi: 10.1192/bjp.163.6.721. [DOI] [PubMed] [Google Scholar]
- Nelson CA, Furtado EA, Fox NA, Zeanah CH. The deprived human brain. American Scientist. 2009;97:222. [Google Scholar]
- Nelson CA, Zeanah CH, Fox NA, Marshall PJ, Smyke AT, Guthrie D. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Developmental science. 2012;15:516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science. 2007;10:464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Rutter M, Beckett C, Keaveney L, Kreppner JM. The effects of global severe privation on cognitive competence: extension and longitudinal follow-up. English and Romanian Adoptees Study Team. Child Development. 2000;71:376–390. doi: 10.1111/1467-8624.00151. [DOI] [PubMed] [Google Scholar]
- O'Kusky JR. Synapse elimination in the developing visual cortex: a morphometric analysis in normal and dark-reared cats. Brain research. 1985;354:81–91. doi: 10.1016/0165-3806(85)90071-9. [DOI] [PubMed] [Google Scholar]
- Ohman A. The role of the amygdala in human fear: Automatic detection of threat. Psychoneuroendocrinology. 2005;10:953–958. doi: 10.1016/j.psyneuen.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. Dev novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology. 2000;109:290–298. [PubMed] [Google Scholar]
- Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biological Psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- Petanjek Z, Judas M, Simic G, Rasin MR, Uylings HB, Rakic PM, Kostovic I. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proceedings of the National Academy of Sciences. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contributions of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Lesions of the dorsal hippocampal formation interfere with background but not foreground contextual fear conditioning. Learning and Memory. 1994;1:34–44. [PubMed] [Google Scholar]
- Pine DS, Fyer A, Grun J, Phelps EA, Szeszko PR, Koda V, Li W, Adrekani B, Maguire EA, Burgess N, Bilder RM. Methods for developmental studies of fear conditioning circuitry. Biological Psychiatry. 2001;50:225–228. doi: 10.1016/s0006-3223(01)01159-3. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Cicchetti D, Klorman R, Brumaghim JT. Cognitive brain event-related potentials and emotion processing in maltreated children. Child Development. 1997;68:773–787. doi: 10.1111/j.1467-8624.1997.tb01961.x. [DOI] [PubMed] [Google Scholar]
- Pollak SD, Klorman R, Thatcher JE, Cicchetti D. P3b reflects maltreated children's reactions to facial displays of emotion. Psychophysiology. 2001;38:267–274. [PubMed] [Google Scholar]
- Pollak SD, Sinha P. Effects of early experience on children's recognition of facial displays of emotion. Development and Psychopathology. 2002;38:784–791. doi: 10.1037//0012-1649.38.5.784. [DOI] [PubMed] [Google Scholar]
- Popoli M, Yan Z, McEwen BS, Sanacora G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature Reviews Neuroscience. 2012;13:22–37. doi: 10.1038/nrn3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D, Lichtman JW. Science. Vol. 210. New York, N.Y: 1980. Elimination of synapses in the developing nervous system; pp. 153–157. [DOI] [PubMed] [Google Scholar]
- Quirk GJ. Memory for extinction of conditioned fear is long-lasting and persists following spontaneous recovery. Learning and Memory. 2002;9:402–407. doi: 10.1101/lm.49602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. Journal of Neuroscience. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. Journal of Neuroscience. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Rincon Cortes M, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: Infant social behavior deficits are followed adolescent depressive-like behaviors mediated by the amygdala. Journal of Neuroscience. 2012;32:7758–7765. doi: 10.1523/JNEUROSCI.5843-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada RD, Kishiyama MMS. Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to levelling the playing field. Frontiers in Human Neuroscience. 2010;4 doi: 10.3389/neuro.09.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raizada RD, Richards TLS, Meltzoff A, Kuhl PK. Socioeconomic status predicts hemispheric specialisation of the left inferior frontal gyrus in young children. NeuroImage. 2008;40:1392–1401. doi: 10.1016/j.neuroimage.2008.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Science. Vol. 232. New York, N.Y: 1986. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex; pp. 232–235. [DOI] [PubMed] [Google Scholar]
- Rao H, Betancourt L, Giannetta J, Brodsky N, Korczykowski M, Avants B, Gee J, Wang J, Hurt H, Detre J, Farah M. Early parental care is important for hippocampal maturation: Evidence from brain morphology in humans. Neuroimage. 2011;49(1):1144–1150. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla L. Spontaneous recovery. Learning and Memory. 2004;11:501–509. doi: 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- Rice CJ, Sandman CA, Lenjavi MR, Baram TZ. A novel mouse model for acute and longlasting consequences of early life stress. Endocrinology. 2008;149:4892–4900. doi: 10.1210/en.2008-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: Neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biological Psychiatry. 2005;57:823–831. doi: 10.1016/j.biopsych.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Sarro EC, Sullivan RM, Barr G. Unpredictable neonatal stress enhances adult anxiety and alters amygdala gene expression related to serotonin and GABA. Neuroscience. doi: 10.1016/j.neuroscience.2013.10.064. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevelinges Y, Mouly A-M, Raineki C, Moriceau S, Forest C, Sullivan RM. Adult depression-like behavior, amygdala and olfactory cortex functions are restored by odor previously paired with shock during infancy. Developmental Cognitive Neuroscience. 2011;1:77–87. doi: 10.1016/j.dcn.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lerch JP, Greenstein D, Sharp W, Clasen L, Evans A, Giedd J, Castellanos X, Rapoport JL. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA. Variation in neural development as a result of exposure to institutionalization early in childhood. Proceedings of the National Academy of Sciences. 2012a;109:12927–12932. doi: 10.1073/pnas.1200041109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Sarsour K, Jutte D, D'Esposito M, Boyce WT. The impact of social disparity on prefrontal function in childhood. PloS One. 2012b;7:e35744. doi: 10.1371/journal.pone.0035744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan M, Coates M, Zhang K, Kiawachi I, Petersen SE, Schlaggar BL. Variation in typical development of neural structure associated with socioeconomic status. (in prep) [Google Scholar]
- Sirin SR. Socioeconomic Status and Academic Achievement: A Meta-Analytic Review of Research. REVIEW OF EDUCATIONAL RESEARCH. 2005;75:417–453. [Google Scholar]
- Smyke AT, Koga S, Johnson DE, Fox NA, Marshall PJ, Nelson CA, Zeanah CH the BEIP Core Group. The caregiving context in institution-reared and family-reared infants and toddlers in Romania. Journal of Child Psychology and Psychiatry. 2007;48:210–218. doi: 10.1111/j.1469-7610.2006.01694.x. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Kim HK, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: correlations with state anxiety. Biological Psychiatry. 2004;55:897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc. Natl. Acad. SciU.SA. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt EG, Friedenberg SL, Swenson CC, Larosa A, De Bellis MD, Macias MM, Summer AP, Hulsey TC, Runyan DK, Brady KT. The Effects of Early Neglect on Cognitive, Language, and Behavioral Functioning in Childhood. Psychology. 2012;3:175–182. doi: 10.4236/psych.2012.32026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proceedings of the National Academy of Sciences. 2012;109:E563–E572. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The St. Petersburg-USA Orphanage Research Team. The effects of early social-emotional and relationship experience on the development of young orphanage children. Monographs for the Society for Research in Child Development. 2008;73 doi: 10.1111/j.1540-5834.2008.00483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Rodriguez M, Sandi C. Stress before puberty exerts a sex-and age-related impact on auditory and contextual fear conditioning in the rat. Neural Plasticity 2007, Article ID 71203. 2007 doi: 10.1155/2007/71203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N. Human amygdala development in the absence of species-typical expected caregiving. Developmental Psychobiology. 2012;54:598–611. doi: 10.1002/dev.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Hare T, Quinn BT, McCarry TW, Nurse M, Gilhooly T, Milner A, Galvan A, Davidson MC, Eigsti I-M, Thomas KM, Freed P, Booma ES, Gunnar MR, Altemus M, Aronson J, Casey BJ. Prolonged institutional rearing is associated with atypically larger amygdala volume and difficulties in emotion regulation. Developmental Science. 2010;13:46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Sheridan MA. A review of adversity, the amygdala, and the hippocampus: A consideration of developmental timing. Frontiers in Human Neuroscience. 2009;3:68. doi: 10.3389/neuro.09.068.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsoory M, Guterman A, Richter-Levin G. Exposure to strssor during juvenility disuprts development-related alterations in the PSA-NCAM to NCAM expression ratio: Potention relevance for mood and anxiety disorders. Neuropsychopharmacology. 2008;33:378–393. doi: 10.1038/sj.npp.1301397. [DOI] [PubMed] [Google Scholar]
- van Marle HJF, Hermans EJ, Qin S, Fernandez G. From specificity to sensitivity: How acute stress affects amygdala processing of biologically salient stimuli. Biological Psychiatry. 2009;66:649–655. doi: 10.1016/j.biopsych.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Voss P, Gougoux F, Zatorre RJ, Lassonde M, Lepore F. Differential occipital responses in early-and late-blind individuals during a sound-source discrimination task. NeuroImage. 2008;40:746–758. doi: 10.1016/j.neuroimage.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Wacker J, Dillon DG, Pizzagalli DA. The role of nucleus accumbens and rostral anterior cingulate cortex in anhedonia: Integration of resting EEG, fMRI, and volumetric techniques. Neuroimage. 2009;46:327–337. doi: 10.1016/j.neuroimage.2009.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisleder A, Fernald A. Talking to children matters: early language experience strengthens processing and builds vocabulary. Psychological science. 2013;24:2143–2152. doi: 10.1177/0956797613488145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Comparison of the effects of unilateral and bilateral eye closure on cortical unit responses in kittens. Journal of neurophysiology. 1965a;28:1029–1040. doi: 10.1152/jn.1965.28.6.1029. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Extent of recovery from the effects of visual deprivation in kittens. Journal of neurophysiology. 1965b;28:1060–1072. doi: 10.1152/jn.1965.28.6.1060. [DOI] [PubMed] [Google Scholar]
- Windsor J, Benigno JP, Wing CA, Carroll PJ, Koga SF, Nelson CA, Fox NA, Zeanah CH. Effect of foster care on young children's language learning. Child Development. 2011;82:1040–1046. doi: 10.1111/j.1467-8624.2011.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe DA, Sas L, Wekerle C. Factors associated with the development of posttraumatic stress disorder among child victims of sexual abuse. Child Abuse and Neglect. 1994;18:37–50. doi: 10.1016/0145-2134(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Woon EL, Hedges DW. Hippocampal and amygdala volumes in children and adolescents with childhood maltreatment related posttraumatic stress disorder: a meta-analysis. Hippocampus. 2008;18:729–736. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- Yeung WJ, Linver MR, Brooks-Gunn J. How money matters for young children's development: parental investment and family processes. Child development. 2002;73:1861–1879. doi: 10.1111/1467-8624.t01-1-00511. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Egger HL, Smyke AT, Nelson CA, Fox NA, Marshall PJ, Guthrie D. Institutional rearing and psychiatric disorders in Romanian preschool children. American Journal of Psychiatry. 2009;166:777–785. doi: 10.1176/appi.ajp.2009.08091438. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Nelson CB, Fox NA, Smyke AT, Marshall PJ, Parker SW, Koga S. Designing research to study the effects of institutionalization on brain and behavioral development: The Bucharest Early Intervention Project. Development and Psychopathology. 2003;15:885–907. doi: 10.1017/s0954579403000452. [DOI] [PubMed] [Google Scholar]